Abstract
Cells depend on polyamines for growth and their depletion represents a strategy for the treatment of cancer. Polyamines assemble de novo through a pathway sensitive to the inhibitor, α-difluoromethylornithine (DFMO). However, the presence of cell-surface heparan sulfate proteoglycans may provide a salvage pathway for uptake of circulating polyamines, thereby sparing cells from the cytostatic effect of DFMO. Here we show that genetic or pharmacologic manipulation of proteoglycan synthesis in the presence of DFMO inhibits cell proliferation in vitro and in vivo. In cell culture, mutant cells lacking heparan sulfate were more sensitive to the growth inhibitory effects of DFMO than wild-type cells or mutant cells transfected with the cDNA for the missing biosynthetic enzyme. Moreover, extracellular polyamines did not restore growth of mutant cells, but completely reversed the inhibitory effect of DFMO in wild-type cells. In a mouse model of experimental metastasis, DFMO provided in the water supply also dramatically diminished seeding and growth of tumor foci in the lungs by heparan sulfate-deficient mutant cells compared with the controls. Wild-type cells also formed tumors less efficiently in mice fed both DFMO and a xylose-based inhibitor of heparan sulfate proteoglycan assembly. The effect seemed to be specific for heparan sulfate, because a different xyloside known to affect only chondroitin sulfate did not inhibit tumor growth. Hence, combined inhibition of heparan sulfate assembly and polyamine synthesis may represent an additional strategy for cancer therapy.
Keywords: metastasis‖proteoglycans‖spermine‖chemotherapy‖xylosides
Polyamines (putrescine, spermidine, and spermine) are present in all mammalian cells, fungi, protozoa, and bacteria (1). As polycations, they bind to nucleic acids and play important roles in DNA conformation, replication, transcription, and translation (2). Because cellular proliferation depends on an adequate supply of polyamines, the polyamine biosynthetic pathway has been an attractive target for the treatment of proliferative disorders, such as cancer and parasitic diseases (2–6). Toward this end, several polyamine synthesis inhibitors have been described that act at specific biosynthetic enzymes [e.g., difluoromethylornithine (DFMO) and methylglyoxal bis(guanylhydrazone)] (see Fig. 1). Unfortunately these compounds have had partly disappointing effects as cancer chemotherapeutics because of the ability of tumor cells to compensate for inhibited de novo synthesis by increased uptake of extracellular polyamines (1, 3, 4, 7). Thus, an effective therapeutic strategy will require inhibition of both de novo polyamine biosynthesis and uptake. To date, a chemotherapy based on combined inhibition of polyamine uptake and synthesis has not been described.
Figure 1.
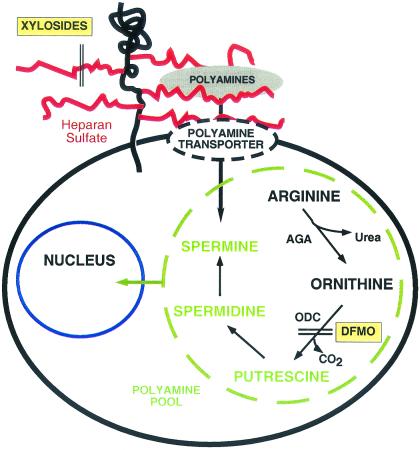
Schematic representation of polyamine assembly and salvage. Arginine is converted by arginase (AGA) to urea and ornithine, which serves as a precursor of the polyamines (shown in green). DFMO inhibits ornithine decarboxylase (ODC), and xylosides block the assembly of heparan sulfate side chains of proteoglycans, which mediate polyamine uptake either directly or indirectly through a separate transporter.
In contrast to what is known about the pathway of de novo polyamine synthesis, the mechanism of uptake in mammalian cells is not well understood, because no transporter has been isolated, cloned, and molecularly characterized (8). Recent studies suggest that uptake of polyamines depends in part on the expression of cell surface proteoglycans, in particular those containing the glycosaminoglycan (GAG), heparan sulfate (9, 10). These highly charged polysaccharides consist of variably sulfated glucosamine residues and glucuronic or iduronic acids. The negatively charged carboxyl and sulfate groups can interact with the positive charges on the polyamines with equal or even higher affinity than DNA (9). Proteoglycans are internalized continuously, along with bound ligands, such as fibroblast growth factor (11, 12). However, it seems unlikely that polyamine uptake is predominantly mediated by endocytosis because this mechanism would not explain the rapidity of the process. Polyamine uptake dependent on proteoglycans may reflect an indirect role of proteoglycans as part of a transport complex in a manner similar to its coreceptor function in fibroblast growth factor signaling (13). Regardless of the mechanism, inhibition of GAG synthesis or exogenous heparan sulfate reduces uptake of exogenous polyamines (10).
