Abstract
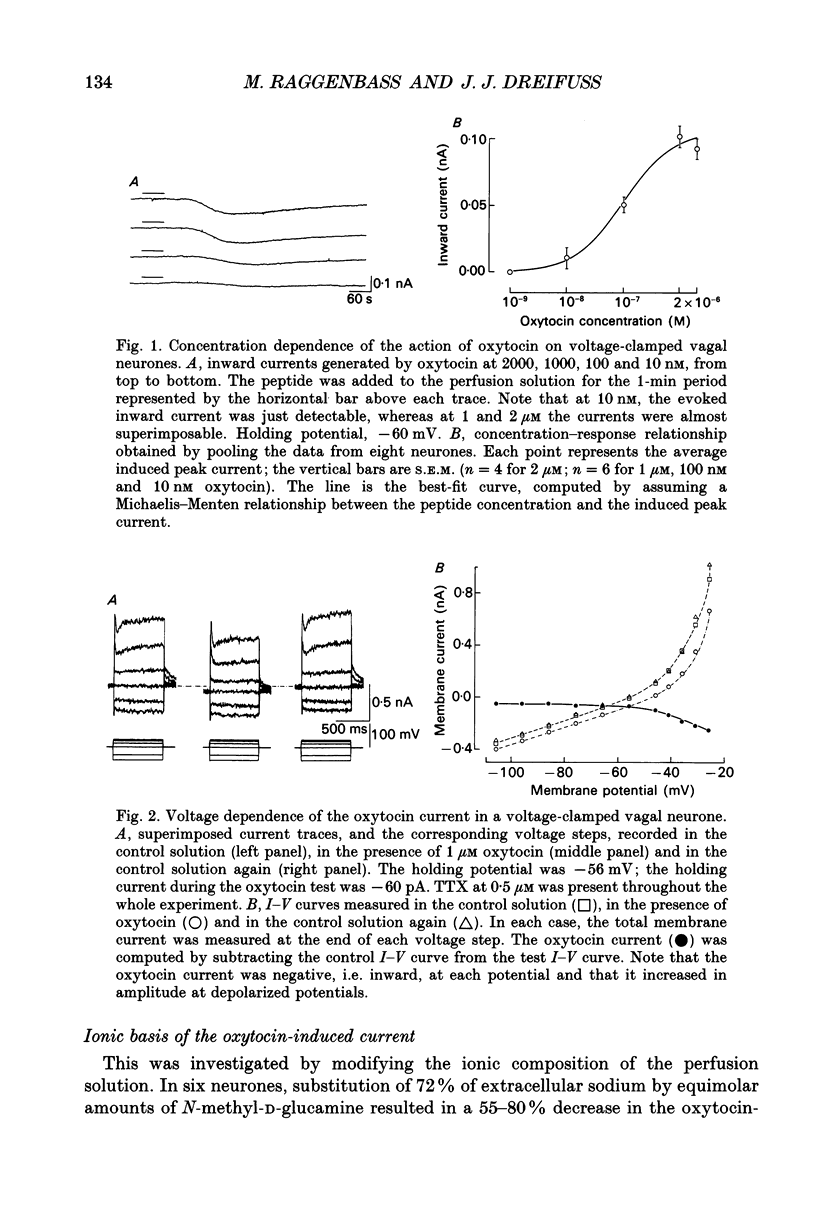
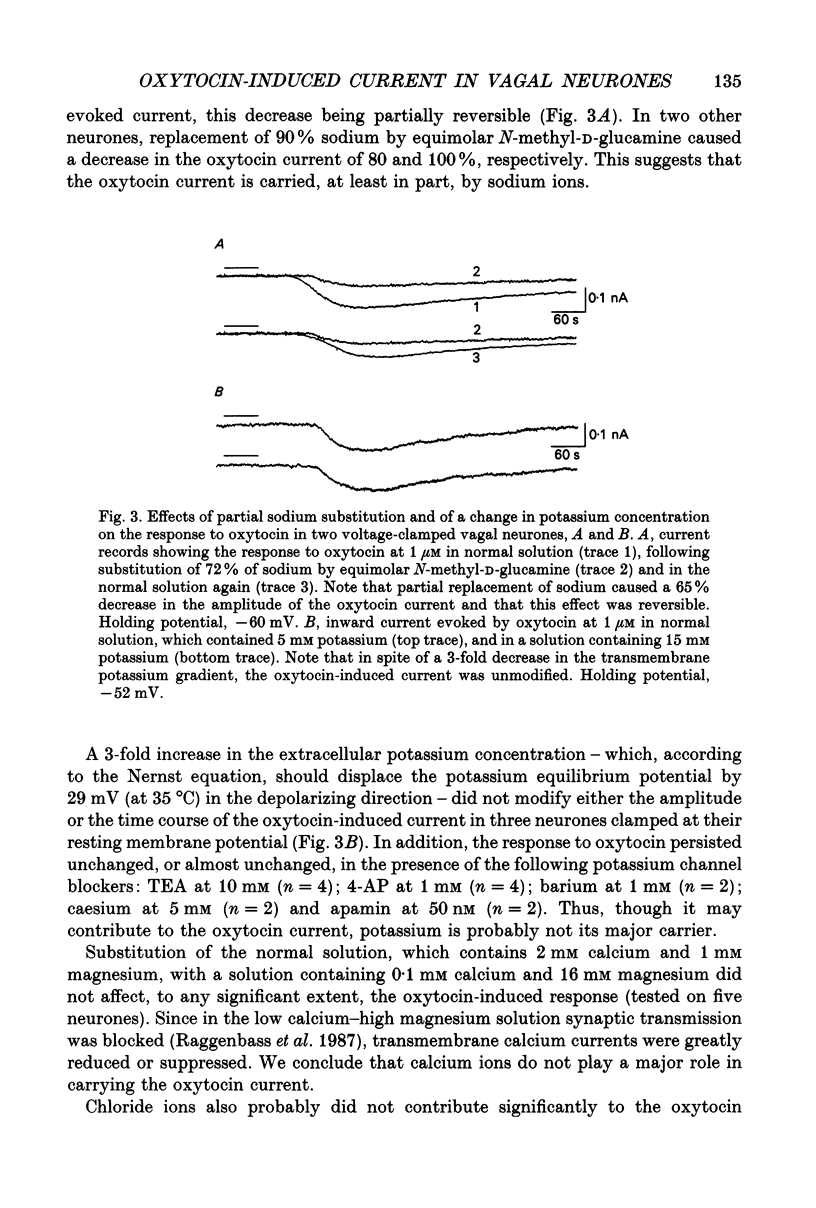
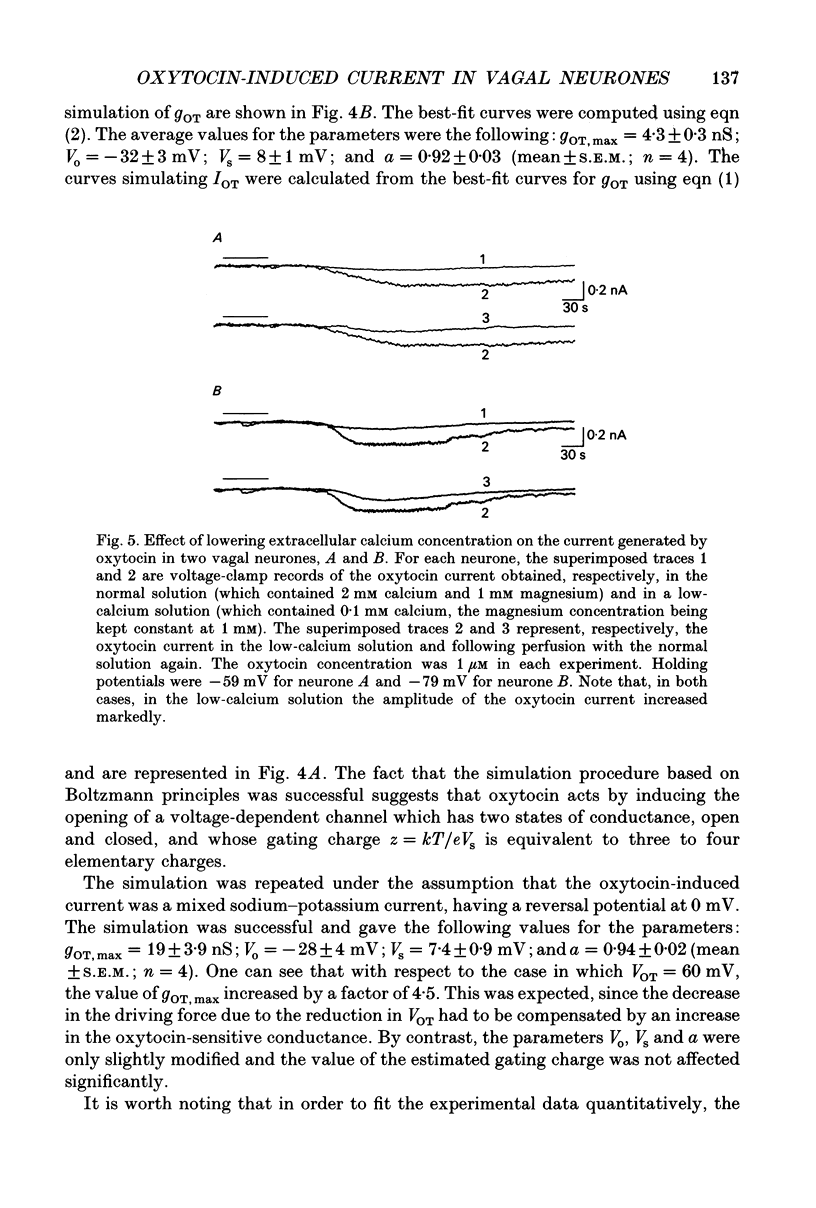
1. The mechanism of action of oxytocin on vagal neurones of the rat was studied using single-electrode voltage-clamp recordings from brainstem slices. The ionic basis of the oxytocin-induced current was examined by changing the composition of the perfusion solution and by making use of channel blockers. 2. In neurones clamped at or near their resting potential, oxytocin generated a sustained, TTX-insensitive inward current whose peak amplitude was concentration related. This current was detectable at 10 nM, was half-maximal at about 100 nM and was maximal at micromolar concentrations of peptide. 