Abstract
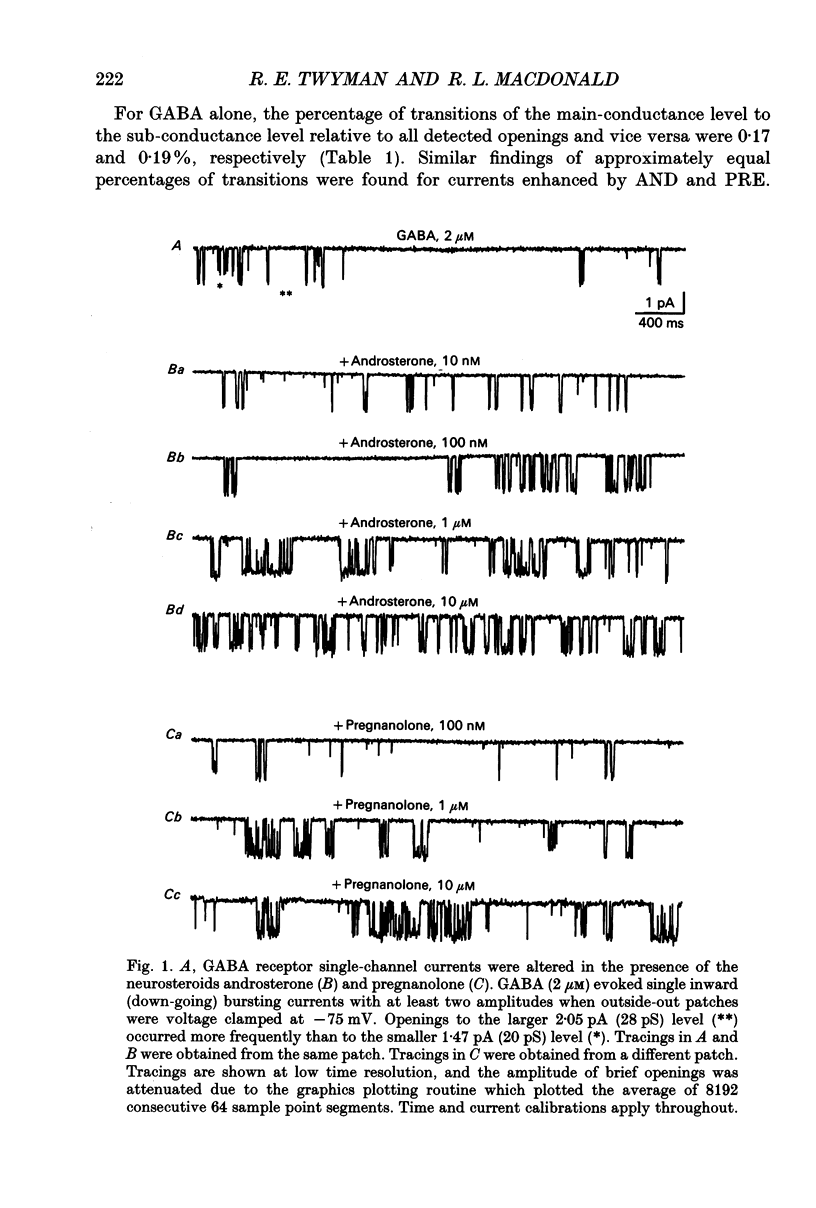
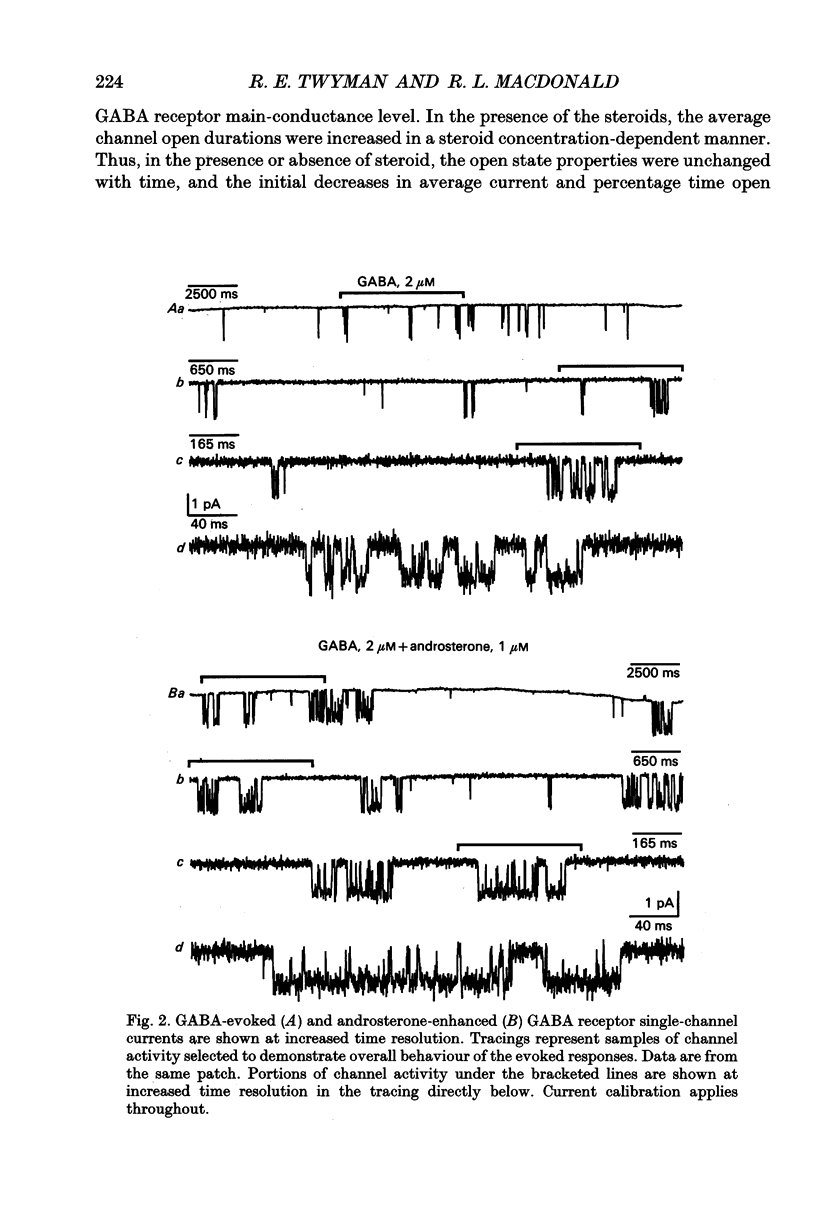
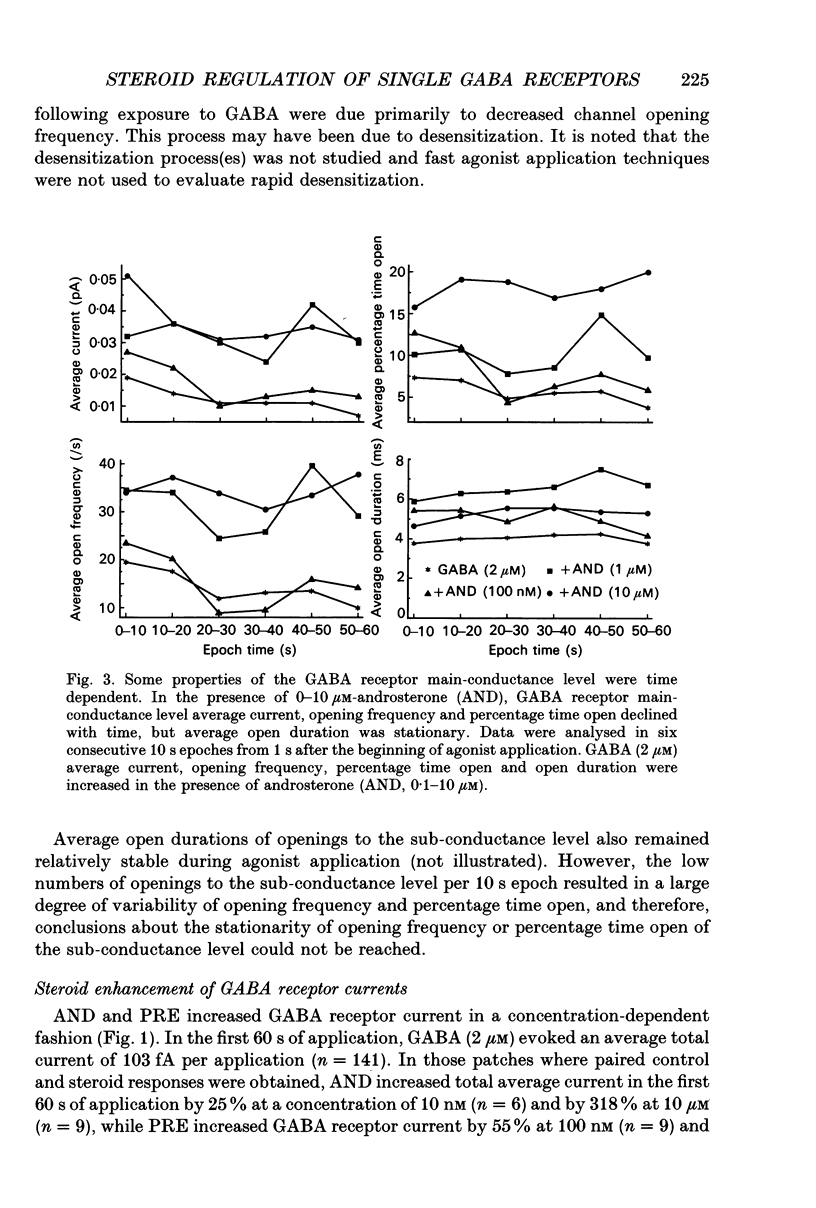
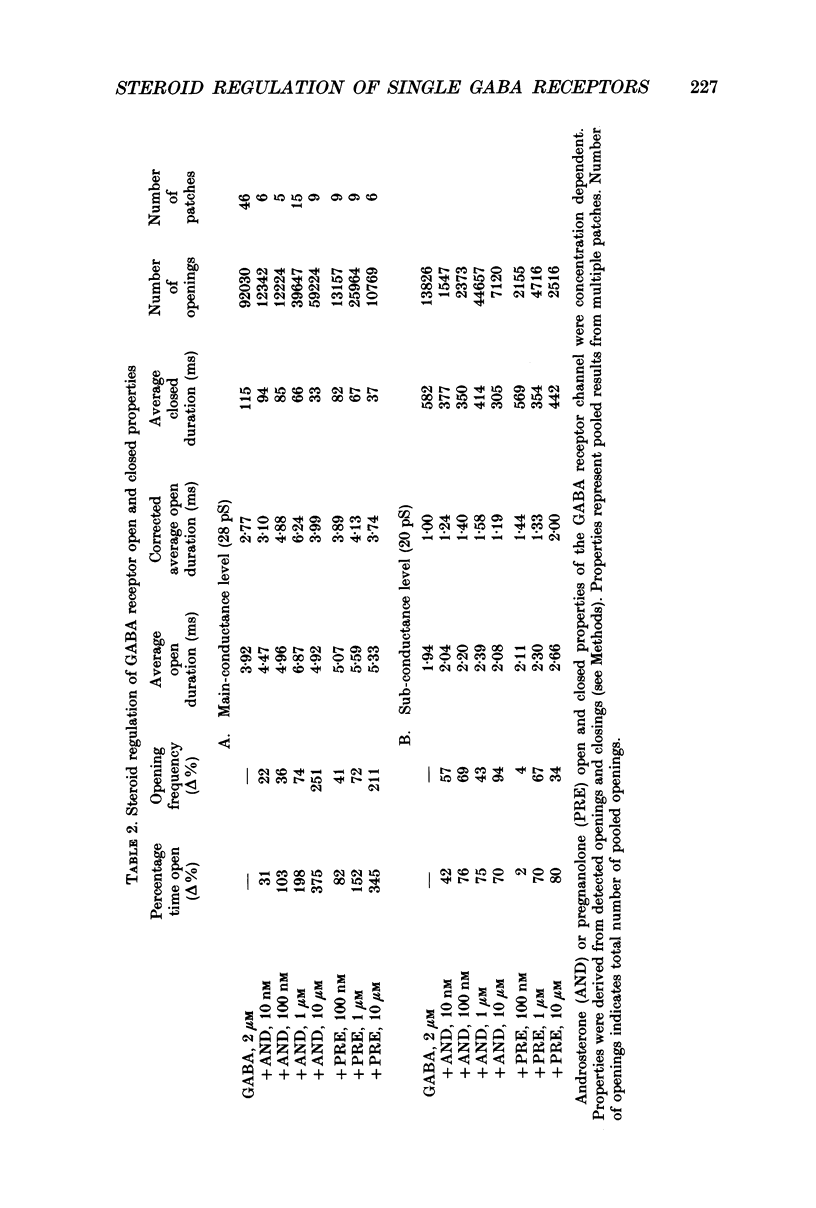
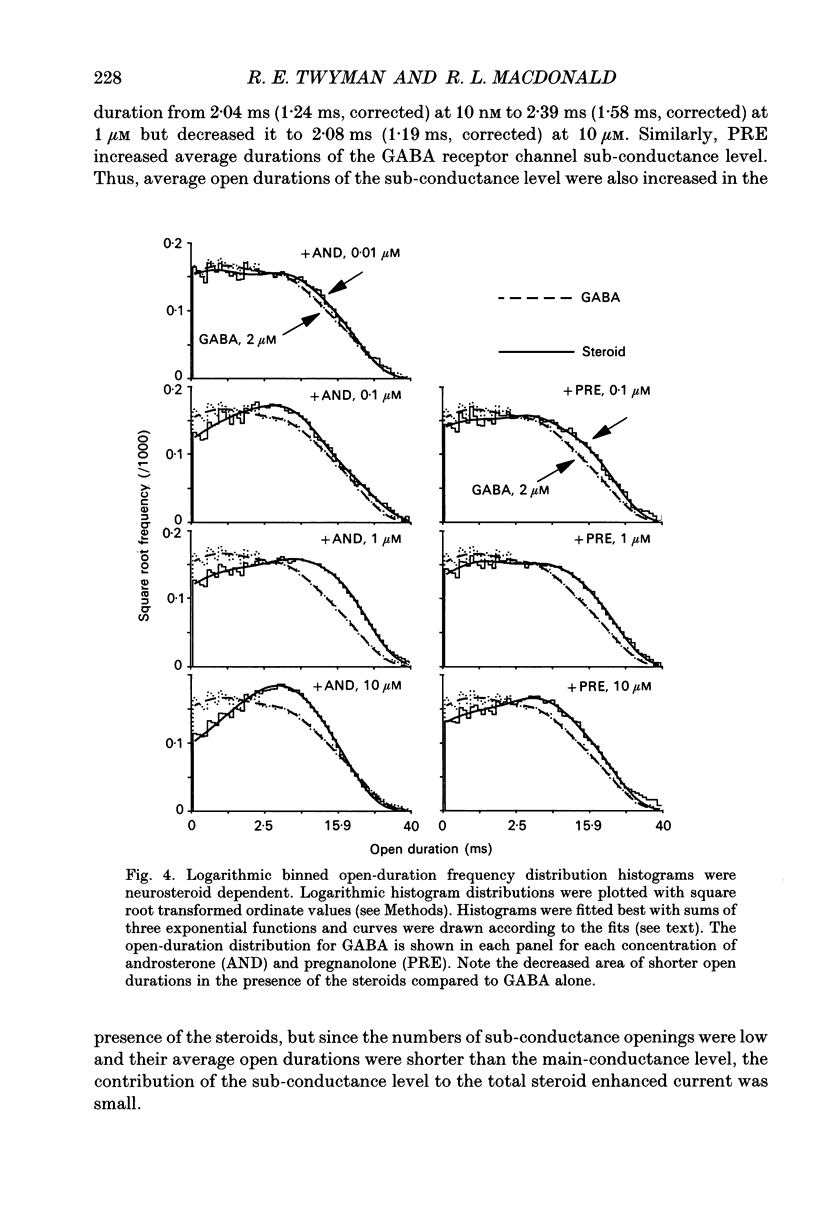
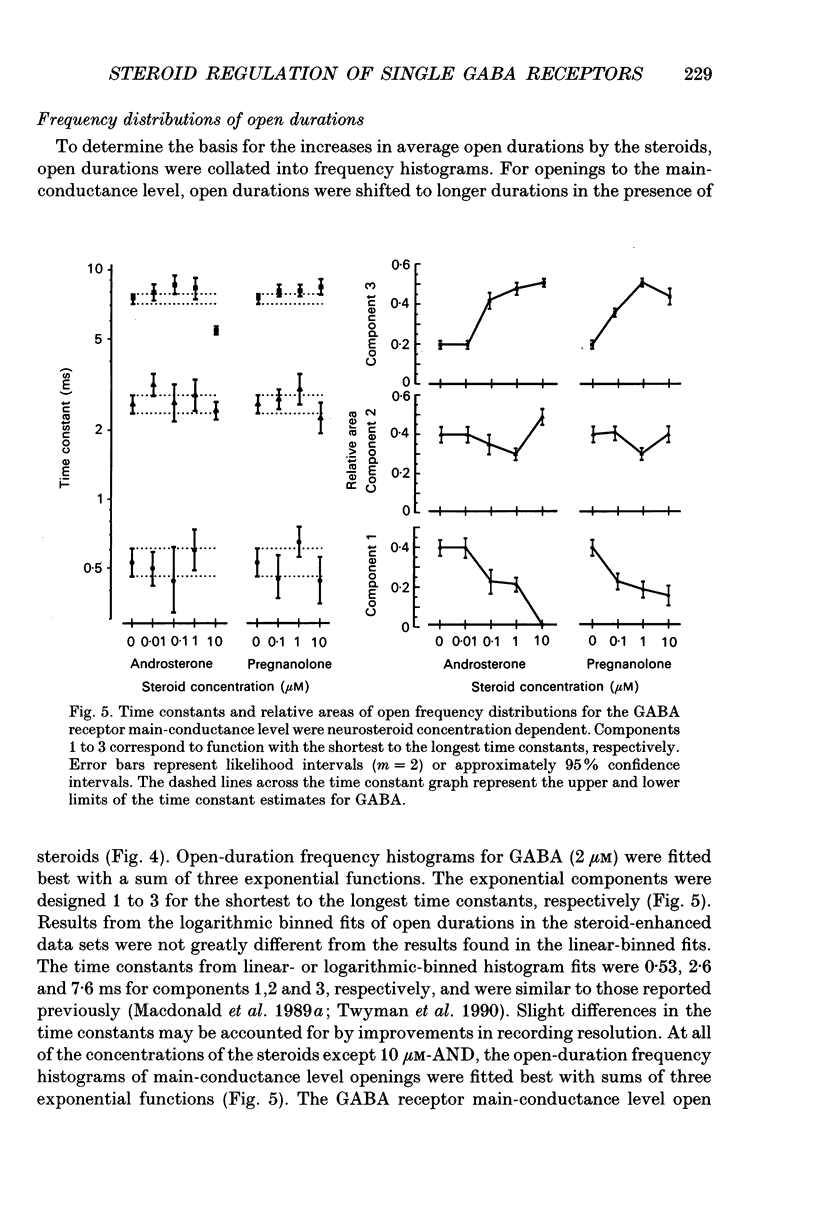
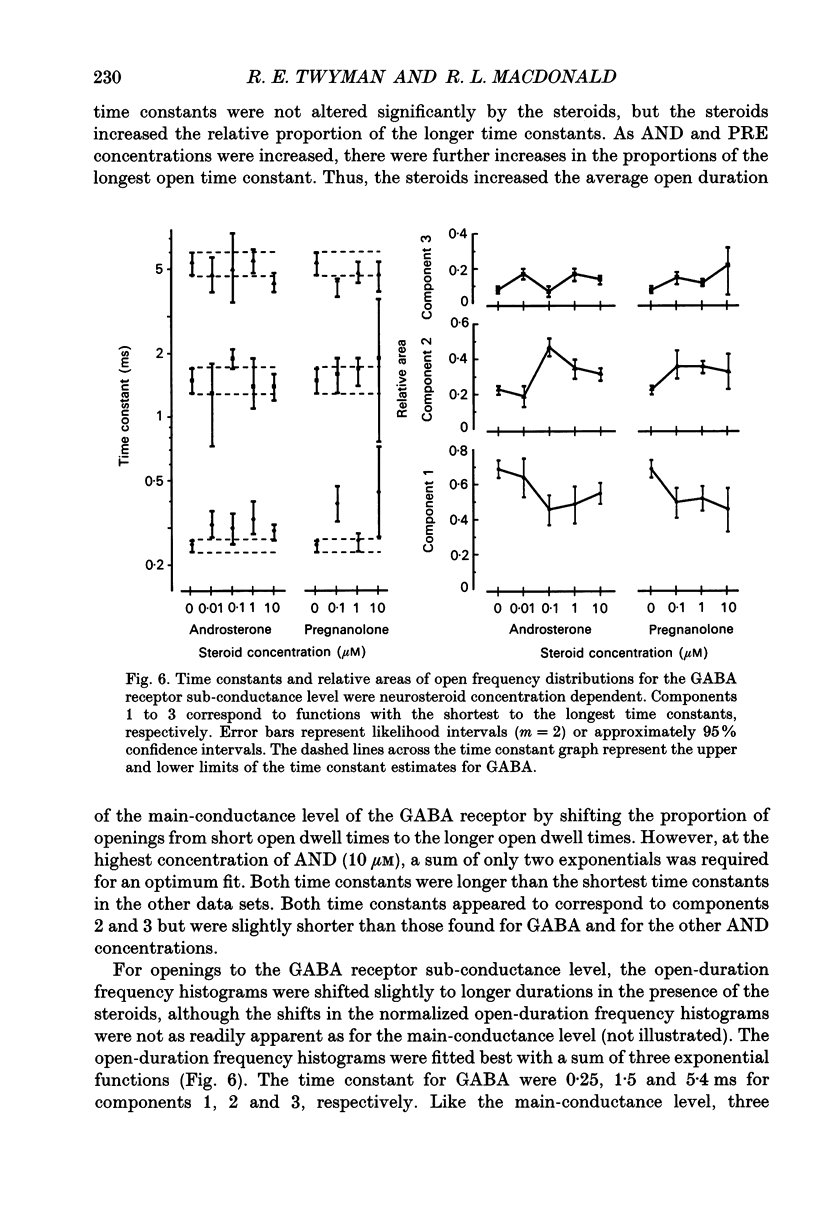
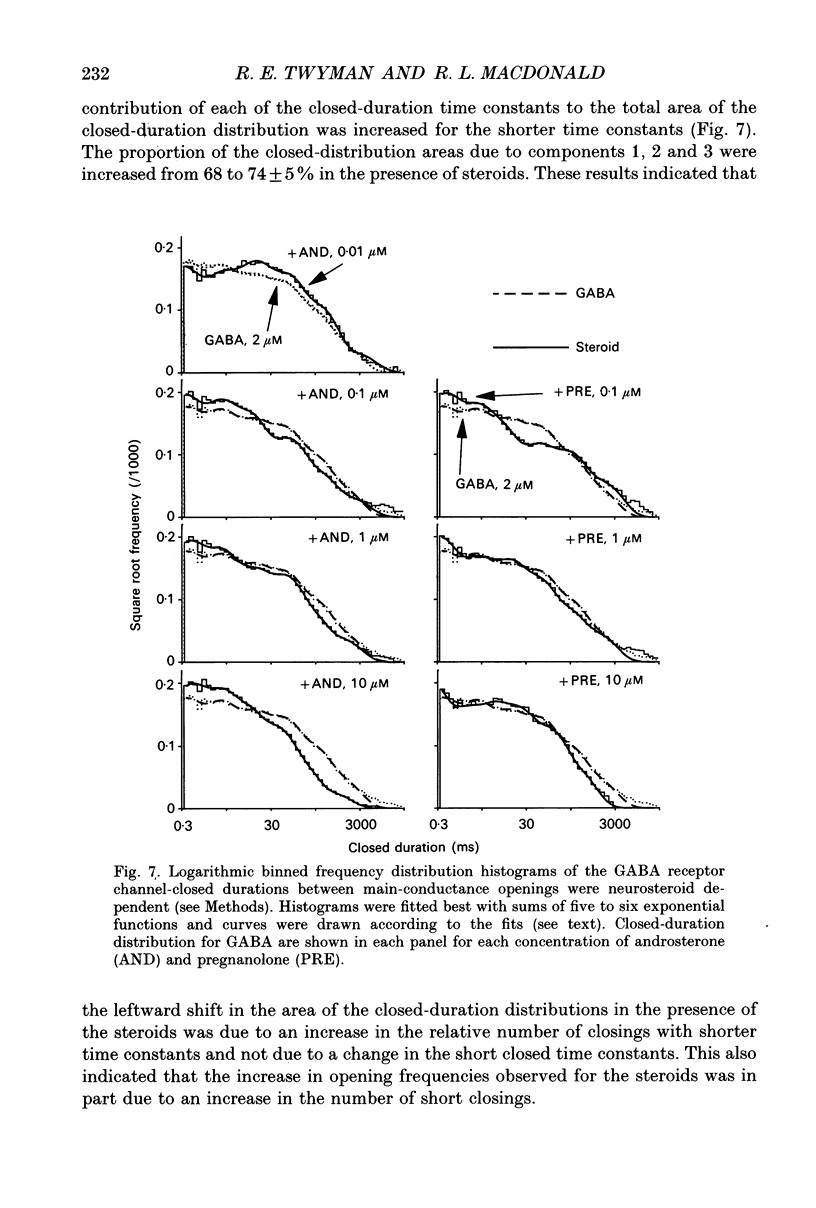
1. Single-channel kinetics of steroid enhancement of single gamma-aminobutyric acidA (GABA) receptor currents obtained from somata of mouse spinal cord neurones in culture were investigated using the excised outside-out patch-clamp recording technique. GABA (2 microM) and GABA (2 microM) plus androsterone (5 alpha-androstan-3 alpha-ol-17-one, AND, 10 nM-10 microM) or pregnanolone (5 beta-pregnan-3 alpha-ol-20-one, PRE, 100 nM-10 microM) applied by pressure ejection from micropipettes evoked inward currents when patches were voltage clamped at -75 mV in symmetrical chloride solutions. Averaged GABA receptor currents were increased in the presence of the steroids. 