In this report we have examined the possibility of combining DFMO, a well tolerated nontoxic inhibitor of polyamine formation, with genetic and pharmacologic methods to diminish heparan sulfate expression as a way to inhibit tumor growth. We demonstrate that inhibition of proteoglycan assembly renders cells hypersensitive to growth inhibition by DFMO and that the combination inhibits tumor formation in vivo.
Materials and Methods
Cell Lines.
Wild-type Chinese hamster ovary cells (CHO, American Type Culture Collection no. CCL61) were cultured in Ham's F-12 medium supplemented with 8% FBS (HyClone) and subcultured every 3–4 days with 0.125% trypsin. The GAG-deficient cell lines used in these studies included pgsA-745 (14), pgsB-618 (15), and pgsG-224 (16). A subline of pgsG cells stably transfected with CHO glucuronosyltransferase I was also used (17). Xylosides were prepared as described (18) and dissolved directly in the drinking water at 2 mM with heat.
Polyamine Uptake Studies.
Cells were seeded at 5 × 104 cells per well in MEM medium and grown for 72 h. The monolayer was rinsed twice with 0.5 ml medium and then incubated with 5 μM [14C]spermine (Amersham Pharmacia; specific activity, 31 Ci/mol). After 10 min at 37°C and 4°C, the cells were chilled and extensively washed with ice-cold medium containing 1 mM spermine. The cells were lysed in a small volume of 0.5 M NaOH, and an aliquot was measured by scintillation counting. The difference in cell-associated polyamine levels obtained at 37°C minus that obtained at 4°C was defined as temperature-dependent polyamine uptake (picomoles per hour per 106 cells).
Growth Studies.
Each well of 96-well microplates received 3,000 cells in DMEM/F-12 supplemented with 10% FBS, 2 mM L-glutamine, and antibiotics. On the next day, fresh growth medium supplemented with DFMO (ILEX Oncology, San Antonio, TX) or spermine was added, and the cells were incubated for 96 h. Cell growth was determined from the amount of crystal violet adsorbed to the cells as measured in a Multiscan 351 photometer (Labsystems, Helsinki) at 595 nm. The data were expressed as the mean ± SD (n = 6). Colony formation was measured by seeding 1 × 103 cells per 75-cm2 culture flask in the absence or presence of 5 mM DFMO. After 4 days, spermine at a final concentration of 2.5 μM or DFMO at 5 mM was added to the medium. After another 6 days in culture, the colonies were fixed, stained, and counted.
Polyamine Deprivation and Tumor Formation in Vivo.
Female Fox chase C.B-17 severe combined immunodeficient (SCID) mice were purchased from Charles River Breeding Laboratories. Mice were kept under specific pathogen-free conditions and fed with autoclaved drinking water with: (i) no additives, (ii) DFMO (0.5–1%, wt/wt), (iii) naphthalenemethanol β-D-xyloside (2 mM), (iv) DFMO (0.5%) and naphthalenemethanol β-D-xyloside (2 mM), or (v) DFMO (0.5%) and cis/trans-decahydro-2-naphthol-β-D-xyloside (2 mM) (n = 5 per group). DFMO and xylosides were provided 2 days before injections and during the full course of the experiments. For injection experiments, cells were harvested with 5 mM EDTA in PBS, extensively washed, and subsequently injected into the lateral tail vein of 5- to 6-week-old mice (2.5 × 105 cells in 100-μl volume per mouse). Animals were killed 10 days after injection. Lungs were fixed in Bouin's solution, and the number of lung surface tumor foci was determined under a dissecting microscope. For histology, samples were transferred to 70% ethanol, embedded in paraffin, sectioned, and stained with hematoxylin/eosin. Representative photomicrographs (magnification, ×200) were taken of samples from each treatment group. Statistical P values were calculated by using Student's unpaired t test.