3. The oxytocin current was inward over membrane potentials ranging from -110 to -20 mV and was voltage dependent, since it increased in magnitude as the membrane was depolarized from the resting potential toward less negative potentials. 4. Partial replacement of extracellular sodium by equimolar N-methyl-D-glucamine reversibly attenuated or suppressed the oxytocin current. By contrast, substituting part of extracellular chloride or blocking calcium currents did not modify it. Increasing the transmembrane potassium gradient was also without effect and none of the potassium channel blockers TEA, 4-amino pyridine (4-AP), apamin, caesium or barium affected the oxytocin current. This current is thus at least in part carried by sodium. 5. The activation of the oxytocin current as a function of the membrane potential could be quantitatively simulated using a Boltzmann equation, suggesting that oxytocin acts by inducing the opening of a voltage-dependent channel which can exist in either of two states, open or closed. 6. Lowering the extracellular calcium concentration from 2 to 0.1 mM, while keeping the magnesium concentration constant at 1 mM, enhanced the response to oxytocin. This low calcium-induced potentiation of the oxytocin current was 1.4-3-fold and was reversible. 7. We conclude that oxytocin increases the excitability of vagal neurones by generating a persistent, voltage-gated current which is sodium dependent, is insensitive to TTX and is modulated by divalent cations.
