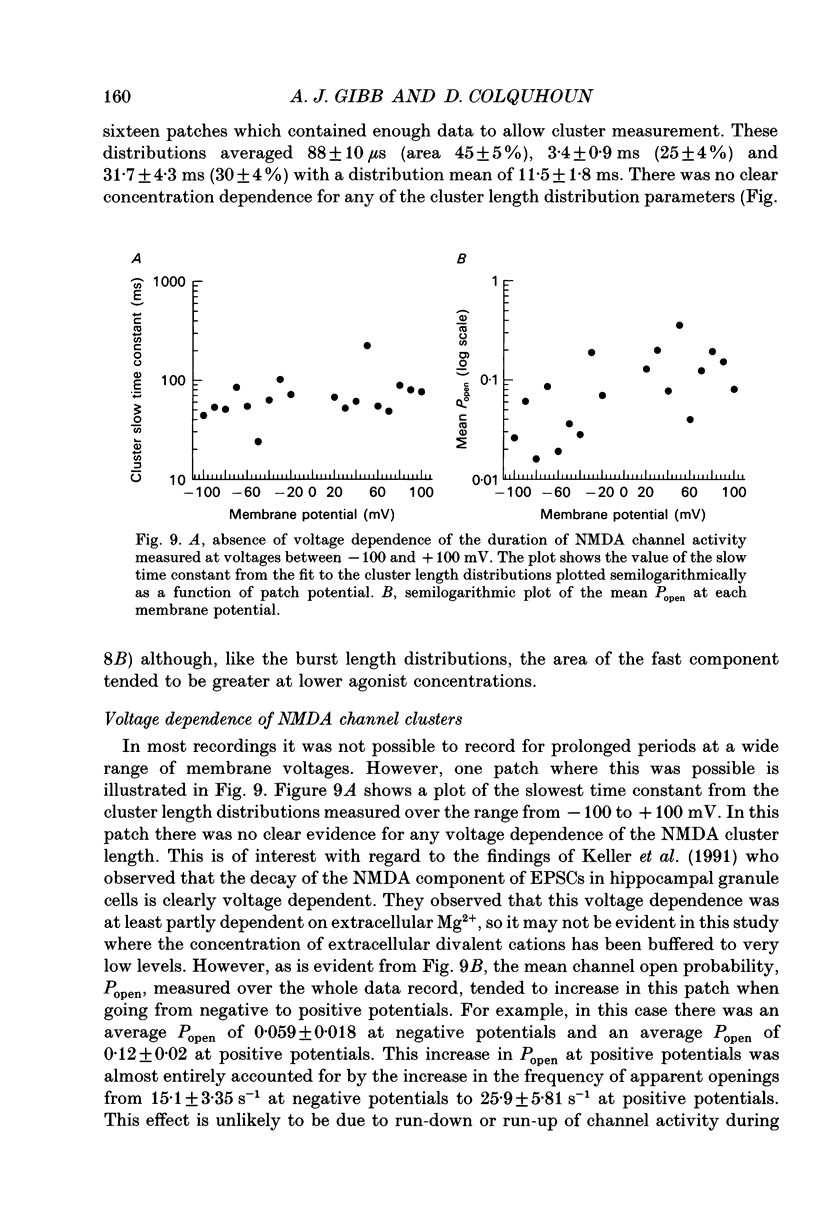
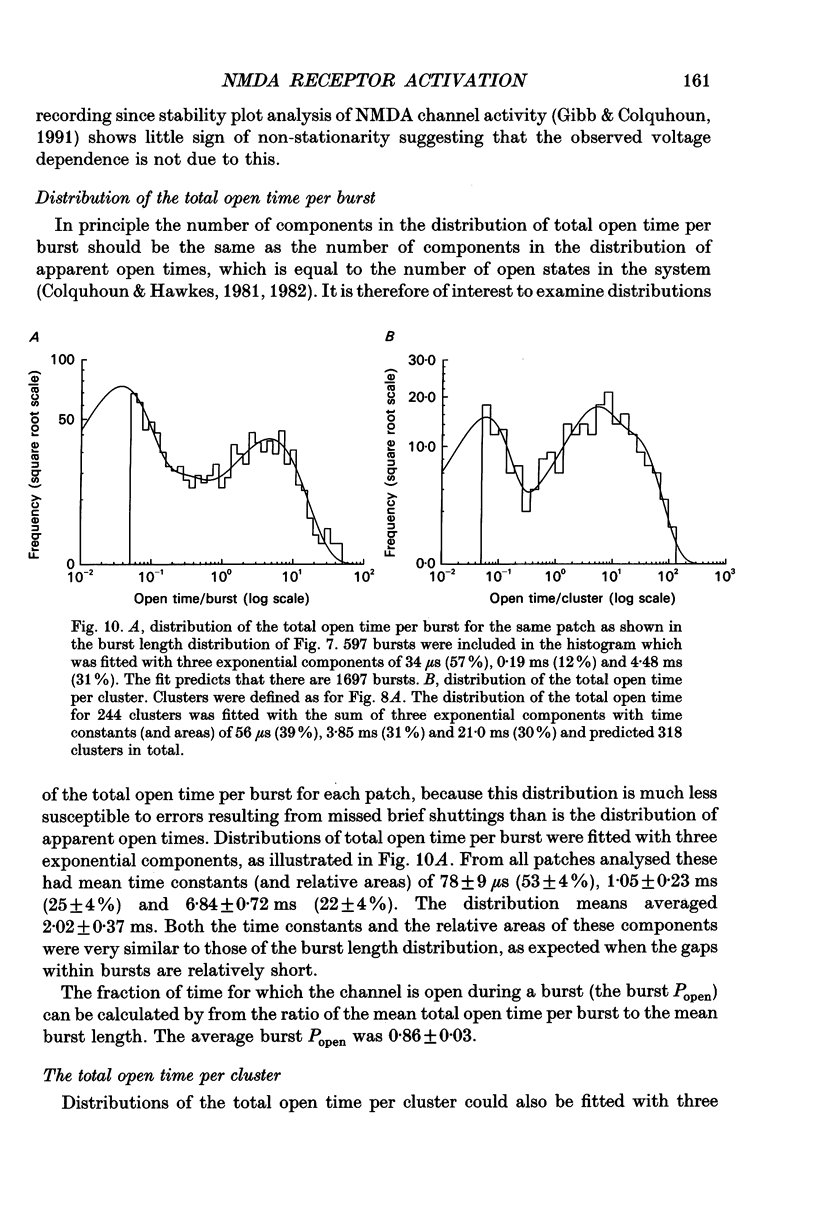
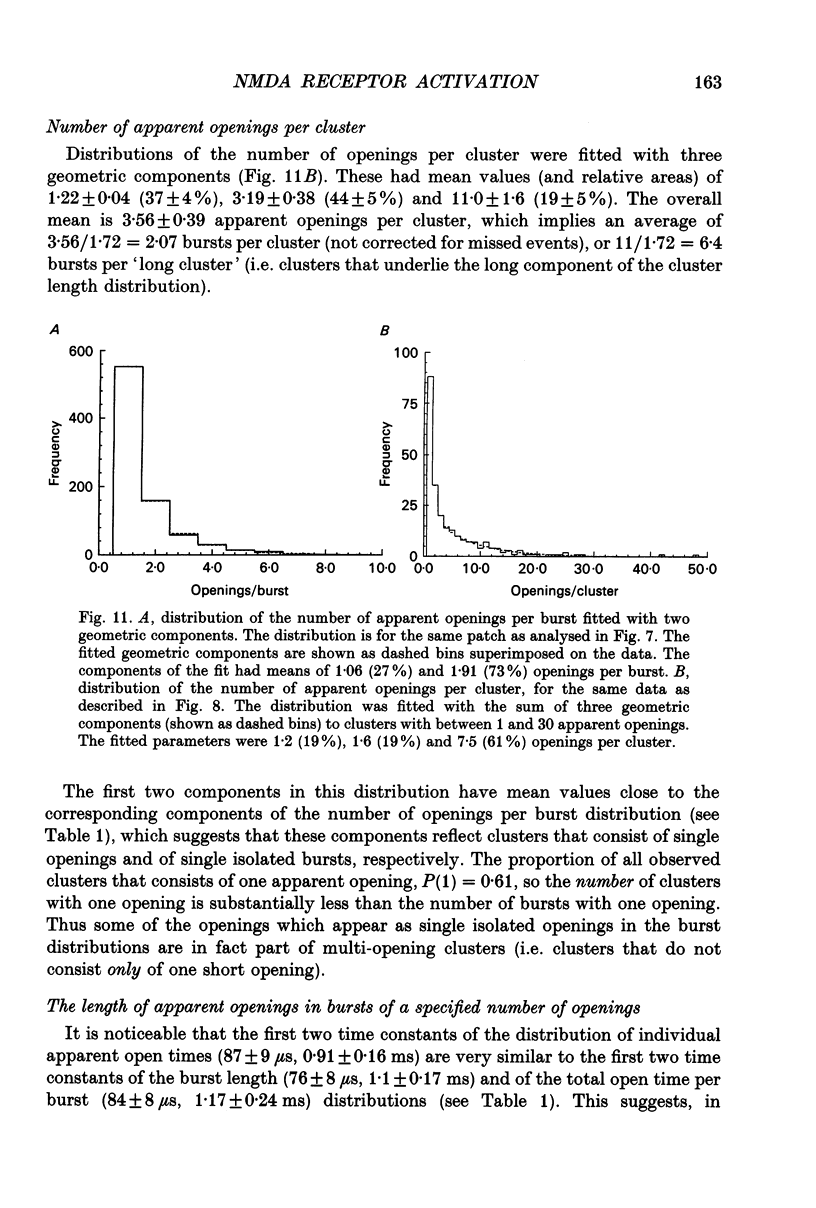
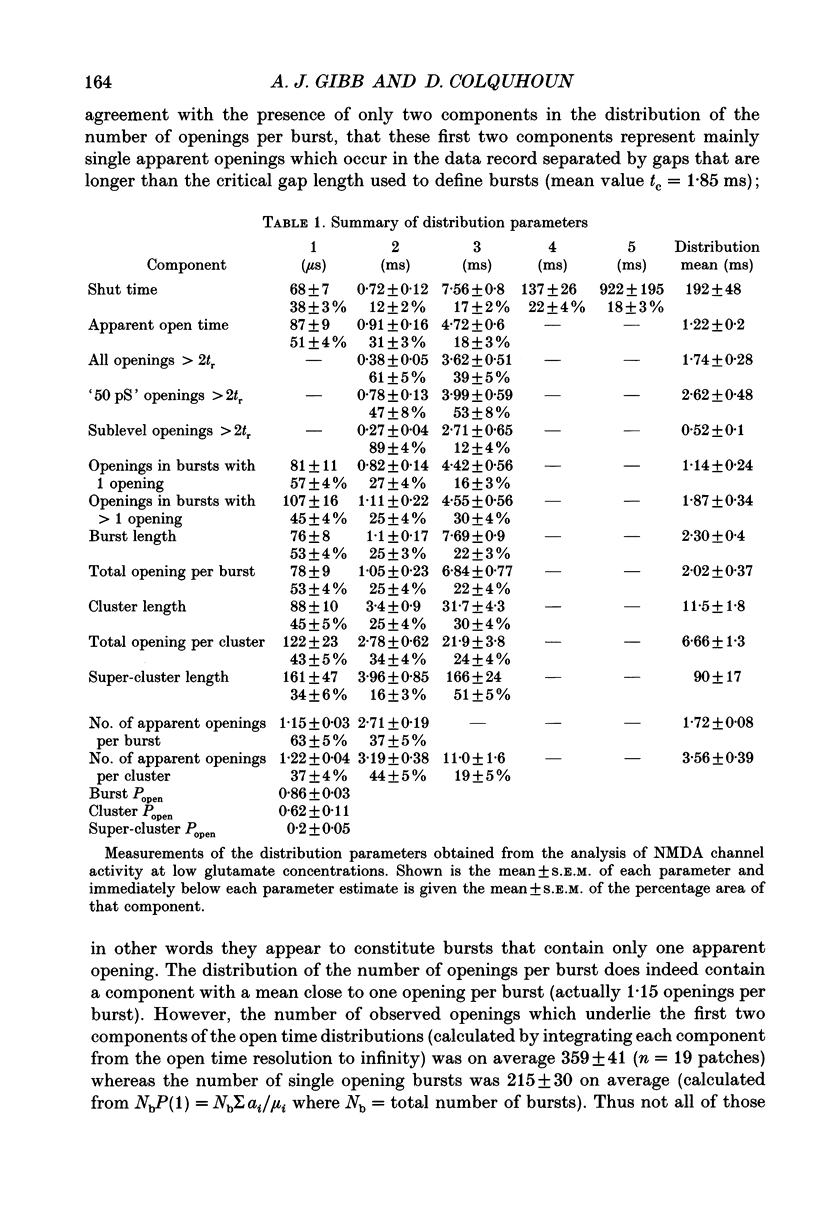
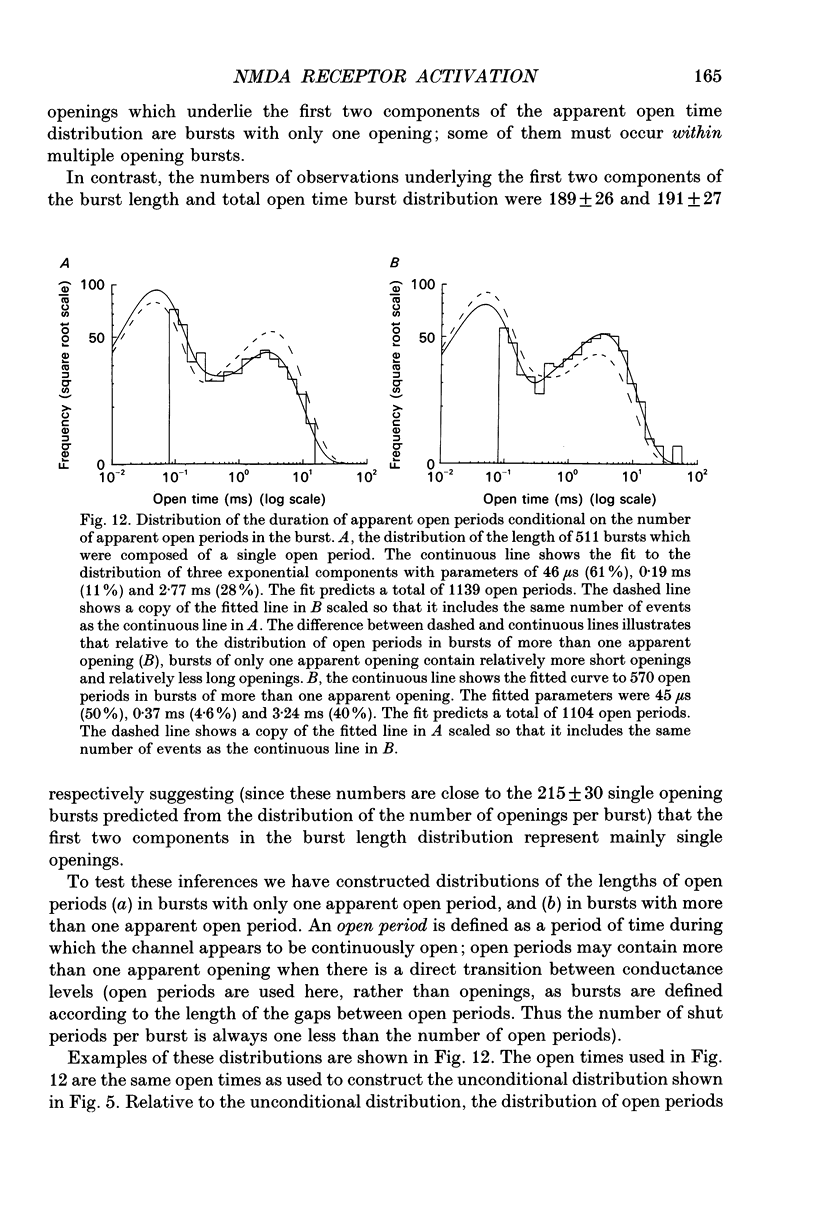
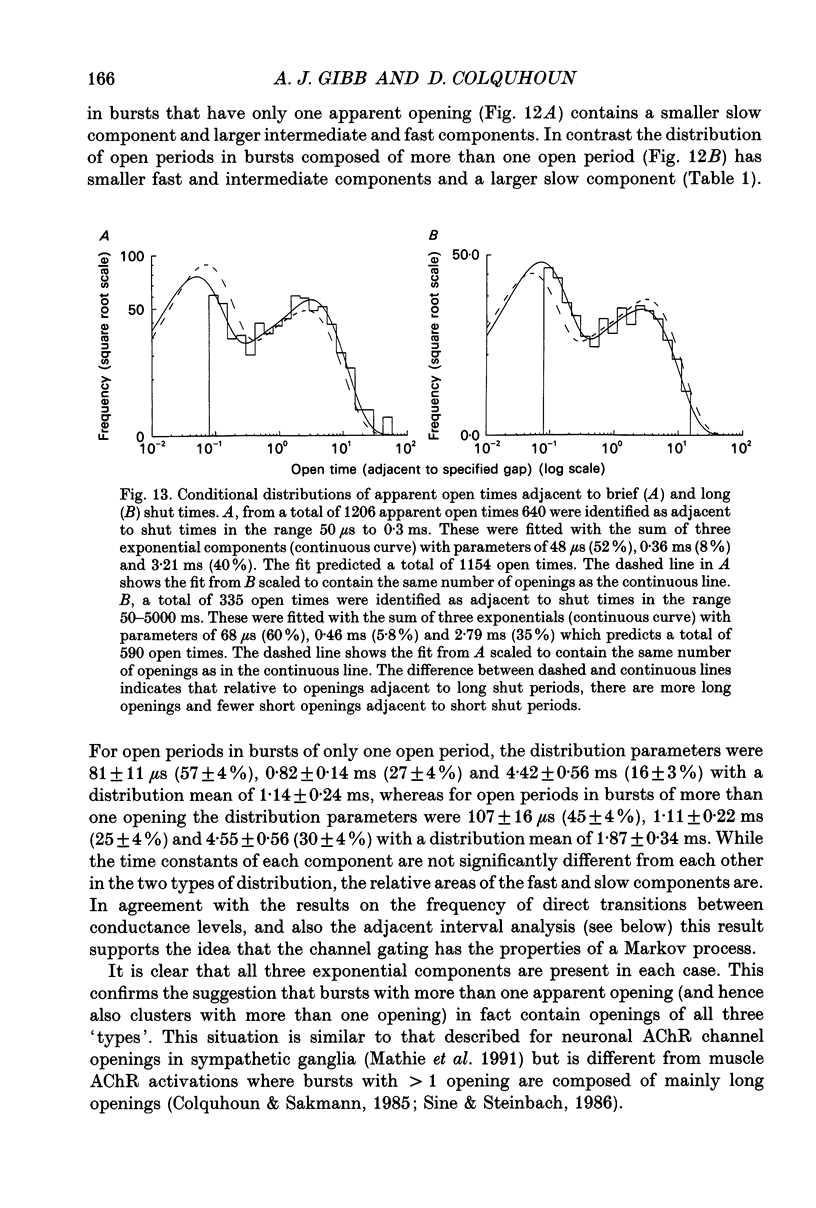
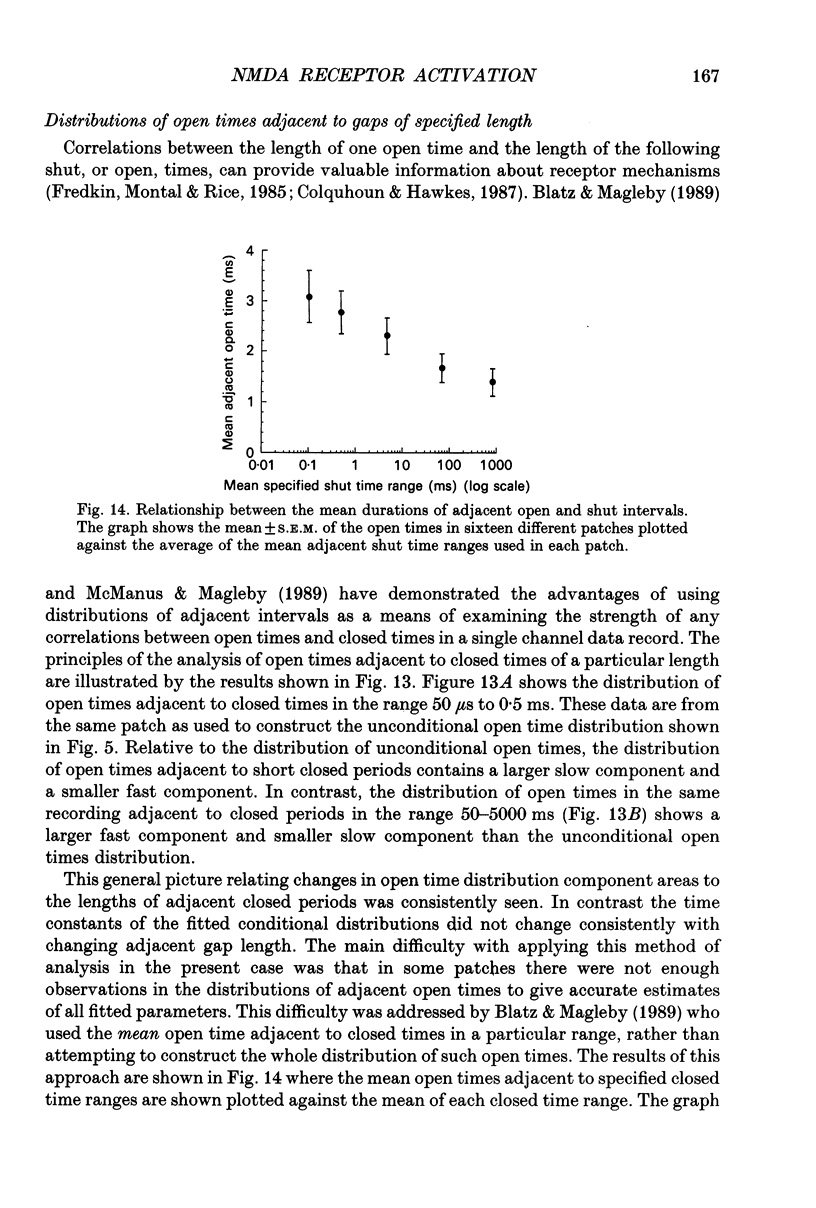
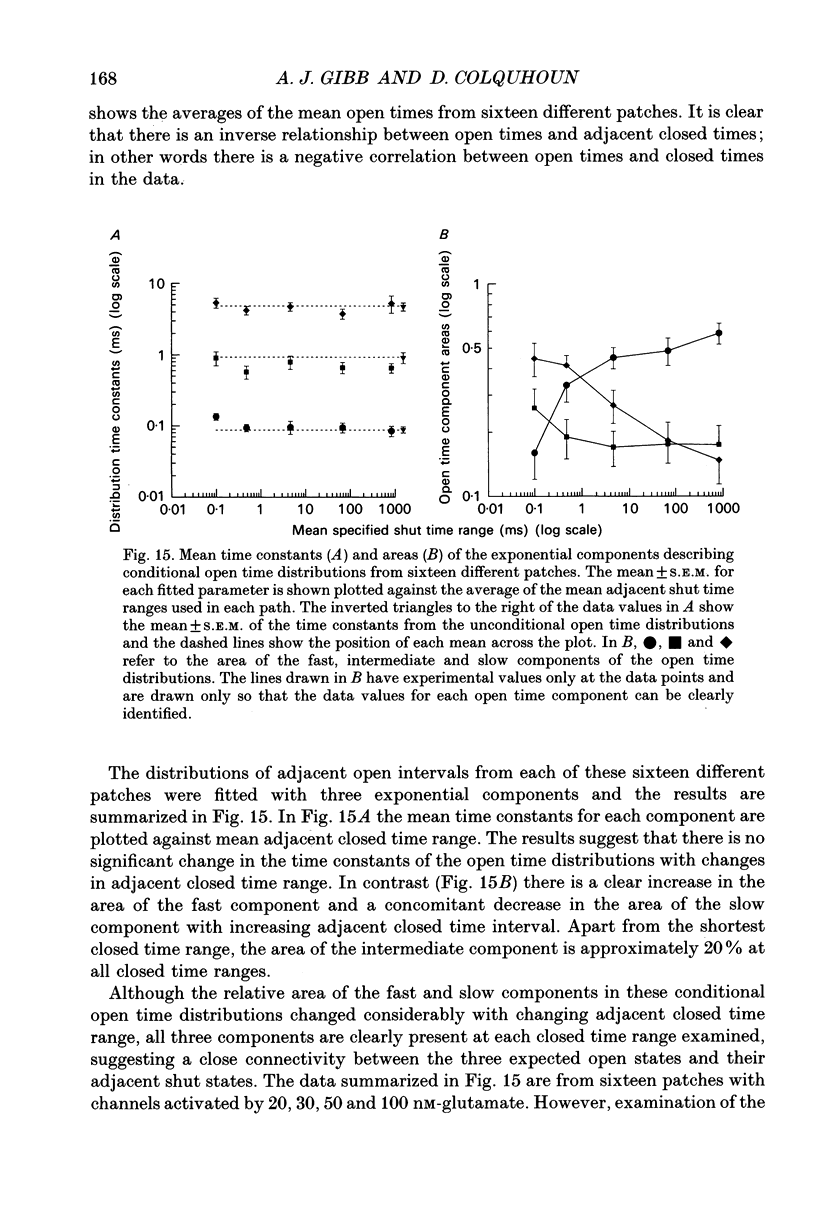
Abstract
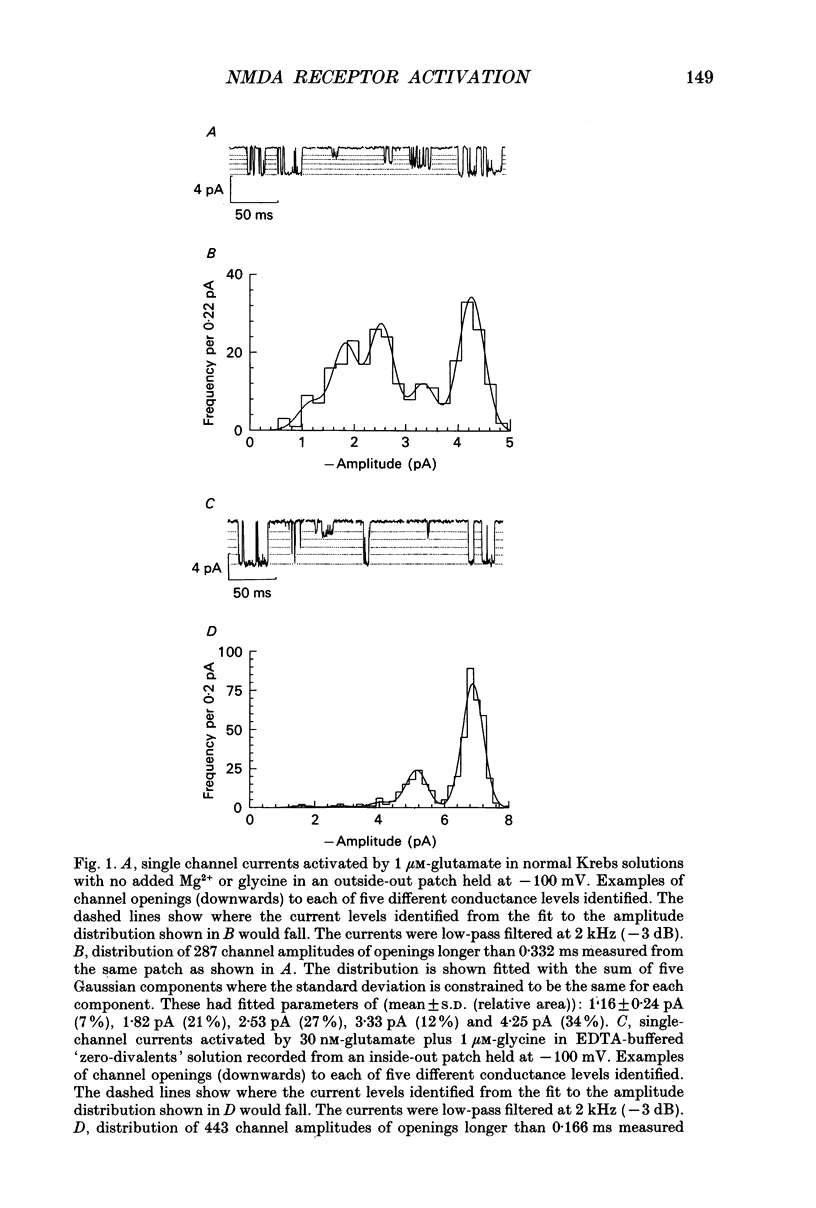
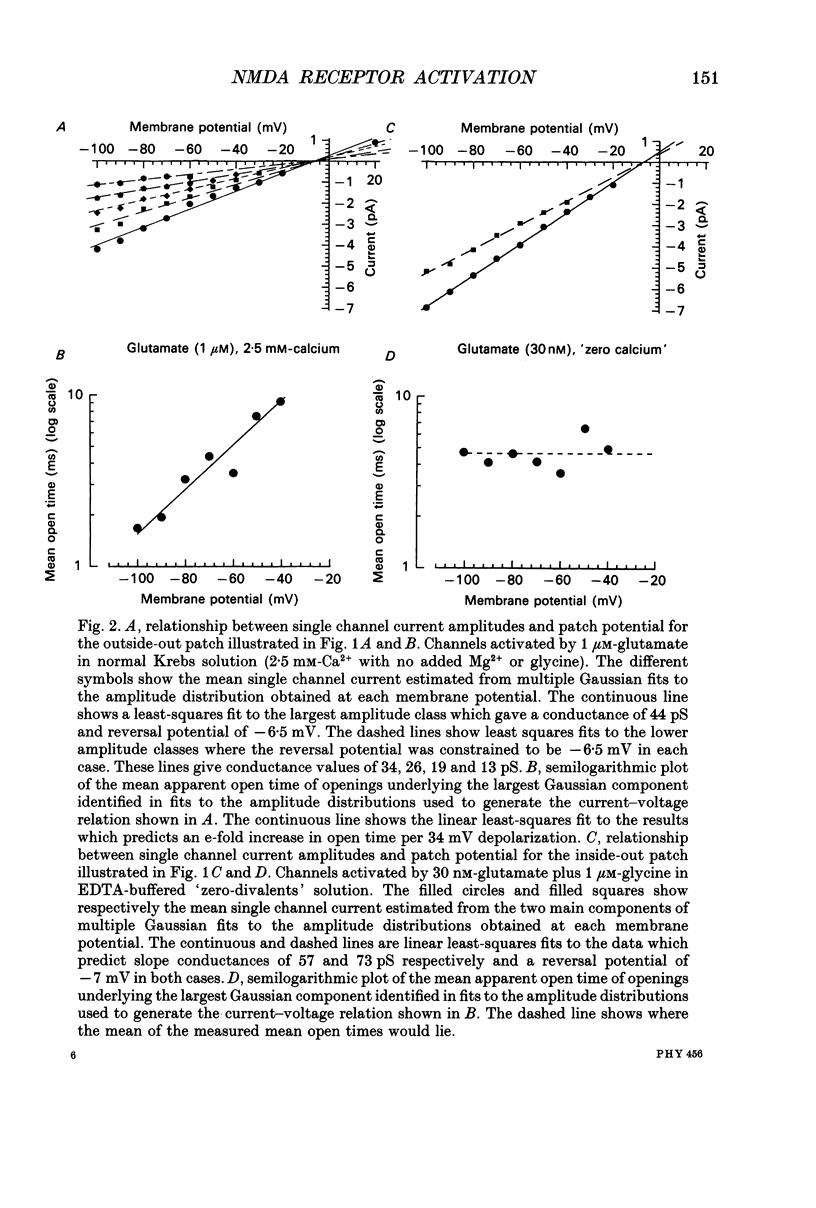
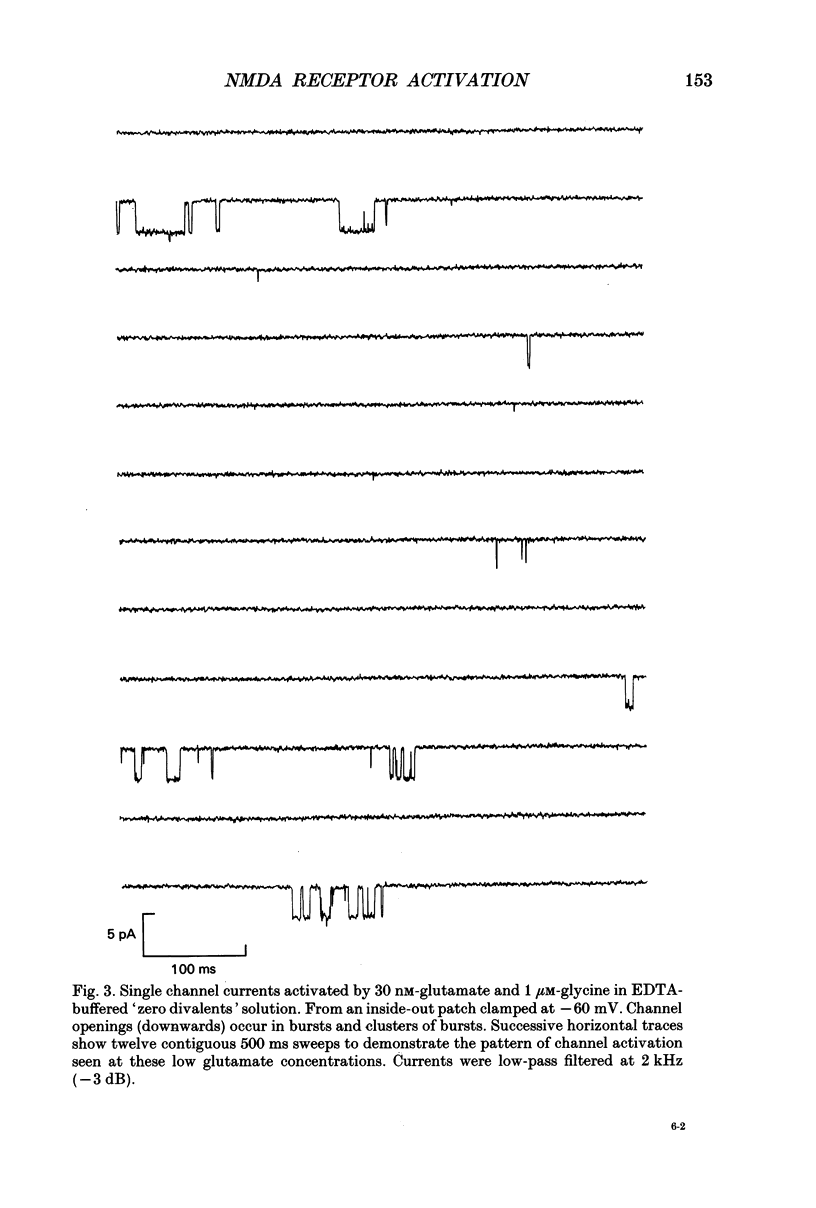
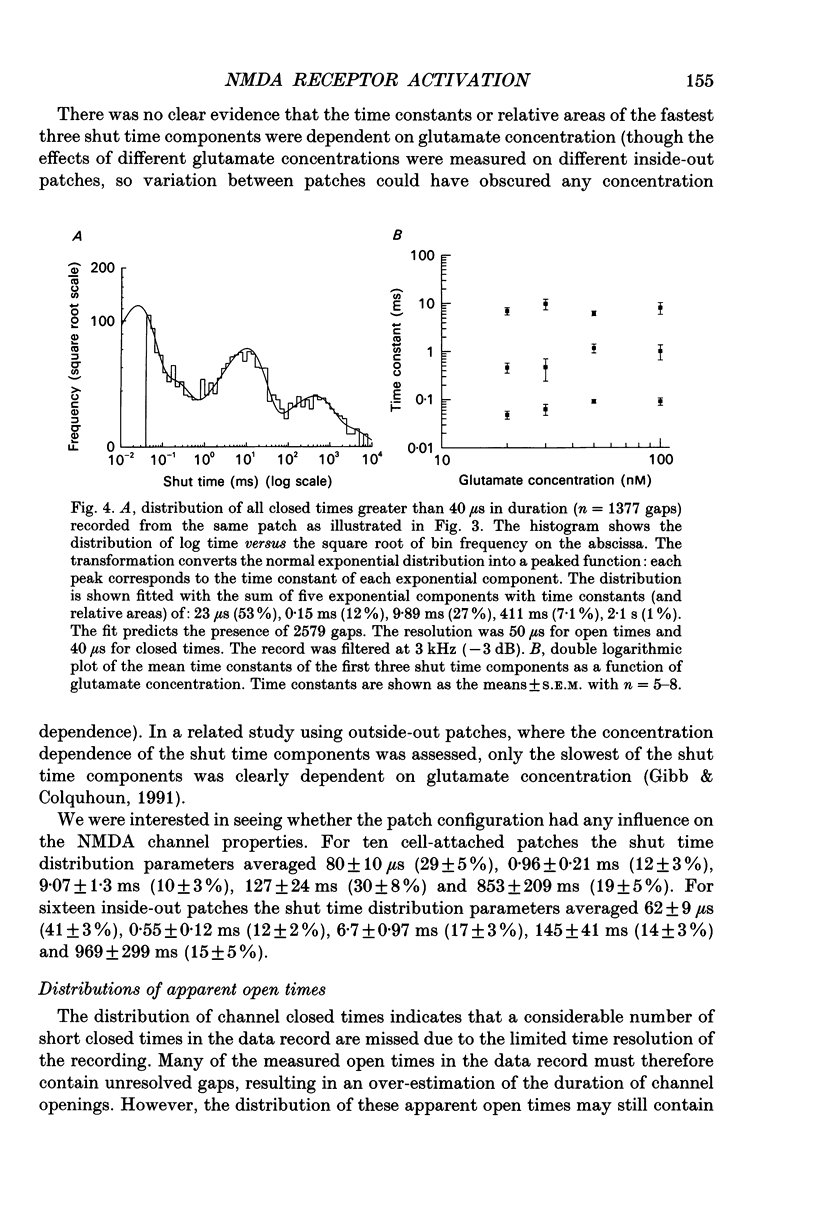
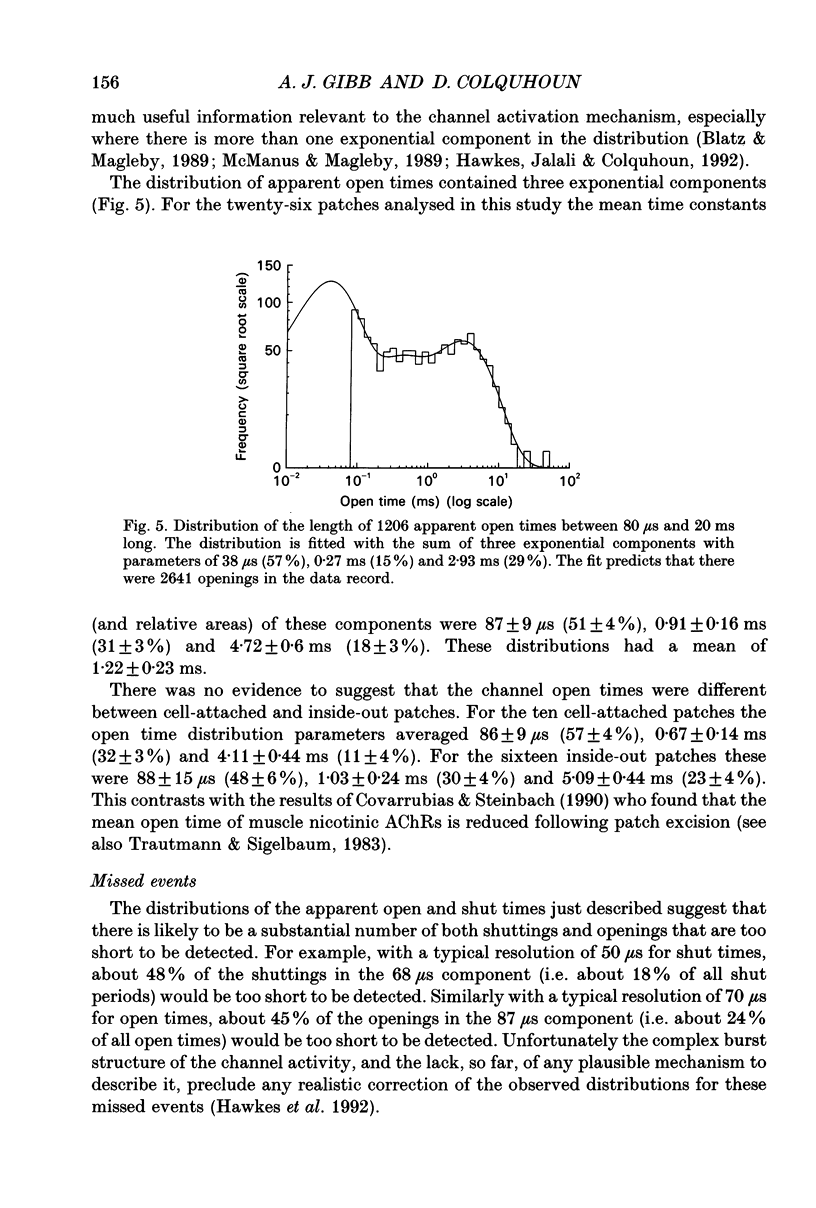
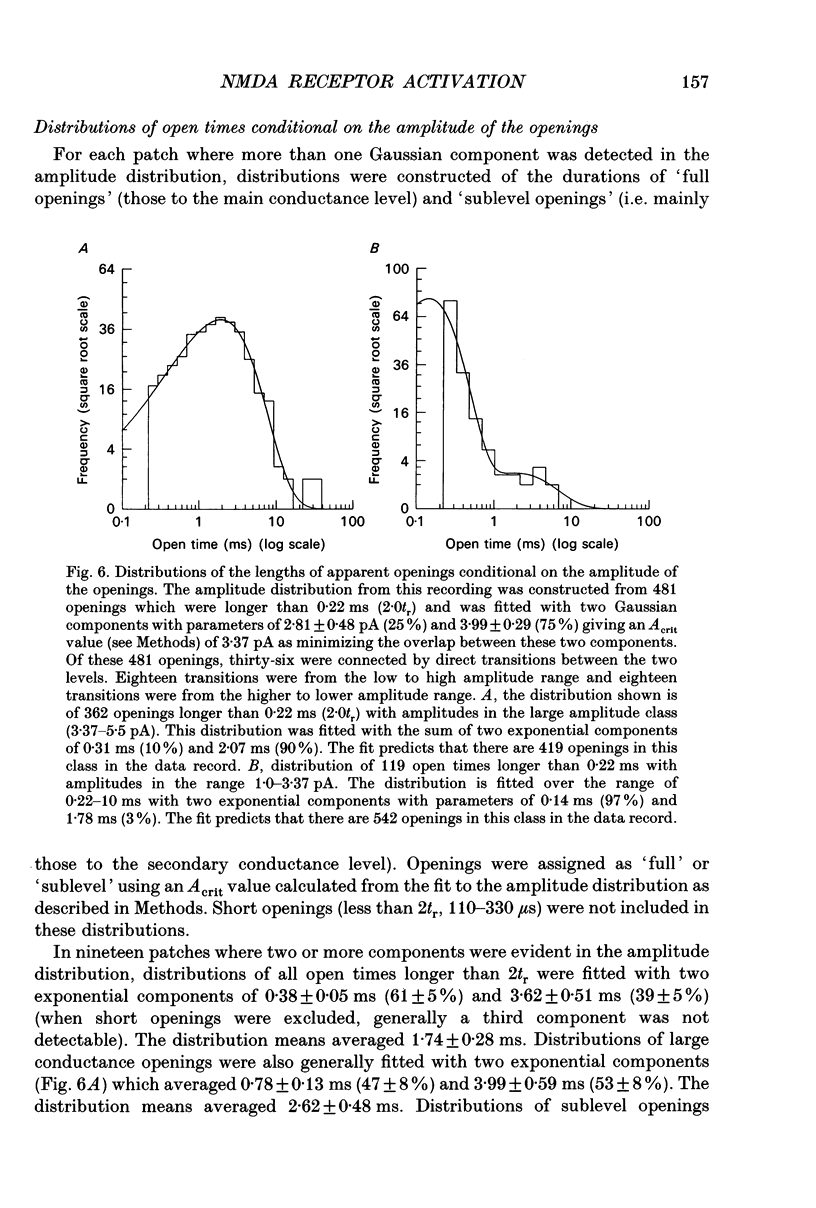
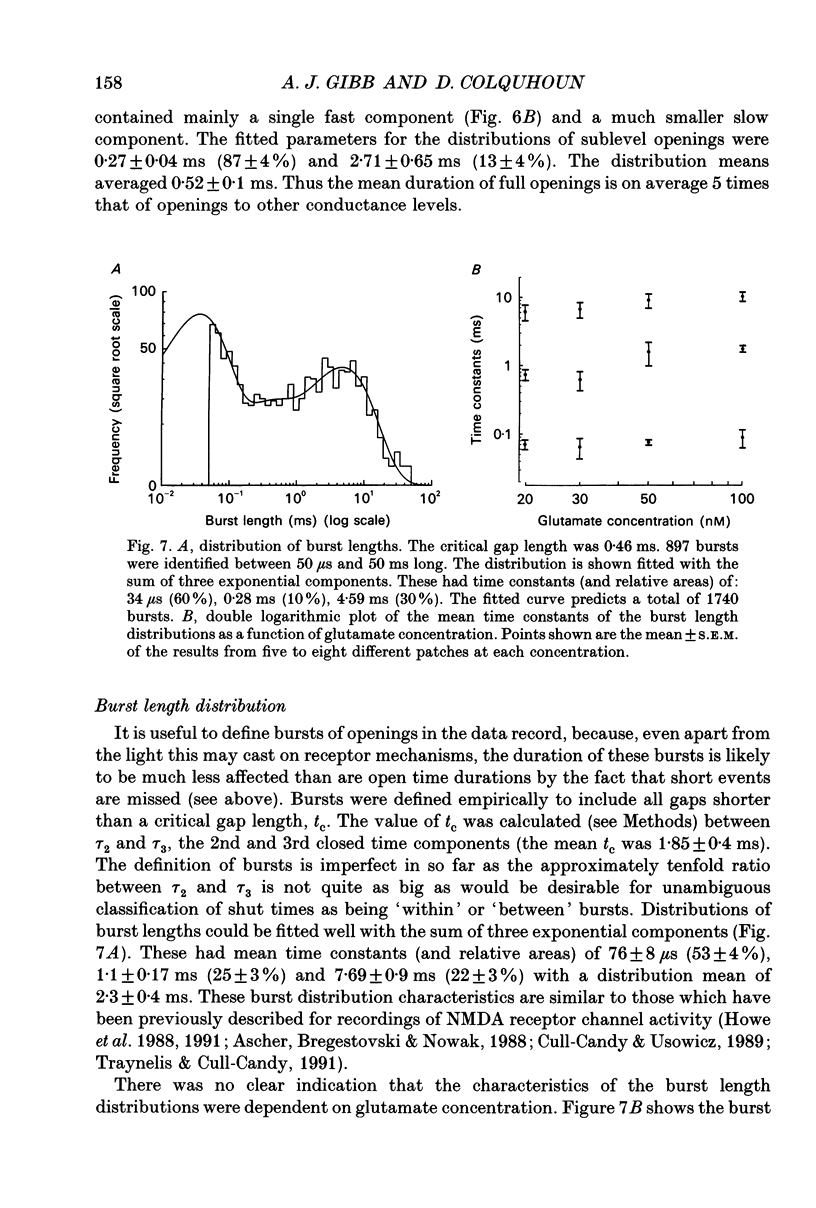
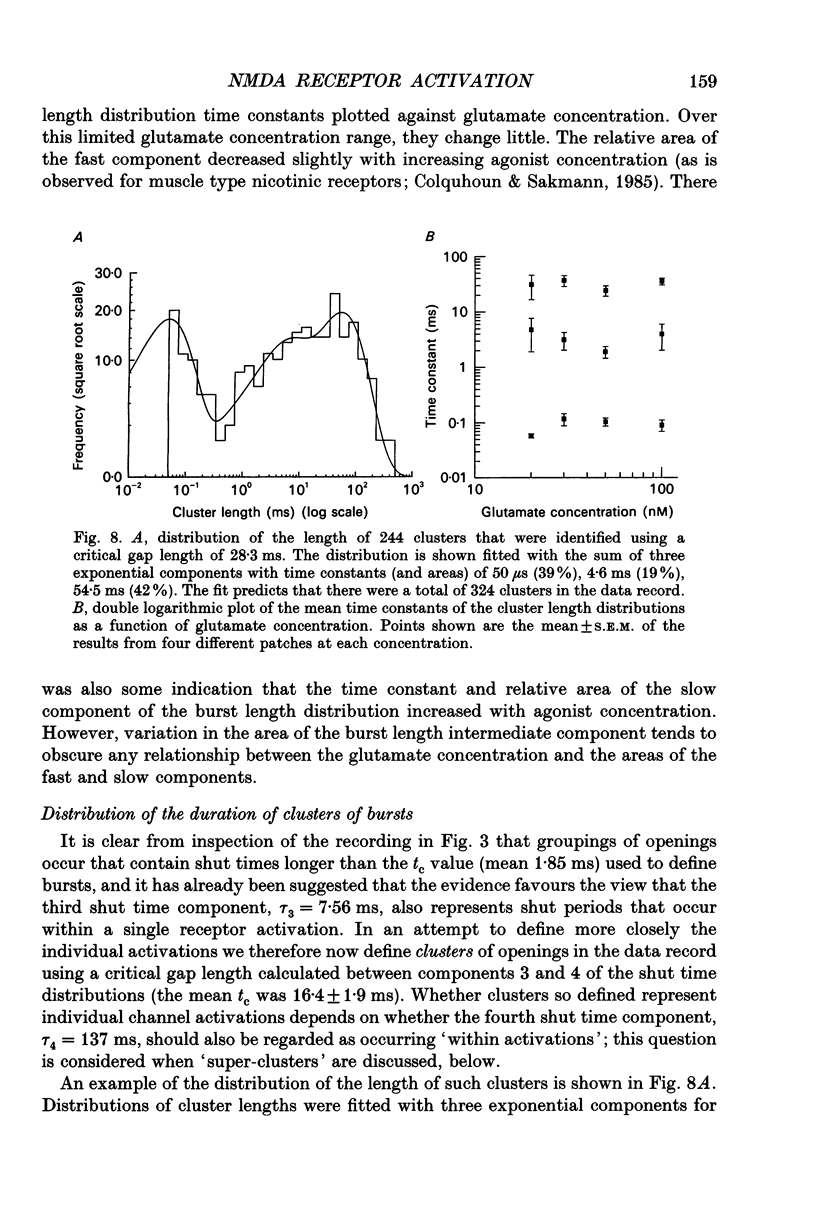
1. Single channel recording techniques were used to study the ion channel openings resulting from activation of N-methyl-D-aspartate (NMDA) receptors by the agonist glutamate. Patches were