Abstract
Imaging reporter gene expression in living subjects is a rapidly evolving area of molecular imaging research. Studies have validated the use of reporter genes with positron emission tomography (PET), single photon emission computed tomography (SPECT), MRI, fluorescence with wild-type and mutants of green fluorescent protein, as well as bioluminescence using Firefly luciferase enzyme/protein (FL). In the current study, we validate for the first time the ability to image bioluminescence from Renilla luciferase enzyme/protein (RL) by injecting the substrate coelenterazine in living mice. A highly sensitive cooled charge-coupled device camera provides images within a few minutes of photon counting. Cells, transiently expressing the Rluc were imaged while located in the peritoneum, s.c. layer, as well as in the liver and lungs of living mice tail-vein injected with coelenterazine. Furthermore, d-luciferin (a substrate for FL) does not serve as a substrate for RL, and coelenterazine does not serve as a substrate for FL either in cell culture or in living mice. We also show that both Rluc and Fluc expression can be imaged in the same living mouse and that the kinetics of light production are distinct. The approaches validated will have direct applications to various studies where two molecular events need to be tracked, including cell trafficking of two cell populations, two gene therapy vectors, and indirect monitoring of two endogenous genes through the use of two reporter genes.
Repetitive monitoring of reporter gene expression in intact living animals is crucial for many applications, including cell trafficking, gene therapy studies, and transgenic models (1). Noninvasive, real-time analysis of molecular events in intact living mammals is an active area of current research (1, 2). Several imaging technologies and new reporter genes are being studied for noninvasive imaging and quantitation of gene expression in living subjects. Some of the imaging modalities and established reporter genes include SPECT using Herpes Simplex Virus Type I thymidine kinase HSV1-tk, Somatostatin Type 2 receptor, and Sodium/Iodide Symporter as reporter genes. PET using HSV1-tk and Dopamine Type 2 Receptor as reporter genes, MRI with various reporter genes, and optical imaging approaches with fluorescence and bioluminescent reporter genes have also been studied. A detailed review of reporter gene approaches for use in living subjects can be found elsewhere (1). For many applications, it would be very useful to have multiple reporter genes. Separate reporter genes can be used with different modalities (e.g., PET, MRI), but would lack convenience and high-throughput, and images would be more difficult to coregister and quantitate.
Reporter genes with optical signatures (e.g., fluorescent and bioluminescent) are a low-cost alternative for real-time analysis of gene expression in small animal models. In fluorescent approaches [e.g., green fluorescent protein (GFP)], an external source of light is required for excitation of the protein. In contrast, bioluminescent reporter proteins can produce light by using appropriate substrates. Recently, several technical advances in developing highly sensitive detection devices have led to the biological use of cooled charge-coupled device (CCD) cameras capable of imaging very low levels of visible light emitted from internal body organs of rodents (3–7).
“Luciferase” is a family of photo-proteins that can be isolated from a large variety of insects, marine organisms, and prokaryotes (8). Luciferase proteins catalyzing the light-emitting reactions of firefly, coelenterates, or bacteria show no nucleotide homology to each other. The substrates “luciferin” of these reactions are also chemically unrelated (9). However, all these bioluminescent reactions are exergonic in nature, where molecular oxygen reacts with “luciferase” and “luciferin,” resulting in formation of a luciferase bound peroxy-luciferin intermediate, which releases photons of visible light (≈50 kcal) (9). The emission spectra ranges between 400 nm and 620 nm (8).
To date, Firefly luciferase enzyme/protein (FL) (4, 7), bacterial luciferase (10), and green fluorescent protein (11) are the three major reporter proteins that have been used for optical reporter gene imaging studies in living rodents. Cells tagged with green fluorescent protein have been used for tracking metastasis (12) and angiogenesis (13, 14) in living rodents. green fluorescent protein-expressing bacteria have been used to study the behavior of spatial migration and infection process in mice (15). In recent years, considerable work with noninvasive imaging of FL has also been carried out (3, 7). Fluc used as a reporter gene for tumor cell growth and metastasis (16), studies of vector mediated gene delivery and expression (5, 7) and bacterial luciferase, used in the study of infection (10), is well documented. The use of bacterial luciferase has been limited to bacteria that express the reporter gene and also produce substrate, in contrast to Fluc that has been expressed in living rodents with exogenous administration of D-luciferin.
In the present investigation, we have explored the potential of a second bioluminescent reporter gene, Rluc, for use in mammalian systems, in addition to the previously validated Fluc isolated from Photinus pyralis. Renilla luciferase enzyme/protein (RL), purified from sea pansy (Renilla reniformis), is a bioluminescent soft coral that displays blue-green bioluminescence upon mechanical stimulation. It is also widely distributed among coelenterates, fishes, squids, and shrimps (8). It has been cloned and sequenced by Lorenz et al. (17) and used as a marker of gene expression in bacteria, yeast, plant, and mammalian cells (18). The enzyme RL catalyzes coelenterazine oxidation leading to bioluminescence. Coelenterazine consists of an imidazolopyrazine structure {2-(p-hydroxybenzyl)-6-(p-hydroxyphenyl)-8-benzylimidazo [1,2-a]pyrazin-3-(7H)-one} that releases blue light across a broad range, peaking at 480 nm upon oxidation by RL in vitro (19).