Results
Chinese hamster ovary cells form solid tumors when injected s.c. in athymic mice, and as shown below, they will form lung nodules in an experimental metastasis model in which the cells are injected in the lateral tail vein. These cells exhibit many of the properties of spontaneous tumors (anchorage-independent growth, low serum requirement, foci formation, tumor growth in athymic mice) and have been used as a model of ovarian cancer (19–21). A major advantage of using this model for tumor formation is that well characterized GAG-deficient CHO mutants exist (14–16, 22), which allowed us to examine the effects of antagonists of polyamine synthesis in the presence and absence of proteoglycans.
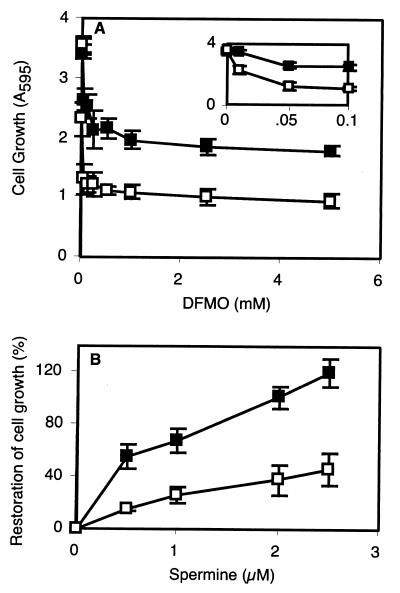
DFMO is a suicide active-site-based inhibitor of ornithine decarboxylase, a key regulatory enzyme in the biosynthesis of polyamines (Fig. 1) (1). Both wild-type and GAG-deficient CHO cells grew more slowly in the presence of DFMO (Fig. 2A), consistent with the known cytostatic effect of polyamine inhibitors (3). GAG-deficient cells exhibited greater sensitivity to DFMO at all concentrations tested, with significant inhibition of growth occurring at 10 μM DFMO (Fig. 2A Inset) and a 4-fold reduction at 5 mM DFMO. It is unlikely that the reduced sensitivity of the wild-type is caused by binding of DFMO to cell surface GAGs, because ornithine (which has the same charge characteristics as DFMO) does not bind to heparin, as determined by affinity chromatography on heparin-agarose (9). The heightened sensitivity of the GAG-deficient cells was independent of mutant genotype because three different strains bearing mutations in different biosynthetic genes behaved identically (pgsA-745, pgsB-618, and pgsG-224). In the absence of DFMO, mutant and wild-type cells grew equally well (Fig. 2A), which indicates that the alteration in growth was due to the reduction of GAGs rather than any secondary mutations that may have been present in these mutants. As shown below, this was confirmed by correction of one of the mutants by transfection.
Figure 2.
DFMO and exogenous polyamines alter the growth of wild-type and GAG-deficient cells. (■, wild-type; □, GAG-deficient pgsB-618.) (A) Growth in the presence of DFMO. (Inset) Response to low concentrations of DFMO. (B) Growth in the presence of 5 mM DFMO and spermine.
The commercial F-12 growth medium used in these experiments contained a low concentration of putrescine (1 μM), a precursor of the major polyamines, spermine and spermidine (Fig. 1). Thus, the increased sensitivity of the mutant cells to DFMO occurred even in the presence of an exogenous polyamine. To examine this sensitivity further, the effect of exogenous spermine on growth of DFMO-treated cells was determined. As shown in Fig. 2B, the addition of 2 μM spermine to the cell culture media restored the growth of DFMO-treated wild-type cells to 100% of the control. However, the effect on GAG-deficient cells was much less, resulting in only ≈40% restoration of growth. The enhanced sparing effect of spermine versus putrescine may reflect differences in uptake or utilization.
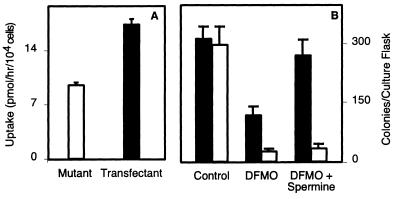
The incomplete reversal of cytostasis by exogenous spermine in the GAG-deficient mutant is consistent with reduced uptake of the polyamines because of the lack of proteoglycans. To verify this idea, GAG-deficient pgsG-224 cells (glucuronosyltransferase I-defective) and a stable line transfected with glucuronosyltransferase I cDNA were compared (16, 17). PgsG cells displayed decreased [14C]spermine uptake (Fig. 3A, open bar), as observed in other mutants [pgsA-745, pgsB-618, and pgsD-677 (10)]. Transfection of pgsG cells completely corrected the enzyme and GAG deficiency (17) and restored uptake to levels comparable with that seen in wild-type cells (Fig. 3A, filled bar).
