Abstract
1. The actions of 5-hydroxytryptamine (5-HT) and noradrenaline (NA) on the membrane properties of facial motoneurones in slices from the adult rat brainstem in vitro were examined using intracellular recording techniques. 2. In voltage clamp recording, hyperpolarizing voltage steps (> 20 mV), from holding potentials at or close to the resting potential, induced a slowly activating, voltage-dependent inward current possessing properties similar to the hyperpolarization-activated current (Ih) seen in other cell types. From tail current analysis two groups of facial motoneurones can be distinguished in terms of the activation range for Ih, one with a half-maximal activation at -81 mV and the other at -94 mV but with similar shapes. 3. 5-HT (120/126) and NA (21/21) depolarized facial motoneurones. The reversal potentials (Em) obtained from peak voltage amplitude I-V plots in varying extracellular potassium concentrations suggested mechanisms involving a decrease in K+ conductance. 4. Under voltage clamp, close to the resting potential, both 5-HT (39/41) and NA (13/13) evoked inward currents. 5. I-V plots and plots of 5-HT-sensitive current at different membrane potentials, obtained from currents evoked by voltage steps and measured before the development of Ih (instantaneous current), indicated that the 5-HT-evoked inward current was predominately associated with a decrease in conductance but with a range of reversal potentials for 5-HT (E5-HT) from close to, to much more negative than the reversal potential for a potassium conductance (EK). In some cases no change or increases in instantaneous conductance were observed. 6. Steady-state I-V relationships and plots of 5-HT-sensitive current, measured after development of Ih, indicated a 5-HT-associated conductance increase with a time and voltage dependence close to that of Ih, which could be abolished by extracellular caesium (2-5 mM). 7. The NA-evoked inward current was always associated with a decrease in conductance. Instantaneous and steady-state I-V relationships as well as plots of NA-sensitive current indicated a reversal potential at EK. 8. The activation curve for Ih was shifted to more positive potentials in the presence of 5-HT. The time constant for activation of Ih showed a similar shift. 9. 