Earlier reports indicate RL to be distinct from FL in terms of its origin, enzyme structure, and substrate requirements (20). Rluc and Fluc have also been commonly used in cell culture with commercial substrate kits (e.g., Dual-Luciferase Reporter Assay System from Promega) to monitor expression of both reporter genes. We therefore hypothesized that it would be possible to image Rluc expression in living mice and also to image both Fluc and Rluc expression in the same living mouse. This study demonstrates the use of Rluc as an in vivo reporter gene and also validates imaging two bioluminescent reporter genes in the same living animal.
Materials and Methods
Cell Lines, Culture Conditions, and Transfection Procedures.
C6 rat glioma cells were maintained in glucose-deficient Minimum Eagle's Medium (MEM) supplemented with 1% penicillin-streptomycin, 1% L-glutamine, and 5% FCS. HeLa (human cervical carcinoma) cells, N2a (mouse neuroblastoma) cells, and 293 (human kidney) cells were maintained in DMEM supplemented with 1% penicillin-streptomycin and 10% FCS, whereas human prostrate adenocarcinoma, PC-3 cells were maintained in RPMI medium 1640 supplemented with 1% antibiotics and 5% FCS.
For assessment of Rluc expression in various cell types, each cell type described above was plated in 12-well plates (Costar) and transfected with pCMV-Rluc plasmid (Promega), using SuperFect Transfection Reagent (Qiagen). Mock-transfected cells were used as control. Cells were lysed in lysis buffer 48 h post transfection, and biochemical studies were carried out using a luminometer as described later.
For additional cell culture studies, C6 cells were plated in 12-well plates and transiently transfected with either pCMV-Fluc (provided by C. H. Contag, Stanford University, Stanford, CA) or pCMV-Rluc plasmid, or mock transfected using SuperFect Transfection Reagent. These C6 cells transiently expressing Fluc and Rluc are referred to as C6-Fluc and C6-Rluc, respectively. Bioluminescent signals from intact cells were detected directly by a cooled CCD camera. C6 cells were also grown in 100-mm plates (Costar), and transfected with pCMV-Rluc under similar conditions. They were collected by trypsinization 48 h post transfection, washed with PBS, counted, and 1 × 106 cells (in 100 μl PBS) were used for in vivo studies described later.
Preparation of Coelenterazine and D-luciferin.
Coelenterazine (also known as “native coelenterazine”), a substrate for RL, was purchased from Biotium (Hayward, CA). The compound (2 mg/ml) was dissolved in methanol. Further dilutions were made in 50 mM sodium phosphate buffer (SPB), pH 7. D-luciferin Firefly potassium salt, the substrate for FL, was purchased from Xenogen (Alameda, CA). A 30-mg/ml stock in PBS was filtered through 0.22-μm filters before use.
Luminometer Measurements.
All bioluminescent assays were performed in a TD 20/20 luminometer (Turner Designs, Sunnyvale, CA). Twenty microliters of crude and clarified cell lysates obtained from C6-Rluc cells and mock-transfected C6 control cells were mixed with 100 μl of coelenterazine solution (50 μg/ml) prepared in SPB, pH 7.0. The reaction was measured over 10 min, every 10 s in the luminometer. The protein content of the cell lysates were determined with Bio-Rad protein assay system (Bio-Rad) in a Beckman DU-50 spectrophotometer (Beckman Instruments) and the luminescence results reported as relative light units (RLU) per milligram of protein.
Coelenterazine Dose Studies in Vitro.
Twenty microliters of C6-Rluc and mock-transfected C6 control cell lysates were mixed with 100 μl of coelenterazine prepared at various concentrations (0.1, 1.0, 10, 50, 100, and 200 μg/ml in SPB) and the dose-dependent RLU were recorded using a luminometer for 10 s. The lysates were collected from three separate wells for each dose and the bioluminescence was normalized to protein content.
Crossreactivity Studies in Cell Culture.
To check the crossreactivity of RL with D-luciferin and FL with coelenterazine, C6-Fluc, and C6-Rluc cells were treated with each substrate and analyzed directly by using the cooled CCD camera. Two 12-well plates, one plated with C6-Fluc and the other with C6-Rluc, were prepared. In each plate, to one row of three wells, 2 μg/ml of coelenterazine, and to the second row 150 μg/ml of D-luciferin was added. The last row consisted of mock-transfected control C6 cells. The substrates were diluted in culture media. The plates were placed in the CCD imaging system (see next paragraph) and the images acquired for 1 min. The cells were lysed with lysis buffer and protein content from each well was determined. Results are reported as bioluminescence normalized to protein content.
Imaging and Quantification of Bioluminescence Data.
