Abstract
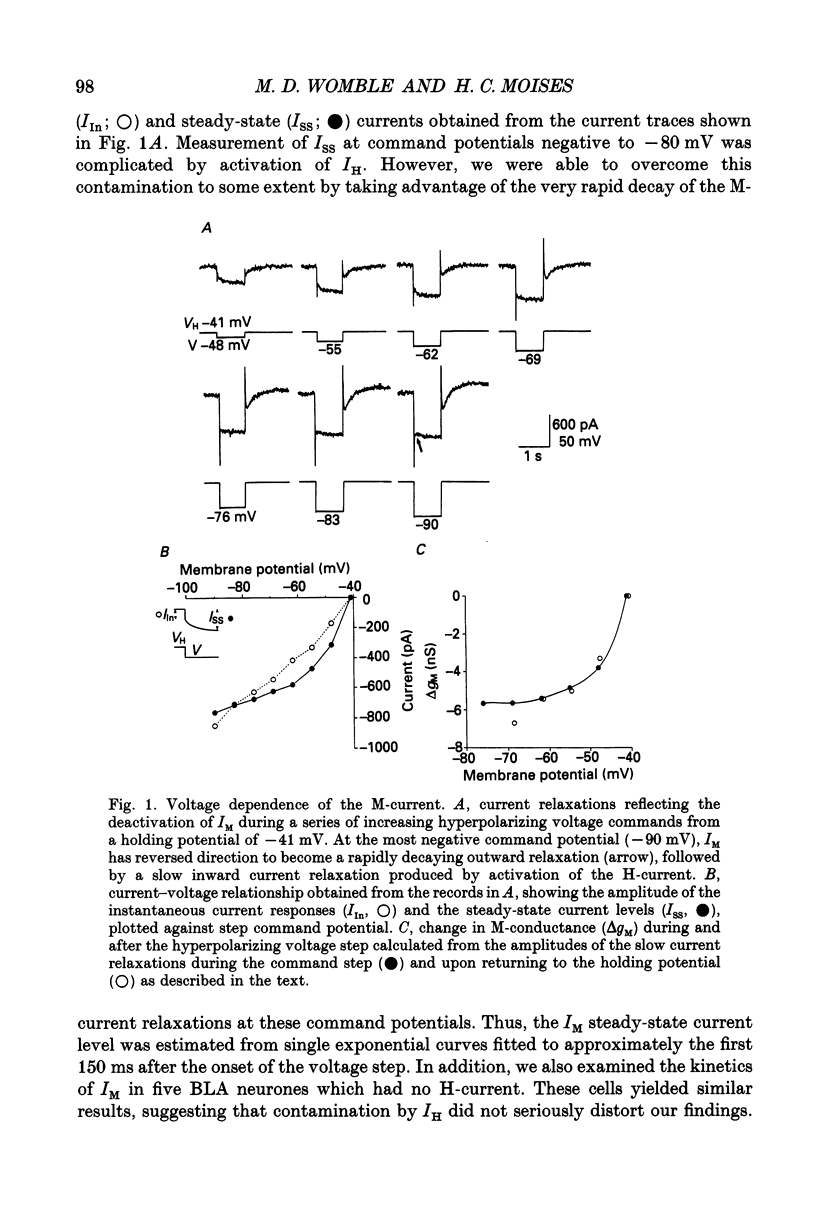
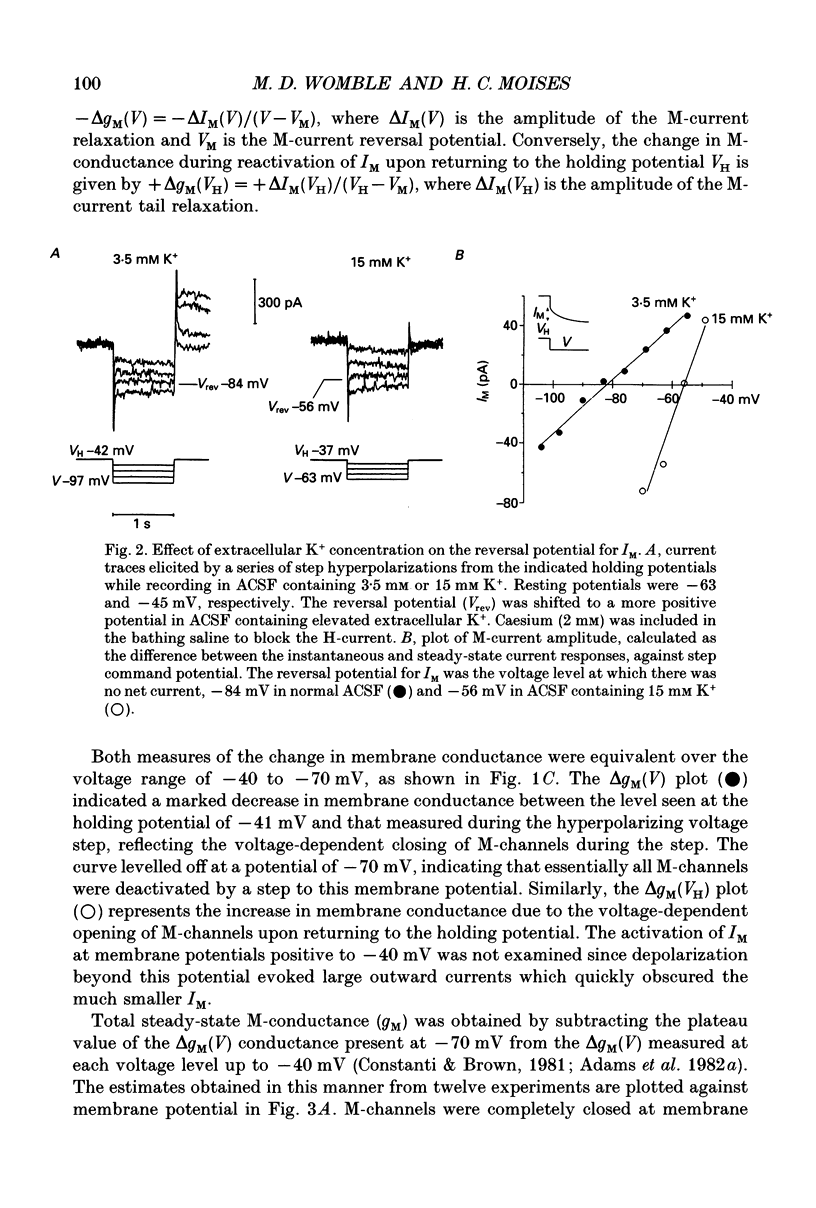
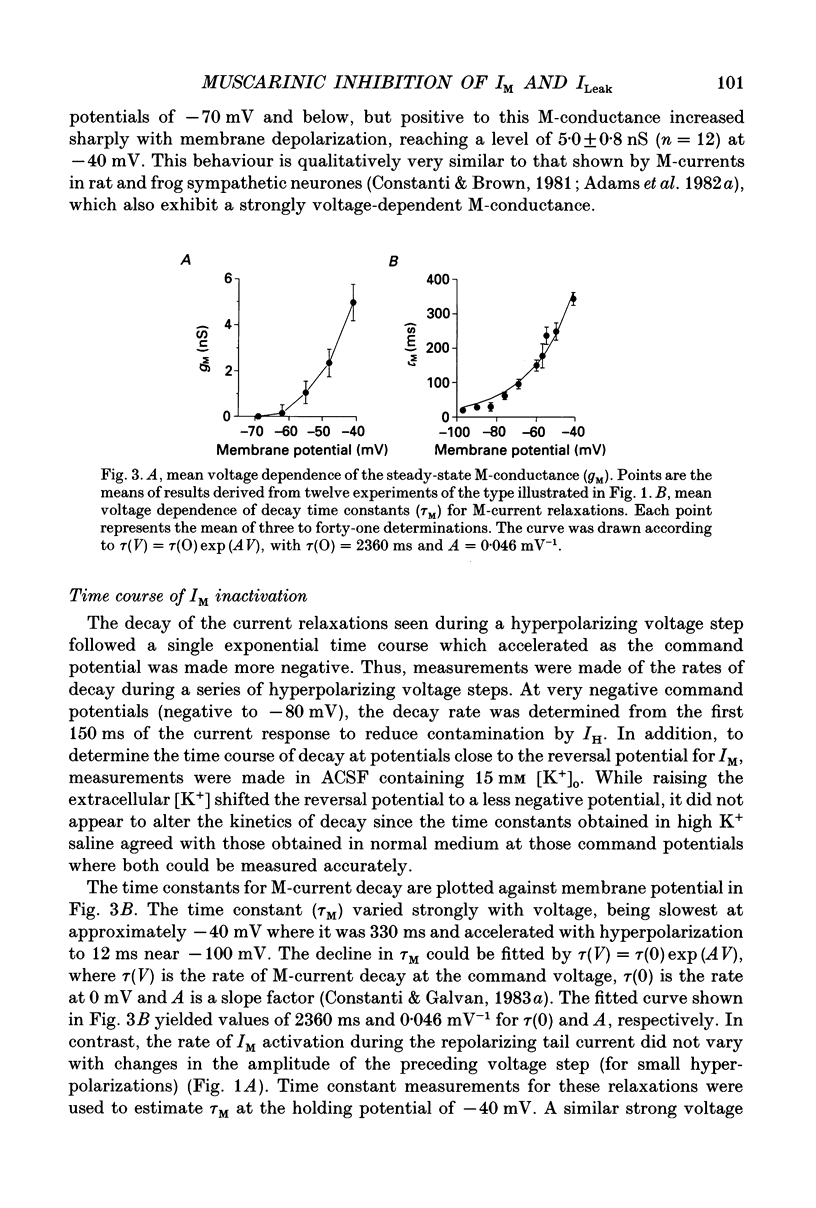
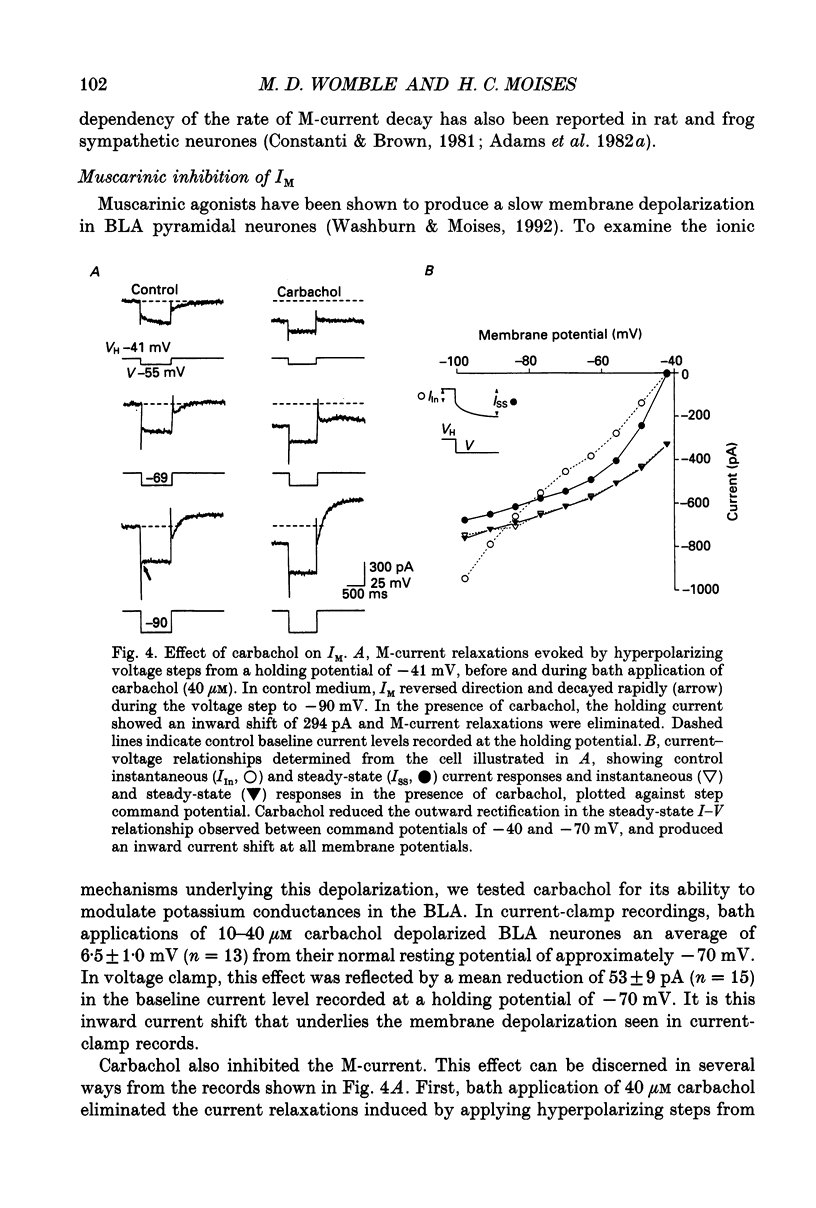
1. Voltage-clamp recordings using a single microelectrode were obtained from pyramidal neurones of the basolateral amygdala (BLA) in slices of the rat ventral forebrain. Slow inward current relaxations during hyperpolarizing voltage steps from a holding potential of -40 mV were identified as the muscarinic-sensitive M-current (IM), a time- and voltage-dependent potassium current previously identified in other neuronal cell types. 2. Activation of IM was voltage dependent with a threshold of approximately -70 mV. At membrane potentials positive to this, the steady-state