Figure 3.
Ectopic expression of glucuronosyltransferase I restores polyamine uptake and polyamine-dependent growth in mutant cells. (A) Spermine uptake in mutant pgsG 224 (GlcATI-deficient) and cells transfected with GlcATI cDNA. Cells were pretreated for 2 days with 5 mM DFMO before measuring transport. (B) Colony formation. (Solid bars, transfected cells; open bars, deficient cells.) The results are expressed as the mean ± SD.
In a more stringent test, we challenged the cells to form colonies from single cells in the presence and absence of DFMO. As shown in Fig. 3B, mutant and transfected cells formed colonies with comparable efficiency in the absence of inhibitor. DFMO inhibited clonal growth of both mutant and transfected cells, but the mutant was somewhat more sensitive to the inhibitor. Adding spermine completely restored the growth of transfected cells to the level observed in the absence of any treatment, but it had virtually no effect on the mutant. Thus, uptake of spermine and cell growth in monolayers or clonal growth from single cells were clearly coupled to the expression of GAGs.
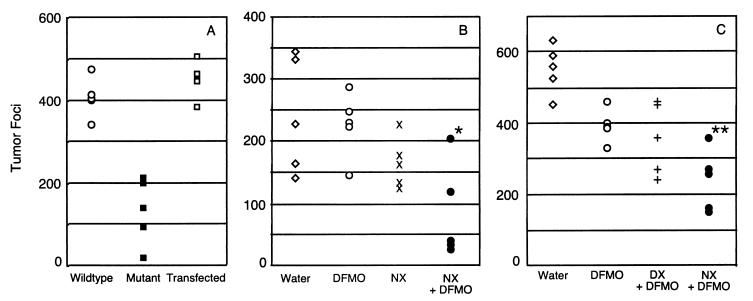
To translate these findings to in vivo conditions, we next examined the tumor-forming properties of wild-type and mutant cells. CHO cells will form lung nodules in an experimental hematogenous metastasis model in which the cells are injected into the tail vein of immunocompromised (SCID) mice. Wild-type and mutant cells formed clearly visible pulmonary tumor foci, and tumors were only occasionally found in other tissues (Fig. 4). The mutant formed tumors less efficiently than wild-type cells both in number and size. This effect was obvious both by visual inspection of the lung surface and by histologic sectioning of representative samples (Fig. 4). The difference in behavior of mutant and wild-type cells was qualitatively similar to that seen when the cells were challenged to form solid tumors after s.c. injection in athymic mice (19), but the effect was less dramatic in the metastasis model.
Figure 4.
Tumor formation in polyamine-deprived mice. Mutant or wild-type cells were injected in the tail vein of SCID mice. Some of the animals received DFMO in their water supply. After 10 days, the animals were killed and the lungs were removed for photography of superficial nodules. They were also sectioned and stained with hematoxylin/eosin. (Magnification, ×200.)
DFMO is relatively nontoxic and is orally bioavailable. As shown in Fig. 4, providing DFMO in the water supply of the mice dramatically depressed the extent of tumor formation by GAG-deficient cells (pgsA-745), both by macroscopic examination of the lungs and by histologic assay of sections. DFMO diffusely reduced the number and size of metastatic seeds, indicating that the effect was not restricted to any particular area of the lung (Fig. 4). In three separate experiments, in which the overall tumor burden varied 10-fold, a striking reduction in tumor nodules was noted in animals injected with mutant cells and fed DFMO (Table 1). In contrast, DFMO had very little effect on tumor formation by wild-type cells.
Table 1.
