Abstract
Neuropilin (NRP) 1, previously identified as a neuronal receptor that mediates repulsive growth cone guidance, has been shown recently to function also in endothelial cells as an isoform-specific receptor for vascular endothelial growth factor (VEGF)165 and as a coreceptor in vitro of VEGF receptor 2. However, its potential role in pathologic angiogenesis remains unknown. In the present study, we first show that VEGF selectively up-regulates NRP1 but not NRP2 via the VEGF receptor 2-dependent pathway. By NRP1 binding analysis, we showed that its induction by VEGF accompanies functional receptor expression. Endothelial proliferation stimulated by VEGF165 was inhibited significantly by antibody perturbation of NRP1. In a murine model of VEGF-dependent angioproliferative retinopathy, intense NRP1 mRNA expression was observed in the newly formed vessels. Furthermore, selective NRP1 inhibition in this model suppressed neovascular formation substantially. These results suggest that VEGF cannot only activate endothelial cells directly but also can contribute to robust angiogenesis in vivo by a mechanism that involves up-regulation of its cognate receptor expression.
Deregulated retinal neovascularization accounts for most angioproliferative ocular diseases including retinopathy of prematurity, diabetic retinopathy, and age-related macular degeneration. Recent studies aimed at elucidating the mechanisms underlying these vision-threatening disorders have focused on the causal cytokines and verified that vascular endothelial growth factor (VEGF) is the key angiogenic factor in these pathologic conditions (1, 2).
Among the diverse angiogenic cytokines, VEGF is distinctive in that its mitogenic effect is highly specific for endothelial cells (ECs; ref. 3) and its expression is up-regulated by hypoxia (4) and hypoglycemia (5). Targeted disruption of even a single allele of this gene in mice is sufficient to cause vascular abnormality and leads to embryonic lethality, indicating that the in vivo level of VEGF is critical for correct vascular development (6, 7). The VEGF receptor (VEGFR) family is comprised of VEGFR1/Flt-1, VEGFR2/KDR, and VEGFR3/Flt-4. Targeted gene disruption for VEGFR1 or VEGFR2 in mice results in embryonic lethality and exhibits lack of tube formation (8) and differentiation of hemangioblasts into ECs (9), respectively, thus verifying the requisite roles of these receptors in embryonic vasculogenesis and angiogenesis.
The neuropilin (NRP) family is comprised of two members. NRP1 was identified originally as a cell surface glycoprotein expressed on axons and has been shown to function as a neuronal receptor for repulsive signals elicited by its ligand, semaphorin 3A (Sema3A; refs. 10 and 11). In contrast, NRP2 binds to Sema3C and Sema3F with high affinity and mediates repulsion of sympathetic neurons (10, 12). Recent studies have demonstrated that NRP1 is expressed also in ECs and functions as an isoform-specific receptor for VEGF165 (13), which is identical to the previously reported receptor mediating inhibition of VEGF165-induced proliferation by exon 7 of VEGF (14). Targeted disruption of this gene in mice resulted in defects in the cardiovascular system in addition to defects of the nervous system (15). However, definition of the in vivo role of NRP1 under pathologic conditions is elusive because of the embryonic lethality that occurs from these genetic alterations. Because NRP1 coexpression has been reported to enhance VEGF binding to VEGFR2 by up to 6-fold (13), it is tempting to consider that NRP1 might govern the substantial effects of the VEGF-VEGFR system. On the other hand, the NRP1 ligand, VEGF165, is deemed to be one of the most biologically active splice variants by virtue of the fact that it is abundant in both physiologic and pathologic angiogenesis (16, 17). These observations seem to underscore the importance of elucidation of NRP1 gene expression in pathologic conditions. In the present study, we first delineated NRP expression in angiogenic circumstances by using VEGF as the stimulus and found selective NRP1 induction that is mediated by VEGFR2. We further demonstrated in vivo a significant role of NRP1 in pathologic angiogenesis by inhibition of this receptor in angioproliferative retinopathy.
Materials and Methods
Materials.
VEGF165, VEGF121, placenta growth factor, fibroblast growth factor-2, hepatocyte growth factor, and VEGFR2 chimeric Ab (VEGFR2-Fc, extracellular domain of VEGFR2 fused to Fc of human IgG1) were purchased from Genzyme. Genistein and GF109203X were purchased from LC Laboratories (Boston). PD098059 was purchased from Upstate Biotechnology (Lake Placid, NY). Sema3A encoding vector, functional blocking NRP1 and NRP2 Abs were provided generously by Alex Kolodkin and David Ginty (Johns Hopkins University School of Medicine, Baltimore; ref. 10). Anti-VEGFR2 and antiphosphorylated VEGFR2 (Tyr-996) mAbs were purchased from Chemicon and Cell Signaling (Beverly, MA), respectively. All other chemicals were purchased from Calbiochem unless otherwise indicated.
Cell Culture and VEGF Treatment.
Primary cultures of bovine retinal ECs (BRECs) and bovine aortic ECs (BAECs) were cultured as described (18). MDA-MB231 breast cancer cells (ATCC no. HTB-26) were cultured in DME supplemented with 10% FBS (ICN). 293EBNA (Invitrogen) and 293T (GenHunter, Nashville, TN) cells were cultured in DME supplemented with 10% FBS and 250 μg/ml geneticin. Human umbilical vein ECs were cultured in EC basal medium-2 (Clonetics) with supplement provided by the manufacturer. To determine the contribution of VEGFRs and downstream signaling molecules, cells were pretreated with the specific inhibitors before the addition of VEGF (25 ng/ml).
