Abstract
Objective(s):
This experiment was carried out to investigate the protective effects of curcumin (CUR) on testicular damage induced by the valproic acid (VPA) administration.
Materials and Methods:
Male Wistar–Albino rats (n=28, 250–300 g) were randomly divided into four groups: Control (1 ml saline, oral), VPA (500 mg/kg, IP), CUR (200 mg/kg, oral), or VPA+CUR (500 mg/kg, VPA, IP plus 200 mg/kg CUR, oral). The treatments were applied for 14 days. Serum testosterone and testis [Janus kinases1 (JAK1), signal transducers and activators of transcription–3 (STAT–3), interleukin–6 (IL–6), malondialdehyde (MDA), tumor necrosis factor-alpha (TNF–α), interleukin–18 (IL–18), and nuclear factor (NF)–κB)] samples were collected for biochemical analyses. Semen samples were subjected to microscopy for spermatological parameters. Testis tissue was also analyzed for histopathological and immunohistochemical methods.
Results:
The VPA administration caused a 37% decrease in serum testosterone concentration and 5.32, 9.51, 2.44, and 3.68–fold increases in testicular tissue JAK1, STAT–3, IL–6, and MDA levels, respectively. There were also 50, 52, and 72% reductions in sperm motility, sperm viability, and the mean testicular biopsy score, respectively, accompanied by considerable degenerative changes and necrosis in seminiferous tubules in the VPA group. There is also an immune-positive reaction for IL–18 and NF–κB in only Leydig cells.
Conclusion:
The CUR treatment may be beneficial in restoring testicular damage through antiinflammatory and anti-oxidant potential.
Key Words: Curcumin, Interleukin–6, Janus kinases, Testicular damage, Valproic acid
Introduction
Valproic acid (VPA) is an antiepileptic and mood stabilizer drug that is commonly used in different psychiatric disorders, including bipolar disorder, post-traumatic stress disorder, treatment-resistant depression, and treatment-resistant schizophrenia, as well as some neurological conditions, including epilepsy, neuropathic pain, tremor, and migraine prophylaxis (1). Although VPA is a very safe drug with wide therapeutic properties, it may exert side effects, such as nausea, vomiting, gastrointestinal, pancreatitis, hematological, hormonal, bleeding, hypotension, tachycardia, respiratory failure, decreased serum carnitine level, and disturbances in lipid, carbohydrate, and urea metabolism (2, 3). Moreover, VPA administration causes atrophy of the testis, epididymis, prostate gland, and seminal vesicles (4), which results in decreased libido and sex hormone levels. The molecular mechanism underlying the adverse effects of VPA on the male reproductive system remains to be elucidated (5).
Considerable proof supports the role of signal transducers and activators of transcription–3 (STAT–3) in the arrangement of apoptosis. STAT–3 belongs to a family of transcription factors activated by Janus kinases (JAK) through phosphorylation of tyrosine705 in response to various cytokines (6, 7). The JAK/STAT signaling pathway is an important cellular signal transduction pathway regulating various cellular physiological processes, including proliferation, differentiation, apoptosis, and death. The JAK1/STAT–3 signaling pathway is activated to regulate the expression of inflammatory factors such as interleukin–6 (IL–6). This may eventually aggravate inflammation and is considered an important therapeutic target for novel drug development (8). Tumor necrosis factor–alpha (TNF–α) is a major mediator of inflammation regulated by the activation of nuclear factor (NF)–κB, a transcription factor. Additionally, most inflammatory cytokines also activate TNF–α and NF–κB. Consequently, agents that downregulate NF–κB levels may influence these diseases (9).
Curcumin (CUR), the main ingredient in turmeric, is one of the most popular phytochemicals (10). It is commonly used in traditional medicine thanks to its antiinflammatory (11), anti–carcinogenesis (12), anti-oxidant (13), and hypocholesterolemic (14) properties. The beneficial effects of supplemental CUR were shown to promote permeability of pancreatitis, gout, inflammatory bowel disease, colorectal cancer, and hepatic fibrosis through various mechanisms, notably by suppressing inflammation (15,16). CUR is capable of suppressing both acute and chronic inflammation. The antiinflammatory mechanism of CUR involves attenuating the inflammatory response in TNF-α stimulated human endothelial cells by interfering with the NF-κB signaling pathway (17). CUR possesses a powerful ability to neutralize superoxide radicals, hydrogen peroxide, and nitric oxide (NO) generated by activated macrophages. It also plays a role in reducing iron complexes and inhibiting lipid peroxidation. CUR effectively scavenges various reactive oxygen species produced by macrophages, including superoxide anions, hydrogen peroxide, and nitrite radicals. These actions are likely key mechanisms through which CUR exerts its anti-oxidant effects (18). This experiment was conducted to determine if CUR has therapeutic effects on testicular damage induced by VPA administration in rats.