Tumor formation in SCID mice
| Cell line | Treatment | Number of surface foci | Average ± SD | P values* |
|---|---|---|---|---|
| Experiment I | ||||
| Wild-type | None | 157, 131, 148, 234 | 168 ± 40 | — |
| Wild-type | DFMO | 124, 41, 26, 149 | 85 ± 52 | NSR |
| Mutant | None | 122, 136, 138, 134 | 133 ± 6 | NSR |
| Mutant | DFMO | 1, 0, 2, 1 | 1 ± 1 | <0.005 |
| Experiment II | ||||
| Wild-type | None | 13, 18, 11, 14, 11 | 13 ± 3 | — |
| Wild-type | DFMO | 34, 24, 18, 37, 35 | 30 ± 7 | NSR |
| Mutant | None | 6, 11, 5, 8, 8 | 8 ± 2 | <0.01 |
| Mutant | DFMO | 0, 2, 1, 2, 3 | 2 ± 1 | <0.005 |
| Experiment III | ||||
| Wild-type | None | 9, 15, 9, 17, 7 | 11 ± 4 | — |
| Wild-type | DFMO | 12, 20, 10, 10, 9 | 12 ± 4 | NSR |
| Mutant | None | 2, 3, 5, 2, 12 | 5 ± 4 | <0.04 |
| Mutant | DFMO | 0, 0, 2, 3, 0 | 1 ± 1 | <0.005 |
Wild-type or GAG-deficient mutant (pgsA-745) cells were injected into the lateral tail vein of SCID mice. The polyamine biosynthesis inhibitor DFMO (1% w/w) was provided in the drinking water. NSR, not significantly reduced compared with control.
P values compare the experimental number of lung foci to the number observed in the control animals that received wild-type cells and no DFMO.
To confirm that the effect seen in vivo was caused by the GAG deficiency in the mutant, tumor formation by pgsG and the transfected cells described above were compared (Fig. 5A). The number of surface nodules formed by the mutant was reduced significantly compared with the wild-type (P < 0.001) cells, and the effect was comparable with that observed with mutant pgsA-745 (Table 1). Correction of the enzyme deficiency completely restored tumor formation demonstrating a clear correlation between tumor growth and GAG synthesis in DFMO-treated animals.
Figure 5.
Inhibition of tumor formation in vivo with DFMO and xylosides. (A) Scatter plot showing the number of superficial lung tumor foci in DFMO-fed mice that were injected with wild-type, pgsG-224 (GlcATI-deficient) or pgsG-224 cells that were stably transfected with glucuronosyltransferase I. (B) Scatter plot showing the number of lung tumor foci in mice injected with wild-type CHO cells and provided with water, 0.5% (wt/wt) DFMO, 2 mM NX, or a combination of NX and DFMO in the water supply. *, P < 0.005 for NX + DFMO vs. DFMO and P < 0.04 for NX + DFMO vs. NX, respectively. (C) Scatter plot showing the number of lung tumor foci in mice injected with wild-type CHO cells and treated with plain water, 0.5% DFMO, a combination of DFMO and 2 mM DX, or a combination of DFMO and NX (2 mM) in the water supply. **, P < 0.004 for NX + DFMO vs. DFMO, and P < 0.05 for NX + DFMO vs. DX + DFMO. n = 5 for each group.
These findings provided compelling evidence that combinations of DFMO and GAG synthesis inhibitors might be used synergistically to block tumor growth of cells expressing a normal complement of GAGs. Polyamine uptake has been shown to occur through proteoglycans containing either heparan sulfate or chondroitin sulfate chains, with greater dependence on heparan sulfate (10). Thus, the most effective inhibitors would be those that target an early step in the biosynthetic pathway common to both heparan sulfate and chondroitin sulfate assembly, i.e., enzymes involved in forming the so-called linkage region on which the chains assemble (23). A variety of xylose-based compounds have been characterized as inhibitors based on their ability to divert GAG synthesis from endogenous proteoglycans by acting as a “primer” (18), and the free GAG chains are secreted from the cells. These compounds are well tolerated when administered to mice and also exhibit oral bioavailability (24, 25).
The combined effect of xyloside and DFMO on tumor growth was therefore assessed in vivo by adding naphthalenemethanol β-D-xyloside (NX) to the water supply with and without DFMO. Mice tolerated the treatment without obvious adverse effects, and they maintained the same daily water intake as mice receiving drug-free water (2–4 ml/day). DFMO or NX individually had only slight effects on the number of tumor nodules (Fig. 5B). However, a combination of NX and DFMO markedly decreased tumor formation relative to DFMO (P < 0.005) or NX alone (P < 0.04). The extent of inhibition was similar to that seen in experiments with DFMO and GAG-deficient cells (Table 1; Fig. 4), consistent with the idea that the xyloside depressed the addition of GAG chains to proteoglycans of the wild-type cells used in these experiments.