Amplification of Human NRP1 and NRP2 cDNAs Using Reverse Transcriptase-PCR.
cDNA templates for PCR were synthesized by reverse transcriptase (first strand kit, Invitrogen) from human umbilical vein ECs. For NRP1, NRP2, and Sema3A cDNA, a standard PCR was performed (PCR optimizer kit, Invitrogen) by using 5′-CAC ATT GGG CGT TAC TGT GGA C-3′ (NRP1 sense primer), 5′-CCT TTG TGG TTG GGG TGT CTA C-3′ (NRP1 antisense primer), 5′-GAG GAG TGG CTT CAG GTA GAT C-3′ (NRP2 sense primer), and 5′-CGG GAC ACC AAC CTA CAT ACT C (NRP2 antisense primer). These cDNAs then were subcloned into a vector (pCR II, Invitrogen) and used for hybridization.
Northern Blot Analysis.
Total RNA was isolated by using acid guanidium thiocyanate, and Northern blot analysis was performed as described (18). Briefly, total RNA (20 μg) was electrophoresed through 1% formaldehyde-agarose gels and then transferred to a nylon membrane (Pall). Radioactive cDNA probes were generated by use of dCTP labeled with 32P (Bio-Rad), and blots were hybridized with the indicated cDNA probe for 24 h. All signals were scanned and analyzed by using a densitometer (BAS-2000 II, Fuji).
Nuclear Run-On Assays.
Nuclear run-on assays were performed as described (18). Briefly, BRECs were lysed, and the nuclear suspension was prepared by incubating the isolated nuclei with ATP, CTP, GTP (50 mM each), and 3.7 MBq of 32P-labeled UTP (Amersham Pharmacia) for 30 min. The samples of equal counts were hybridized with nitrocellulose filters (Schleicher & Schüll) deposited with cDNA probes. The filters then were washed, and the radioactivity was measured by using a densitometer (BAS-2000II). The levels of NRP1 mRNA were normalized to that of 36B4. Empty plasmid vector was used as a control for nonspecific hybridization.
Analysis of NRP1 mRNA Half-Life.
BRECs were treated with 25 ng/ml VEGF for 2 h before mRNA stability experiments. Thereafter, half the plates were returned to DME without VEGF, and actinomycin D (10 μg/ml) was added to all plates. Total RNA was isolated at 0, 2, and 4 h after the addition of actinomycin D, and Northern blot analysis was performed.
Western Blot Analysis.
Cells were washed three times with cold PBS and then solubilized with lysis buffer (1% Triton X-100/50 mM Hepes/10 mM EDTA/10 mM sodium pyrophosphate/100 mM sodium fluoride/1 mM sodium orthovanadate/1 mg/ml aprotinin/1 mg/ml leupeptin/2 mM PMSF). An equal amount of protein (30 μg) from each sample was subjected to 7.5% SDS gel electrophoresis and transferred to polyvinylidene difluoride membrane (Schleicher & Schüll). After blocking with PBS containing 0.1% Tween 20 (PBS-T) and 3% BSA for 1 h at room temperature, the membrane was incubated with primary Ab for 1.5 h. After washing with PBS-T, the membrane was incubated with horseradish peroxidase-conjugated secondary Ab followed by further washes with PBS-T. The signals were detected by using the ECL Western blot analysis system (Amersham Pharmacia) and x-ray films.
Generation of Alkaline Phosphatase (AP)-Fusion Proteins.
To establish stable 293EBNA cell lines expressing Sema3A-AP (N terminus) fusion protein, the Sema3A coding sequence was inserted into pAPtag4 vector (GenHunter), and the entire Sema3A-AP sequence was then subcloned to pCEP4 vector (Invitrogen) followed by hygromycin B selection. The amount of AP activity in the conditioned medium was titrated to correspond to the activity of known amounts of AP. For competition analysis in binding experiments, the Sema3A coding sequence subcloned into pcDNA6/His (Invitrogen) was transfected to 293T cells to produce unfused Sema3A protein.
Quantitative Cell Surface Binding Analysis.
Quantitative cell surface binding analysis was performed essentially as described (19). Briefly, BRECs were washed with PBS and then incubated for 1 min with PBS supplemented with 5 mg/ml BSA/20 mM acetic acid, pH 3.75, to dissociate cell surface receptor-bound VEGF (20). Cells then were washed with Hanks' balanced salt solution with 0.5 mg/ml BSA/0.1% NaN3/20 mM Hepes, pH 7.0, followed by incubation for 90 min at room temperature with either Sema3A-AP (for total binding) or Sema3A-AP plus 100 nM unfused Sema3A (for nonspecific binding). After solubilization with 1% Triton X-100 and 10 mM Tris⋅HCl, pH 8.0, nuclei were spun out. The supernatants were incubated at 72°C for 3 h to inactivate endogenous AP activity. The samples then were incubated with the phosphatase reaction mixture (AP assay reagent A, GenHunter), and AP activity was determined colorimetrically by measuring OD at 405 nm. Specific binding was calculated by subtracting nonspecific from total binding.