Materials and Methods
Experimental design
Atatürk University Local Ethics Committee for Animal Experiments approved this experimental protocol (2021/56). A total of 28 male Wistar–Albino rats, weighing 250–300 g, were kept at standard housing facilities (21–22°C, 60±5% humidity, and 12 hr light:12 hr dark cycle) and fed a standard laboratory chow diet and water ad libitum.
The rats were randomly divided into four groups: 1 ml of saline via oral gavage (Control), 500 mg/kg VPA (dissolved in distilled water) by intraperitoneally (5) (VPA), 200 mg/kg CUR via oral gavage (19) (CUR), or 500 mg/kg VPA plus 200 mg/kg CUR (VPA+CUR). The treatments were applied for 14 days. The VPA (catalog no: 1069–66–5) and CUR (catalog no: 458–37–7) were purchased from Sigma Aldrich (Sigma Chemical Co., St. Louis, MO, USA).
Blood and testis samples were collected for biochemical analyses and histopathological evaluations. Prior to blood sampling intracardially, rats were administered ketamine (80 mg/kg; Ketalar®, 50 mg/ml, Eczacibasi, Istanbul, Turkey) and xylazine (10 mg/kg; Rompun®, 2%, Bayer, Istanbul, Turkey) at the end of the experiment. Then, they were sacrificed. After opening the abdominal wall, the testicles were exposed and removed.
Biochemical analysis
Serum testosterone (SunRed, Biological Technology Co. Ltd, Shanghai, China) and testis tissue STAT–3 (Biocompare, South San Francisco, CA, USA), JAK1 (Biocompare), and IL–6 (SunRed) levels were measured by the Sandwich-ELISA method, based on a specific antigen and antibody reaction according to the manufacturer’s protocol. An enzyme is used as a marker to prepare the labeled conjugate. After completion of the reaction, separation was achieved by adding a substrate to the medium, and enzyme activity was measured spectrophotometrically.
Testis tissue malondialdehyde (MDA) levels were measured based on a reaction with thiobarbituric acid at 90–95°C to yield a pink-colored chromogen (20). After 15 min, the absorbance values of the rapidly cooled samples were read spectrophotometrically at 532 nm. The MDA level was expressed as nmol/g tissue protein. Protein was assayed by the method of Bradford et al. (21), with serum bovine albumin as standard.
Semen evaluation
One cauda epididymis was used to obtain semen samples for each animal. Medley-selected cauda epididymidis was chopped in a Petri dish, including 5 ml of physiological saline. To provide the migrations of spermatozoa from cauda epididymidis to fluid, A 5–minute incubation period was acquired on the warmed stage at 35 °C. Then, following the incubation period, cauda epididymidis residue was eliminated using anatomical tweezers from the Petri dish. The fluid remaining in the Petri dish was used as a semen sample. Evaluation of semen was conducted using routine spermatological parameters, including motility and dead sperm rate (22). To evaluate the percentage of sperm motility, A light microscope (Primo Star; Carl Zeiss, Oberkochen, Germany) equipped with the heated stage was used to measure the percentage of sperm motility. Briefly, a slide was placed on a heated stage warmed up to 35 °C placed on a conventional light microscope. Approximately 20 ll of semen sample was dropped on the slide. The percentage of sperm motility was determined by visual examination of the sample. To evaluate sperm motility, randomly selected three areas from each sample were also assessed to predict sperm motility. The average of three field estimates ions was counted as the final motility score of the sample (23, 24).
Determination of the dead sperm rate was observed under the light microscope. According to the staining of sperm heads, they were classified as dead (having a stained head) or live (having an unstained head) sperm cells. Randomly selected 300 sperm cells for each sample were investigated, and dead sperm rates counted as the percentage (25).
Histopathological and immunohistochemical analysis
Testes tissue samples were fixed with Bouin’s solution for 36 hr, dehydrated through a graded alcohol series, cleared with xylene, and embedded in paraffin wax. Sections were cut at 4 μm and mounted on slides. Sections were deparaffinized with xylene, rehydrated through graded alcohol solutions, and stained with hematoxylin and eosin (26).
Tissue sections were evaluated by high–power light microscopy (Olympus Bx51 with a DP72 camera system (Olympus Corp., Tokyo, Japan). Each specimen was examined in 10 randomly selected areas of approximately at 40x. The inflammation scores were graded as absent, mild, moderate, strong, and very strong if there was no staining (score 0), mild staining (score 1), moderate staining (score 2), strong staining (score 3), and very strong staining (score 4) (27).
The mean testicular biopsy score criteria were determined histopathologically (27). A score of 0–10 was given to each tubule according to epithelial maturation (1: No cells, 2: Sertoli cells without germ cells, 3: Only spermatogonia, 4: Only a few spermatocytes, 5: Many spermatocytes, 6: Only a few early spermatids, 7: Many early spermatids, 8: Few late spermatids, 9: Many late spermatids, and 10: Full spermatogenesis).
