Abstract
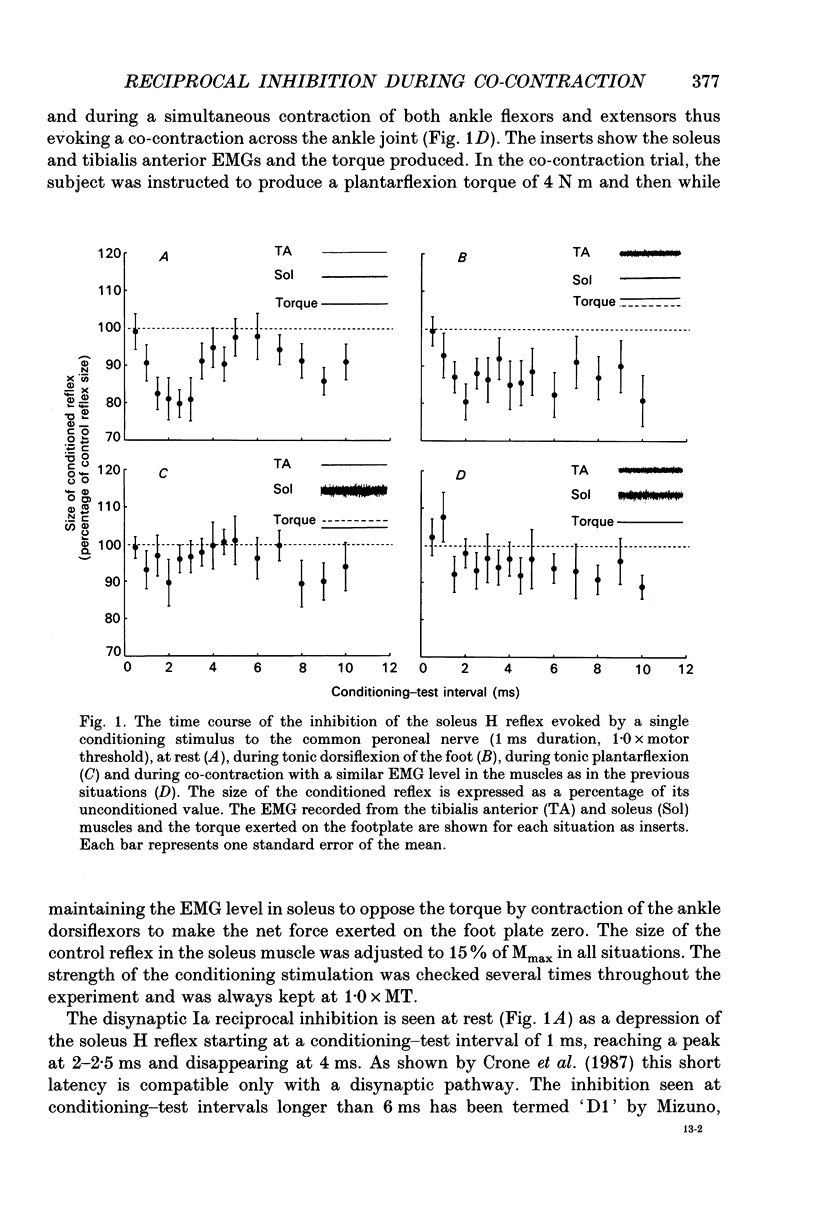
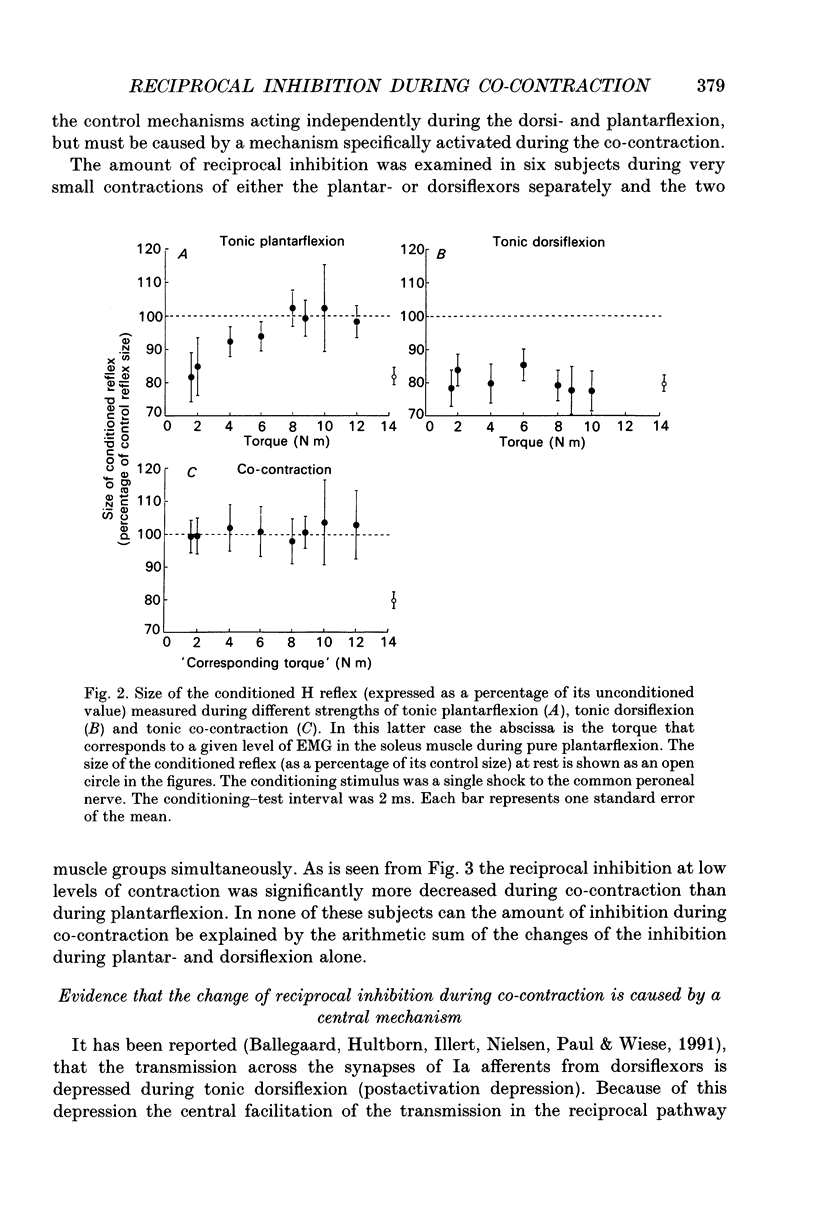
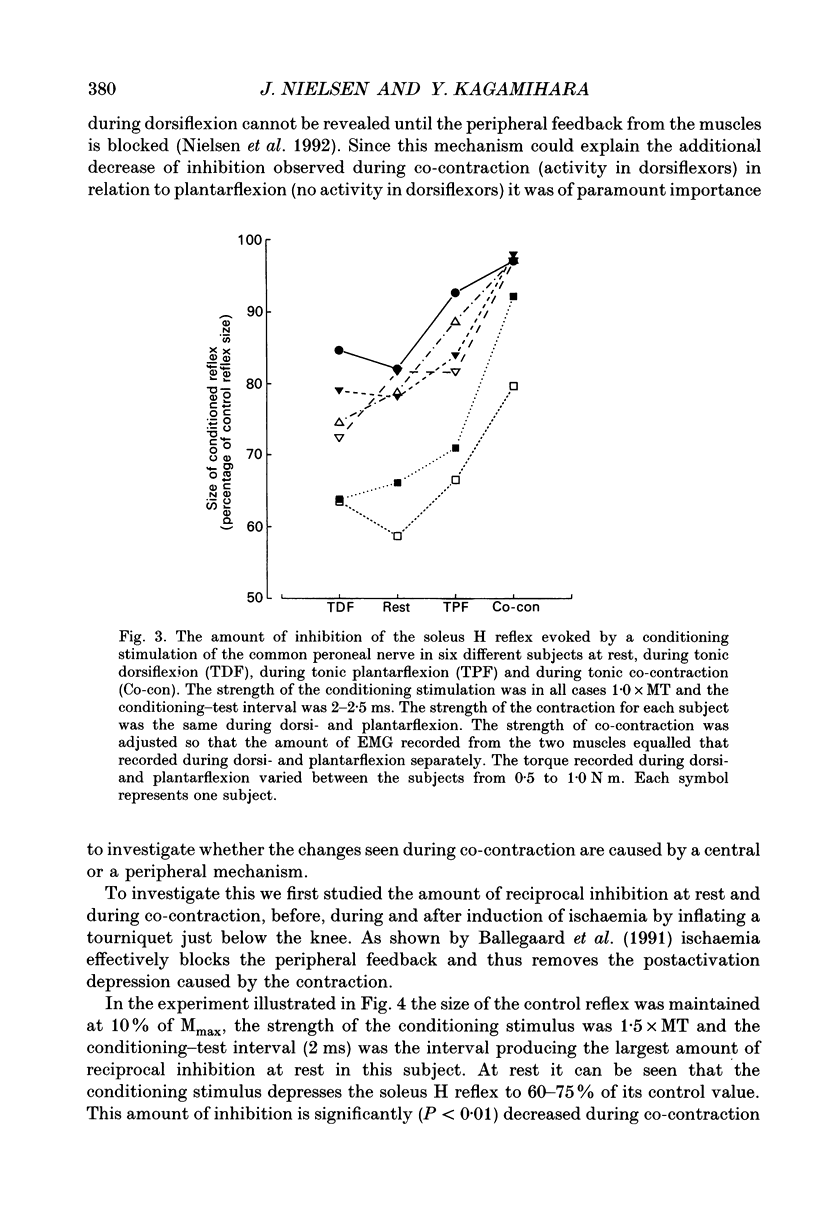
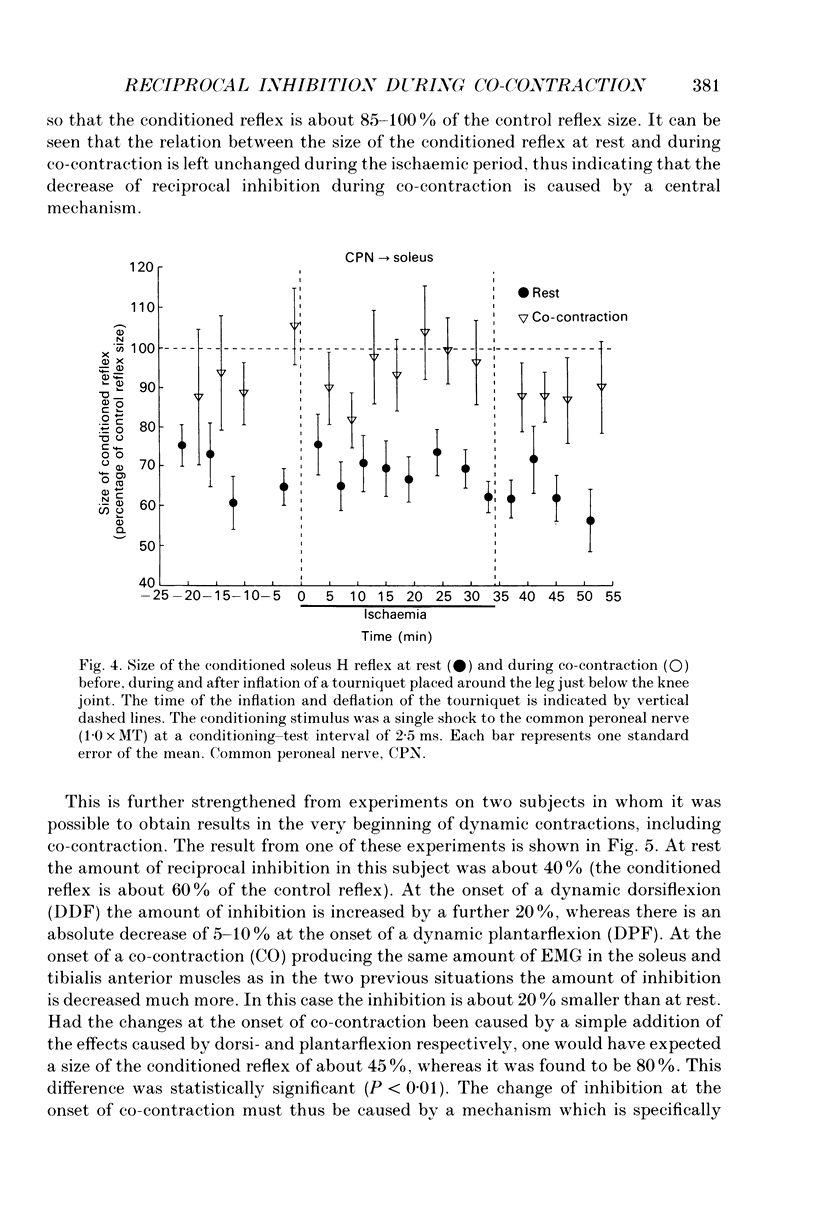
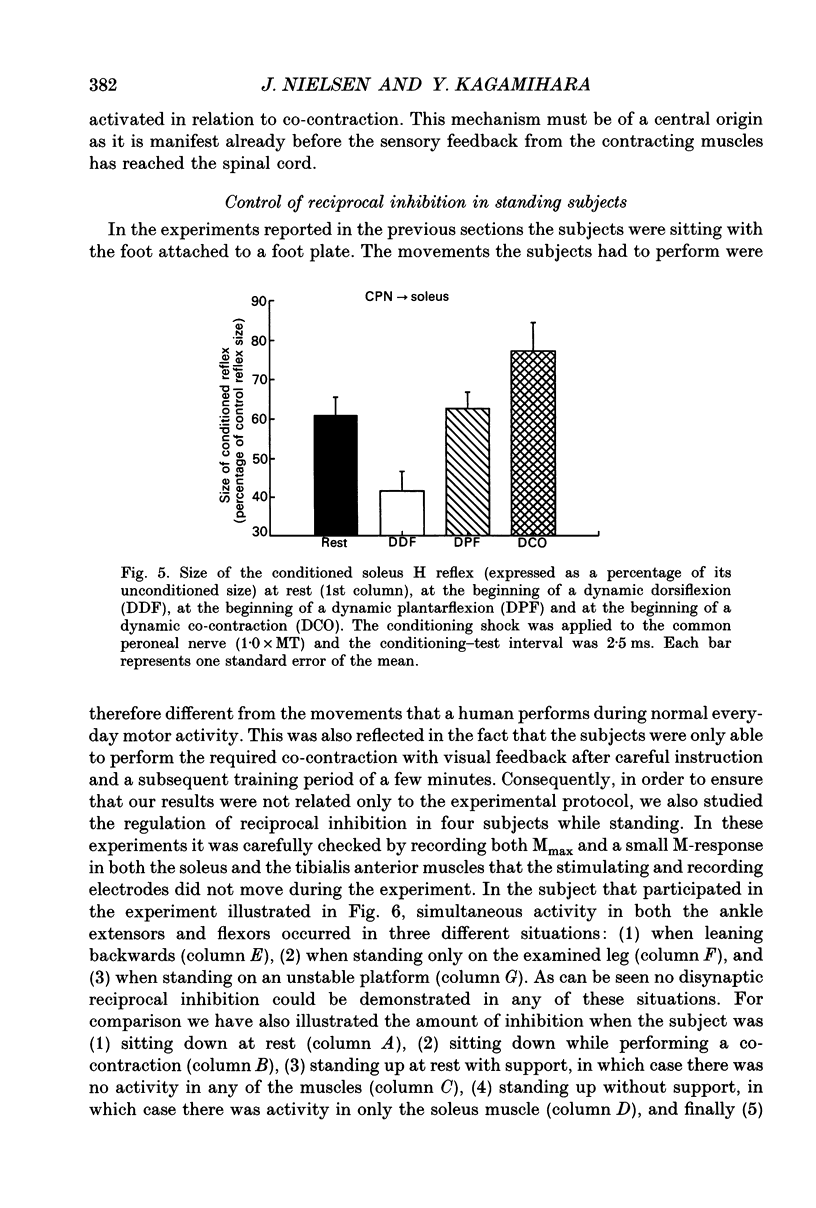
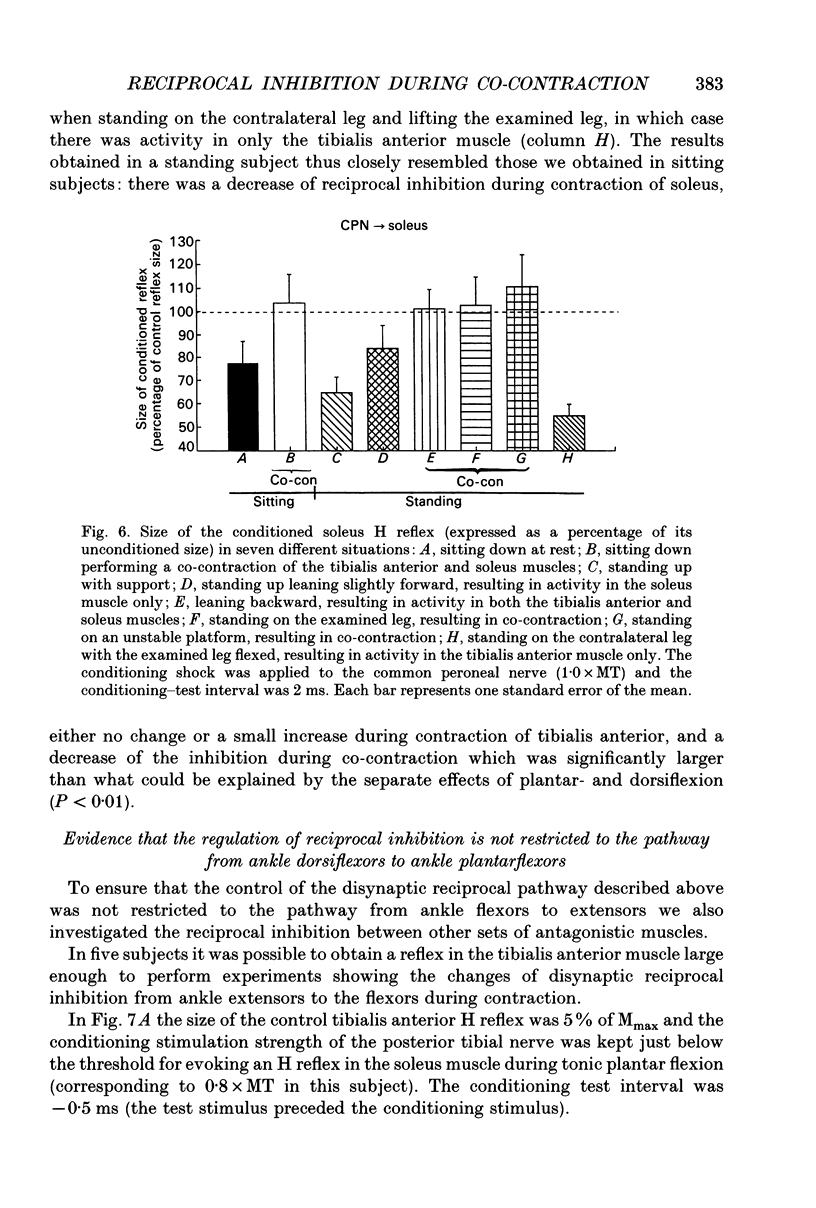
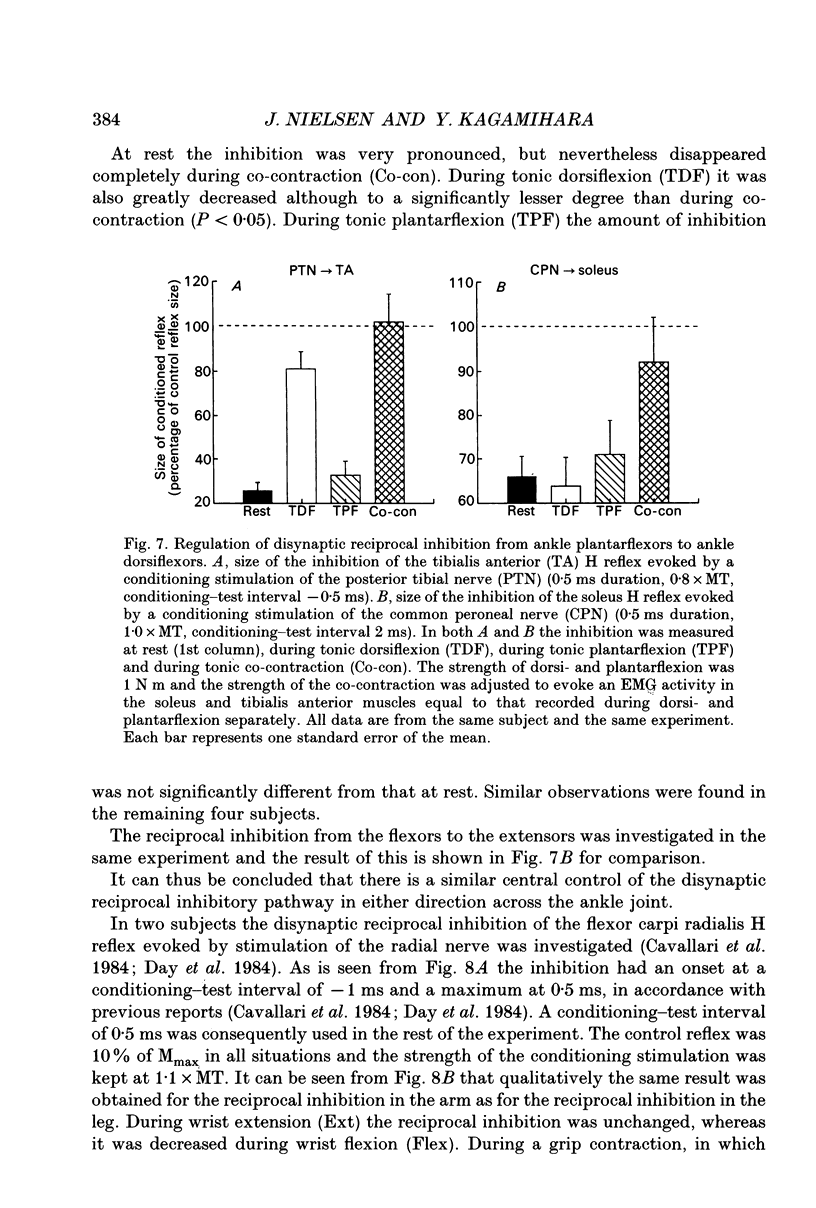
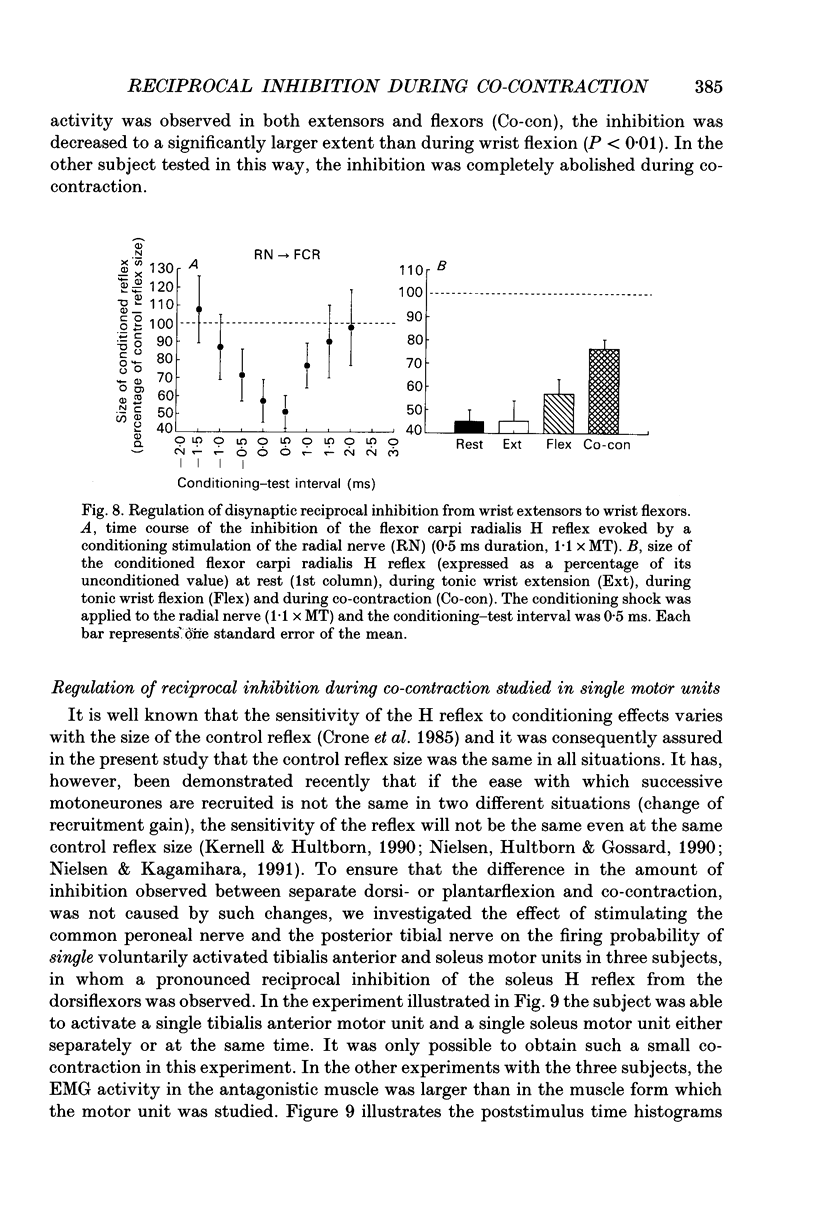
1. The disynaptic reciprocal inhibition from ankle dorsiflexors to ankle plantarflexors was investigated at rest, during tonic plantar- and dorsiflexion and during co-contraction. In relation to rest, it was found to be decreased during plantarflexion and co-contraction, but unchanged during dorsiflexion. 2. When increasing the strength of plantarflexion the amount of inhibition became progressively smaller. Already during weak co-contraction, the amount of inhibition was very small and it did not become smaller during stronger contraction. The decrease of inhibition during co-contraction could not be explained by an addition of the changes of inhibition observed during plantar- and dorsiflexion individually. 