Full text
PDF


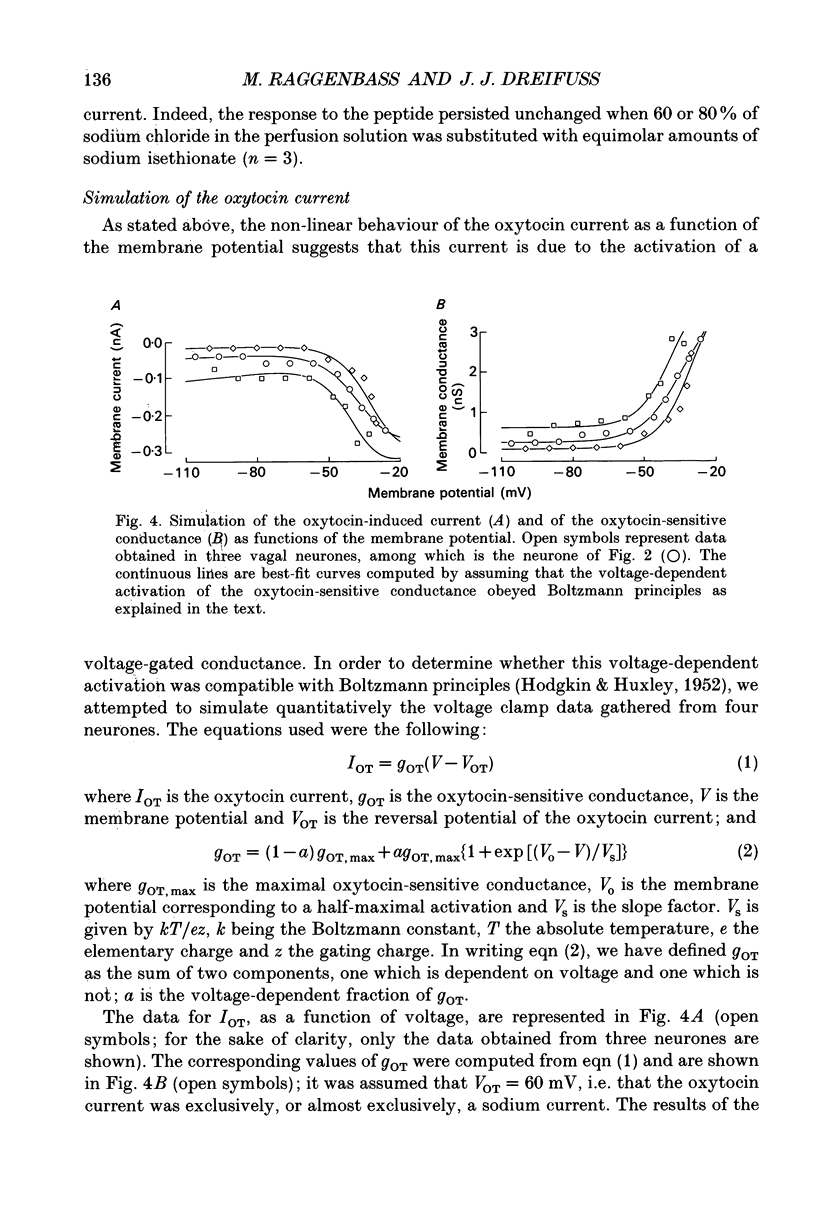





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charpak S., Armstrong W. E., Mühlethaler M., Dreifuss J. J. Stimulatory action of oxytocin on neurones of the dorsal motor nucleus of the vagus nerve. Brain Res. 1984 May 21;300(1):83–89. doi: 10.1016/0006-8993(84)91342-8. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Raggenbass M., Charpak S., Dubois-Dauphin M., Tribollet E. A role of central oxytocin in autonomic functions: its action in the motor nucleus of the vagus nerve. Brain Res Bull. 1988 Jun;20(6):765–770. doi: 10.1016/0361-9230(88)90089-5. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M., Raggenbass M., Widmer H., Tribollet E., Dreifuss J. J. Morphological and electrophysiological evidence for postsynaptic localization of functional oxytocin receptors in the rat dorsal motor nucleus of the vagus nerve. Brain Res. 1992 Mar 13;575(1):124–131. doi: 10.1016/0006-8993(92)90431-8. [DOI] [PubMed] [Google Scholar]
- Elands J., Beetsma A., Barberis C., de Kloet E. R. Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist. J Chem Neuroanat. 1988 Nov-Dec;1(6):293–302. [PubMed] [Google Scholar]
- Funase K. Oxytocin-induced sodium current is mediated by cAMP-dependent protein phosphorylation in an identified snail neuron. Brain Res. 1990 May 28;517(1-2):263–268. doi: 10.1016/0006-8993(90)91036-g. [DOI] [PubMed] [Google Scholar]
- Gillette R., Green D. J. Calcium dependence of voltage sensitivity in adenosine 3',5'-cyclic phosphate-stimulated sodium current in Pleurobranchaea. J Physiol. 1987 Dec;393:233–245. doi: 10.1113/jphysiol.1987.sp016821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M., McAdoo D. J. The voltage-dependent, slow inward current induced by the neuropeptide FMRFamide in Aplysia neuron R14. J Neurosci. 1988 Oct;8(10):3891–3900. doi: 10.1523/JNEUROSCI.08-10-03891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Cyclic AMP-induced slow inward current in depolarized neurons of Aplysia californica. J Neurosci. 1990 Oct;10(10):3194–3207. doi: 10.1523/JNEUROSCI.10-10-03194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Sasaki K., Sato M., Shozushima M., Takashima K. Dopamine-induced depolarizing responses associated with negative slope conductance in LB-cluster neurones of Aplysia. J Physiol. 1988 Dec;407:199–213. doi: 10.1113/jphysiol.1988.sp017410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann M. J., Rogers R. C. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990 Sep;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosfeldt Laursen A., Rekling J. C. Electrophysiological properties of hypoglossal motoneurons of guinea-pigs studied in vitro. Neuroscience. 1989;30(3):619–637. doi: 10.1016/0306-4522(89)90156-5. [DOI] [PubMed] [Google Scholar]