Crosslinking Experiments.
BRECs were incubated with binding buffer (1.2 ml of DME/20 mM Hepes, pH 7.2/0.1% gelatin) containing the indicated concentrations of 125I-VEGF165 (Amersham Pharmacia) for 2 h at 4°C (21). At the end of the incubation, bound 125I-VEGF165 was crosslinked to the cells with disuccinimidyl suberate. All the buffers used after the crosslinking reaction contained the following protease inhibitors: phenylmethylsulfonyl fluoride (1 mM), leupeptin (1 μg/ml), aprotinin (1.5 μg/ml), and EDTA (1 mM). Cells then were solubilized in 50 μl of cold lysis buffer (10 mM Tris⋅HCl, pH 7/1% Nonidet P-40 and protease inhibitors). Samples containing equal amounts of protein were boiled for 3 min and analyzed by SDS/PAGE using 6% polyacrylamide gels. Signals were scanned by using a densitometer (BAS-2000 II).
Cell Proliferation Assay.
Approximately 2 × 103 BRECs per well were seeded in complete medium onto a fibronectin-coated 96-well microplate. The medium was replaced by DME with 1% platelet-derived horse serum the next day. After 24 h, cells were treated with the indicated Ab for 30 min before the addition of specific cytokines. The cells were incubated further for 72 h, and then proliferation was determined by measuring absorbance at OD at 450 nm by using a tetrazolium dye-based assay kit (Chemicon).
Murine Model of Angioproliferative Retinopathy.
The well established murine model of angioproliferative retinopathy was created as described (22). All procedures involving animals were conducted in accordance with both the guidelines for animal experiments of Kyoto University and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Briefly, litters of 7-day-old [postnatal day 7 (P7)] C57BL/6J mice were exposed to 75 ± 2% oxygen for 5 days and then returned to room air at age P12 to produce retinal neovascularization. Mice of the same age maintained in room air served as controls. For histologic studies, mice were anesthetized deeply with i.p. injection of sodium pentobarbital and killed by perfusion through the left ventricle with 4% paraformaldehyde in PBS. Eyes were enucleated, fixed in 4% RNase-free paraformaldehyde, and embedded in paraffin. Neovascular quantification was performed as described (22) in a double-blind manner.
In Situ Hybridization of NRP-1 mRNA Expression.
Slides were processed as described (18) and then hybridized with digoxigenin-labeled NRP1 cRNA probes (DIG RNA labeling kit, Roche Molecular Biochemicals) at 45°C for 16 h. The hybridization product was detected after incubation with an alkaliphosphatase-conjugated anti-digoxigenin Ab (Roche Molecular Biochemicals) overnight at 4°C followed by development in 4-tetrazolium chloride (Roche Molecular Biochemicals) overnight at room temperature.
Intraocular Injection.
Intravitreal injections were performed as described (23) with a 32-gauge needle and syringe (Hamilton) to deliver Ab solution diluted in balanced salt solution (BSS, Alcon; final concentration, 30 μg/ml). To exclude the potential effects of Ab diluent on retinal neovascularization, additional animals were injected unilaterally with the same amount of BSS. In some experiments, NRP1 Ab solution containing VEGF165 (final concentration, 2 or 10 ng/ml) or VEGFR2-Fc Ab (final concentration, 26 μg/ml) was injected. Repeat injections were performed through a previously unmanipulated section of limbus 2 days later.
Statistical Analysis.
For in vitro data, results are expressed as mean ± SD. All determinations were performed in triplicate, and experiments were repeated three times unless otherwise indicated. For Northern blot analysis, representative blots and data of three independent experiments performed in triplicate are shown. One-way ANOVA followed by Fisher's least significant difference test was used to evaluate significant differences. In vivo data of Ab-treated groups were analyzed by using Wilcoxon signed rank test. Other in vivo data were analyzed by using the Student's t or Mann–Whitney rank sum test for data with unequal variance. A P value of <0.05 was considered statistically significant.
Results
VEGF Selectively Increases NRP1 Expression in BRECs.
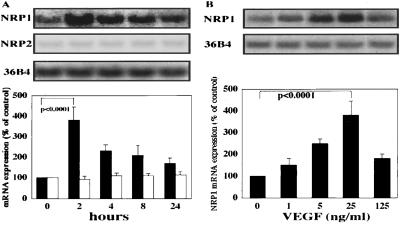
To investigate the effect of VEGF treatment, Northern blot analyses of NRP1 and NRP2 expression were performed with BRECs. VEGF (25 ng/ml) increased NRP1 mRNA expression by 3.8 ± 0.6-fold (P < 0.0001) after 2 h of stimulation (Fig. 1A). In contrast to NRP1, NRP2 mRNA expression was much weaker compared with NRP1 and remained stable after VEGF treatment. To analyze dose dependence, cells were treated with various concentrations of VEGF for 2 h. A dose-dependent increase of NRP1 mRNA was observed with an EC50 of ≈12.5 ng/ml and peaked at 25 ng/ml (P < 0.0001; Fig. 1B). These data suggested that VEGF selectively increases NRP1 mRNA expression in both a time- and dose-dependent manner. To study whether a similar effect of VEGF exists in macrovascular ECs, we stimulated BAECs with VEGF (25 ng/ml). The resultant response revealed a 2.1 ± 0.23-fold (P < 0.0001) increase of NRP1 mRNA expression after 2 h of treatment (data not shown). In contrast, we did not observe a noticeable amount of NRP2 mRNA in BAECs. Additionally, we could not detect Sema3A mRNA in either cell type (data not shown).