Variation of the aglycone portion of a xyloside can markedly affect its relative ability to prime heparan sulfate versus chondroitin sulfate chains and to inhibit the formation of the corresponding proteoglycans (18, 26). Because studies have suggested that polyamine uptake was more dependent on heparan sulfate than chondroitin sulfate (10), we compared lung colonization in mice treated with either NX, which primes both heparan sulfate and chondroitin sulfate, or cis/trans-decahydro-2-naphthol-β-D-xyloside (DX), which primes only chondroitin sulfate (18). The combination of NX and DFMO again led to inhibition of tumor growth, whereas the number of tumor foci seen in mice fed DX and DFMO was not significantly different than the number observed in mice treated with DFMO alone (Fig. 5C).
Discussion
In this study we have shown that (i) uptake of polyamines depends in part on the expression of proteoglycans, (ii) cell growth can be made dependent on polyamine uptake through proteoglycans by inhibiting de novo synthesis of polyamines, (iii) tumor formation in vivo exhibits similar characteristics, and (iv) pharmacologic inhibition of both proteoglycan and polyamine synthesis represents a potential strategy for treating metastasis and tumor formation. Many genetic studies have indicated an important role for heparan sulfate in solid tumor formation (19, 27–31). In general, these effects have been attributed to the well known propensity of cell surface heparan sulfate to bind a variety of growth factors, chemokines, and cytokines, which facilitates downstream signaling events leading to cell proliferation and angiogenesis (13, 32, 33). The findings presented here demonstrate that heparan sulfate also plays a role in the salvage of polyamines in the circulation. Thus, altering the expression of heparan sulfate in tumor cells may diminish cell growth by multiple mechanisms.
Because polyamines play an essential role in cell proliferation, both salvage and de novo pathways exist to ensure an adequate supply of polyamines. The initial step in polyamine formation involves conversion of L-ornithine (derived from arginine) to putrescine and CO2, a reaction catalyzed by ornithine decarboxylase, a key regulatory enzyme in the pathway (Fig. 1). In addition, all cells possess an energy-dependent, facilitated transport system for salvage of polyamines from the extracellular environment (8). The gastrointestinal tract is considered to be the main source of exogenous polyamines, i.e., from dietary sources and intestinal bacteria (34, 35). Polyamines are also excreted from cells, and therefore recapture from the circulation may be an important recycling mechanism (6). Cells that cannot produce ornithine cannot proliferate in serum-free medium, unless ornithine or polyamines are provided, indicating that the salvage pathway has sufficient capacity to supply all of the polyamines necessary for growth (36).
Although polyamine transport has been studied extensively, a transporter protein or gene has not yet been identified in mammalian cells. The affinity of substrates for the transporter increases with the number of positive charges (spermine > spermidine > putrescine) and Km values in the range of 10-4–10−6 M have been described, depending on the tissue or cell type studied (8). These kinetic characteristics are consistent with electrostatic binding of polyamines to the negatively charged carboxyl and sulfate groups on GAG chains, but it may also reflect an indirect involvement of proteoglycan in forming a functional transport complex. Proteoglycans act as “coreceptors” in fibroblast growth factor and vascular endothelial growth factor signaling pathways, acting as templates to approximate ligands and receptors in functional signaling complexes (13). Cell surface heparan sulfate proteoglycans can also interact with heparin-binding epidermal growth factor precursor protein expressed on the surface of cells, creating a binding site for diphtheria toxin (37). Thus, by analogy to these systems, the functional polyamine transporter may consist of a heparan sulfate proteoglycan in complex with a polyamine carrier protein. The requirement for a proteoglycan might explain some of the difficulties encountered in the purification of the transporter (8).