The in vivo Imaging System (IVIS, Xenogen), consisting of a cooled CCD camera mounted on a light-tight specimen chamber (dark box), a camera controller, a camera cooling system, and a Windows computer system, was used for data acquisition and analysis (7). Each 12-well plate sample or supine mouse was placed in the specimen chamber mounted with the CCD camera cooled to −120°C, with a field of view (FOV) set at 25 cm above the sample shelf. The photon emission, transmitted from cell samples and mice was measured. The gray scale photographic images and bioluminescence color images were superimposed using the LIVINGIMAGE V. 2.11 software overlay (Xenogen) and IGOR image analysis software (V. 4.02 A, WaveMetrics, Lake Oswego, OR). A region of interest (ROI) was manually selected over the signal intensity. The area of the ROI was kept constant and the intensity was recorded as maximum [photons⋅s−1⋅cm−2⋅sr−1 (steradian)] within a ROI.
Imaging RL Bioluminescence in Various Tissues.
All animal handling was performed in accordance with University of California, Los Angeles, and Animal Research Committee guidelines. Three sets of CD-1 mice, 4 weeks old (≈30 g; Charles River Breeding Laboratories) in duplicates, were anesthetized by i.p. injection of ≈40 μl of a ketamine and xylazine (4:1) solutions. To check for background signal from animals not expressing Rluc, one set of mice was tail-vein injected with 0.7 mg/kg body weight of coelenterazine. Of the two other sets, the first mouse set was injected with C6-Rluc cells (1 × 106 cells in 100 μl of PBS) directly into the peritoneal cavity and the second set had the same number of cells injected via tail-vein. One hundred micro liters of 0.36 mg/kg body weight of coelenterazine was injected immediately via tail-vein to observe the bioluminescence from the peritoneum. A higher dose of 2.8 mg/kg body weight of coelenterazine was injected after 90 min of cell injection to the second set, to obtain sufficient bioluminescence from deep tissues such as the liver and lung. A whole-body image was acquired using the cooled CCD camera.
Effects of Coelenterazine Dose in Mouse Studies.
A group of 4-week-old CD-1 mice were anesthetized followed by s.c. implantation of C6-Rluc cells (1 × 106 cells in 100 μl of PBS) in the left forearm region and C6 (control cells) in the right thigh region. Different doses of coelenterazine (0.07, 0.36, 0.7, 1.4, 2.1, 2.8, and 3.5 mg/kg body weight) were injected via tail-vein in duplicate mice. Bioluminescence was measured from both C6 control and C6-Rluc sites over a 10-min time period by using ten 1-min acquisition scans.
Substrate Crossreactivity Studies and Comparison of Fluc and Rluc Expression in Living Mice.
Four sets of anesthetized mice (three mice in each set) were injected at three sites with C6-Rluc in the left forearm, C6-Fluc in the right forearm, and C6 control cells at the right thigh region. To the first set, 100 μl of coelenterazine (0.7 mg/kg body weight) was injected via tail-vein and the mice were scanned with fifteen 1-min scans using the cooled CCD camera. To the second set, 100 μl of D-luciferin (150 mg/kg body weight) was injected via tail-vein and scanned with fifteen 1-min scans. To the third set, a mouse with implanted C6 cells was first injected with 100 μl of coelenterazine solution (0.7 mg/kg body weight) via tail-vein and bioluminescence was recorded using the cooled CCD camera with a 1-min acquisition time. After 3 h, 100 μl of D-luciferin solution (150 mg/kg body weight) was injected again, via tail-vein to the same mouse and an image obtained for 1 min. One mouse in each set was also imaged again by injecting each substrate i.p. instead of via tail-vein.
To study the kinetics of light production from FL and RL in vivo, 200 μl of a mixture of D-luciferin (150 mg/kg body weight) and coelenterazine (0.7 mg/kg body weight; 1:1) was injected via tail-vein to the fourth set of mice. Bioluminescence was measured using ten 1-min acquisition scans.
Results
Different Cell Lines Can Be Successfully Transfected with the pCMV-Rluc Plasmid.
Cell lines from different tissue origins (C6, HeLa, N2a, 293, PC-3) were transiently transfected with the pCMV-Rluc plasmid to check the expression of Rluc. All cell lines shows significantly higher (P < 0.05) levels of gene expression compared with the mock-transfected control cells as assessed by the luminometer by using triplicate samples (data not shown). Successful transfection in different cell lines indicates that Rluc can be expressed in different tissues.
Coelenterazine Induces Flash Kinetics Within the First 10 s in C6-Rluc Cell Lysates, Which Rapidly Decays with Time.
We first determined the time kinetics of light production from RL with the substrate coelenterazine. Cell lysates from transiently transfected C6-Rluc cells were mixed with 50 μg/ml of coelenterazine and the bioluminescent emission was recorded in the luminometer. The light intensity is highest within the first 10 s of the reaction and drops significantly over the course of the next 10 min (Fig. 1). Mock-transfected control C6 cells show a negligible bioluminescence (0.96 × 103 ± 0.14 × 102 RLU/mg).