Figure 1.
Kinetic studies of gene expression of NRPs in response to VEGF treatment. (A) Time course of Northern blotting on total RNA of BRECs stimulated with VEGF165 (25 ng/ml). cDNA probes for NRP1, NRP2, and 36B4 were used. (B) Concentration dependence of NRP1 mRNA regulation by VEGF stimulation. BRECs were treated with the indicated concentration of VEGF for 2 h. Results were quantified by densitometric analysis of the autoradiogram derived from the Upper panels after normalization to the 36B4 control mRNA signals. Values are presented as a percentage of the control (Lower panels). Black and white bars indicate NRP1 and NRP2 mRNA levels, respectively.
VEGF Increases the Rate of NRP1 mRNA Transcription.
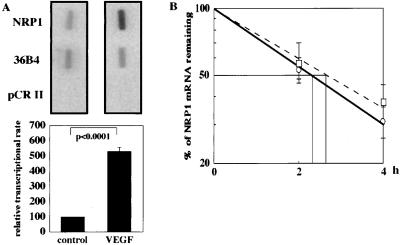
We investigated whether the VEGF-induced increase in NRP1 mRNA is derived from up-regulation of transcription or increased mRNA stability. Nuclear run-on assays were used to determine whether VEGF leads to an increase in transcriptional initiation rate. VEGF treatment increased the rate of NRP1 gene transcription by 5.3 ± 0.26-fold (P < 0.0001) compared with that of the control (Fig. 2A). To determine whether VEGF affects the stability of NRP1 mRNA, we examined the half-life of NRP1 mRNA with the aid of actinomycin D to inhibit de novo gene transcription. The half-life of NRP1 mRNA was 2.6 ± 0.18 h when treated with VEGF and 2.3 ± 0.15 h in unstimulated controls (P > 0.05; Fig. 2B). These findings clearly demonstrate that the VEGF-induced increase in NRP1 mRNA is derived mainly from an increase in the transcriptional rate.
Figure 2.
Effect of VEGF on the transcriptional rate and mRNA stability of NRP1. (A) Nuclear run-on assay for NRP1 mRNA from BRECs. Nuclear extracts were prepared after treatment with or without 25 ng/ml VEGF for 2 h, and 32P-labeled RNA probes were hybridized to nitrocellulose filters deposited with plasmid DNAs containing cDNAs for NRP1, 36B4, and empty vector (pCRII). The representative blots of three independent experiments are shown (Upper). Results were quantified by densitometric analysis of the autoradiogram after normalization to the radioactivity of 36B4. Values are presented as a percentage of the control (Lower). (B) Decay of NRP1 mRNA in the presence of actinomycin D in BRECs. Total RNA was isolated at the indicated time points after administration of actinomycin D, and Northern blot analysis was performed. The values shown represent the percentage of initial NRP1 mRNA signal remaining under the specified conditions and are plotted in logarithmic scale. ○, control cells; □, cells in the presence of VEGF.
Role of VEGFRs in VEGF-Induced NRP1 Expression.
To determine which VEFGR mediates VEGF-induced NRP1 expression, we first tested the effect of SU-1498, a VEGFR2-selective tyrosine kinase inhibitor (24). SU-1498 (20 μM) abolished VEGF-induced NRP1 mRNA expression (P < 0.0001), whereas SU-1498 alone did not exert a significant effect on basal NRP1 expression (Fig. 6A, which is published as supporting information on the PNAS web site, www.pnas.org). Because VEGFR1 expression is reported to be much less than that of VEGFR2 in BRECs (25), we next studied the role of this receptor by using human umbilical vein ECs, which express both VEGFR1 and VEGFR2 at a comparable level (26). As for BRECs, VEGF significantly increased NRP1 mRNA expression by 3.2 ± 0.25-fold in human umbilical vein ECs (P < 0.0001; Fig. 6B). In contrast, placenta growth factor-1, a selective VEGFR1 agonist, did not affect NRP1 expression significantly, although concentrations of up to 50 ng/ml were tested. In addition, NRP2 expression was weaker than NRP1 as in the case of BRECs and remained unchanged by VEGF and placenta growth factor stimulation. Because of the lack of known natural ECs that express NRP1 alone among receptors for VEGF, we further studied the peculiar role of NRP1 by using MDA-MB231 cells, a tumor cell line that has been shown to express NRP1 but not NRP2 or other VEGFRs (13, 27). VEGF did not exert a significant effect on NRP1 expression in MDA-MB231 cells (data not shown). These results demonstrate the predominant role of VEGFR2 in NRP1 induction by VEGF.
Role of Signaling Molecules Downstream of VEGFR2 in VEGF-Induced NRP1 Expression.