current-voltage (I-V) relationship showed substantial outward rectification, reflecting the time- and voltage-dependent opening of M-channels. The underlying conductance (gM) also increased sharply with depolarization. 3. The reversal potential for IM was -84 mV in medium containing 3.5 mM K+. This was shifted positively by 27 mV when the external K+ concentration was raised to 15 mM. 4. The time courses of M-current activation and deactivation were fitted by a single exponential. The time constant for IM decay, measured at 24 degrees C, was strongly dependent on membrane potential, ranging from 330 ms at -40 mV to 12 ms at -100 mV. 5. Bath application of carbachol (0.5-40 microM) inhibited IM, as evidenced by the reduction or elimination of the slow inward M-current relaxations evoked during hyperpolarizing steps from a holding potential of -40 mV. The outward rectification of the steady-state I-V relationship at membrane potentials positive to -70 mV was also largely eliminated. The inhibition of IM by carbachol was dose dependent and antagonized by atropine. 6. Carbachol produced an inward current shift at a holding potential of -40 mV that was only partially attributable to inhibition of IM. An inward current shift was also produced by carbachol at membrane potentials negative to -70 mV, where IM is inactive. These effects were dose dependent and antagonized by atropine. They were attributed to the muscarinic inhibition of a voltage-insensitive potassium leak conductance (ILeak). 