Abstract
Identification of mutations in the ABCA1 transporter (ABCA1) as the genetic defect in Tangier disease has generated interest in modulating atherogenic risk by enhancing ABCA1 gene expression. To investigate the role of ABCA1 in atherogenesis, we analyzed diet-induced atherosclerosis in transgenic mice overexpressing human ABCA1 (hABCA1-Tg) and spontaneous lesion formation in hABCA1-Tg × apoE-knockout (KO) mice. Overexpression of hABCA1 in C57BL/6 mice resulted in a unique anti-atherogenic profile characterized by decreased plasma cholesterol (63%), cholesteryl ester (63%), free cholesterol (67%), non-high density lipoprotein (HDL)-cholesterol (53%), and apolipoprotein (apo) B (64%) but markedly increased HDL-cholesterol (2.8-fold), apoA-I (2.2-fold), and apoE (2.8-fold) levels. These beneficial changes in the lipid profile led to significantly lower (65%) aortic atherosclerosis in hABCA1-Tg mice. In marked contrast, ABCA1 overexpression had a minimal effect on the plasma lipid profile of apoE-KO mice and resulted in a 2- to 2.6-fold increase in aortic lesion area. These combined results indicate that overexpression of ABCA1 in C57BL/6 mice on a high cholesterol diet results in an atheroprotective lipoprotein profile and decreased atherosclerosis, and thus provide previously undocumented in vivo evidence of an anti-atherogenic role for the ABCA1 transporter. In contrast, overexpression of ABCA1 in an apoE-KO background led to increased atherosclerosis, further substantiating the important role of apoE in macrophage cholesterol metabolism and atherogenesis. In summary, these results establish that, in the presence of apoE, overexpression of ABCA1 modulates HDL as well as apoB-containing lipoprotein metabolism and reduces atherosclerosis in vivo, and indicate that pharmacological agents that will increase ABCA1 expression may reduce atherogenic risk in humans.
Plasma high density lipoprotein cholesterol (HDL-C) levels are inversely correlated with the incidence of cardiovascular disease (1–3). A major mechanism by which HDL may protect against the development of atherosclerosis is reverse cholesterol transport, the process by which excess cholesterol is transferred from peripheral tissues to the liver, where it is excreted from the body directly or after conversion into bile acids (4, 5). A major advance in our understanding of the reverse cholesterol transport pathway occurred with the discovery of the ATP binding cassette transporter A1 (ABCA1), which was identified by the analysis of patients with Tangier disease and selected kindreds with familial hypoalphalipoproteinemia (6–12). Tangier disease patients are characterized by orange tonsils, decreased plasma cholesterol, low density lipoproteins (LDL) and HDL, and increased triglycerides, cholesterol accumulation in tissues, including macrophages, and cardiovascular disease (13, 14).
Several studies have established that the ABCA1 transporter facilitates the efflux of cellular phospholipids and cholesterol to apolipoprotein acceptors, such as apolipoproteins (apo) A-I and apoE, and results in the formation of nascent or pre-β HDL (6, 11, 15–18). The ABCA1 transporter not only is present on the cell surface but also has a recycling vesicular pathway to the late endocytic compartment of the cell, which may play a pivotal role in mediating intracellular trafficking of cholesterol to the cell surface for efflux (19). The human ABCA1 gene is 175 kb with 50 exons interrupted by 49 introns (12, 20, 21). Recently, oxysterols were identified as important modulators of transcription of the ABCA1 gene through a liver X receptor (LXR)/retinoid X receptor (RXR) response element in the promoter of the ABCA1 gene (22–25).
Similar to the clinical phenotype of Tangier disease patients, inactivation of the ABCA1 gene in mice resulted in a marked decrease of total plasma cholesterol and phospholipids, and the virtual absence of HDL (17, 26, 27). However, the effect of ABCA1 transporter deficiency on the susceptibility to diet-induced atherosclerosis in mice has not been reported to date.