from cells acutely dissociated from adult rat hippocampus (CA1). Channel activity was studied at low glutamate concentrations (20-100 nM) with 1 microM-glycine, in the absence of extracellular divalent cations. 2. Channel openings were to two main conductance levels corresponding to 50 pS and 40 pS openings in extracellular solution with 1 mM-Ca2+. Around 80% of openings were to the large conductance level. The single channel conductances increased as extracellular Ca2+ was reduced. 3. Distributions of channel open times were described by three exponential components of 87 microseconds, 0.91 ms and 4.72 ms (relative areas of 51, 31 and 18%). Most long openings were to the large conductance level. 4. The channel closed time distribution was complex, requiring five exponential components to describe it adequately. Of these five components, at least three, with time constants of 68 microseconds, 0.72 ms and 7.6 ms (relative areas of 38, 12 and 17%) represent gaps within single activations of the receptor. The presence of a component with a mean of 7.6 ms is notable because gaps of this length have not previously been identified as being within single NMDA receptor channel activations. 5. Channel activations were identified as including gaps underlying at least the first three closed time components. Activations consisted of clusters of channel openings. Distributions of the length of these clusters had mean time constants of 88 microseconds, 3.4 ms and 32 ms (relative areas of 45, 25 and 30%). Long clusters contained short, intermediate and long duration openings as well as subconductance openings. The open probability within clusters averaged 0.62. Three components were evident in distributions of the number of openings per cluster. These had mean values of 1.22, 3.2 and 11 openings per cluster. 