The sections from the testes samples (4 μm) were cut and prepared for immunohistochemical analysis of testicular cells by a standard avidin–biotin–peroxidase method (28). Briefly, rabbit polyclonal antibodies that react with rat TNF–α (catalog number: sc–52746651), IL–6 (catalog number: sc–57315), IL–18 (catalog number: sc–133127), and NF–κβ (catalog number: sc–8008) at the dilutions of 1:100, 1:100, 1:100, and 1:100, respectively were applied for 60 min (Santa Cruz Biotechnology, Dallas, US). An exposed mouse and rabbit-specific horseradish peroxidase/3,3–diaminobenzidine chromogen solution (HRP/DAB) estimation kit (ab80436; Abcam, Cambridge, UK). After three washes with 0.1% Tween 20 in phosphate-buffered saline, the sections were incubated with 3,3–diaminobenzidine (Dako Cytomation, Santa Clara, CA, USA) and counterstained with Mayer’s hematoxylin (Dako Cytomation). Each specimen was examined in 10 randomly selected areas of approximately at 40x. Immune positivity was graded as absent [no staining (score 0)], mild [mild staining (score 1)], moderate [moderate staining (score 2)], strong [strong staining (score 3)], and very strong [strong staining (score 4)] (29).
Statistical analysis
The continuous data were subjected to one–way ANOVA using the GLM Procedure (Statistical Analysis of the System, SAS, Version 9.0, SAS Institute Inc., Cary, NC, USA). The mean differences among the four groups were attained by the LSD option. The discrete data were subjected to the Wilcoxon rank sum test using the NPAR1WAY Procedure (SAS). Values for P≤0.05 were considered statistically significant.
Results
Biochemistry
The VPA administration caused a 37% decrease in serum testosterone concentration compared to the control group (Table 1). The CUR treatment restored serum testosterone concentration. Compared to the control group, the VPA administration resulted in 5.32, 9.51, 2.44, and 3.68–fold increases in testicular tissue JAK1, STAT–3, IL–6, and MDA levels, respectively. The CUR treatment completely alleviated testicular tissue JAK1 level and partially alleviated testicular tissue STAT–3, IL–6, and MDA levels.
Table 1.
Effect of curcumin treatment on serum testosterone concentrations and testicular tissue transcription and antiinflammatory markers in rats exposed to valproic acid-induced testicular damage
| Parameters1 | |||||
|---|---|---|---|---|---|
| Groups2 | Testosterone (pg/ml) | JAK1 (ng/ml) | STAT–3 (ng/ml) | IL–6 (pg/ml) | MDA (nmol/g protein) |
| Control | 80.2±0.4a | 3.26±0.18a | 1.81±0.04c | 24.9±2.5c | 5.95±0.70c |
| VPA | 50.3±0.6b | 17.3±2.4b | 17.2±3.2a | 60.7±6.2a | 21.9±2.5a |
| CUR | 70.9±0.6a | 3.43±0.12a | 1.86±0.07c | 36.3±3.8b | 9.57±0.74b |
| VPA+CUR | 90.2±0.6a | 6.60±1.33a | 6.36±0.80b | 37.6±1.3b | 9.21±1.13b |
Data are the least square means±SE. Different superscripts within columns differ (P<0.05).
1STAT–3 = Signal transducers and activators of transcription–3; JAK1 = Janus kinases, IL–6 = Interleukin–6, MDA = Malondialdehyde.
2Treatments were applied for 14 days. Control = rats given 1 ml of normal saline via oral gavage, VPA = 500 mg/kg valproic acid (dissolved in distilled water) intraperitoneally, CUR = 200 mg/kg curcumin via oral gavage, and VPA+CUR = 500 mg/kg VPA plus 200 mg/kg CUR.
a Significantly different from the VPA group (P<0.05).
b Significantly different compared to the control group (P<0.05).
c Significantly different compared to the VPA+CUR group (P<0.05).
CUR: Curcumin; VPA: Valproic acid; JAK: Janus kinases; STAT-3: Transcription–3; MDA: Malondialdehyde
Spermatology
The VPA administration decreased sperm motility and viability by 50% and 52%, respectively (Table 2). The CUR treatment partially improved sperm motility and viability in the VPA group. The median testicular biopsy score for the rats administered with VPA dramatically decreased from 8 to 2. Treatment with CUR partially abolished destruction in testicular cells (Table 2).
Table 2.
Effect of curcumin treatment on sperm characteristics and testicular biopsy score in rats exposed to valproic acid-induced testicular damage
| Parameters | |||
|---|---|---|---|
| Groups1 | Motility (%) | Viable (%) | Testicular Biopsy Score2 |
| Control | 70.0±3.3a | 57.0 ± 2.9a | 8 (7–10)a |
| VPA | 35.0±3.1b | 27.3 ± 4.4b | 2 (1–4)d |
| CUR | 65.7±3.2a | 23.9 ± 4.5b | 7 (6–8)b |
| VPA+CUR | 42.9±2.1b | 34.3 ± 5.6b | 5 (1–7)c |
Data are the least square means±SE, except for testicular biopsy score [median (minimum–maximum]. Different superscripts within columns differ (P<0.05).