Figure 1.
Kinetics of light production with C6-Rluc and C6 cell extracts exposed to coelenterazine. The graph shows a peak signal within the first 10 s that goes down steadily for next 10 min. The values plotted were integrated every 10 s. Control C6 cells do not show any significant signal. The values are normalized to mg of total protein. The error bars represents standard error of mean (SEM) of triplicates.
Bioluminescence from C6-Rluc Cell Lysates Increases with Higher Doses of Coelenterazine.
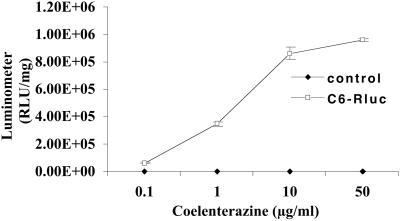
To determine the effects of coelenterazine dose on light yield, a study with coelenterazine and C6-Rluc cell lysates was performed using the luminometer. Coelenterazine, as low as 0.1 μg/ml is able to produce detectable light with cell lysates (Fig. 2). An approximately linear relationship between the doses of coelenterazine (0.1–10 μg/ml) and signal intensity is observed. The peak signal is found with 50 μg/ml of coelenterazine within the first 10 s of reaction. With increasing doses (>50 μg/ml), there is a significant decrease in the signal that is probably due to absorption of light in the buffer that becomes yellow in color with higher concentration of coelenterazine (data not shown). The optimum dose of coelenterazine for further in vitro studies was chosen to be 50 μg/ml.
Figure 2.
Effects of coelenterazine dose on measured light from C6-Rluc and control C6 cell lysates. A dose range of 0.1–50 μg/ml coelenterazine produces a maximum of 9.6 × 105 ± 1.1 × 104 RLU/mg, as measured in the luminometer. A near linear increase with dose is observed between 0.1–10 μg/ml, with some plateauing between 10–50 μg/ml. Control C6 cell lysates do not show any significant signal. The signal from C6-Rluc cell lysates is significantly different (P < 0.05) from control with a dose as low as 0.1 μg/ml. The values are from triplicate wells normalized to mg protein. The error bar represents SEM.
Coelenterazine and d-luciferin Do Not Exhibit Crossreactivity to FL or RL, Respectively, in Cell Culture.
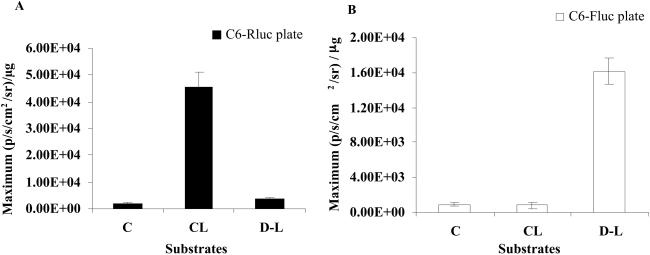
We next checked the crossreactivity of D-luciferin with C6-Rluc cells and coelenterazine with C6-Fluc cells directly in cell culture, using the cooled CCD camera. Fig. 3A shows data from the C6-Rluc cells with negligible bioluminescence when D-luciferin is added to the cell media or when mock transfected C6 cells without addition of substrate are imaged. C6-Rluc cells exposed to coelenterazine show a significantly higher signal (P < 0.01). Similarly, in the C6-Fluc cell plate (Fig. 3B), three wells treated with coelenterazine exhibited minimal signal with C6-Fluc cells as did control cells untreated with substrate, but cells exposed to D-luciferin show a significantly higher signal (P < 0.01).
Figure 3.
Crossreactivity of d-luciferin with RL and coelenterazine with FL in cell culture. (A) The wells with C6-Rluc cells show bioluminescence with coelenterazine (CL) and not with d-luciferin (D-L). (B) The C6-Fluc cells showed bioluminescence with d-luciferin (D-L), whereas CL produces no significant signal from these cells. Control cells, untreated with substrate, show negligible signal in both. All RLU values are normalized to μg of total protein. The error bar represents the SEM for triplicate wells.
C6-Rluc Cells Present in Various Tissues in Living Mice Can Be Imaged in the Cooled CCD Camera After Injection of Coelenterazine.