- Nitzan R., Segev I., Yarom Y. Voltage behavior along the irregular dendritic structure of morphologically and physiologically characterized vagal motoneurons in the guinea pig. J Neurophysiol. 1990 Feb;63(2):333–346. doi: 10.1152/jn.1990.63.2.333. [DOI] [PubMed] [Google Scholar]
- Raggenbass M., Dubois-Dauphin M., Charpak S., Dreifuss J. J. Neurons in the dorsal motor nucleus of the vagus nerve are excited by oxytocin in the rat but not in the guinea pig. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3926–3930. doi: 10.1073/pnas.84.11.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M., Goumaz M., Sermasi E., Tribollet E., Dreifuss J. J. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991 Jun;11(6):1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M., Tribollet E., Dubois-Dauphin M., Dreifuss J. J. Vasopressin receptors of the vasopressor (V1) type in the nucleus of the solitary tract of the rat mediate direct neuronal excitation. J Neurosci. 1989 Nov;9(11):3929–3936. doi: 10.1523/JNEUROSCI.09-11-03929.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. Depolarization of nonmyelinated fibers of the rat vagus nerve produced by activation of protein kinase C. J Neurosci. 1988 Jul;8(7):2606–2617. doi: 10.1523/JNEUROSCI.08-07-02606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982 Mar 1;205(3):260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Siaud P., Denoroy L., Assenmacher I., Alonso G. Comparative immunocytochemical study of the catecholaminergic and peptidergic afferent innervation to the dorsal vagal complex in rat and guinea pig. J Comp Neurol. 1989 Dec 15;290(3):323–335. doi: 10.1002/cne.902900302. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Charpak S., Schmidt A., Dubois-Dauphin M., Dreifuss J. J. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci. 1989 May;9(5):1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E., Goumaz M., Raggenbass M., Dubois-Dauphin M., Dreifuss J. J. Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Brain Res Dev Brain Res. 1991 Jan 15;58(1):13–24. doi: 10.1016/0165-3806(91)90232-8. [DOI] [PubMed] [Google Scholar]
- Voorn P., Buijs R. M. An immuno-electronmicroscopical study comparing vasopressin, oxytocin, substance P and enkephalin containing nerve terminals in the nucleus of the solitary tract of the rat. Brain Res. 1983 Jun 27;270(1):169–173. doi: 10.1016/0006-8993(83)90809-0. [DOI] [PubMed] [Google Scholar]
- Yarom Y., Sugimori M., Llinás R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985 Dec;16(4):719–737. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]