1Treatments were applied for 14 days. Control = rats given 1 ml of normal saline via oral gavage, VPA = 500 mg/kg valproic acid (dissolved in distilled water) intraperitoneally, CUR = 200 mg/kg curcumin via oral gavage, and VPA+CUR = 500 mg/kg VPA plus 200 mg/kg CUR.
2Based on Johnsen (1970). 1: No cells, 2: Sertoli cells without germ cells, 3: Only spermatogonia, 4: Only a few spermatocytes, 5: Many spermatocytes, 6: Only a few early spermatids, 7: Many early spermatids, 8: Few late spermatids, 9: Many late spermatids, and 10: Full spermatogenesis.
a Significantly different from the VPA group (P<0.05).
b Significantly different compared to the control group (P<0.05).
c Significantly different compared to the VPA+CUR group (P<0.05).
d Significantly different compared to the control group (P<0.05).
CUR: Curcumin; VPA: Valproic acid
Histopathology
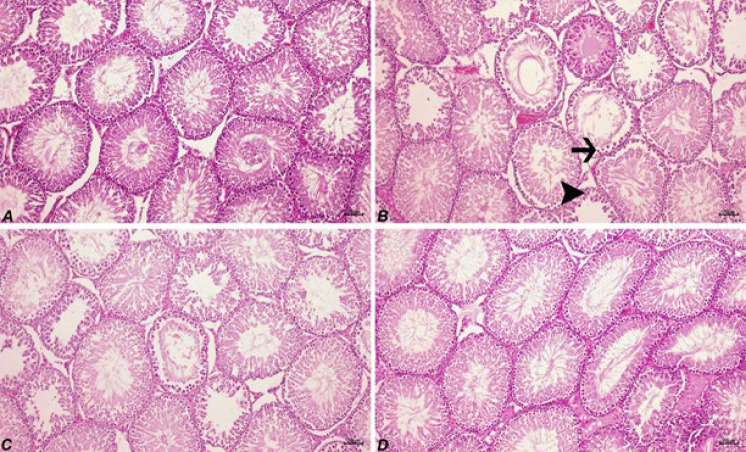
Histopathological examination of the control group exhibited a normal testis tissue histology (Figure 1A; Table 3). In the VPA group (Figure 1B), there were intense degenerative changes and necrosis in seminiferous tubules. Also, atrophy in some seminiferous tubules was seen. There were irregular views in spermatogenetic cells, the loss of spermatogenic cells, and edema and hyperemia in interstitial areas. The CUR treatment partially reduced the severity of the lesions in the VPA group (Figure 1D; Table 3).
Table 3.
Effect of curcumin treatment on histopathology of seminiferous
| Parameters2 | |||
|---|---|---|---|
| Groups1 | Degeneration | Necrosis | |
| Control | 0 (0–1)d | 0 (0–0)c | |
| VPA | 4 (3–4)a | 3 (2–3)a | |
| CUR | 1 (1–2)c | 0 (0–1)c | |
| VPA+CUR | 3 (1–4)b | 1 (0–2)b | |
tubules epithelial cells on rats
Data are the median value (minimum-maximum). Different superscripts within columns differ
(P<0.05).
1 The treatments were applied for 14 days. Control = rats given 1 ml of normal saline via oral gavage, VPA = 500 mg/kg valproic acid (dissolved in distilled water) intraperitoneally, CUR = 200 mg/kg curcumin via oral gavage, and VPA+CUR = 500 mg/kg VPA plus 200 mg/kg CUR.
2 Based on Apaydin–Yildirim et al. (2017). 0: No staining, 1: Mild staining, 2: Moderate staining, 3: Strong staining, and 4: Very strong staining.
a Significantly different when compared with the control group (P<0.05).
b Significantly different when compared with the VPA group (P<0.05).
c Significantly different compared to the VPA+CUR group (P<0.05).
d Significantly different from the CUR group (P<0.05).
CUR: Curcumin; VPA: Valproic acid
Figure 1.
Degenerative (arrow) and necrotic (arrowhead) changes in seminiferous tubules epithelial cells, H&E, 70 µm
A: Normal appearance of testicular tissue in the control group. B: Intense in the VPA group, C: Mild degeneration in the CUR group, D: Moderate in the VPA+CUR group. Control = rats given 1 ml of normal saline via oral gavage, VPA = 500 mg/kg valproic acid (dissolved in distilled water) intraperitoneally, CUR = 200 mg/kg curcumin via oral gavage, and VPA+CUR = 500 mg/kg VPA plus 200 mg/kg CUR.