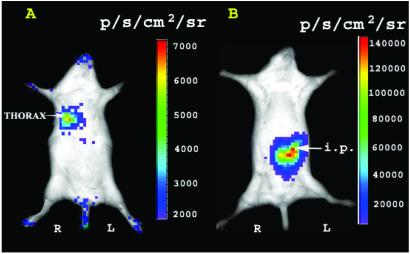
To check the background signal from control mice, duplicate mice were tail-vein injected with 0.7 mg/kg body weight of coelenterazine. They show a relatively low background bioluminescence of 2.78 × 103 ± 0.46 × 103 maximum (photons⋅s−1⋅cm−2·sr−1). To further check whether bioluminescence could be detected from various tissues, we injected 1.0 × 106 C6-Rluc cells suspended in 100 μl of PBS via tail-vein (for cell trafficking to liver and lungs) and into the peritoneal cavity in separate sets of animals. The bioluminescent signal is detected from the thorax region when coelenterazine is tail-vein injected 90 min after cell injection (Fig. 4A). Earlier imaging showed cells initially trafficking to liver region (data not shown). The signal increases with time (peaking at ≈5 min) and subsides within 7–10 min after injection of coelenterazine. Sacrifice of mice and luminometer assessment of tissue homogenates reveled that more signal was present in the right vs. left lung (data not shown). Bioluminescent signal is also detected from cells in the peritoneum after tail-vein injection of coelenterazine (Fig. 4B). The peak signal was seen ≈3 min after injection of coelenterazine and retained for ≈10–12 min. C6-Rluc cells implanted s.c. show a peak signal at ≈1 min after tail-vein injection of coelenterazine (described later). These results were consistent across two different mice.
Figure 4.
RL bioluminescence from C6-Rluc cells present in various tissues in living mice. (A) The C6-Rluc cells (1.0 × 106) were injected via tail-vein and coelenterazine was tail-vein injected 90 min later. The bioluminescence seen represents the thorax region of the mouse where C6-Rluc cells are trapped in the lungs. (B) C6-Rluc cells (1.0 × 106) were implanted in the peritoneum of a different mouse and coelenterazine was tail-vein injected immediately. Bioluminescence is seen only from the i.p. region. R and L represent the right and left side of the mouse resting in supine position.
RL Signal Enhances with Increasing Coelenterazine Dose in Living Mice.
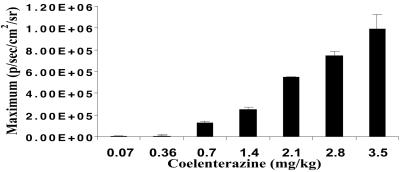
C6-Rluc cells (1 × 106 cells in 100 μl PBS) were s.c. implanted into the left shoulder of a mouse while the mock-transfected C6 cells were implanted in the right thigh. When the mouse is injected with coelenterazine (0.07 mg/kg body weight) via tail-vein there is a detectable bioluminescence of ≈8.3 × 103 ± 0.15 × 103 maximum (photons⋅s−1⋅cm−2⋅sr−1) from an ROI drawn over the site of implantation at the left shoulder area. This signal subsides within 5 min. The C6 control site at the right thigh region shows a signal of ≈3.1 × 103 ± 0.5 × 103 maximum (photons⋅s−1⋅cm−2⋅sr−1). There is a progressive increase in the bioluminescence from the implanted cells with increasing coelenterazine dose from 0.07–3.5 mg/kg body weight (Fig. 5). The duration of bioluminescence also increases with the dose of substrate (data not shown). We kept the dose in the range of 0.36–0.7 mg/kg body weight in further studies to minimize costs.
Figure 5.
RL bioluminescence in living mice depends on the dose of coelenterazine injected. A dose range of coelenterazine from 0.07–3.5 mg/kg body weight was injected via tail-vein in two mice s.c. implanted with C6-Rluc cells. The ROI signal increases as a function of higher coelenterazine dose. The error bar represents mean ± SEM.
There Is Minimal Signal from C6-Rluc and C6-Fluc Cells Implanted Subcutaneously in Mice upon Tail-Vein Injection of d-luciferin and Coelenterazine, Respectively.
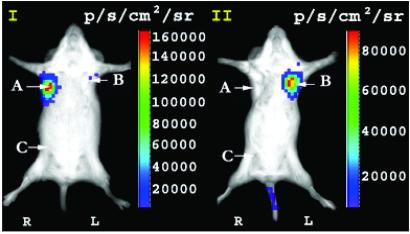
To check for any crossreactivity between the two proteins and substrates in vivo we implanted C6 control, C6-Rluc, and C6-Fluc cells at right thigh, left forearm, and right forearm, respectively, and tail-vein injected coelenterazine followed later by D-luciferin. We observe a significant level of bioluminescence only from the C6-Fluc implanted site when D-luciferin is injected (Fig. 6, mouse I, site A) and background signal from C6-Rluc and control cell sites (Fig. 6, mouse I, sites B and C). Bioluminescence is seen only from the C6-Rluc site when coelenterazine is injected (Fig. 6, mouse II, site B). There is no sign of any crossreactivity up to 15 min of repetitive scanning. We also injected each substrate into the same mouse, 3 h apart, giving sufficient time for the first signal from coelenterazine to dissipate completely, followed by D-luciferin injection, and also found a lack of crossreactivity (data not shown). These results are consistent across three different mice. Similar results are obtained when mice had substrates injected i.p., except that there is a delay in the time to peak bioluminescent signal (data not shown).
Figure 6.
Crossreactivity of RL for d-luciferin and FL for coelenterazine in living mice. Both C6-Fluc (A) and C6-Rluc (B) cells were implanted s.c. at right forearm and left forearm sites respectively in the same mouse with control C6 cell (C) implanted in the right thigh region. Injection of d-luciferin via tail-vein in the mouse I shows bioluminescence from site A and minimal signal from the B and C sites. Injection of coelenterazine via tail-vein in mouse II produce bioluminescence from site B but minimal signal from the A or C sites. R and L represent the right and left side of the mouse resting in supine position.