5-Carboxamidotryptamine (5-CT), a 5-HT receptor agonist, was selective for the enhancement of Ih and only evoked an inward current when the holding potential was within the activation range of Ih.(ABSTRACT TRUNCATED AT 400 WORDS)
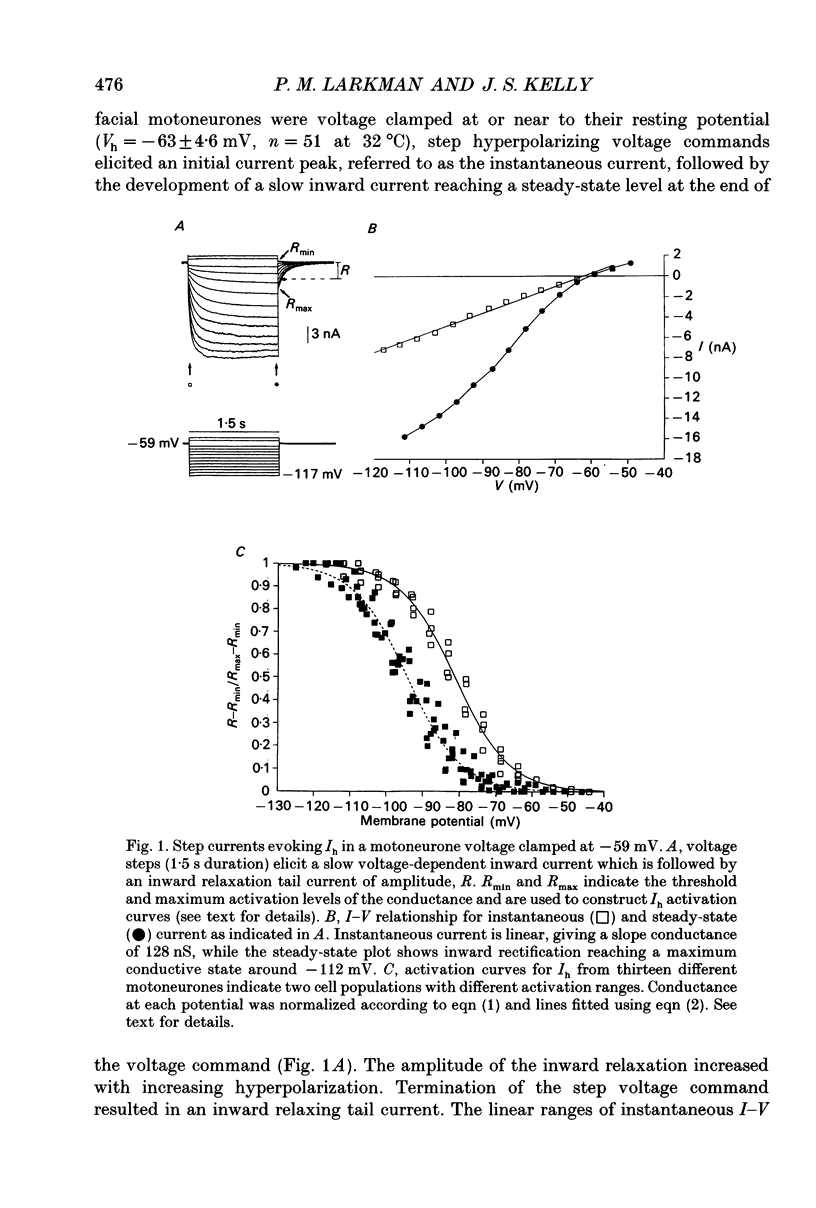
Full text
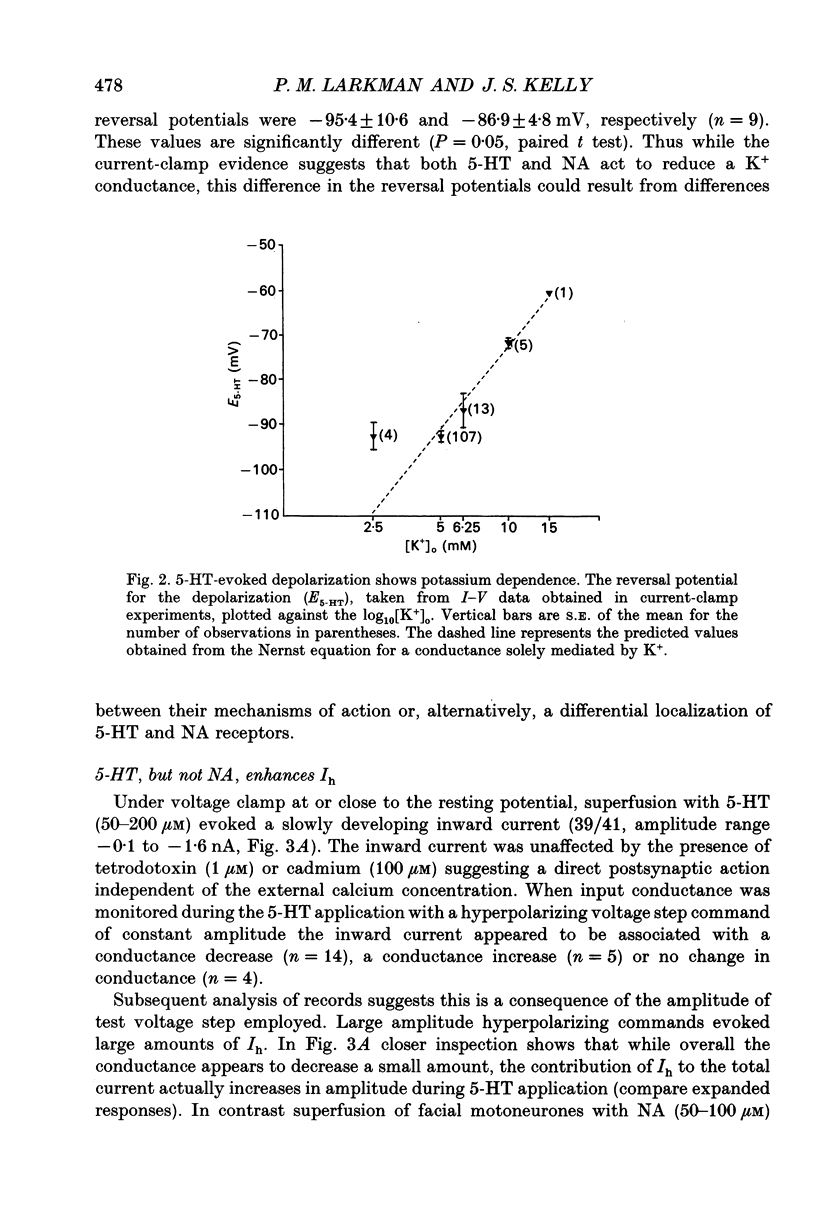
PDF



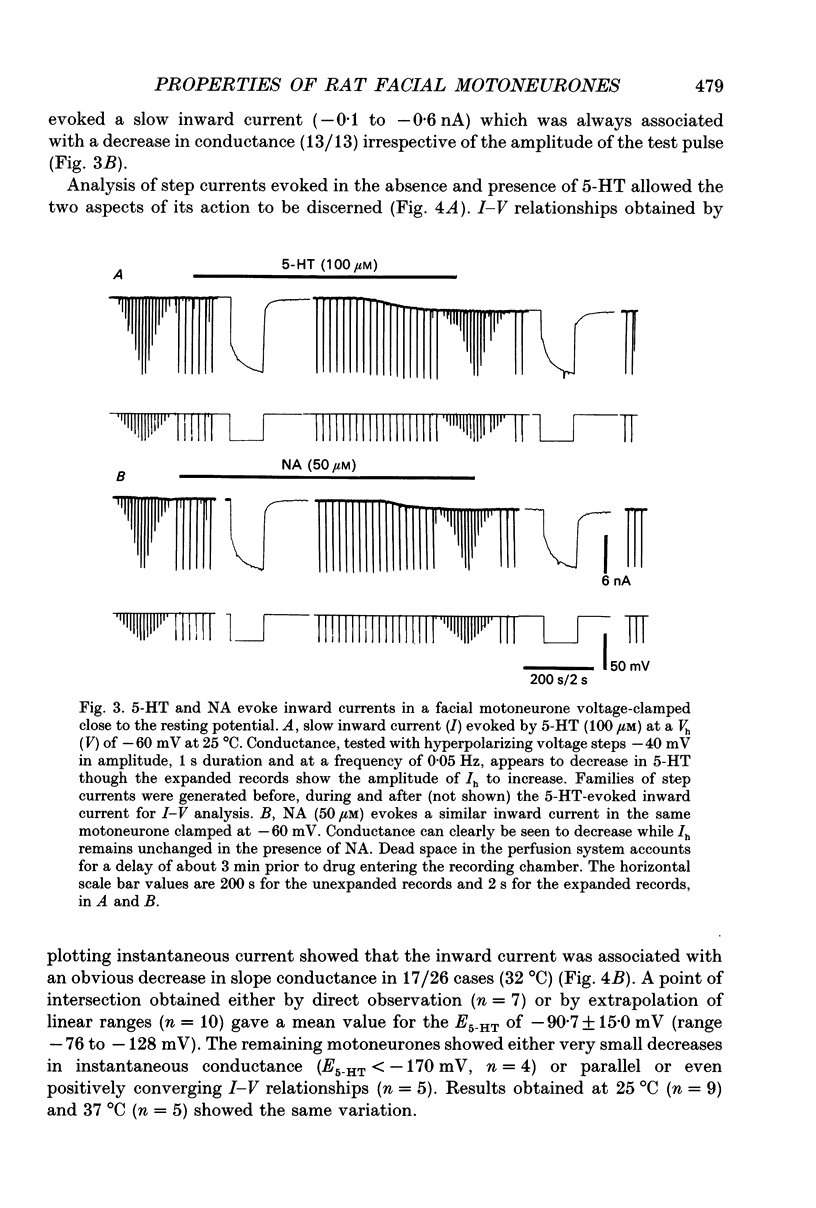







Selected References
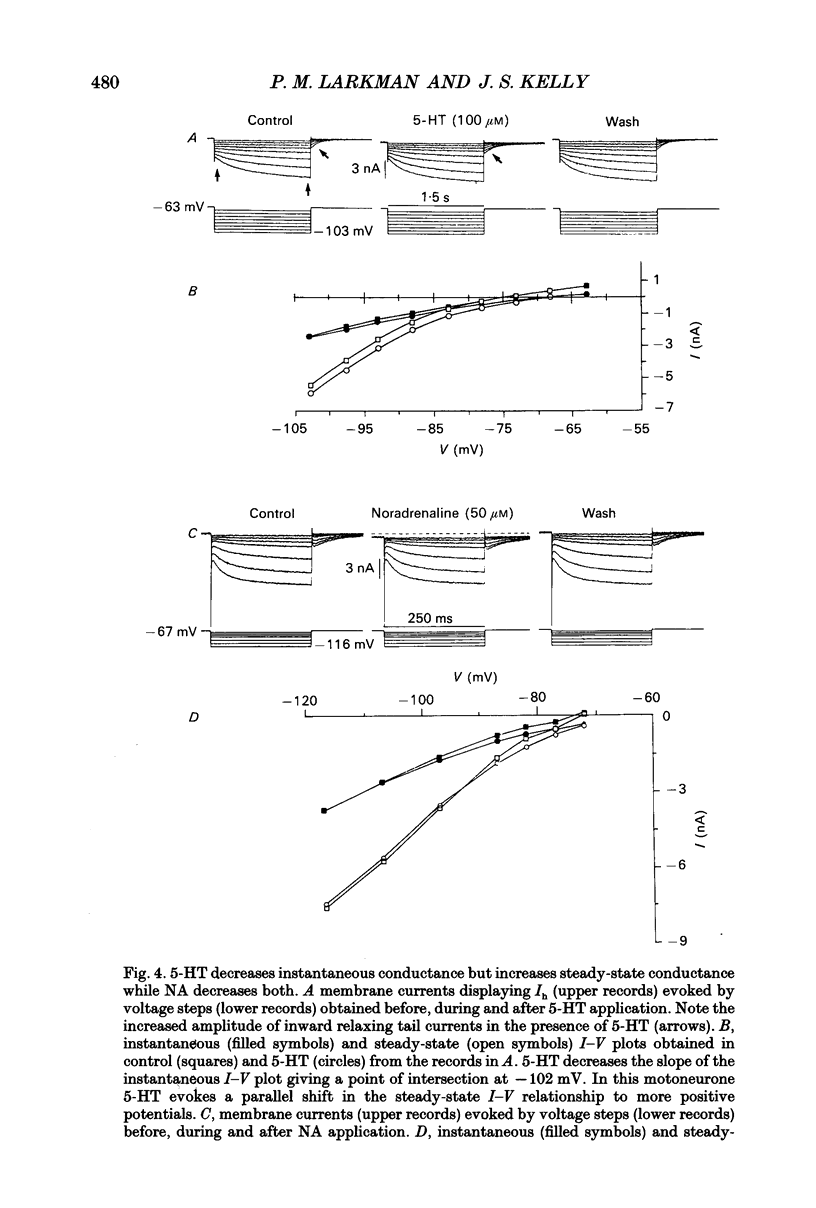
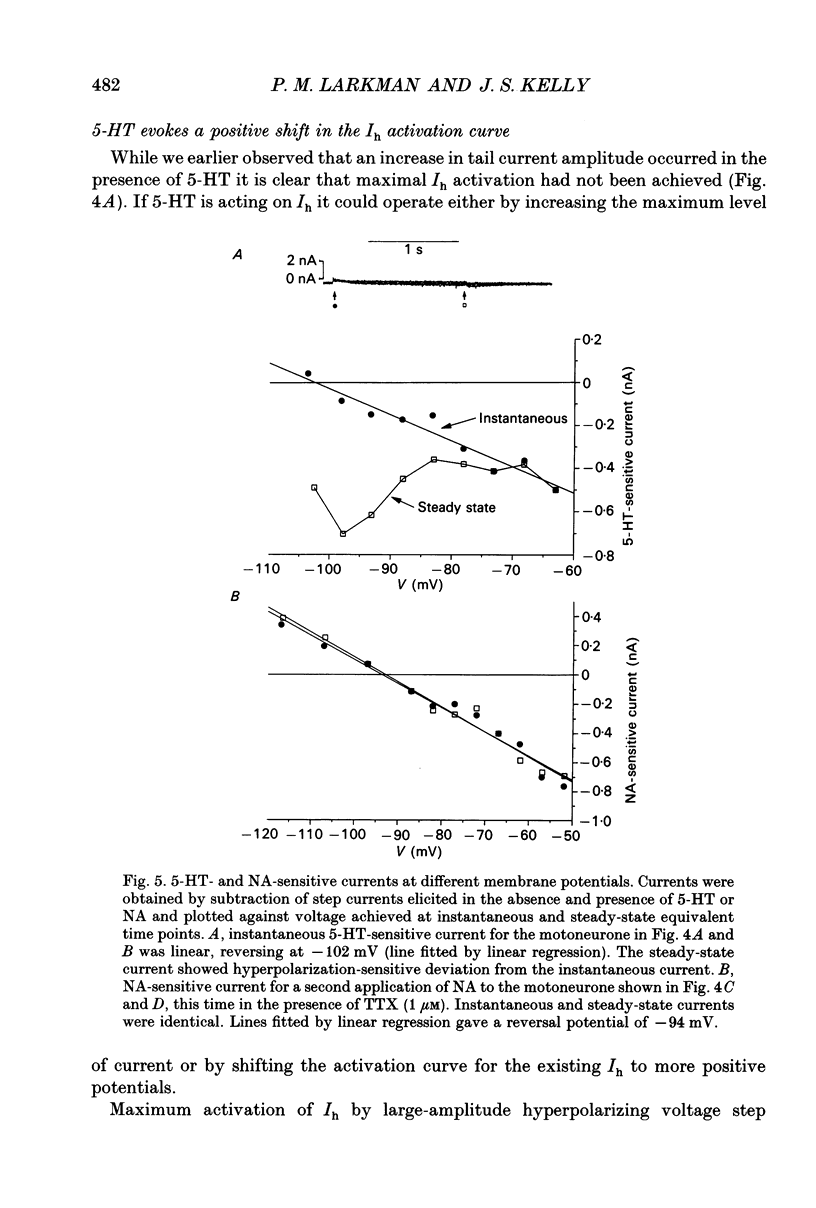
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
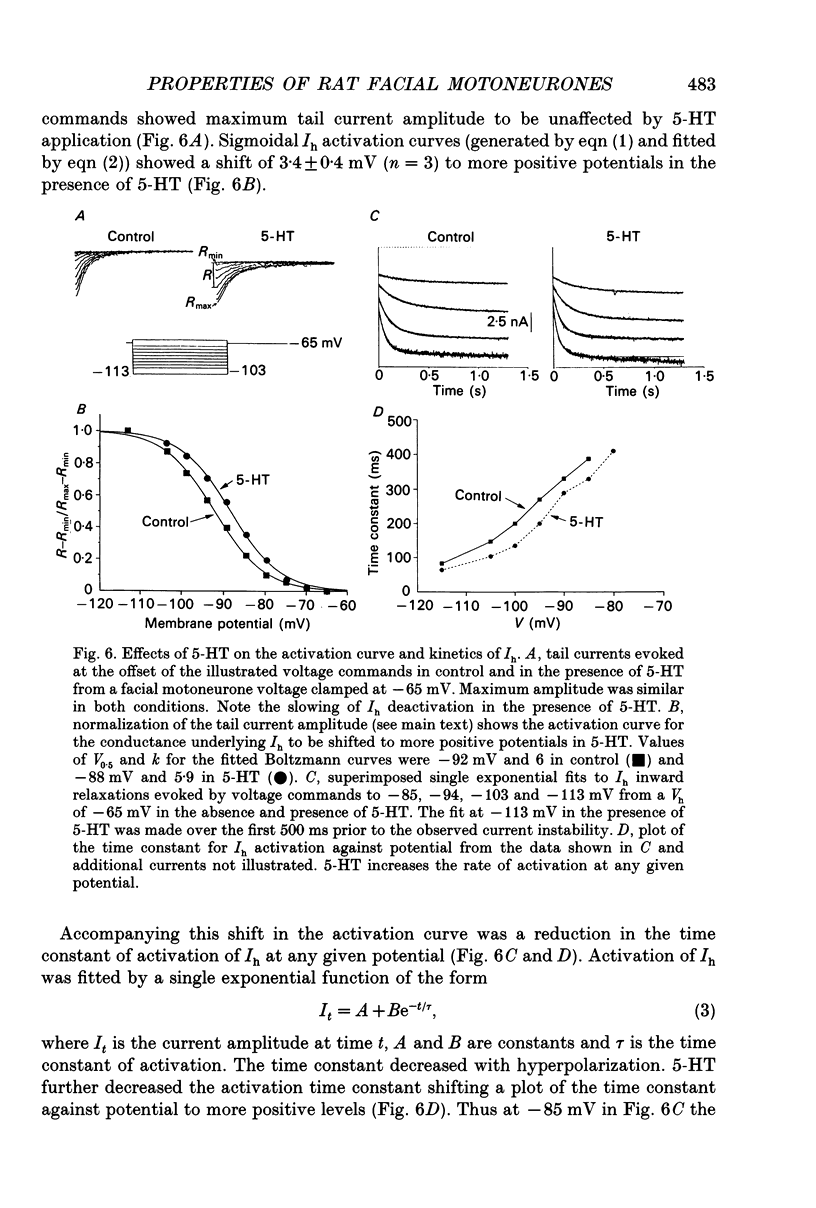
- Anwyl R. Neurophysiological actions of 5-hydroxytryptamine in the vertebrate nervous system. Prog Neurobiol. 1990;35(6):451–468. doi: 10.1016/0301-0082(90)90031-b. [DOI] [PubMed] [Google Scholar]
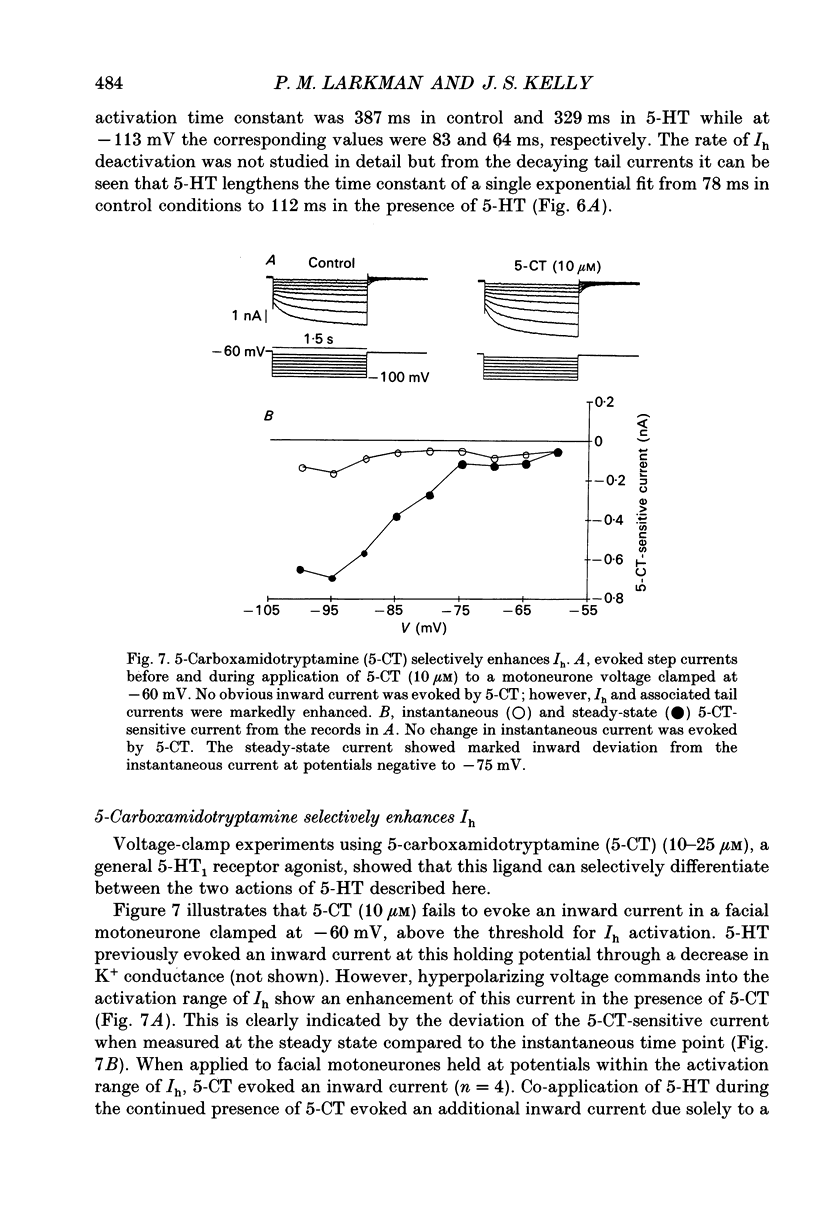
- Araneda R., Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40(2):399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. M., Blitzer R. D., Landau E. M. An analysis of the depolarization produced in guinea-pig hippocampus by cholinergic receptor stimulation. J Physiol. 