CUR: Curcumin; VPA: Valproic acid
Immunohistochemistry
No immune positive reaction for TNF–α, IL–6, IL–18, and NF–κB was observed in the Sertoli cell, germ cells (spermatid, spermatocyte, and spermatogonia), and Leydig cell of the control rats (Table 4; Figure 2). The VPA administration did not trigger the expression of TNF–α and IL–6 in any of the cells but did of IL–18 and NF–κB in only Leydig cells. The CUR treatment was ineffective in altering the expression of TNF–α, IL–6, IL–18, and NF–κB in the Sertoli cell, germ cells (spermatid, spermatocyte, spermatogonia), and Leydig cell of the rats exposed to testicular damage.
Table 4.
Effect of curcumin treatment on immunohistochemistry of seminiferous tubules epithelial cells on rats
| Cells | ||||||
|---|---|---|---|---|---|---|
| Parameters1 | Groups2 | Sertoli | Spermatid | Spermatocyte | Spermatogonia | Leydig |
| TNF-α | Control | 0 (0–1)b | 0 (0–1)b | 0 (0–1)b | 0 (0–1)b | 0 (0–1)c |
| VPA | 1 (1–2)a | 1 (1–2)a | 1 (1–2)a | 1 (1–2)a | 2 (2–3)a | |
| CUR | 0 (0–1)b | 0 (0–1)b | 0 (0–1)b | 0 (0–1)b | 1 (0–1)b | |
| VPA+CUR | 1 (0–2)a | 1 (0–2)a | 1 (0–2)a | 1 (0–2)a | 1 (1–2)b | |
| IL-6 | Control | 0 (0–0)b | 0 (0–0)b | 0 (0–0)b | 0 (0–0)b | 0 (0–1)b |
| VPA | 1 (0–2)a | 1 (0–2)a | 1 (0–2)a | 1 (0–1)a | 1 (1–2)a | |
| CUR | 0 (0–0)b | 0 (0–0)b | 0 (0–0)b | 0 (0–0)b | 1 (0–1)a | |
| VPA+CUR | 0 (0–1)b | 0 (0–1)b | 0 (0–1)b | 0 (0–1)b | 1 (1–2)a | |
| IL-18 | Control | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1)b |
| VPA | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (1–2)a | |
| CUR | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 1 (0–1)a | |
| VPA+CUR | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (1–2)a | |
| NF-κB | Control | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1)b |
| VPA | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (1–2)a | |
| CUR | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 1 (0–1)b | |
| VPA+CUR | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (1–2)a | |
Data are the median value (minimum-maximum). Different superscripts within columns differ (P<0.05).
1TNF- α: Tumor necrosis factor-alpha; IL-6: Interleukin 6; IL-18: Interleukin 18; NF-κβ: Nuclear factor kappa beta.
2Treatments were applied for 14 days. Control = rats given 1 ml of normal saline via oral gavage, VPA = 500 mg/kg valproic acid (dissolved in distilled water) intraperitoneally, CUR = 200 mg/kg curcumin via oral gavage, and VPA+CUR = 500 mg/kg VPA plus 200 mg/kg CUR.
a Significantly different from the VPA group (P<0.05).
b Significantly different compared to the control group (P<0.05).
CUR: Curcumin; VPA: Valproic acid
Discussion
Despite considerable side effects, VPA is commonly used in the treatment of epilepsy, an episodic cerebral disorder caused by the increased stimulation of various nerve cells in the brain for various reasons (30). There is increasing concern about the potential effects of VPA on reproductive endocrine function. It decreases the level of testosterone in male patients with epilepsy. Moreover, it causes atrophies of the testes and prostate as well as suppresses spermatogenesis (31). Sperm motility as a reproductive final stage and sperm motility evaluations are an integral part of some reproductive toxicity test guidelines. In the literature, there are studies on the protective properties of various plant-derived and anti-oxidant substances to prevent the damage caused by VPA administration (32-34). This study examined the potential protective effects of CUR on VPA–induced testis damage, where the JAK1/STAT–3/IL–6 pathway and oxidative stress are involved in the pathogenesis.
Mitochondria are required for the energy generation of the cells, and motility and stability of the sperm are necessary for normal mitochondrial function (35), which are compromised by the VPA administration (5). It may also impair cellular mechanisms in different ways that can lead to toxicity by inducing free radical formation and lipid peroxidation. Testicular membranes are structures rich in polyunsaturated fatty acids that are not resistant to oxidative degradation. Lipid peroxidation causes the membranes to lose their functions. MDA levels in tissues and serum increase. The increased MDA level indicates the formation of oxidative stress (36, 37).