7. In most cells, carbachol reduced the slope of the instantaneous I-V relationship obtained from a holding potential of -70 mV so that it crossed the control I-V plot at the reversal potential for ILeak. This was found to be -108 mV in 3.5 mM K+ saline, shifting to -66 mV in 15 mM K+ saline.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
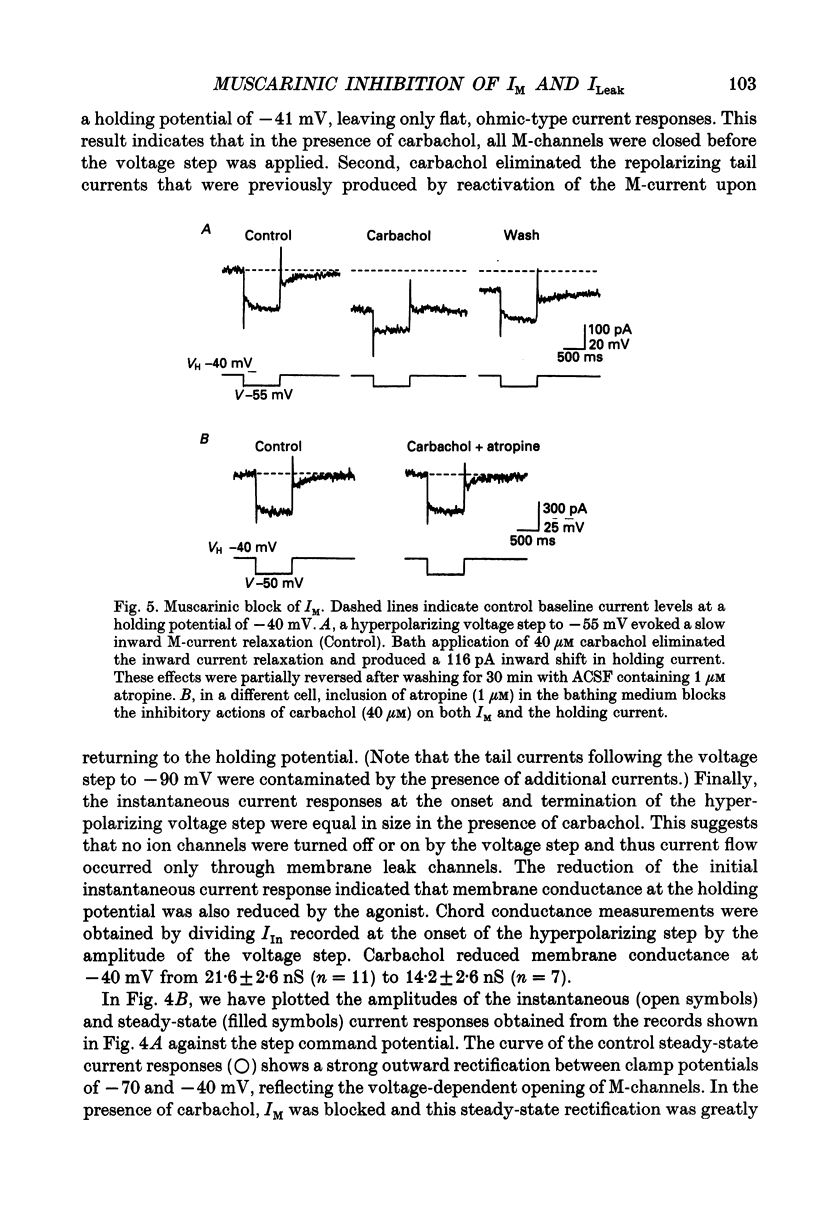
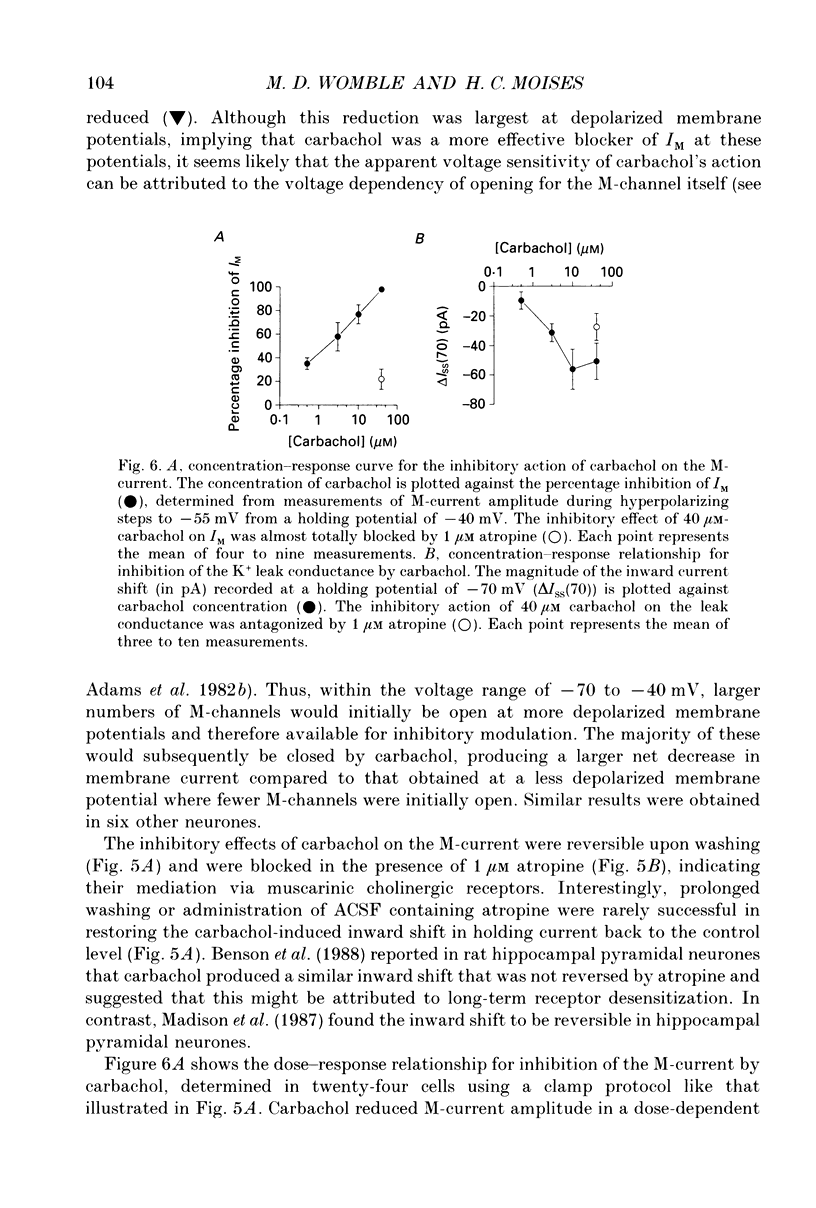
These references are in PubMed. This may not be the complete list of references from this article.
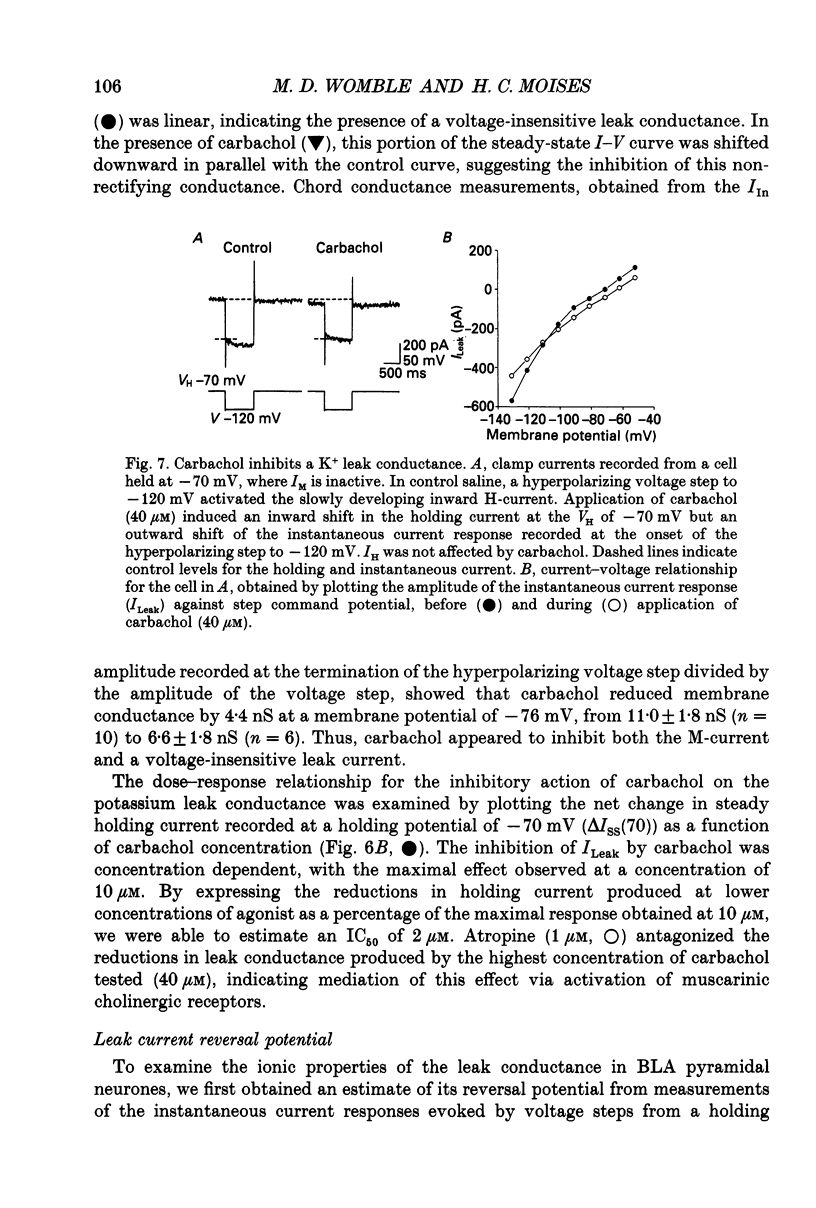
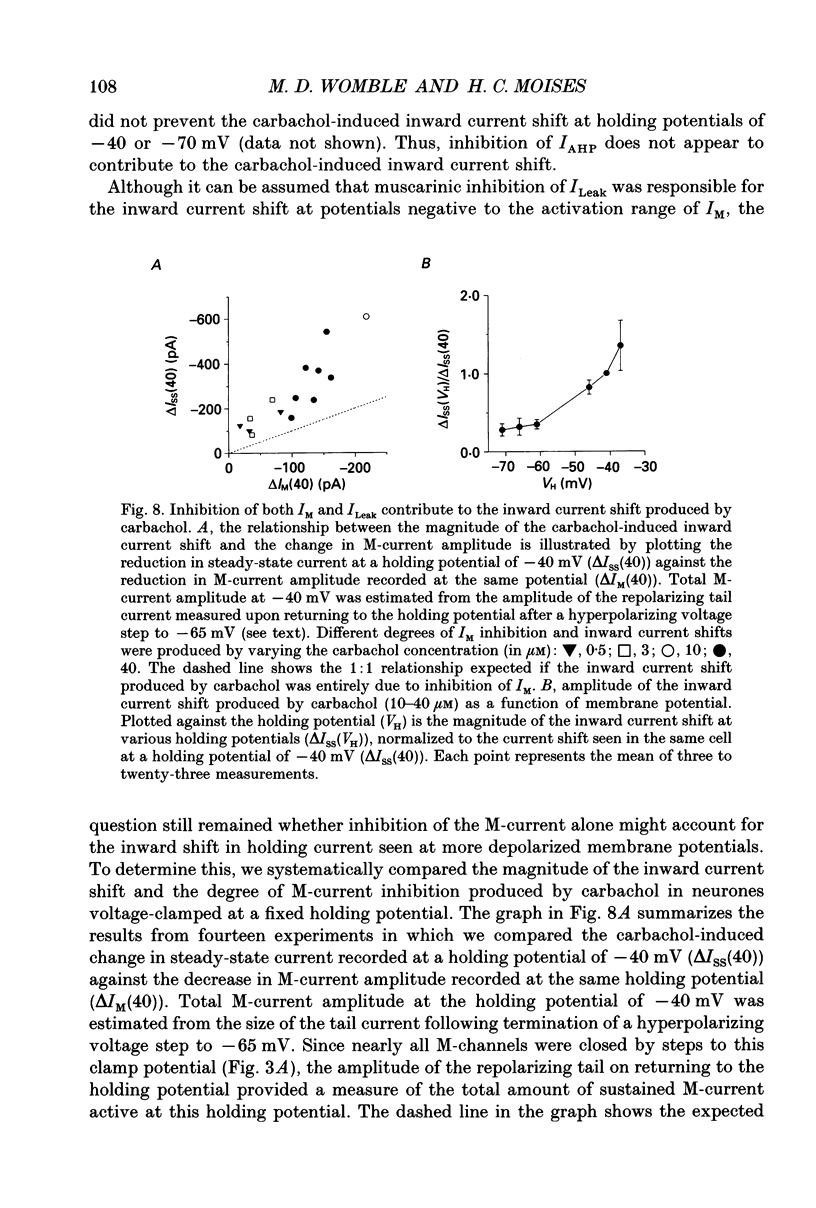
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Galvan M. Voltage-dependent currents of vertebrate neurons and their role in membrane excitability. Adv Neurol. 