3. The disynaptic reciprocal inhibition was also found to be decreased when the peripheral feedback from the muscles was blocked by inducing ischaemia in the leg and at the beginning of a dynamic co-contraction before sensory feedback could interfere. This implies that the observed decrease is caused by a central inhibition of the transmission in the pathway. 4. The amount of disynaptic reciprocal inhibition was also investigated during standing. No significant difference in the amount of inhibition was found when the subjects were standing up at rest as compared to sitting down at rest. When the subjects were forced to make a co-contraction in order to maintain balance, i.e. when they were standing on one leg, leaning backwards or standing on an unstable platform, a decrease of disynaptic reciprocal inhibition was seen. When the subjects leaned forward, thus forcing a contraction of the soleus muscle, a decrease was also seen, but it was smaller than in the co-contraction tasks. Finally, when the subjects lifted the examined leg, thus contracting the tibialis anterior muscle, either no change or a small increase of inhibition was seen. 5. A similar control of the disynaptic reciprocal inhibition as described for the pathway from ankle dorsiflexors to ankle plantarflexors was also observed for the pathway from ankle plantarflexors to dorsiflexors and from wrist extensors to wrist flexors. 6. It is concluded that when co-contraction is used in order to stabilize a joint, i.e. to maintain posture, a specific co-contraction motor programme is activated that depresses the transmission in the disynaptic reciprocal pathway thereby ensuring a high excitability level in the motoneurones of both antagonistic muscles.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby P., Labelle K. Effects of extensor and flexor group I afferent volleys on the excitability of individual soleus motoneurones in man. J Neurol Neurosurg Psychiatry. 1977 Sep;40(9):910–919. doi: 10.1136/jnnp.40.9.