Inflammatory (JAK1, STAT–3, and IL–6) and oxidative status (MDA) markers increased considerably upon the VPA administration, which can be explained by changes in membrane integrity and fatty acid composition and the increased sensitivity of cells to oxidative damage (38). In the study by Ourique et al. (39), it was observed that the testicular MDA value increased and sperm motility decreased in oxidative stress-related damage to the testis in rats administered with VPA. Sukhorum and Iamsaard (40) indicated that VPA treatment changed the expression of testicular proteins, which are accountable for spermatogenesis and testosterone generation that lead to infertility. Testicular oxidative stress appears as a common feature in most of the underlying causes of male infertility. It is considered that this situation may benefit the development of anti-oxidant treatments in the relevant cases of hypospermatogenesis (41). With a decrease in sperm motility, damaged sperm cell percentage increased upon the VPA administration, which was associated with decreased testosterone level and destructive histopathology of testicular tissues as well as inflammatory reactions in Leydig cells. In the study by Roste et al. (42), testicular atrophy and spermatogenesis arrest were detected in rats administered with VPA at a daily dose of 400 mg/kg. In comparison, no pathology was detected when a daily dose of 200 mg/kg was administered. In this study, rats administered with VPA exhibited degenerative changes, necrosis, and atrophy in the seminal tubules.
The JAK/STAT pathway is a therapeutic target to cure spermatogenesis deterioration (43, 44) because it plays a key role in the occurrence and regulation of the inflammatory response via the transmission of intercellular cytokine signals to the nucleus. Intracellular STAT–3 activation is provided by stimulating IL–6, IL–10, various growth factors, and their receptors (45). This is important for spermatogonial stem cell production and regeneration, which are necessary processes to ensure male fertility. The CUR treatment increased serum testosterone levels and partially restored testicular MDA and JAK1, STAT–3, and IL–6 levels, as well as sperm motility and stability. The CUR treatment partially alleviated the degenerative and necrosis effect of the VPA administration on the seminiferous tubuli, which might result in the loss of spermatogenic cells. The protective effect of CUR could be related to its anti-oxidant property, which alleviates the VPA–induced tissue toxicity (46). Moreover, when used at a higher dose and for a longer time, VPA causes testicular damage by inflammation, which is mediated by NF–κB phosphorylation and increased oxidative stress. The CUR treatments block NF–κB activation through increasing inflammatory stimuli (47). However, the CUR treatment failed to suppress TNF–α, IL–6, IL–18, and NF–κB in Sertoli cells.
Conclusion
VPA administration exerted destructive effects on testicular tissues. The CUR treatment partially restored the histopathology of testicular damage by restoring antiinflammatory and transcription markers and sperm motility and stability. Further studies should consider testing the CUR treatment at different doses and time periods.
Acknowledgment
This work was supported by the Coordinator of Scientific Research Projects [2020.M84.02.01] at Artvin Coruh University, Turkey.
Authors’ Contributions
E D designed the study and took part in data interpretation. E D and H I contributed to article writing; I B and B M contributed to animal experiments; H I and E D contributed to biochemical analysis and interpretation. A D O contributed to sperm analysis. KT K and I B contributed to histopathological analysis, and A H contributed to statistical analysis.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Declaration
We have not used any AI tools or technologies to prepare this manuscript.
References
- 1.Zhu MM, Li HL, Shi LH, Chen XP, Luo J, Zhang ZL. The pharmacogenomics of valproic acid. J Hum Genet. 2017;62:1009–1014. doi: 10.1038/jhg.2017.91. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg–Stern H, Yaacobi E, Phillip M, de Vries L. Endocrine effects of valproic acid therapy in girls with epilepsy: A prospective study. Eur J Paediatr Neurol. 2014;18:759–765. doi: 10.1016/j.ejpn.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, Calabresi P, Costa C. Valproic acid and epilepsy: From molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019;17:926–946. doi: 10.2174/1570159X17666181227165722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura T, Sakai M, Yonezawa H. Effects of valproic acid on fertility and reproductive organs in male rats. J Toxicol Sci. 2000;25:85–93. doi: 10.2131/jts.25.85. [DOI] [PubMed] [Google Scholar]