We (28), as well as other laboratories (29, 30), have recently reported on the generation of transgenic mice that overexpress human ABCA1 (ABCA1-Tg). ABCA1 transgenic mice were shown to have increased total plasma and HDL-cholesterol, as well as apoA-I, and apoA-II levels, when compared with control C57BL/6 mice. The increased plasma HDL-C levels in ABCA1-Tg mice were due to decreased apoA-I catabolism, and the net hepatic flux of HDL-C was increased in the ABCA1-Tg mice (28). These combined data indicate that the ABCA1 transporter is intimately involved in the process of reverse cholesterol transport, as well as HDL maturation, and suggest that therapeutic up-regulation of ABCA1 expression may be beneficial in the prevention and regression of atherosclerosis.
In the present study, we directly address this question by evaluating the effect of ABCA1 overexpression on the development of diet-induced atherosclerosis in C57BL/6 mice and the development of spontaneous aortic lesions in apoE-KO mice.
Materials and Methods
Animals.
Human ABCA1-Tg mice (28) were crossed with apoE-knockout (KO) mice (31) with C57BL/6 background (The Jackson Laboratory) to generate ABCA1-Tg × apoE-KO mice. Expression of human ABCA1 was determined by dot blot hybridization, using an ABCA1 cDNA [32P]dCTP-labeled probe generated from ABCA1 cDNA with Ready-To-Go DNA Labeling Beads (Amersham Pharmacia Biotech). Mouse genomic DNA was isolated from white blood cells, by using Promega Wizard PCR Preps DNA Purification System, and the genotype was confirmed by PCR amplification, using ABCA1-specific PCR primers ABCA1-242F (5′-GTT TCC GTT ACC CGA CTC CT-3′) and ABCA1-942R (5′-GCC CGC AGA CAA TAC GA-3′). ApoE genotype was determined by PCR screening, using primers (forward primer 180, 5′-GCC TAG CCG AGG GAG AGC CG-3′; reverse primer 181, 5′-TGT GAC TTG GGA GCT CTG CAG C-3′; and reverse primer 182, 5′-GCC GCC CCG ACT GCA TCT-3′) indicated by The Jackson Laboratory web page (http://jaxmice.jax.org). Human apoE-KO and ABCA1 × apoE-KO mice were maintained on a regular chow diet (NIH-31 chow diet; Ziegler Brothers, Gardner, PA) from weaning until sacrifice at 3 months of age. Age- and sex-matched ABCA1-Tg mice and C57BL/6 controls were placed on a Cocoa Butter diet (TD88051; Harlan Teklad; Madison, WI) containing 1.25% cholesterol and 0.5% cholic acid at 2–3 months of age and maintained on the diet for 15 weeks before sacrifice. The mice were housed under protocols approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute.
Quantification of Plasma Lipids, Lipoproteins, and Apolipoproteins.
Fasting plasma samples were collected at 0, 3, 7, 10, 13, and 15 weeks after initiation of the Cocoa Butter diet in ABCA1-Tg mice. After a 4-h fast, blood was obtained from the retro-orbital plexus of mice anesthetized with methoxyflurane (Pitman–Moore, Mundelein, IL), placed into precooled tubes containing EDTA (final concentration 4 mM), and centrifuged at 2500 × g for 20 min at 4°C, and aliquots of plasma were stored at −70°C. Total cholesterol (TC), triglycerides (TG), phospholipids (PL), and free cholesterol (FC) were assayed as previously described (32). HDL-cholesterol was determined as the cholesterol remaining in the plasma after precipitation of apoB-containing lipoproteins with dextran sulfate (Ciba-Corning, Oberlin, OH). Plasma levels of murine apoA-I, apoB, and apoE were quantified by a sandwich ELISA, using polyclonal antibodies raised in rabbits against synthetic peptides of mouse apoA-I, apo-AII, and apoE, as previously reported (33).
Analyses of Plasma Lipoproteins.