1986;44:137–170. [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Benson D. M., Blitzer R. D., Landau E. M. An analysis of the depolarization produced in guinea-pig hippocampus by cholinergic receptor stimulation. J Physiol. 1988 Oct;404:479–496. doi: 10.1113/jphysiol.1988.sp017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Carlsen J., Záborszky L., Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985 Apr 8;234(2):155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. M-current in voltage-clamped olfactory cortex neurones. Neurosci Lett. 1983 Aug 19;39(1):65–70. doi: 10.1016/0304-3940(83)90166-0. [DOI] [PubMed] [Google Scholar]
- Constanti A., Sim J. A. Calcium-dependent potassium conductance in guinea-pig olfactory cortex neurones in vitro. J Physiol. 1987 Jun;387:173–194. doi: 10.1113/jphysiol.1987.sp016569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahwiler B. H., Brown D. A. Functional innervation of cultured hippocampal neurones by cholinergic afferents from co-cultured septal explants. Nature. 1985 Feb 14;313(6003):577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V. M-current in human neocortical neurones. Neurosci Lett. 1986 Jun 6;67(1):1–6. doi: 10.1016/0304-3940(86)90198-9. [DOI] [PubMed] [Google Scholar]
- Hellendall R. P., Godfrey D. A., Ross C. D., Armstrong D. M., Price J. L. The distribution of choline acetyltransferase in the rat amygdaloid complex and adjacent cortical areas, as determined by quantitative micro-assay and immunohistochemistry. J Comp Neurol. 1986 Jul 22;249(4):486–498. doi: 10.1002/cne.902490405. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Johnston D. Passive cable properties of hippocampal CA3 pyramidal neurons. Cell Mol Neurobiol. 1981 Mar;1(1):41–55. doi: 10.1007/BF00736038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987 Nov;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol. 1986 Jun;375:169–194. doi: 10.1113/jphysiol.1986.sp016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L. M., Macdonald R. L. Muscarine-sensitive voltage-dependent potassium current in cultured murine spinal cord neurons. Neurosci Lett. 1983 Jan 31;35(1):85–91. doi: 10.1016/0304-3940(83)90531-1. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Development of post-tetanic potentiation at identified inhibitory and excitatory synapses in Aplysia. J Physiol. 1982 Jan;322:223–240. doi: 10.1113/jphysiol.1982.sp014034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie D. G., Asprodini E. K., Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol. 1991 Sep;66(3):986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- Storm J. F. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989 Feb;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Washburn M. S., Moises H. C. Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. J Physiol. 1992 Apr;449:121–154. doi: 10.1113/jphysiol.1992.sp019078. [DOI] [PMC free article] [PubMed] [Google Scholar]