- 5.Iamsaard S, Sukhorum W, Arun S, Phunchago N, Uabundit N, Boonruangsri P, Namking M. Valproic acid induces histologic changes and decreases androgen receptor levels of testis and epididymis in rats. Int J Reprod Biomed. 2017;15:217–224. [PMC free article] [PubMed] [Google Scholar]
- 6.Kowshik J, Baba AB, Giri H, Deepak–Reddy G, Dixit M, Nagini S. Astaxanthin inhibits JAK/STAT–3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS One. 2014;9:e109114. doi: 10.1371/journal.pone.0109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba AB, Nivetha R, Chattopadhyay I, Nagini S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial–mediated apoptosis by abrogating the JAK/STAT–3 signalling pathway. Food Chem Toxicol. 2017;109:534–543. doi: 10.1016/j.fct.2017.09.054. [DOI] [PubMed] [Google Scholar]
- 8.Chang X, Hu LF, Ma XJ, Yin J, Liu XY, Li JB. Influence of roflumilast on sepsis mice through the JAK/STAT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:1335–1341. doi: 10.26355/eurrev_201902_17028. [DOI] [PubMed] [Google Scholar]
- 9.Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. doi: 10.3389/fphar.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yalçın AS, Yılmaz AM, Altundağ EM, Koçtürk S. Anti-cancer effects of curcumin, quercetin and tea catechins. Marmara Pharmaceutical J. 2017;21:19–29. [Google Scholar]
- 12.Park J, Conteas CN. Anti–carcinogenic properties of curcumin on colorectal cancer. World J Gastrointest Oncol. 2010;2:169–176. doi: 10.4251/wjgo.v2.i4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadabady S, Beheshti F, Shahidpour F, Khordad E, Hosseinid MA. Protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem Biophys Rep. 2021;25:100908. doi: 10.1016/j.bbrep.2021.100908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewlings SJ, Kalman DS. Curcumin: A review of its effects on human health. Foods. 2017;6:92–102. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarıyer ET, Aksu BM. Curcumin and gastrointestinal system diseases. J Biotechnol Strategic Health Res. 2020;4:194–205. [Google Scholar]
- 16.Iqbal U, Anwar H, Quadri AA. Use of curcumin in achieving clinical and endoscopic remission in ulcerative colitis: A systematic review and meta-analysis. Am J Med Sci. 2018;356:350–356. doi: 10.1016/j.amjms.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Alok A, Singh ID, Singh S, Kishore M, Jha PC. Curcumin – pharmacological actions and its role in oral submucous fibrosis: A review. J Clin Diagn Res. 2015 Oct;9:ZE01–ZE03. doi: 10.7860/JCDR/2015/13857.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin–cellular and molecular mechanisms of action. Crit Rev Food Sci Nut. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 19.Aksu EH, Kandemir FM, Yıldırım S, Küçükler S, Dörtbudak MB, Çağlayan C, Benzer F. Palliative effect of curcumin on doxorubicin–induced testicular damage in male rats. Biochem Mol Toxicol. 2019;33:e22384. doi: 10.1002/jbt.22384. [DOI] [PubMed] [Google Scholar]
- 20.Esterbauer X H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4–hidroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976;72:24854. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Ömür AD, Apaydın Yıldırım B, Kandemir MF, Akman O, Aktaş Şenocak E, Aksu EH. Can taraxacum officinale (dandelion) extract be an alternative of paracetamol in inflammatory and painful cases? An evaluation with regard to biochemical and reproductive parameters. Kafkas Univ Vet Fak Derg. 2017;23:47–54. [Google Scholar]
- 23.Turk G, Atessahin A, Sonmez M, Ceribasi AO, Yuce A. Improvement of cisplatin–induced injuries to sperm quality, the oxidant–antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril. 2008;89:1474–1481. doi: 10.1016/j.fertnstert.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 24.Aksu EH, Akman O, Ozkaraca M, Omur AD, Ucar O. Effect of maclura pomifera extract against cisplatin–induced damage in reproductive system of male rats. Kafkas Univ Vet Fak Derg. 2015;21:397–403. [Google Scholar]
- 25.Aksu EH, Özkaraca M, Kandemir FM, Ömür AD, Eldutar E, Küçükler S, Çomaklı S. Mitigation of paracetamol-induced reproductive damage by chrysin in male rats via reducing oxidative stress. Andrologia. 2016;48:1145–1154. doi: 10.1111/and.12553. [DOI] [PubMed] [Google Scholar]
- 26.Dokumacioglu E, Iskender H, Yenice G, Kapakin KAT, Sevim C, Hayirli A, et al. Effects of astaxanthin on biochemical and histopathological parameters related to oxidative stress on testes of rats on high fructose regime. Andrologia. 2018;50:e13042. doi: 10.1111/and.13042. [DOI] [PubMed] [Google Scholar]
- 27.Apaydin–Yildirim B, Kordali S, Terim–Kapakin KA, Yildirim F, Aktas–Senocak E, Altun S. Effect of Helichrysum plicatum DC subsp plicatum ethanol extract on gentamicin–induced nephrotoxicity in rats. J Zhejiang Univ Sci B. 2017;18:501–511. doi: 10.1631/jzus.B1500291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnsen SG. Testicular biopsy score count a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 29.Terim–Kapakin K, Imik H, Gumus R, Kapakin S, Sağlam Y. Effect of Vit E on secretion of HSP–70 in testes of broilers exposed to heat stress. Kafkas Univ Vet Fak Derg. 2013;19:305–310. [Google Scholar]