Previous reports have shown that tyrosine kinases and other signaling molecules have significant roles in the downstream of VEGFR2 (28, 29). Inhibition of protein tyrosine kinase by chemically distinct inhibitors, genistein or herbimycin A, almost abolished VEGF-induced NRP1 expression (both P < 0.0001; Fig. 6C). Ligand-activated VEGFR2 interacts with phospholipase C (PLC)-γ and leads to subsequent PKC activation (29). We therefore tested the PLC-γ inhibitor, U73122, and the protein kinase C (PKC) inhibitor, GF109203X. As expected, these inhibitors also suppressed the effect of VEGF on NRP1 expression significantly by 74 ± 7.4% (P < 0.0001) and 70 ± 8.7% (P < 0.0001), respectively. Recent reports have shown that phosphatidylinositol 3-kinase (PI3-kinase) also contributes substantially to the signaling initiated by VEGF (28, 29). Inhibition of PI3-kinase by wortmannin reduced the increase in NRP1 expression by 80 ± 5.8% (P < 0.0001). Finally, we investigated the contribution of mitogen-activated protein kinase (MAPK), which functions as a signaling molecule downstream of both PLC-γ-PKC and PI3-kinase in the VEGF signaling pathway (28, 30). The specific inhibitor of MAPK, PD098059, abolished the response of NRP1 to VEGF treatment (P < 0.0001). In contrast, H89, a protein kinase A selective inhibitor used as positive control, had no significant effect. These data indicate that tyrosine phosphorylation and MAPK play a major role in VEGF-induced NRP1 expression and that PLC-γ, PKC, and PI3-kinase also contribute, although to a lesser extent, to NRP1 induction.
VEGF Selectively Increases NRP1 Protein Synthesis and Cell Surface Binding Sites.
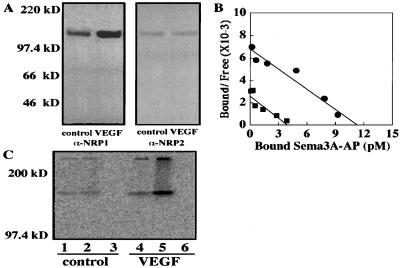
To determine whether the increase in NRP1 mRNA expression was accompanied by an increase of de novo protein synthesis, we performed Western blot analysis. VEGF (25 ng/ml) stimulation for 24 h elicited a remarkable increase in NRP1 protein synthesis (Fig. 3A). In contrast, the NRP2 protein level was less abundant in comparison with NRP1 and remained unchanged by VEGF stimulation. Because Sema3A is the only known ligand that binds exclusively to NRP1 with high affinity among VEGFRs and NRPs expressed on ECs, we performed cell surface binding analyses by using Sema3A-AP as the ligand to determine whether VEGF treatment could modulate the amount of functional NRP1 expression. By Scatchard analysis, we observed that VEGF significantly increased Sema3A-AP binding sites from 2.4 ± 0.3 × 104 per cell to 6.8 ± 0.2 × 104 per cell (P < 0.0001) but had no significant effect on binding affinity (1.5 ± 0.1 and 1.7 ± 0.2 nM, P > 0.05; Fig. 3B). To further confirm whether the observed increase in Sema3A-AP binding sites actually reflects increased VEGF165 bindings to NRP1, we performed crosslinking experiments by using 125I-VEGF165. Fig. 3C shows that 125I-VEGF165 is crosslinked to two cell surface receptors in BRECs. The lower (175 kDa) and higher (220 kDa) molecular mass complexes correspond to VEGF165-NRP1 and VEGF165-VEGFR2 complexes, respectively (25). An increase in crosslinked complexes of VEGF165-NRP1 was observed at both concentrations in VEGF-stimulated cells in comparison with control cells. In addition, VEGF165-VEGFR2 complexes also increased in VEGF-treated cells. This result is consistent with our data showing enhanced NRP1 expression by VEGF stimulation, because coexistence of NRP1 has been shown to increase VEGF165 bindings to VEGFR2 (13). These data suggest that VEGF selectively stimulated NRP1 protein synthesis and induced a quantitative increase in functional receptor expression.
Figure 3.
NRP1 protein expression induced by VEGF. (A) Western blot analysis of NRP protein expression in BRECs treated with or without VEGF (25 ng/ml) for 24 h. The blot was probed first with NRP1 Ab and then reprobed with NRP2 Ab with an equal exposure duration to films. The position and size in kilodaltons (kD) of molecular mass markers are indicated to the left. The size of both NRP1 (Left) and NRP2 (Right) protein corresponded to ≈130 kDa. (B) Scatchard analysis of quantitative cell surface binding of Sema3A to BRECs. Cells pretreated with (●) or without (■) VEGF (25 ng/ml) for 24 h were exposed to conditioned medium containing the indicated concentration of Sema3A-AP. AP activity was measured colorimetrically (OD at 405 nm). (C) Binding and crosslinking of 125I-VEGF165 to NRP1 and VEGFR2. BRECs were stimulated with (lanes 4–6) or without (lanes 1–3) VEGF165 (25 ng/ml) for 24 h and then incubated with 2 ng/ml (lanes 1 and 4) or 10 ng/ml of 125I-VEGF165 (lanes 2 and 5). Competition was performed by incubating cells with 10 ng/ml 125I-VEGF165 in the presence of 1 μg/ml unlabeled VEGF165 (lanes 3 and 6).
NRP1 mRNA Expression Is Up-Regulated in the Murine Model of Angioproliferative Retinopathy.