1988 Oct;404:479–496. doi: 10.1113/jphysiol.1988.sp017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Ion conductances affected by 5-HT receptor subtypes in mammalian neurons. Trends Neurosci. 1990 May;13(5):169–173. doi: 10.1016/0166-2236(90)90042-9. [DOI] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989 Jun;2(6):1535–1540. doi: 10.1016/0896-6273(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Chaput Y., Araneda R. C., Andrade R. Pharmacological and functional analysis of a novel serotonin receptor in the rat hippocampus. Eur J Pharmacol. 1990 Jul 17;182(3):441–456. doi: 10.1016/0014-2999(90)90041-4. [DOI] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987 Jul 2;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Connell L. A., Wallis D. I. 5-Hydroxytryptamine depolarizes neonatal rat motorneurones through a receptor unrelated to an identified binding site. Neuropharmacology. 1989 Jun;28(6):625–634. doi: 10.1016/0028-3908(89)90142-1. [DOI] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G., Kennett G. A. m-CPP: a tool for studying behavioural responses associated with 5-HT1c receptors. Trends Pharmacol Sci. 1990 May;11(5):181–182. doi: 10.1016/0165-6147(90)90109-l. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Factors determining the variation of the afterhyperpolarization duration in cat lumbar alpha-motoneurones. Brain Res. 1985 Feb 11;326(2):392–395. doi: 10.1016/0006-8993(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The interaction of potassium with the activation of anomalous rectification in frog muscle membrane. J Physiol. 1981 Aug;317:497–508. doi: 10.1113/jphysiol.1981.sp013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Larkman P., Penington N. J., Rainnie D. G., McAllister-Williams H., Hodgkiss J. Serotonin receptor heterogeneity and the role of potassium channels in neuronal excitability. Adv Exp Med Biol. 1991;287:177–191. doi: 10.1007/978-1-4684-5907-4_15. [DOI] [PubMed] [Google Scholar]
- Larkman P. M., Penington N. J., Kelly J. S. Electrophysiology of adult rat facial motoneurones: the effects of serotonin (5-HT) in a novel in vitro brainstem slice. J Neurosci Methods. 1989 May;28(1-2):133–146. doi: 10.1016/0165-0270(89)90018-6. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall R. B., Aghajanian G. K. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979 Jun 15;169(1):11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol. 1989 Oct;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K., Aghajanian G. K. Serotonin excitation of facial motoneurons: receptor subtype characterization. Synapse. 1990;5(4):324–332. doi: 10.1002/syn.890050409. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Crill W. E. Influence of anomalous rectifier activation on afterhyperpolarizations of neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1988 Feb;59(2):468–481. doi: 10.1152/jn.1988.59.2.468. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Berger A. J. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990 Apr;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Inward rectification in neonatal rat spinal motoneurones. J Physiol. 1990 Apr;423:47–62. doi: 10.1113/jphysiol.1990.sp018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N., Cherubini E., North R. A. Inward rectification in rat nucleus accumbens neurons. J Neurophysiol. 1989 Dec;62(6):1280–1286. doi: 10.1152/jn.1989.62.6.1280. [DOI] [PubMed] [Google Scholar]
- VanderMaelen C. P., Aghajanian G. K. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980 Sep 25;287(5780):346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- Vandermaelen C. P., Aghajanian G. K. Serotonin-induced depolarization of rat facial motoneurons in vivo: comparison with amino acid transmitters. Brain Res. 1982 May 6;239(1):139–152. doi: 10.1016/0006-8993(82)90838-1. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., Connell L. A., Kvaltinova Z. Further studies on the action of 5-hydroxytryptamine on lumbar motoneurones in the rat isolated spinal cord. Naunyn Schmiedebergs Arch Pharmacol. 1991 Apr;343(4):344–352. doi: 10.1007/BF00179038. [DOI] [PubMed] [Google Scholar]
- Walton K., Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983 Nov 14;278(1-2):387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]
- Wang M. Y., Dun N. J. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990 Nov;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Nakajima Y., Nakajima S., Stanfield P. R. Modulation of inwardly rectifying channels by substance P in cholinergic neurones from rat brain in culture. J Physiol. 1990 Jul;426:499–520. doi: 10.1113/jphysiol.1990.sp018151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]