Plasma lipoproteins either from individual or from pooled mouse plasma were separated by gel filtration, using two Superose 6 h 10/30 columns connected in series (Amersham Pharmacia Biotech). Lipoproteins were eluted at a constant flow rate of 0.3 ml/min with PBS containing 0.02% EDTA and 0.04% sodium azide. Native gel electrophoresis of pooled plasma was performed by using the Titan Gel Lipoprotein Electrophoresis System (Helena Laboratories) under native conditions, as specified by the manufacturer. Two-dimensional electrophoresis of plasma lipoproteins was performed as previously described (34). Plasma lipoproteins were also separated on 4–20% Tris–glycine gels and 3–8% Tris–acetate gels (NOVEX, San Diego, CA) and transferred to polyvinylidene difluoride microporous membranes (Millipore). Mouse apolipoproteins A-I, A-II, B, and E were identified by immunostaining with polyclonal rabbit anti-mouse IgG (Biodesign International, Kennebunkport, ME) and visualized by using a biotinylated secondary antibody, according to the Vectastain Elite ABC Kit (Vector Laboratories) (35).
Evaluation of Aortic Atherosclerosis.
The proximal aorta was collected after saline perfusion through the left ventricle. Aortas were placed in 4% phosphate buffered formaldehyde for 24 h and then transferred to 10% phosphate-buffered formaldehyde solution for 5 days. Specimens were embedded in 25% gelatin and sectioned with a cryostat at −25°C. The aortic root and ascending aorta were sectioned at a thickness of 10 mm, and alternate sections were saved on slides and stained with oil-red-O for neutral lipids and hematoxylin. Five sections/animal were evaluated for the cross-sectional area of lesions from the aortic root for a distance of 350 mm at 80-mm intervals, as previously reported (36).
Statistical Analyses.
Values are reported as mean ± SEM. Comparisons between control and transgenic mice were made by using the Student t test for independent samples (two-tailed) and ANOVA. Differences in mean aortic lesion areas were evaluated by using the Mann–Whitney test (Instat; GraphPad, San Diego).
Results
ABCA1 Overexpression Alters the Plasma Lipid Profile of hABCA1-Tg Mice in Response to a Dietary Cholesterol Challenge.
The plasma lipid, lipoprotein, and apolipoprotein concentrations of hABCA1-Tg and control C57BL/6 mice 15 weeks after initiating the high cholesterol atherogenic diet are summarized in Table 1. As previously reported (28), on a regular chow, low cholesterol diet, hABCA1-Tg mice had significantly higher plasma TC, PL, FC, cholesteryl esters (CE), HDL-C, and apoA-I concentrations than control C57BL/6 mice. Thus, ABCA1 overexpression results in the accumulation of lipids primarily in HDL of hABCA1-Tg mice (28).
Table 1.
Lipid, lipoprotein, and apolipoprotein analyses of hABCA1-Tg and C57BL/6 mice
| C57BL/6 (females) n = 11 15 wk | hABCA1 (females) n = 14 15 wk | |
|---|---|---|
| TC | 389 ± 33 | 247 ± 17* |
| TG | 49 ± 4 | 43 ± 3 |
| PL | 158 ± 10 | 178 ± 6 |
| CE | 327 ± 28 | 205 ± 15** |
| FC | 62 ± 6 | 42 ± 3* |
| HDL-C | 18 ± 1 | 51 ± 4* |
| Non-HDL-C | 371 ± 37 | 196 ± 20* |
| ApoB | 168.6 ± 16.3 | 108.8 ± 4.8** |
| ApoE | 0.5 ± 0.05 | 1.9 ± 0.5** |
| Apo-AI | 67.3 ± 5.9 | 144.8 ± 4.6* |
Data expressed as mean (mg/dl) ± SEM.
, P < 0.0005,
, P < 0.005; hABC1-Tg vs. C57BL/6.
After 15 weeks on the atherogenic diet, the plasma HDL-C, apoA-I and apoE levels were significantly increased (2.8-, 2.2-, and 3.0-fold, respectively) in hABCA1-Tg mice when compared with controls (Table 1). In marked contrast, hABCA1-Tg mice had significantly decreased plasma levels of TC, CE, FC, non-HDL-C, and apoB concentrations when compared with control C57BL/6 mice (63%, 63%, 67%, 53%, and 64%, respectively; Table 1). The decrease in the plasma TC and non-HDL-C in hABCA1-Tg mice relative to control mice was first evident 7 weeks after starting the atherogenic diet and was sustained throughout the duration of the diet study (data not shown). The reduction in the non-HDL-C in hABCA1-Tg mice was accompanied by a decrease in the apoB plasma concentrations, indicating an overall reduction in the number of apoB-containing lipoprotein particles.
