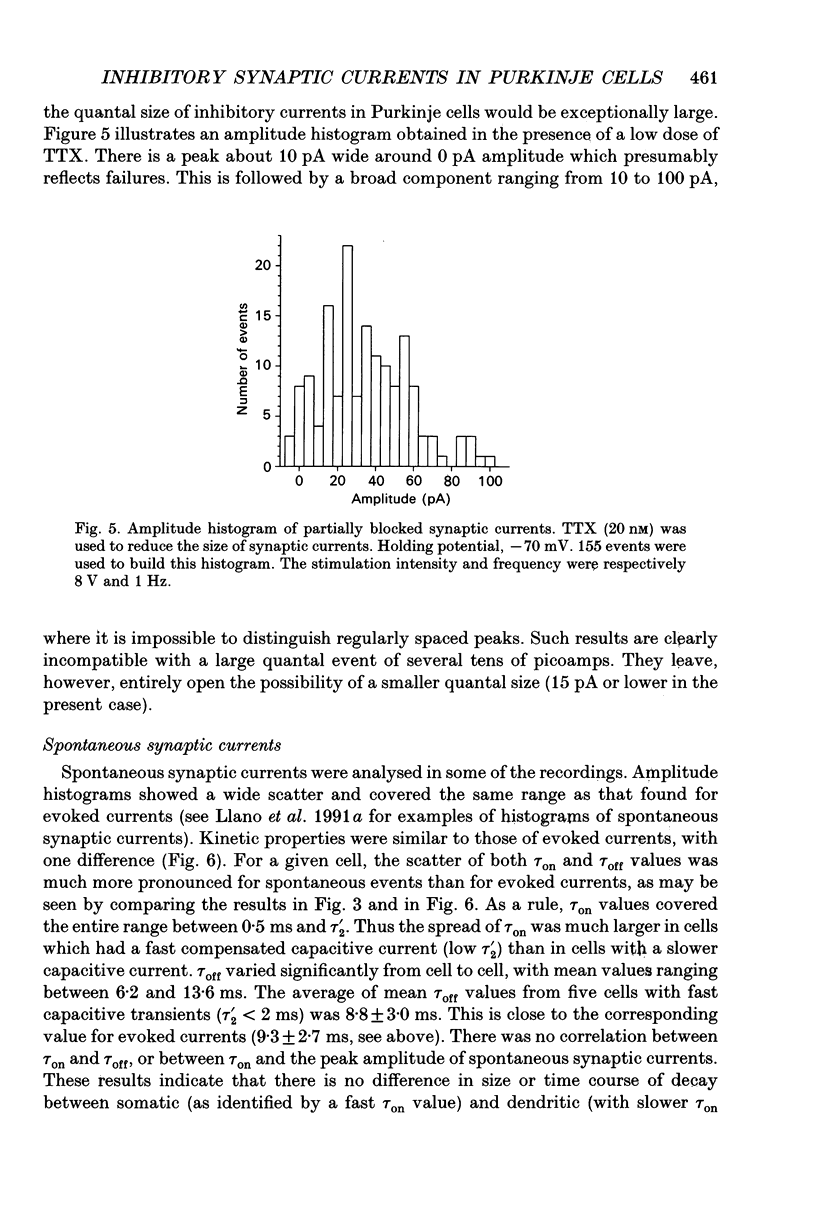
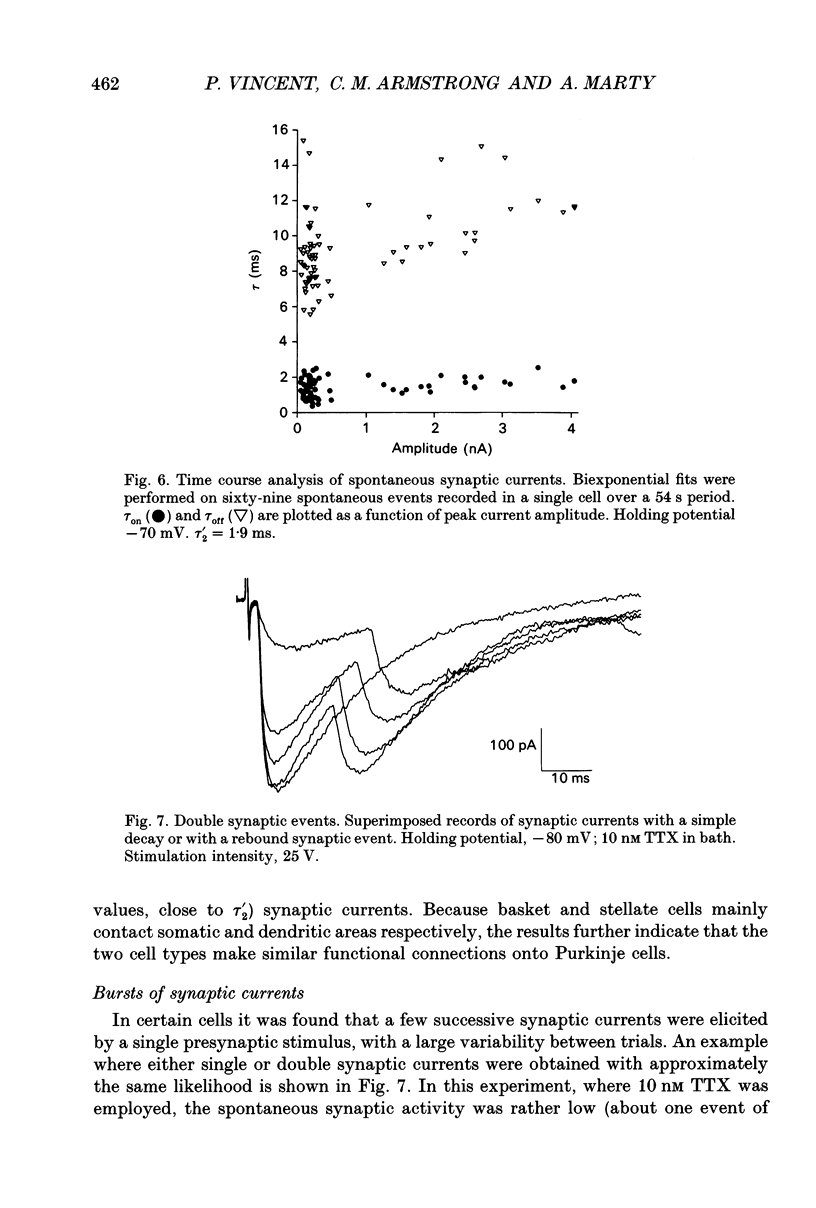
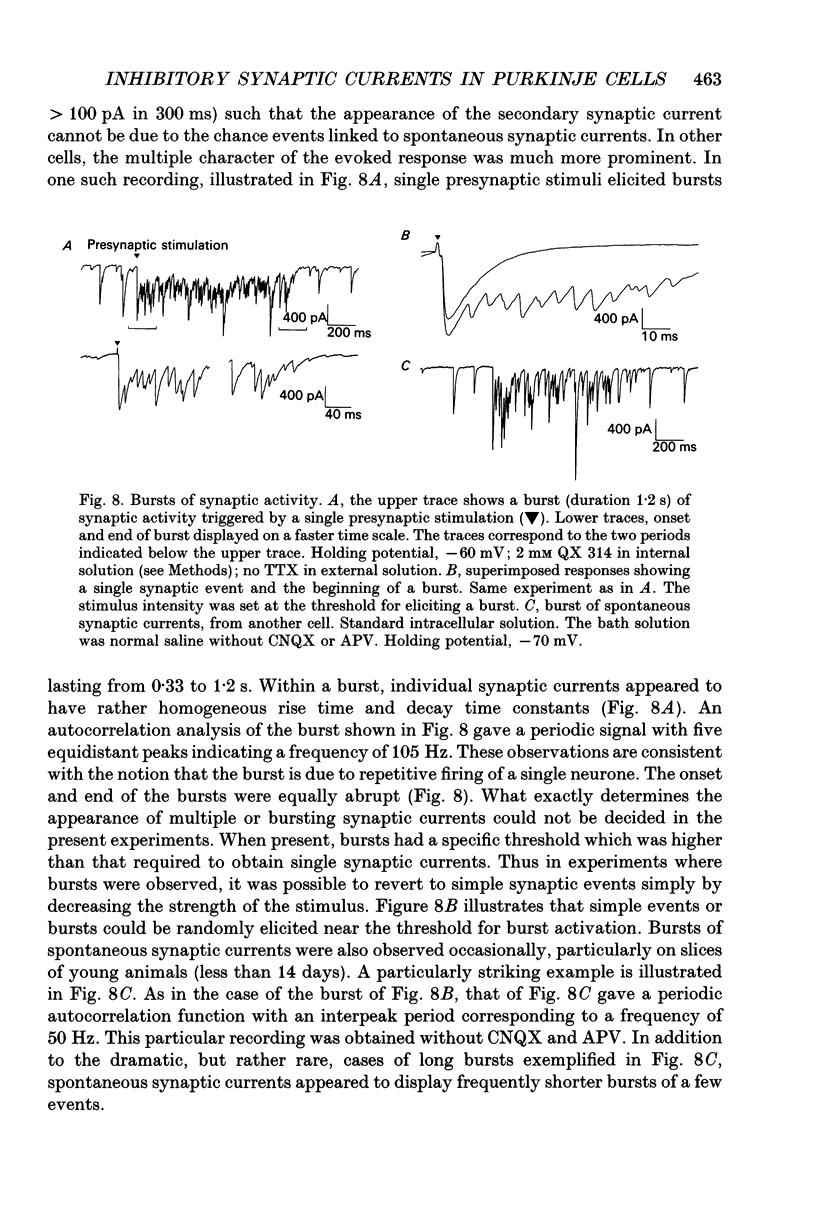
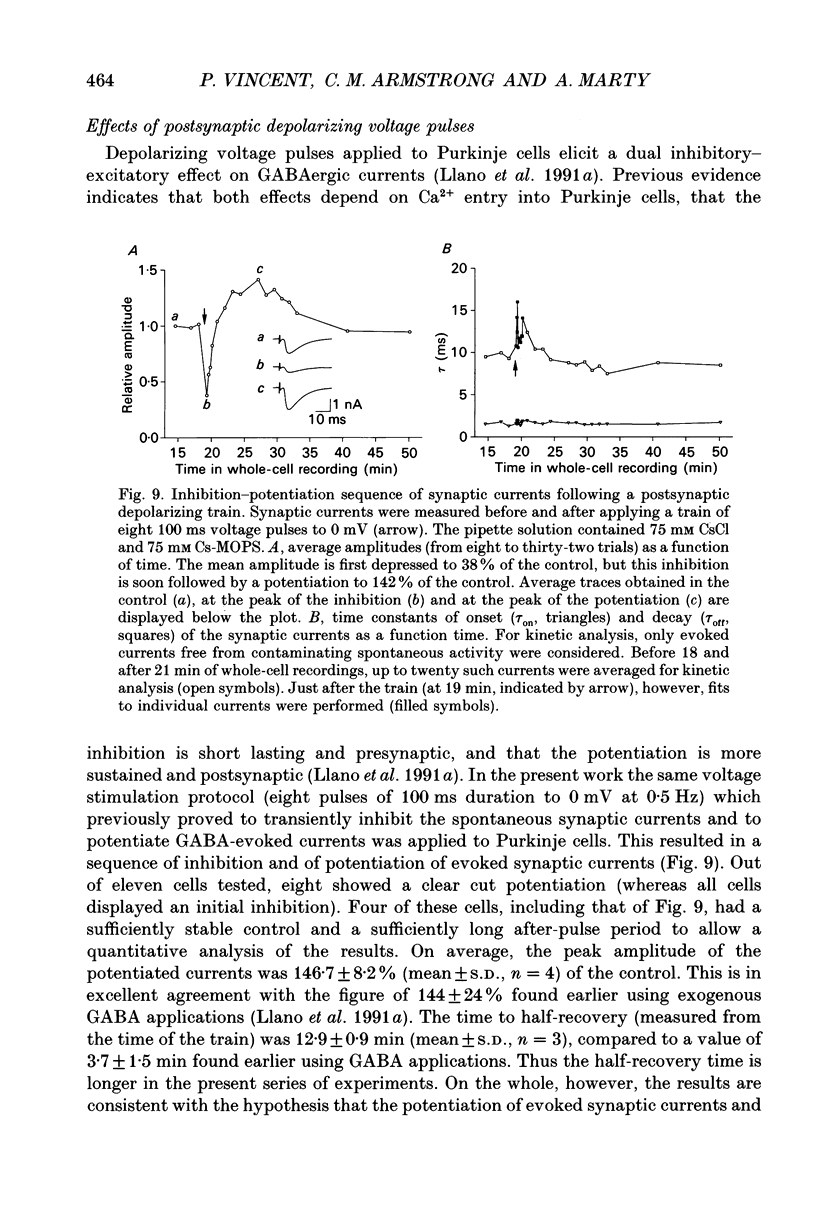
Abstract
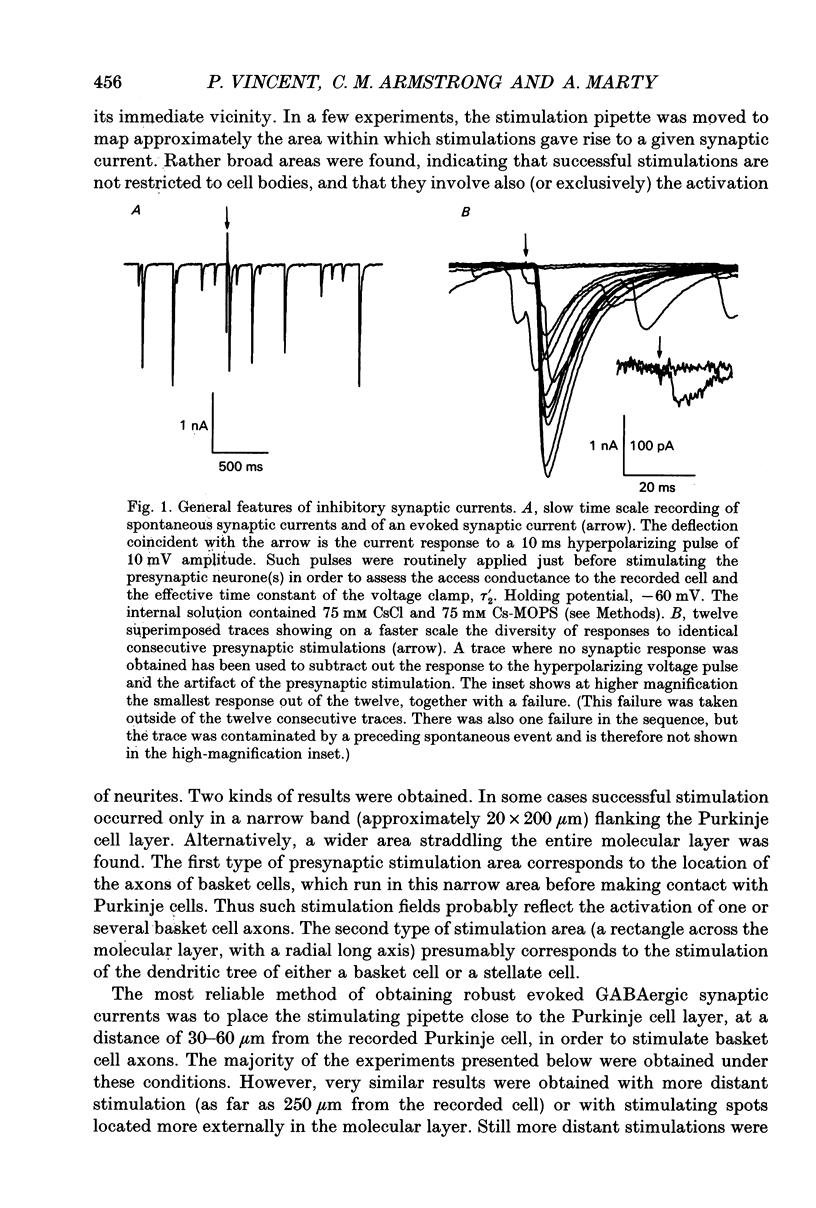
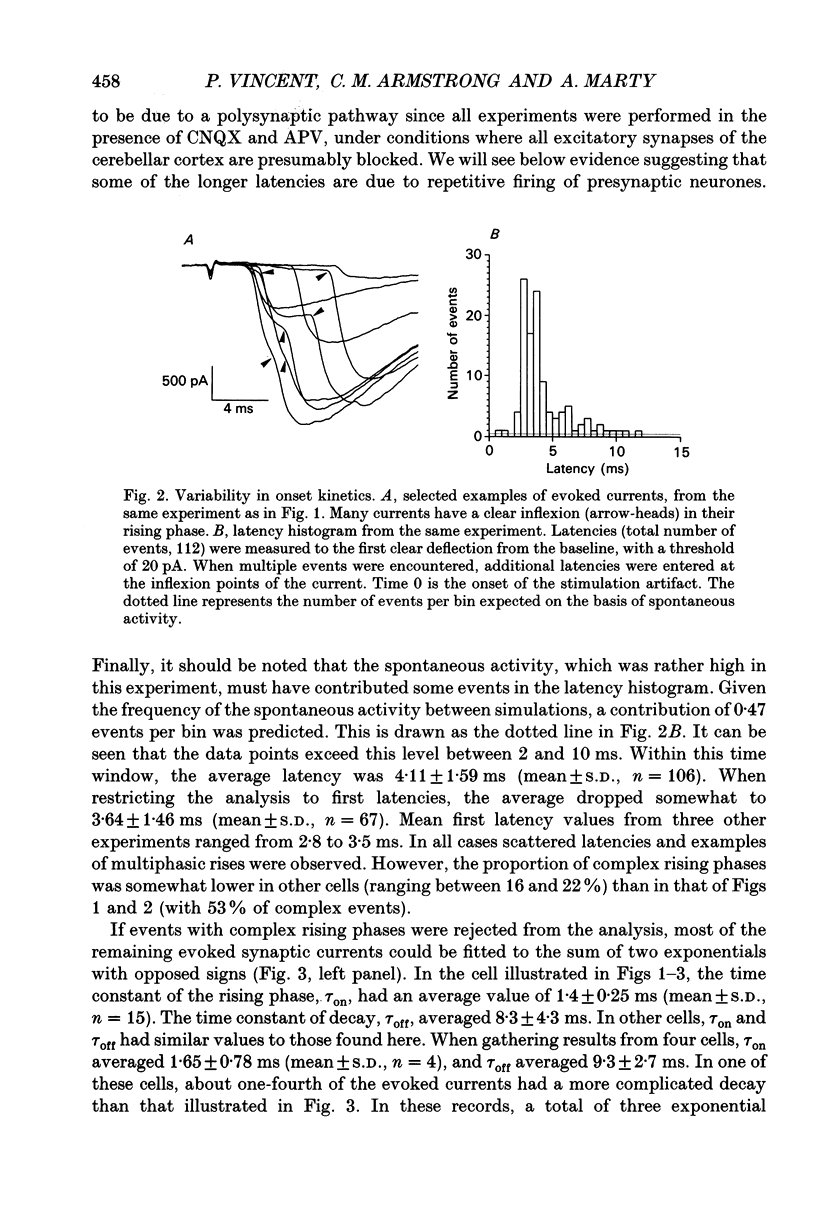
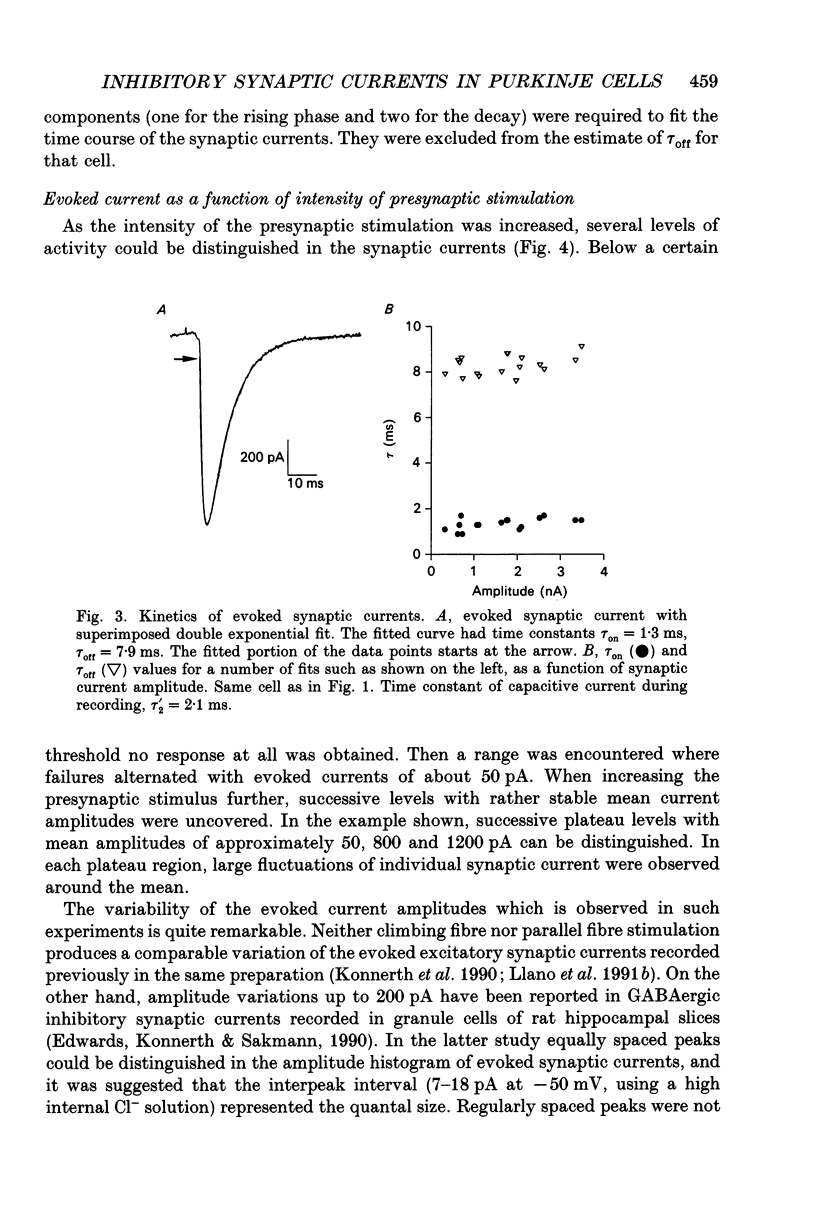
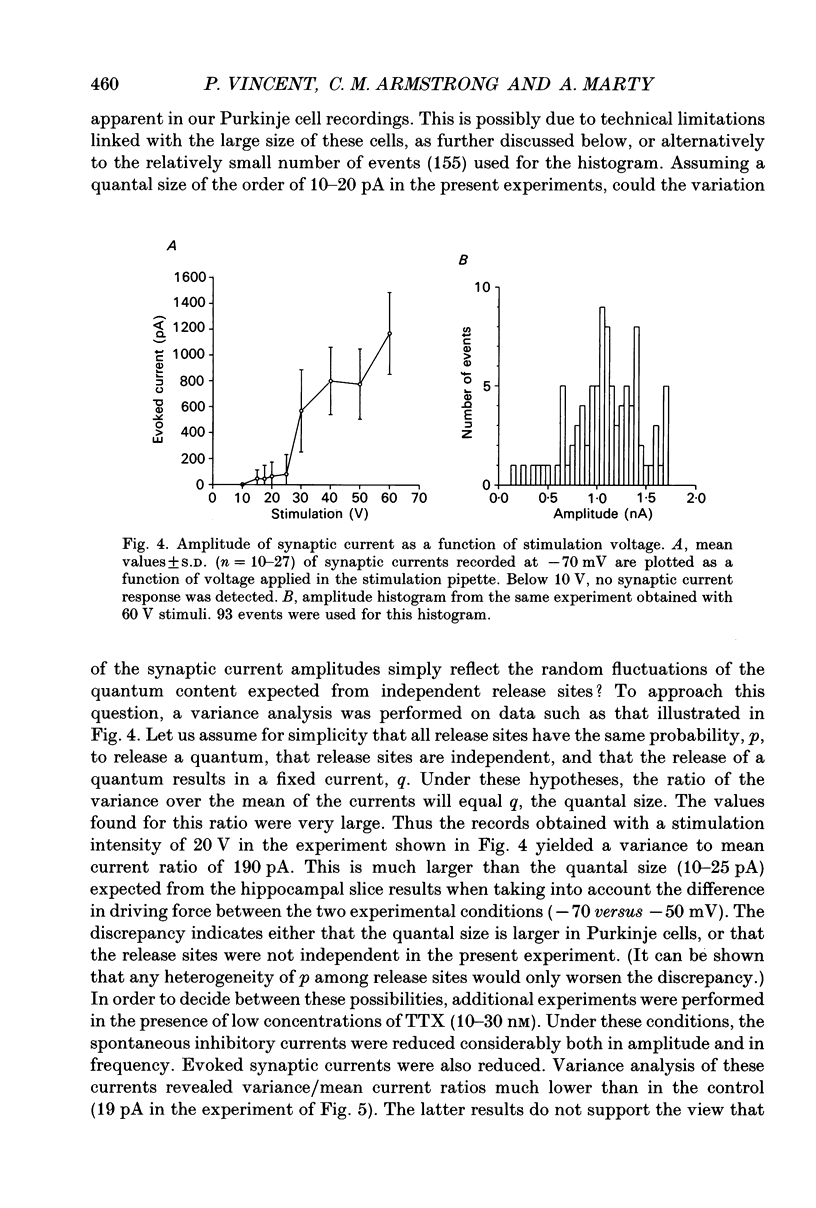
1. Synaptic currents were recorded in voltage-clamped cerebellar Purkinje cells using the tight-seal whole-cell recording technique. Cells were dialysed with a CsCl solution and were held at -60 