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner F. D., Baker J. R., Stephens J. A. Correlation between the discharges of motor units recorded from the same and from different finger muscles in man. J Physiol. 1991 Jan;432:355–380. doi: 10.1113/jphysiol.1991.sp018389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner F. D., Baker J. R., Stephens J. A. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol. 1991 Jan;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P., Fournier E., Katz R., Pierrot-Deseilligny E., Shindo M. Changes in reciprocal Ia inhibition from wrist extensors to wrist flexors during voluntary movement in man. Exp Brain Res. 1984;56(3):574–576. doi: 10.1007/BF00237999. [DOI] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Jespersen B., Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987 Aug;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Jespersen B. Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res. 1985;59(2):418–422. doi: 10.1007/BF00230924. [DOI] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Mazières L., Morin C., Nielsen J., Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81(1):35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Crone C., Nielsen J. Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol. 1989 Sep;416:255–272. doi: 10.1113/jphysiol.1989.sp017759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Marsden C. D., Obeso J. A., Rothwell J. C. Reciprocal inhibition between the muscles of the human forearm. J Physiol. 1984 Apr;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman A. G. Superposition of motor programs--I. Rhythmic forearm movements in man. Neuroscience. 1980;5(1):81–90. doi: 10.1016/0306-4522(80)90073-1. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Functional relations between primate motor cortex cells and muscles: fixed and flexible. Ciba Found Symp. 1987;132:98–117. doi: 10.1002/9780470513545.ch7. [DOI] [PubMed] [Google Scholar]
- Fournier E., Meunier S., Pierrot-Deseilligny E., Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986 Aug;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C., Stark L. Interactions between voluntary and postural mechanisms of thehuman motor system. J Neurophysiol. 