To study in vivo NRP1 expression in angiogenic conditions, the well established murine model of angioproliferative retinopathy was used. In this model, preformed retinal vasculature regresses during hyperoxic treatment, and subsequent retinal neovascularization, for which VEGF has been shown to be the predominant inducer (23, 31), follows ischemic conditions induced by returning the mice to room air. Hybridization with an antisense probe revealed basal levels of signal located in the ganglion cell layer, the inner nuclear layer, and the outer nuclear layer at P12 just before removal of the animals from oxygen in both age-matched control retinas and ischemic retinas (Fig. 7 A and B, which is published as supporting information on the PNAS web site). Hybridization of P12 ischemic retinas with a sense probe did not reveal an appreciable signal (Fig. 7C). At P17, the time at which retinal neovascularization has been shown to peak (31), no change in the NRP1 signal level was observed in control retinas (Fig. 7E). In contrast, prominent NRP1 signals were observed in the newly formed vessels, termed neovascular tufts, that grew into the vitreous cavity through the inner limiting membrane of the retina (Fig. 7D). Some intense signals were detected also in the intraretinal vessels. To determine whether NRP1 up-regulation is actually derived from the enhanced VEGF expression in this model, we injected VEGFR2-Fc antibodies to inhibit VEGF activity. Inactivation of VEGF remarkably suppressed neovascular tufts formation, and the intensity of NRP1 signals in the P17 ischemic retina was reduced to the level similar to that of the control retinas (Fig. 7F). These data suggest that NRP1 expression is up-regulated by VEGF during in vivo retinal neovascularization.
NRP1 Inhibition Attenuates the Mitogenic Effect of VEGF165 on BRECs.
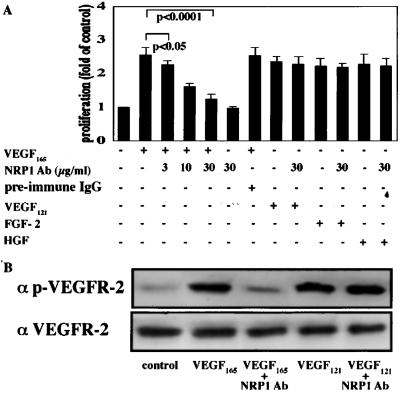
Although coexistence of NRP1 has been shown to increase the binding of VEGF to VEGFR2 (13), the effect of NRP1 on endothelial mitogenesis has not been well studied. We examined the role of NRP1 in VEGF-induced endothelial proliferation by using the anti-NRP1 blocking Ab. This Ab does not crossreact with NRP2 (32) and has been shown to inhibit effectively Sema3A-induced responses in both neuronal and endothelial cells (10, 33). As shown in Fig. 4A, NRP1 Ab significantly suppressed the mitogenic effects of VEGF165 by up to 85% (P < 0.0001). To further confirm the specificity of NRP1 Ab in inhibition of VEGF165, we tested VEGF121 and other cytokines including fibroblast growth factor-2 and hepatocyte growth factor. NRP1 Ab (30 μg/ml) did not inhibit proliferation stimulated by any of these cytokines and thus verified its specificity in suppression of VEGF165-induced endothelial proliferation. We next performed Western blot analysis to determine whether VEGFR2 signaling is actually attenuated by NRP1 Ab. As shown in Fig. 4B, NRP1 Ab pretreatment remarkably reduced the amount of phosphorylated VEGFR2 stimulated by VEGF165. In contrast, the Ab did not alter the extent of VEGFR2 phosphorylation elicited by VEGF121.
Figure 4.
(A) Effect of NRP1 inhibition in VEGF165-induced endothelial proliferation. BRECs seeded in 96-well plates (2 × 103 cells per well) were pretreated with NRP1 Ab (0–30 μg/ml) or preimmune IgG Ab (30 μg/ml) followed by incubation with VEGF165 (25 ng/ml), VEGF121 (25 ng/ml), fibroblast growth factor-2 (FGF-2, 10 ng/ml), or hepatocyte growth factor (HGF, 25 ng/ml). After 72 h, a tetrazolium-based proliferation assay was performed, and the proliferation of ECs was determined by measuring OD at 450 nm. The average values of four wells were expressed as the percentage of control. Representative results of three independent experiments are shown. (B) The effect of NPR1 Ab on VEGFR2 signaling stimulated by VEGF. BRECs were pretreated with or without NRP1 Ab (30 μg/ml) for 1 h and then stimulated with VEGF165 or VEGF121 (25 ng/ml) for 5 min. (Upper) Western blot was performed by using a specific mAb against phosphorylated VEGFR2. (Lower) The blot was reprobed to detect total VEGFR2 protein. The representative data of three independent experiments are shown.
NRP1 Inhibition Suppressed Angioproliferative Retinopathy in the Mouse.