or -70 mV. Inhibitory interneurones (basket and stellate cells) were stimulated using an extracellular pipette positioned in the molecular layer. Blockers of excitatory glutamatergic synapses were included in the bath solution. 2. Evoked synaptic currents were observed after a latency of 3-4 ms. The time course of synaptic currents could in most cases be fitted to a biexponential curve, with a rise time constant, tau on, of 1-3 ms and a decay time constant, tau off, of 7-13 ms. These currents were blocked by bicuculline. 3. The mean amplitude of evoked synaptic currents increased in discrete steps when the voltage applied to the stimulating pipette was increased. At each level, very prominent fluctuations of the amplitude were observed among trials. 4. Complex synaptic currents corresponding to repetitive activity of the presynaptic interneurone were occasionally observed, particularly with high intensity presynaptic stimulation. This repetitive activity could lead to bursts of synaptic currents lasting for several seconds. 5. Following a depolarizing voltage train in the postsynaptic Purkinje cell, the amplitude of evoked synaptic currents was first inhibited, and then potentiated. The inhibition was accompanied by a small but consistent increase in tau off and by no alteration in tau on. When using small intensity presynaptic stimuli, it was found that the probability of failures was greatly enhanced. The inhibitory phase lasted for about 1 min before giving way to potentiation. The potentiation returned to the control with a time to half-decay of 12.9 +/- 0.9 min. 6. The