1970 May;33(3):365–381. doi: 10.1152/jn.1970.33.3.365. [DOI] [PubMed] [Google Scholar]
- Hongo T., Lundberg A., Phillips C. G., Thompson R. F. The pattern of monosynaptic Ia-connections to hindlimb motor nuclei in the baboon: a comparison with the cat. Proc R Soc Lond B Biol Sci. 1984 May 22;221(1224):261–289. doi: 10.1098/rspb.1984.0034. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Meunier S., Pierrot-Deseilligny E., Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987 Aug;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Transmission in the pathway of reciprocal Ia inhibition to motoneurones and its control during the tonic stretch reflex. Prog Brain Res. 1976;44:235–255. doi: 10.1016/S0079-6123(08)60736-0. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R., Reed D. J. Separate cortical systems for control of joint movement and joint stiffness: reciprocal activation and coactivation of antagonist muscles. Adv Neurol. 1983;39:347–372. [PubMed] [Google Scholar]
- Iles J. F. Reciprocal inhibition during agonist and antagonist contraction. Exp Brain Res. 1986;62(1):212–214. doi: 10.1007/BF00237419. [DOI] [PubMed] [Google Scholar]
- Jongen H. A., Denier van der Gon J. J., Gielen C. C. Inhomogeneous activation of motoneurone pools as revealed by co-contraction of antagonistic human arm muscles. Exp Brain Res. 1989;75(3):555–562. doi: 10.1007/BF00249906. [DOI] [PubMed] [Google Scholar]
- Katz R., Morin C., Pierrot-Deseilligny E., Hibino R. Conditioning of H reflex by a preceding subthreshold tendon reflex stimulus. J Neurol Neurosurg Psychiatry. 1977 Jun;40(6):575–580. doi: 10.1136/jnnp.40.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990 Jan 15;507(1):176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Mao C. C., Ashby P., Wang M., McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56(2):341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Tanaka R., Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971 Nov;34(6):1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Muir R. B., Lemon R. N. Corticospinal neurons with a special role in precision grip. Brain Res. 1983 Feb 21;261(2):312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Kagamihara Y., Crone C., Hultborn H. Central facilitation of Ia inhibition during tonic ankle dorsiflexion revealed after blockade of peripheral feedback. Exp Brain Res. 1992;88(3):651–656. doi: 10.1007/BF00228194. [DOI] [PubMed] [Google Scholar]
- Shindo M., Harayama H., Kondo K., Yanagisawa N., Tanaka R. Changes in reciprocal Ia inhibition during voluntary contraction in man. Exp Brain Res. 1984;53(2):400–408. doi: 10.1007/BF00238170. [DOI] [PubMed] [Google Scholar]
- Smith A. M. The coactivation of antagonist muscles. Can J Physiol Pharmacol. 1981 Jul;59(7):733–747. doi: 10.1139/y81-110. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Usherwood T. P., Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976 Sep 23;263(5575):343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21(5):529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]