Bioluminescence in Living Mice Implanted with C6-Rluc and C6-Fluc Shows Distinct Kinetics from Each of the Two Reporter Proteins.
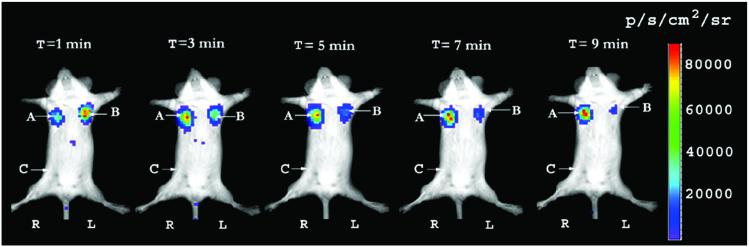
As shown above, RL and FL do not crossreact with each of their respective substrates in vitro or in vivo. Therefore to determine the kinetics of light production when both substrates are injected simultaneously, a mixture of D-luciferin and coelenterazine was injected via tail-vein into the same mouse implanted with both C6-Rluc and C6-Fluc at two s.c. forearm sites and control C6 cells at the thigh region (Fig. 7). It should be noted that the amount of coelenterazine injected is very low (0.7 mg/kg body weight) compared with D-luciferin (150 mg/kg body weight). The C6-Rluc implanted site (left forearm) shows a quick peak in the signal within 1 min, which consistently decreases over the 10-min period. On the other hand, the C6-Fluc site (right forearm) shows a progressive increase of the signal until ≈3–4 min after tail-vein injection, followed by a progressive decrease in the signal. There is strong bioluminescence from C6-Fluc site even at 10 min after injection of both substrates. There is a clear distinction in the pattern of light kinetics with each reporter maintaining its individual characteristics. The control site shows background level of signal. The results are consistent across three different mice.
Figure 7.
Kinetics of light production from mice carrying s.c. C6-Fluc and C6-Rluc cells after simultaneous tail-vein injection of both d-luciferin and coelenterazine. A mouse was injected s.c. with C6-Fluc (A), C6-Rluc (B), and C6 control cells (C) on right forearm, left forearm, and right thigh regions, respectively. Simultaneous injection of both coelenterazine and d-luciferin mixture via tail-vein shows bioluminescence from both the sites simultaneously but with distinct kinetics. A series of image at 2-min intervals is shown from the same mouse. Each image represents a scan time of 1 min. The signal from C6-Rluc cells (B) peaks early and is near extinguished within 10 min. Bioluminescence from C6-Fluc cells (A) shows a relatively strong signal beyond 10 min. The region of control cells does not show any significant bioluminescence. R and L represent the right and left side of the mouse resting in supine position.
Discussion
In the current study we show that Rluc can be used in living mice by measuring light from RL bioluminescence in a cooled CCD camera after mice are tail-vein or i.p. injected with coelenterazine. We initially showed that Rluc can be transiently transfected into a variety of cell types consistent with the literature in which Rluc has been used in numerous different types of cell culture experiments. We showed that the kinetics of light production from cell culture lysates are rapid, with a quick peak in the initial 10 s followed by a rapid decline over 10 min. We show that the peak signal can be increased up to a limit with increasing coelenterazine dose and then decreases. This decrease was unexpected but is thought to be due to color changes in the buffer, which probably lead to greater absorption of bioluminescent light. There is no significant bioluminescence when cells in culture transiently expressing Rluc are exposed to D-luciferin or when cells transiently expressing Fluc are exposed to coelenterazine. These in vitro and cell culture data support the unique characteristics of Rluc and also provide a basis for using both Rluc and Fluc in the same living animal due to the lack of significant crossreactivity of RL and FL for their respective substrates.
The results in living mice show the ability to image C6-Rluc cells implanted in the liver, lungs, peritoneum, and s.c. regions. The ability to image cells in deeper tissue was achieved by injecting cells via the tail-vein and letting them naturally traffic to the lungs via the liver as we and others have previously reported. We find that higher doses of coelenterazine injected via tail-vein have to be used to detect C6-Rluc cells from deeper tissues as compared with superficial tissues. This result is likely due to less transmitted light from deeper tissues and formal quantitative relationships between depth and light transmission will help to better characterize this in future studies. Although most of the bioluminescent light is likely to be scattered and absorbed, enough escapes from the animal to be detected by the highly sensitive cooled CCD camera. The results show that bioluminescence can also be produced while injecting coelenterazine i.p., although the kinetics of light production from nonperitoneal sites are slower. This observation is probably due to a slower transit time for coelenterazine to get into the blood from the peritoneal space. The kinetics of light production from regions containing C6-Rluc cells depends on the route of coelenterazine injection as well as on the specific site of the cells. Increasing the dose of tail-vein injected coelenterazine in mice s.c. implanted with C6-Rluc cells shows an increase in the CCD measured light. Studies to compare the sensitivity in terms of photon yield/concentration of bioluminescent protein/concentration of substrate will also be needed to better understand the differences between Fluc and Rluc. It is also important to note that C6 control cells implanted in mice show only background signal when mice were tail-vein or i.p. injected with coelenterazine. Control mice with no cells implanted and injected with coelenterazine also only show background signal. The background signal is due to various factors including low levels of light emitted from the mice even though there is no bioluminescent light, low levels of photons in the “light-tight” box, as well as noise from the CCD camera due in part to thermal drift.