In almost every cell culture system tested, including normal and transformed cells, DFMO has a cytostatic effect (3), but loss of cellular viability occurs on a more complete depletion of polyamines (7). The dramatic antiproliferative effect of DFMO on numerous transformed cells has led to clinical studies with cancer patients. Although DFMO has shown antitumor effects in established human brain tumors (38), most recent human trials have focused on its use as a chemopreventive agent in at-risk individuals with cervical, colon, or esophageal premalignant changes (39). Unfortunately, the effect of DFMO has not been as effective in vivo, where the antiproliferative activity is substantially diminished. Two major mechanisms are considered to be responsible for this discrepancy: amplification of ornithine decarboxylase, resulting in resistance to DFMO, and up-regulation of polyamine import from the extracellular compartment (40–42). Although other inhibitors are available that target different enzymes in the pathway {e.g., methylglyoxal bis(guanyl hydrazone), CGP 48664, S-(5′-deoxy-5′-adenosyl)-methylthioethyl-hydroxylamine, 1-aminooxy-3-aminopropane, and ([4-amino-2-butenyl]methylamino)-5′-deoxyadenosine}, the enhanced uptake of polyamines caused by decreased de novo synthesis remains a problem. Furthermore, several of these inhibitors have less potency or less specificity than DFMO. In this report, we have shown that xylosides will render tumor cells more sensitive to the cytostatic effects of one of these inhibitors (DFMO) by inhibiting proteoglycan biosynthesis. This study emphasizes the need for continued development of pharmacologic agents that block proteoglycan formation, which in conjunction with conventional inhibitors of de novo polyamine formation may provide new avenues for treating cancer.
Acknowledgments
This work was supported by grants from The Swedish Foundation for International Cooperation in Research and Higher Education, International Union Against Cancer, Swedish Cancer Fund, and Medical Faculty, Lund University (to M.B.), the Tobacco-Related Disease Research Program at the University of California (to M.F.), Swedish Medical Research Council, Technical Research Council, and Cancer Fund (to L-Å.F. and L.P.), and National Institutes of Health Grants GM33063 and CA91290 (to J.D.E.).
Abbreviations
- DFMO
α-difluoromethylornithine
- GAG
glycosaminoglycan
- CHO
Chinese hamster ovary
- SCID
severe combined immunodeficient
- NX
naphthalenemethanol β-d-xyloside
- DX
cis/trans-decahydro-2-naphthol-β-d-xyloside
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tabor C W, Tabor H. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 2.Thomas T, Thomas T J. Cell Mol Life Sci. 2001;58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pegg A E. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 4.Heby O, Persson L. Trends Biochem Sci. 1990;15:153–158. doi: 10.1016/0968-0004(90)90216-x. [DOI] [PubMed] [Google Scholar]
- 5.McCann P P, Pegg A E. Pharmacol Ther. 1992;54:195–215. doi: 10.1016/0163-7258(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 6.Marton L J, Pegg A E. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 7.Pegg A E. Biochem J. 1986;234:249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seiler N, Delcros J G, Moulinoux J P. Int J Biochem Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Belting M, Havsmark B, Jönsson M, Persson S, Fransson L-Å. Glycobiology. 1996;6:121–129. doi: 10.1093/glycob/6.2.121. [DOI] [PubMed] [Google Scholar]
- 10.Belting M, Persson S, Fransson L-Å. Biochem J. 1999;338:317–323. [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagishita M, Hascall V. J Biol Chem. 1992;267:9451–9454. [PubMed] [Google Scholar]
- 12.Williams K J, Fuki I V. Curr Opin Lipidol. 1997;8:253–262. doi: 10.1097/00041433-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Park P W, Reizes O, Bernfield M. J Biol Chem. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- 14.Esko J D, Stewart T E, Taylor W H. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esko J D, Weinke J L, Taylor W H, Ekborg G, Rodén L, Anantharamaiah G, Gawish A. J Biol Chem. 1987;262:12189–12195. [PubMed] [Google Scholar]