6. An inverse correlation was evident between the length of adjacent open and closed times. When open intervals were separated into groups based on the length of adjacent gaps, the time constants of the exponential components in these conditional open time distributions were independent of the length of the adjacent gap. This supports the idea that the NMDA receptor channel gating has the properties of a discrete Markov process. 7. The long duration of NMDA receptor channel clusters suggests that they contribute to the slow time course of the NMDA receptor-mediated synaptic current.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball F. G., Sansom M. S. Single-channel autocorrelation functions: the effects of time interval omission. Biophys J. 1988 May;53(5):819–832. doi: 10.1016/S0006-3495(88)83161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys J. 1991 Mar;59(3):560–573. doi: 10.1016/S0006-3495(91)82272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Adjacent interval analysis distinguishes among gating mechanisms for the fast chloride channel from rat skeletal muscle. J Physiol. 1989 Mar;410:561–585. doi: 10.1113/jphysiol.1989.sp017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Steinbach J. H. Excision of membrane patches reduces the mean open time of nicotinic acetylcholine receptors. Pflugers Arch. 1990 Jun;416(4):385–392. doi: 10.1007/BF00370744. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Parker I. Rapid kinetics of single glutamate-receptor channels. Nature. 1982 Feb 4;295(5848):410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc Biol Sci. 1991 Jan 22;243(1306):39–45. doi: 10.1098/rspb.1991.0007. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawkes A. G., Jalali A., Colquhoun D. Asymptotic distributions of apparent open times and shut times in a single channel record allowing for the omission of brief events. Philos Trans R Soc Lond B Biol Sci. 1992 Sep 29;337(1282):383–404. doi: 10.1098/rstb.1992.0116. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Sah P., Nicoll R. A. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990 Sep;5(3):247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Cull-Candy S. G., Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991 Jan;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986 May;16(3):227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin N. I., Krishtal O. A., Tsyndrenko A. Ya. Cross-desensitization Reveals Pharmacological Specificity of Excitatory Amino Acid Receptors in Isolated Hippocampal Neurons. Eur J Neurosci. 1990;2(5):461–470. doi: 10.1111/j.1460-9568.1990.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Waddell P. J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991 Apr;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Conductance and kinetic properties of single nicotinic acetylcholine receptor channels in rat sympathetic neurones. J Physiol. 1991 Aug;439:717–750. doi: 10.1113/jphysiol.1991.sp018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Inverse relationship of the durations of adjacent open and shut intervals for C1 and K channels. Nature. 1985 Oct 17;317(6038):625–627. doi: 10.1038/317625a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittman S., Taylor W. R., Copenhagen D. R. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol. 1990 Sep;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., Walker V. E., Flynn D. M. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 1989 Mar 30;338(6214):422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol. 1991 Feb;433:727–763. doi: 10.1113/jphysiol.1991.sp018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991 Apr;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Intraburst kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1990 Apr;423:193–220. doi: 10.1113/jphysiol.1990.sp018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D. S., Magleby K. L. Gating scheme for single GABA-activated Cl- channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J Neurosci. 1989 Apr;9(4):1314–1324. doi: 10.1523/JNEUROSCI.09-04-01314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