Results from mice carrying both the C6-Rluc and C6-Fluc cells implanted s.c. show that the RL and FL signal can be distinguished by separate injections of D-luciferin and coelenterazine as was also seen in cell culture studies. Although the FL:D-luciferin signal was much higher, it must be noted that the amount of coelenterazine used was relatively low compared with D-luciferin to minimize costs. D-luciferin was purchased at a cost of $5/mg and coelenterazine at a cost of $190/mg. However, the effective cost per mouse study for D-luciferin was $15 (3 mg/mouse), and $19 for the highest dose of coelenterazine used (100 μg/mouse). With increasing use of coelenterazine it is likely that improved methods for its synthesis and demand will further reduce costs.
In mice carrying both C6-Rluc and C6-Fluc cells implanted s.c., which are injected simultaneously with D-luciferin and coelenterazine via tail-vein, distinct light kinetics from RL and FL are observed. Light from the C6-Rluc cells quickly peaks and is rapidly extinguished, whereas light from C6-Fluc cells peaks later and persists longer. The difference in light kinetics may allow separation of reporter protein signal through obtaining multiple images in the same mouse after co-injection with both D-luciferin and coelenterazine.
Several issues could have hindered the success of imaging Rluc expression with coelenterazine in living mice. These issues include instability of coelenterazine in plasma, insufficient delivery of coelenterazine to target sites, as well as insufficient bioluminescent light yield. Rluc may have some distinct advantages over Fluc that deserve specific mention. RL is a 36-kDa monomeric enzyme that catalyzes the oxidation of coelenterazine in presence of oxygen to generate a flash of blue luminescence with a wavelength centering at 482 nm. The oxidative decarboxylation of coelenterazine by RL in the presence of oxygen yields “oxyluciferin” CO2 and blue light (λmax = 480 nm) in vitro (9). The more commonly used FL is a 61-kDa single-subunit protein that catalyzes D-luciferin to produce oxyluciferin in the presence of oxygen, cofactors, Mg+2, and ATP to give a flash of green light at 562 nm (21). It should be noted that the two proteins RL and FL and the two substrates coelenterazine and D-luciferin are structurally unrelated. The RL–coelenterazine reaction is simpler compared with the FL–D-luciferin reaction (20). RL has a significant advantage in that it does not need cofactors or ATP, and therefore Rluc is ideal because it likely causes less perturbation to the cells in which it is expressed. Also, the rapid light kinetics of RL in living mice may be quite useful in animal experiments where a quick signal is needed that does not persist over time. Future studies need to look at the half-life of Rluc mRNA and RL to better understand the ability to repetitively image changes in reporter gene expression. Furthermore, quantitative relationships between levels of Rluc mRNA, RL, coelenterazine delivery, and bioluminescence will also be useful for building fully quantitative assays.
The current study has not addressed the biodistribution of coelenterazine in mice. Previous studies with Fluc and D-luciferin have also not addressed the biodistribution of D-luciferin but have found through placing cells in various sites, using various gene delivery vectors, and transgenic models the high accessibility of D-luciferin to various tissues, including the brain. It is likely that coelenterazine will also be accessible to many tissues because of its diffusable nature (18), but this will require further detailed investigation. We are currently pursuing biodistribution studies by attempting to radiolabel both coelenterazine and D-luciferin. It will be important to perform these studies with different doses of cold and radiolabeled substrate to study changes in biodistribution (if any). Furthermore potential differences in biodistribution between mice and rats will need to be studied. The current study has also not addressed any potential toxicities of repetitively using coelenterazine in living mice. We did not observe any direct toxicity in our mice studies, but formal toxicology studies will need to be performed. The stable expression of Rluc gene in C5 fibroblast has indirectly demonstrated the nontoxicity of the Rluc gene product in mammalian cells as reported (18). Toxicology studies have also not yet been reported for D-luciferin, even though many published studies use this substrate in living rodents.
The use of Rluc in various paradigms in living subjects including gene delivery, cell trafficking, and transgenic models is becoming possible. Pending further kinetic, biodistribution, and toxicology studies, it should be feasible to use both Rluc and Fluc in numerous models in which two independent optical signals are desired for measurement of the location, magnitude, and persistence of expression of two different genes. Additional future studies with Rluc may be able to use mutants of RL that have significant wavelength differences from the wild-type, and synthetic versions of Rluc better optimized for efficient expression in mammalian cells. RL in combination with FL and its mutants may allow multiplexing approaches in which several molecular events can be simultaneously studied through the use of multiple reporter genes, each with proteins that have distinct signals due to wavelength, substrate specificity, or both.