- 16.Bai X M, Wei G, Sinha A, Esko J D. J Biol Chem. 1999;274:13017–13024. doi: 10.1074/jbc.274.19.13017. [DOI] [PubMed] [Google Scholar]
- 17.Wei G, Bai X M, Sarkar A K, Esko J D. J Biol Chem. 1999;274:7857–7864. doi: 10.1074/jbc.274.12.7857. [DOI] [PubMed] [Google Scholar]
- 18.Fritz T A, Lugemwa F N, Sarkar A K, Esko J D. J Biol Chem. 1994;269:300–307. [PubMed] [Google Scholar]
- 19.Esko J D, Rostand K S, Weinke J L. Science. 1988;241:1092–1096. doi: 10.1126/science.3137658. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman R M. Cancer Metastasis Rev. 1998–1999;17:271–277. doi: 10.1023/a:1006188412324. [DOI] [PubMed] [Google Scholar]
- 21.Fink D, Nebel S, Norris P S, Baergen R N, Wilczynski S P, Costa M J, Haas M, Cannistra S A, Howell S B. Int J Cancer. 1998;77:741–746. doi: 10.1002/(sici)1097-0215(19980831)77:5<741::aid-ijc13>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Lidholt K, Weinke J L, Kiser C S, Lugemwa F N, Bame K J, Cheifetz S, Massagué J, Lindahl U, Esko J D. Proc Natl Acad Sci USA. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esko J D, Zhang L. Curr Opin Struct Biol. 1996;6:663–670. doi: 10.1016/s0959-440x(96)80034-0. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy F, Barberousse V, Martin N, Masson P, Millet J, Samreth S, Sepulchre C, Theveniaux J, Horton D. Eur J Med Chem. 1995;30:101S–115S. [Google Scholar]
- 25.Martin N B, Masson P, Sepulchre C, Theveniaux J, Millet J, Bellamy F. Semin Thromb Hemostasis. 1996;22:247–254. doi: 10.1055/s-2007-999015. [DOI] [PubMed] [Google Scholar]
- 26.Lugemwa F N, Esko J D. J Biol Chem. 1991;266:6674–6677. [PubMed] [Google Scholar]
- 27.Mathiak M, Yenisey C, Grant D S, Sharma B, Iozzo R V. Cancer Res. 1997;57:2130–2136. [PubMed] [Google Scholar]
- 28.Adatia R, Albini A, Carlone S, Giunciuglio D, Benelli R, Santi L, Noonan D M. Ann Oncol. 1997;8:1257–1261. doi: 10.1023/a:1008243115385. [DOI] [PubMed] [Google Scholar]
- 29.Kleeff J, Wildi S, Kumbasar A, Friess H, Lander A D, Korc M. Pancreas. 1999;19:281–288. doi: 10.1097/00006676-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Marchisone C, Del Grosso F, Masiello L, Prat M, Santi L, Noonan D M. Pathol Oncol Res. 2000;6:10–17. doi: 10.1007/BF03032652. [DOI] [PubMed] [Google Scholar]
- 31.Alexander C M, Reichsman F, Hinkes M T, Lincecum J, Becker K A, Cumberledge S, Bernfield M. Nat Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 32.Lindahl U, Kusche-Gullberg M, Kjellén L. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 33.Lander A D, Selleck S B. J Cell Biol. 2000;148:227–232. doi: 10.1083/jcb.148.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hessels J, Kingma A W, Ferwerda H, Keij J, van den Berg G A, Muskiet F A. Int J Cancer. 1989;43:1155–1164. doi: 10.1002/ijc.2910430632. [DOI] [PubMed] [Google Scholar]
- 35.Sarhan S, Knodgen B, Seiler N. Anticancer Res. 1989;9:215–223. [PubMed] [Google Scholar]
- 36.Holtta E, Pohjanpelto P. Biochim Biophys Acta. 1982;721:321–327. doi: 10.1016/0167-4889(82)90085-4. [DOI] [PubMed] [Google Scholar]
- 37.Shishido Y, Sharma K D, Higashiyama S, Klagsbrun M, Mekada E. J Biol Chem. 1995;270:29578–29585. doi: 10.1074/jbc.270.49.29578. [DOI] [PubMed] [Google Scholar]
- 38.Levin V A, Prados M D, Yung W K, Gleason M J, Ictech S, Malec M. J Natl Cancer Inst. 1992;84:1432–1437. doi: 10.1093/jnci/84.18.1432. [DOI] [PubMed] [Google Scholar]
- 39.Meyskens F L, Jr, Gerner E W. Clin Cancer Res. 1999;5:945–951. [PubMed] [Google Scholar]
- 40.Choi J H, Scheffler I E. J Biol Chem. 1983;258:12601–12608. [PubMed] [Google Scholar]
- 41.Pohjanpelto P, Holtta E, Janne O A, Knuutila S, Alitalo K. J Biol Chem. 1985;260:8532–8537. [PubMed] [Google Scholar]
- 42.Persson L, Holm I, Ask A, Heby O. Cancer Res. 1988;48:4807–4811. [PubMed] [Google Scholar]