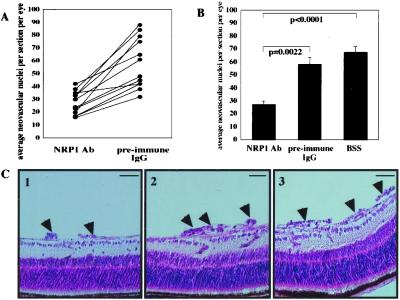
To further determine the role of NRP1 in pathologic angiogenesis in vivo, we examined the effect of selective NRP1 inhibition in the murine model of angioproliferative retinopathy. NRP1 Ab treatment resulted in a significant reduction in retinal neovascularization compared with either injections with preimmune IgG Ab or Ab diluent (BSS) alone (P = 0.0022 and P < 0.0001, respectively; Fig. 5 A and B). In contrast, preimmune IgG Ab treatment did not exert a significant effect compared with injection of BSS alone (P = 0.21). Because VEGFR2 can transduce signals in the absence of NRP1 (13), the addition of exogenous VEGF165 is expected to restore neovascularization if NRP1 Ab in vivo exclusively inhibits NRP1. We observed that exogenous VEGF165 could restore retinal neovascularization in a dose-dependent manner (data not shown). Suppression and restoration of the neovascular response was evident by histologic examination of paraffin-embedded ocular cross-section (Fig. 5C and data not shown). No retinal toxicity or inflammation was apparent by light microscopy.
Figure 5.
Inhibition of NRP1 in the murine model of angioproliferative retinopathy. Mice of the Ab-treated group (n = 12) were injected intravitreally with either NRP1 Ab (right eye) or preimmune IgG (left eye). Additional mice were injected unilaterally with Ab diluent (BSS) for comparison (n = 14). (A) Average neovascular cell nuclei per 6-μm retinal section per eye were determined with mice treated with Ab alone. Eyes from the same animal are connected by solid lines. (B) Comparison between groups classified by treatment. Error bars indicate SEM for all animals in each group. (C) Typical histologic sections of eyes injected with NRP1 Ab (1), preimmune IgG Ab (2), and Ab diluent (3) are shown. Arrowheads indicate neovascular tufts. (Bars, 50 μm.)
Discussion
The NRP family is comprised of two structurally related neuronal receptors, NRP1 and NRP2, that have been reported to mediate the chemorepulsive effect elicited by Semas (10, 12, 32). Interestingly, both NRPs have been shown recently to function as unique VEGFRs in that they can bind to two biologically distinct groups of cytokines. In ECs, NRP1 functions as a receptor for VEGF165 (13), a potent angiogenic cytokine that is abundant in pathologic angiogenesis. To explore whether NRP expression might be modulated under angiogenic conditions, we examined whether VEGF could alter NRP expression in ECs. By using BRECs, we demonstrated that VEGF selectively up-regulates NRP1. The difference in the magnitude of NRP1 induction by VEGF between BRECs and BAECs may be explained by the fact that BRECs have more VEGFR2s per cell than BAECs (25). For elucidation of the underlying mechanism of rapid NRP1 induction upon VEGF stimulation, we performed run-on assays and mRNA stability analyses. Our results revealed that transcriptional activation is the primary mechanism for this response.
Our data of NRP1 binding using Sema3A fusion protein revealed that up-regulation of NRP1 after VEGF stimulation is accompanied by an increase in cell surface binding, thus reflecting the de novo NRP1 protein synthesis as demonstrated by Western blot analysis. The Kd of Sema3A-AP binding to NRP1 in unstimulated BRECs, 1.5 nM, is comparable to the previously reported values ranging from 0.33 to 1.5 nM (10, 11). Furthermore, our crosslinking experiments revealed that VEGF165 binding to NRP1 also was increased, which is consistent with previous reports showing that both Sema3A and VEGF165 bind to the same domain of NRP1 (13, 32). To confirm NRP1 induction in vivo under angiogenic conditions, we examined NRP1 mRNA expression in the murine model of angioproliferative retinopathy, where VEGF is the predominant inducer (23, 31). Our data demonstrated up-regulation of NRP1 transcripts in several retinal layer and intense NRP1 signals in neovascular tufts at P17.
The fact that NRP1 coexpression enhances VEGF165 binding to VEGFR2 by up to 6-fold (13), together with our data showing selective NRP1 up-regulation after VEGF treatment, prompted us to explore the role of this receptor in VEGF-induced angiogenesis. We first examined the effect of NRP1 inhibition on EC proliferation stimulated with VEGF. Our studies revealed that NRP1 Ab reduced VEGF-induced mitogenesis by up to 85%, which is comparable to the 80% inhibition of Sema3A-elicited reduction of endothelial motility reported in the previous study using this Ab (33). We further performed Western blot analysis to examine the effect of NRP1 inhibition on VEGFR2 phosphorylation and revealed that it selectively suppresses VEGF165- but not VEGF121-induced VEGFR2 signaling. This result also suggests that the inhibitory effect of NRP1 Ab is not mediated by direct suppression of VEGFR2 but by interaction with NRP1. We further studied in vivo the effect of NRP1 inhibition by intravitreal injection of this Ab in the murine model of angioproliferative retinopathy. Although there is scatter among individual mice, the 53% reduction in retinal neovascularization observed in the present study suggests that NRP1 inhibition is relatively effective in comparison with previous studies reporting the effect of VEGF inhibition in this model (23, 34). Taken together, our results indicate that, as in the case of the Sema3A-NRP1 system (13), NRP1 inhibition is effective in suppressing VEGF-elicited angiogenic effects both in vitro and in vivo. Although we did not examine the role of NRP2 in VEGF-induced angiogenesis, our results showing the predominant NRP1 expression in ECs, together with a recent report of a lack of VEGF-induced cellular response in ECs expressing NRP2 alone (27), this receptor might have only a minimal contribution. In addition, NRP2 seems to have only a minor contribution in embryonic vasculogenesis and angiogenesis, because its expression is not detected in the heart or capillaries of the embryo (12). However, because the interaction between NRP2 and other VEGFRs is still unknown, yet has the possibility of contributing to VEGF signaling, further studies regarding this point are required.