Acknowledgments
We thank Mani Berenji and Meera Iyer for helping with early phases of the project, and Xiaoman Lewis for the tail-vein injections. We thank David Stout for help with the CCD camera calibration. This work is supported in part by National Institutes of Health Grants P50 CA86306, R0-1 CA82214, SAIRP R24 CA92865, Department of Energy Contract DE-FC03-87ER60615, and CaP Cure.
Abbreviations
- CCD
charged-coupled device
- FL
firefly luciferase enzyme/protein
- GFP
green fluorescent protein
- RL
Renilla luciferase enzyme/protein
- RLU
relative light units
- ROI
region of interest
References
- 1.Ray P, Bauer E, Iyer M, Barrio J R, Satyamurthy N, Phelps M E, Herschman H R, Gambhir S. Semin Nucl Med. 2001;31:321–320. doi: 10.1053/snuc.2001.26209. [DOI] [PubMed] [Google Scholar]
- 2.Bremer C, Weissleder R. Acad Radiol. 2001;8:15–23. doi: 10.1016/s1076-6332(03)80739-0. [DOI] [PubMed] [Google Scholar]
- 3.Contag C H, Spilman S D, Contag P R, Oshiro M, Eames B, Dennery P, Stevenson D K, Benaron D A. Photochem Photobiol. 1997;66:523–531. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 4.Contag P R, Olomu I N, Stevenson D K, Contag C H. Nat Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 5.Lipshutz G S, Gruber C A, Cao Y, Hardy J, Contag C H, Gaensler K M L. Mol Ther. 2001;3:284–292. doi: 10.1006/mthe.2001.0267. [DOI] [PubMed] [Google Scholar]
- 6.Honigman A, Zeira E, Ohana P, Abramovitz R, Tavor E, Bar I, Zilberman Y, Rabinovsky R, Gazit D, Joseph A, et al. Mol Ther. 2001;4:239–249. doi: 10.1006/mthe.2001.0437. [DOI] [PubMed] [Google Scholar]
- 7.Wu J C, Sunderasan G, Iyer M, Gambhir S. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 8.Hastings J W. Gene. 1996;173:5–11. doi: 10.1016/0378-1119(95)00676-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilson T, Hastings J W. Annu Rev Cell Dev Biol. 1998;14:197–230. doi: 10.1146/annurev.cellbio.14.1.197. [DOI] [PubMed] [Google Scholar]
- 10.Contag C H, Contag P R, Mullins J I, Spilman S D, Stevenson D K, Benaron D A. Mol Microbiol. 1995;18:593–603. doi: 10.1111/j.1365-2958.1995.mmi_18040593.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Baranov E, Moossa A R, Penman S, Hoffman R M. Proc Natl Acad Sci USA. 2000;97:12278–12282. doi: 10.1073/pnas.97.22.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chishima T, Miyagi Y, Wang X, Tan Y, Shimada H, Moossa A, Hoffman R M. Anticancer Res. 1997;17:2377–2384. [PubMed] [Google Scholar]
- 13.Hoffman R M. Cancer Metastasis Rev. 1998;17:271–277. doi: 10.1023/a:1006188412324. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa S, Yang M, Chishima T, Miyagi Y, Shimada H, Moossa A R, Hoffman R M. Cancer Gene Ther. 2000;7:1336–1340. doi: 10.1038/sj.cgt.7700244. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Yanh M, Baranov E, Wang X, Penman S, Moossa A R, Hoffman R M. Proc Natl Acad Sci USA. 2001;98:9814–9818. doi: 10.1073/pnas.161275798. . (First Published July 31, 2001; 10.1073/pnas.161275798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edinger M, Sweeney T J, Tucker A A, Olomu A B, Negrin R S, Contag C H. Neoplasia. 1999;1:303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz W W, McCann R O, Longiaru M, Cormier M J. Proc Natl Acad Sci USA. 1991;88:4438–4442. doi: 10.1073/pnas.88.10.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz W W, Cormier M J, O'Kane D J, Hua D, Escher A A, Szalay A A. J Biolumin Chemilumin. 1996;11:31–37. doi: 10.1002/(SICI)1099-1271(199601)11:1<31::AID-BIO398>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Matthews J C, Hori K, Cormier M J. Biochemistry. 1977;16:5217–5220. doi: 10.1021/bi00643a009. [DOI] [PubMed] [Google Scholar]
- 20.Inouye S, Shimomura O. Biochem Biophys Res Commun. 1997;233:349–353. doi: 10.1006/bbrc.1997.6452. [DOI] [PubMed] [Google Scholar]
- 21.DeLuca M, McElroy W D. Biochemistry. 1974;13:921–925. doi: 10.1021/bi00702a015. [DOI] [PubMed] [Google Scholar]