- 30.Mirza R, Sharma B. Beneficial effects of pioglitazone, a selective peroxisome proliferator–activated receptor–γ agonist in prenatal valproic acid–induced behavioral and biochemical autistic like features in Wistar rats. Int J Dev Neurosci. 2019;76:6–16. doi: 10.1016/j.ijdevneu.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Cansu A, Ekinci Ö, Serdaroglu A, Gürgen S G, Ekinci Ö, Erdogan D, Coskun ZK, Tunc L. Effects of chronic treatment with valproate and oxcarbazepine on testicular development in rats. Seizure. 2011;20:203–207. doi: 10.1016/j.seizure.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed N, Aljuhani N, Al–Hujaili HS, Al–Hujaili MA, Elkablawy MA, Noah MM, Abo-Haded H, El-Agamy DS. Agmatine protects against sodium valproate induced hepatic injury in mice via modulation of nuclear factor–κB/inducible nitric oxide synthetase pathway. J Biochem Mol Toxicol. 2018;32:e22227. doi: 10.1002/jbt.22227. [DOI] [PubMed] [Google Scholar]
- 33.Gai Z, Krajnc E, Samodelov SL, Visentin M, Kullak–Ublick GA. Obeticholic acid ameliorates valproic acid–induces hepatic steatosis and oxidative stress. Mol Pharmacol. 2020;97:314–323. doi: 10.1124/mol.119.118646. [DOI] [PubMed] [Google Scholar]
- 34.Turkyilmaz IB, Altas N, Arisan I, Yanardag R. Effect of vitamin B6 on brain damage in valproic acid induced toxicity. J Biochem Mol Toxicol. 2021;35:e22855. doi: 10.1002/jbt.22855. [DOI] [PubMed] [Google Scholar]
- 35.Bairy L, Paul V, Rao Y. Reproductive toxicity of sodium valproate in male rats. Indian J Pharmacol. 2010;42:90–94. doi: 10.4103/0253-7613.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dabrowska Z, Dąbrowska E, Onopiuk B, Onopiuk P, Orywal K, Mroczko B, Pietruska M. The protective impact of black chokeberry fruit extract (Aronia melanocarpa L ) on the oxidoreductive system of the parotid gland of rats exposed to cadmium. Oxid Med Cell Longev. 2019;2019:1–11. doi: 10.1155/2019/3403264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Çelik Ç, Bayrak BB, Hacıhasanoğlu Çakmak N, Yanardağ R. Protective effect of edaravone on rat testis after valproic acid treatment. J Res Pharm. 2022;26:52–62. [Google Scholar]
- 38.Sharma S, Sharma V, Pracheta SSH. Therapeutic potential of hydromethanolic root extract of Withania somnifera on neurological parameters in Swiss Albino mice subjected to lead nitrate. Int J Current Pharm Res. 2011;3:52–56. [Google Scholar]
- 39.Ourique GM, Saccol EM, Pês TS, Glanzner WG, Schiefelbein SH, Woehl VM, Baldisserotto B, Pavanato MA, Gonçalves PB, Barreto KP. Protective effect of vitamin E on sperm motility and oxidative stress in valproic acid-treated rats. Food Chem Toxicol. 2016;95:159–167. doi: 10.1016/j.fct.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Sukhorum W, Iamsaard S. Changes in testicular function proteins and sperm acrosome status in rats treated with valproic acid. Reprod Fertil Dev. 2017;29:1585–1592. doi: 10.1071/RD16205. [DOI] [PubMed] [Google Scholar]
- 41.Turner TT, Lysiak JJ. Oxidative stress: A common factor in testicular dysfunction. J Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 42.Roste L S, Tauboll E, Berner A, Berg KA, Aleksandersen M, Gjerstad L. Morphological changes in the testis after long–term valproate treatment in male Wistar rats. Seizure. 2001;10:559–565. doi: 10.1053/seiz.2001.0545. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Zuo QS, Li D, Lian C, Ahmed EK, Tang BB, Song SJ, Zhang YN, Li BC. Study on the role of JAK/STAT signaling pathway during chicken spermatogonial stem cells generation based on RNA–Seq. J Integr Agric. 2015;14:939–948. [Google Scholar]
- 44.Li J, Zhang L, Li B. Correlative study on the JAK–STAT/PSMβ3 signal transduction pathway in asthenozoospermia. Exp Ther Med. 2016;13:127–130. doi: 10.3892/etm.2016.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alves–Silva T, Freitas GA, Húngaro TGR, Arruda AC, Oyama LM, Avellar MCW, Araujo RC. Interleukin–6 deficiency modulates testicular function by increasing the expression of suppressor of cytokine signaling 3 (SOCS3) in mice. Sci Rep. 2021;11:11456. doi: 10.1038/s41598-021-90872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savran M, Ascı H, Armagan I, Erzurumlu Y, Azırak S, Kaya–Ozer M, Bilgic S, Korkmaz DT. Thymoquinone could be protective against valproic acid-induced testicular toxicity by antioxidant and antiinflammatory mechanisms. Andrologia. 2020;52:e13623. doi: 10.1111/and.13623. [DOI] [PubMed] [Google Scholar]
- 47.Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental–Mendia LE, Majeed M, Sahebkar A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post–hoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016;82:578–582. doi: 10.1016/j.biopha.2016.05.037. [DOI] [PubMed] [Google Scholar]