In summary, the present study provides a persuasive argument for a molecular mechanism by which VEGF selectively up-regulates its homologous receptor, NRP1. This result indicates that VEGF-induced robust angiogenesis is derived not only from the direct effects on ECs but also from NRP1 up-regulation stimulated by VEGF itself. Furthermore, we demonstrated that selective perturbation of NRP1 under angiogenic conditions is effective in inhibition of angioproliferative retinopathy and thus verified that NRP1 is a viable therapeutic target for the suppression of pathologic angiogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Alex Kolodkin and Dr. David Ginty for kindly providing anti-NRP1 and anti-NPR2 antibodies and the Sema3A sequence-containing vector. This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, and Culture (11694267) and the Ministry of Health and Welfare of the Japanese Government.
Abbreviations
- VEGF
vascular endothelial growth factor
- EC
endothelial cell
- VEGFR
VEGF receptor
- NRP
neuropilin
- Sema
semaphorin
- BREC
bovine retinal EC
- BAEC
bovine aortic EC
- AP
alkaline phosphatase
- P
postnatal day
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Aiello L P, Avery R L, Arrigg P G, Keyt B A, Jampel H D, Shah S T, Pasquale L R, Thieme H, Iwamoto M A, Park J E, et al. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 2.Adamis A P, Miller J W, Bernal M T, D'Amico D J, Folkman J, Yeo T K, Yeo K T. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 3.Gospodarowicz D, Abraham J A, Schilling J. Proc Natl Acad Sci USA. 1989;86:7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 5.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 8.Fong G H, Rossant J, Gertsenstein M, Breitman M L. Nature (London) 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 9.Shalaby F, Rossant J, Yamaguchi T P, Gertsenstein M, Wu X F, Breitman M L, Schuh A C. Nature (London) 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 10.Kolodkin A L, Levengood D V, Rowe E G, Tai Y T, Giger R J, Ginty D D. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 11.He Z, Tessier-Lavigne M. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Chedotal A, He Z, Goodman C S, Tessier-Lavigne M. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 13.Soker S, Takashima S, Miao H Q, Neufeld G, Klagsbrun M. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 14.Soker S, Gollamudi-Payne S, Fidder H, Charmahelli H, Klagsbrun M. J Biol Chem. 1997;272:31582–31588. doi: 10.1074/jbc.272.50.31582. [DOI] [PubMed] [Google Scholar]
- 15.Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- 16.Bacic M, Edwards N A, Merrill M J. Growth Factors. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]
- 17.Berkman R A, Merrill M J, Reinhold W C, Monacci W T, Saxena A, Clark W C, Robertson J T, Ali I U, Oldfield E H. J Clin Invest. 1993;91:153–159. doi: 10.1172/JCI116165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. J Biol Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Nakamura F, Strittmatter S M. J Neurosci. 1997;17:9183–9193. doi: 10.1523/JNEUROSCI.17-23-09183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omura T, Miyazawa K, Ostman A, Heldin C H. J Biol Chem. 1997;272:23317–23322. doi: 10.1074/jbc.272.37.23317. [DOI] [PubMed] [Google Scholar]
- 21.Vaisman N, Gospodarowicz D, Neufeld G. J Biol Chem. 1990;265:19461–19466. [PubMed] [Google Scholar]
- 22.Smith L E, Wesolowski E, McLellan A, Kostyk S K, D'Amato R, Sullivan R, D'Amore P A. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 23.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strawn L M, McMahon G, App H, Schreck R, Kuchler W R, Longhi M P, Hui T H, Tang C, Levitzki A, Gazit A, et al. Cancer Res. 1996;56:3540–3545. [PubMed] [Google Scholar]
- 25.Thieme H, Aiello L P, Takagi H, Ferrara N, King G L. Diabetes. 1995;44:98–103. doi: 10.2337/diab.44.1.98. [DOI] [PubMed] [Google Scholar]
- 26.Takagi H, King G L, Aiello L P. Diabetes. 1996;45:1016–1023. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- 27.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 28.Thakker G D, Hajjar D P, Muller W A, Rosengart T K. J Biol Chem. 1999;274:10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 29.Xia P, Aiello L P, Ishii H, Jiang Z Y, Park D J, Robinson G S, Takagi H, Newsome W P, Jirousek M R, King G L. J Clin Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L W, Mayo L D, Dunbar J D, Kessler K M, Baerwald M R, Jaffe E A, Wang D, Warren R S, Donner D B. J Biol Chem. 2000;275:5096–5103. doi: 10.1074/jbc.275.7.5096. [DOI] [PubMed] [Google Scholar]
- 31.Pierce E A, Avery R L, Foley E D, Aiello L P, Smith L E. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giger R J, Urquhart E R, Gillespie S K, Levengood D V, Ginty D D, Kolodkin A L. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 33.Miao H Q, Soker S, Feiner L, Alonso J L, Raper J A, Klagsbrun M. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson G S, Pierce E A, Rook S L, Foley E, Webb R, Smith L E. Proc Natl Acad Sci USA. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.