2. GABA receptor currents were recorded with at least two conductance levels, a predominant or main-conductance level of about 28 pS (which contributed 96% of the current evoked) and a minor or sub-conductance level of about 20 pS. The current amplitudes of the two conductance levels were unchanged by the steroids. The gating (opening and closing) kinetics of both of the conductance levels were analysed. Findings for the main-conductance level are summarized below. 3. Both steroids increased the average GABA receptor channel open duration. Consistent with the increased GABA receptor channel average open duration, the steroids shifted frequency histograms of GABA receptor channel open durations to longer durations. Three exponential functions were required to fit best the frequency histograms of GABA open durations, consistent with at least three kinetic open states of the main-conductance level. Time constants obtained from the GABA receptor channel open-duration frequency histograms were unchanged in the presence of the steroids. The basis for the increased average GABA receptor channel open durations by the steroids was due to an increased relative proportion of the two longer open-duration time constants. The GABA receptor channel average open durations were increased by AND and PRE in a concentration-dependent manner by shifting the proportion of openings to the longer open time constants. At a concentration of 10 microM, the prolongation of the average open duration was decreased, suggesting that the GABA receptor channel was blocked by these steroids. 4. GABA receptor channel opening frequency was increased and average channel-closed duration was decreased by AND or PRE. Consistent with this, areas of the frequency histograms of channel closed durations were shifted to shorter durations. Closed frequency distributions were fitted best with five to six exponential functions, suggesting that the channel had multiple kinetic closed states. The three briefest time constants were not greatly altered by the steroids.