present results give further evidence to a previously proposed hypothesis that the inhibition produced by Purkinje cell depolarization is mainly presynaptic. The longer lasting potentiation, on the other hand, has most probably a postsynaptic origin. Cerebellar Purkinje cells receive inhibitory GABAergic inputs from two classes of interneurones located in the molecular layer (reviewed in Palay & Chan-Palay, 1974; Ito, 1984). Basket cells are closest to the Purkinje cell layer and address their inhibitory signal primarily to the soma and to the main dendrites. Stellate cells are more externally located and have contacts with the more distal part of the dendritic arborization of Purkinje cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
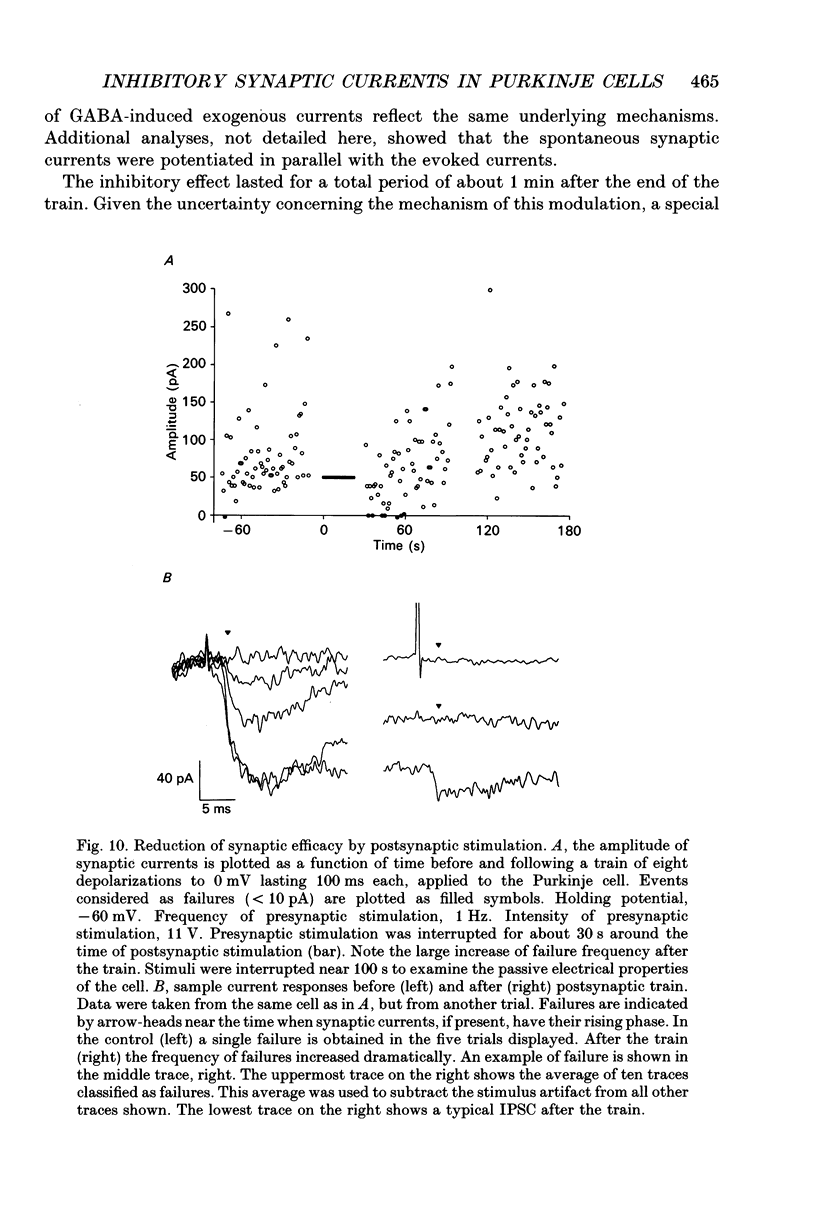
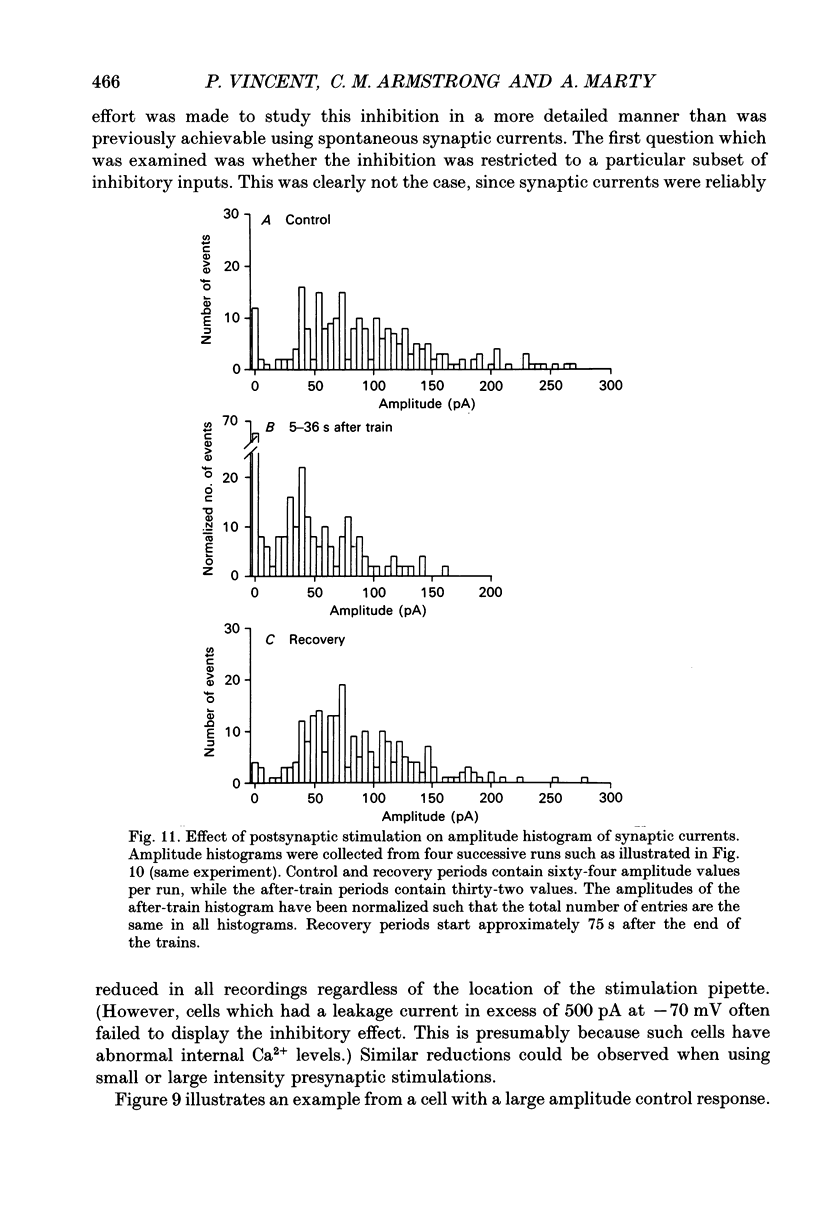
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., VOORHOEVE P. E. POSTSYNAPTIC INHIBITION OF CEREBELLAR PURKINJE CELLS. J Neurophysiol. 1964 Nov;27:1138–1153. doi: 10.1152/jn.1964.27.6.1138. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., Gaiarsa J. L., Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991 Dec;14(12):515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Intracellularly recorded responses of the cerebellar Purkinje cells. Exp Brain Res. 1966;1(2):161–183. doi: 10.1007/BF00236869. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp Brain Res. 1966;1(1):1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Farrant M., Cull-Candy S. G. Excitatory amino acid receptor-channels in Purkinje cells in thin cerebellar slices. Proc Biol Sci. 1991 Jun 22;244(1311):179–184. doi: 10.1098/rspb.1991.0067. [DOI] [PubMed] [Google Scholar]
- Frazier D. T., Narahashi T., Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970 Jan;171(1):45–51. [PubMed] [Google Scholar]
- Konnerth A., Llano I., Armstrong C. M. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991 Apr;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropert N., Miles R., Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol. 1990 Sep;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]