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Harrison N. L., Lange G. D., Owen D. G. Potentiation of gamma-aminobutyric-acid-activated chloride conductance by a steroid anaesthetic in cultured rat spinal neurones. J Physiol. 1987 May;386:485–501. doi: 10.1113/jphysiol.1987.sp016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H., Cottrell G. A., Hather N. Y., Lambert J. J., Nooney J. M., Peters J. A. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987 Aug 21;231(1264):359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Stochastic properties of ion channel openings and bursts in a membrane patch that contains two channels: evidence concerning the number of channels present when a record containing only single openings is observed. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1299):453–477. doi: 10.1098/rspb.1990.0048. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Lambert J. J., Peters J. A. Modulation of GABAA receptor activity by alphaxalone. Br J Pharmacol. 1987 Mar;90(3):491–500. doi: 10.1111/j.1476-5381.1987.tb11198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee K. W., Bolger M. B., Brinton R. E., Coirini H., McEwen B. S. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988 Aug;246(2):803–812. [PubMed] [Google Scholar]
- Harrison N. L., Majewska M. D., Harrington J. W., Barker J. L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984 Dec 10;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Hu Z. Y., Bourreau E., Jung-Testas I., Robel P., Baulieu E. E. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im W. B., Blakeman D. P., Davis J. P., Ayer D. E. Studies on the mechanism of interactions between anesthetic steroids and gamma-aminobutyric acidA receptors. Mol Pharmacol. 1990 Mar;37(3):429–434. [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Kirkness E. F., Turner A. J. The stimulatory effects of secobarbital and pregnanolone on the GABAA receptor can be blocked selectively. Eur J Pharmacol. 1988 Jun 10;150(3):385–388. doi: 10.1016/0014-2999(88)90024-6. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M. D., Ford-Rice F., Falkay G. Pregnancy-induced alterations of GABAA receptor sensitivity in maternal brain: an antecedent of post-partum 'blues'? Brain Res. 1989 Mar 20;482(2):397–401. doi: 10.1016/0006-8993(89)91208-0. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Mienville J. M., Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988 Aug 1;90(3):279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Parsons B. Gonadal steroid action on the brain: neurochemistry and neuropharmacology. Annu Rev Pharmacol Toxicol. 1982;22:555–598. doi: 10.1146/annurev.pa.22.040182.003011. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Mistry D. K., Cottrell G. A. Actions of steroids and bemegride on the GABAA receptor of mouse spinal neurones in culture. Exp Physiol. 1990 Mar;75(2):199–209. doi: 10.1113/expphysiol.1990.sp003394. [DOI] [PubMed] [Google Scholar]
- Morrow A. L., Suzdak P. D., Paul S. M. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987 Oct 27;142(3):483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Kirkness E. F., Callachan H., Lambert J. J., Turner A. J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988 Aug;94(4):1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Santi M. R., Vicini S., Pritchett D. B., Purdy R. H., Paul S. M., Seeburg P. H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990 May;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy R. H., Morrow A. L., Blinn J. R., Paul S. M. Synthesis, metabolism, and pharmacological activity of 3 alpha-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990 Jun;33(6):1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Rościszewska D., Buntner B., Guz I., Zawisza L. Ovarian hormones, anticonvulsant drugs, and seizures during the menstrual cycle in women with epilepsy. J Neurol Neurosurg Psychiatry. 1986 Jan;49(1):47–51. doi: 10.1136/jnnp.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Green R. M., MacDonald R. L. Kinetics of open channel block by penicillin of single GABAA receptor channels from mouse spinal cord neurones in culture. J Physiol. 1992 Jan;445:97–127. doi: 10.1113/jphysiol.1992.sp018914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991 Apr;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989 Mar;25(3):213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Intraburst kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1990 Apr;423:193–220. doi: 10.1113/jphysiol.1990.sp018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci Lett. 1989 Jan 2;96(1):89–95. doi: 10.1016/0304-3940(89)90248-6. [DOI] [PubMed] [Google Scholar]
- Vicini S., Mienville J. M., Costa E. Actions of benzodiazepine and beta-carboline derivatives on gamma-aminobutyric acid-activated Cl- channels recorded from membrane patches of neonatal rat cortical neurons in culture. J Pharmacol Exp Ther. 1987 Dec;243(3):1195–1201. [PubMed] [Google Scholar]
- Weiss D. S., Magleby K. L. Gating scheme for single GABA-activated Cl- channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J Neurosci. 1989 Apr;9(4):1314–1324. doi: 10.1523/JNEUROSCI.09-04-01314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