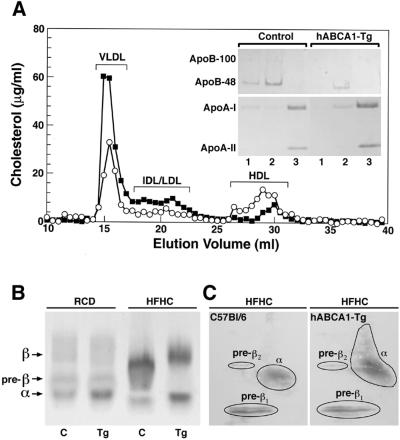
Further analyses of the plasma lipoproteins by FPLC and native agarose gel electrophoresis (Fig. 1a and b) confirmed these findings. By FPLC, the majority of the reduction in plasma cholesterol in hABCA1-Tg mice occurred in fractions corresponding to VLDL, intermediate density lipoproteins (IDL), and LDL (Fig. 1A). Immunoblot analysis of FPLC fractions from hABCA1-Tg mice revealed decreased apoB in the VLDL and IDL/LDL fractions and increased apoA-I and apoA-II in HDL (Fig. 1A Inset). Native agarose gel electrophoresis also demonstrated a substantial decrease in the cholesterol present in pre-β and β-lipoproteins in hABCA1-Tg mice compared with control mice (Fig. 1b). Increased α- and pre-β-migrating HDL lipoproteins were detected in the plasma of hABCA1-Tg mice by two-dimensional gel electrophoresis followed by immunoblotting with an anti-human apoA-I antibody (Fig. 1C). Thus, ABCA1 overexpression resulted in a significant increase in HDL and pre-β HDL lipoproteins and a decrease in the apoB-containing lipoproteins. These combined findings establish that ABCA1 modulates the response to dietary cholesterol, resulting in a less atherogenic profile in hABCA1-Tg mice.
Figure 1.
Analyses of the plasma lipoproteins in hABCA1-Tg and C57BL/6 control mice maintained on the atherogenic diet. (A) The distribution of cholesterol after separation of the plasma lipoproteins present in pooled plasma from female C57BL/6 (■; n = 5) and hABCA1-Tg (○; n = 5) mice by FPLC. ApoA-I, apoA-II, and apoB present in FPLC fractions corresponding to VLDL (lanes 1), IDL/LDL (lanes 2), and HDL (lanes 3) elution volumes (15, 21, and 29 ml, respectively) were determined by immunoblot analysis (Inset). (B) Two microliters of plasma from C57BL/6 (C) and hABCA1-Tg (Tg) mice were analyzed by native agarose gel electrophoresis followed by staining with fat red B. (C) Detection of apoA-I by immunoblotting after two-dimensional gel electrophoresis of plasma from C57BL/6 and hABCA1-Tg mice.
ABCA1 Overexpression Reduces Diet-Induced Aortic Atherosclerosis in hABCA1-Tg Mice.
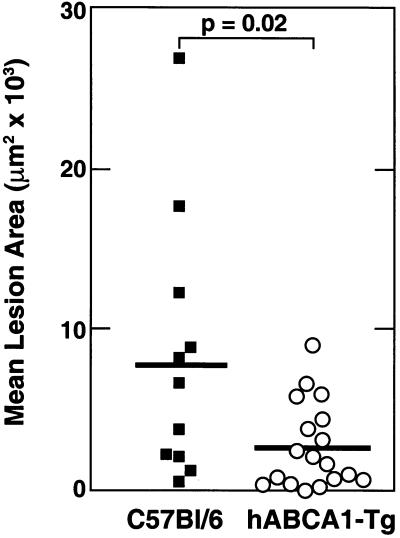
ABCA1-Tg and control mice were killed and their proximal aortas harvested for lesion analyses after 15 weeks on the atherogenic diet (Fig. 2). When compared with control mice, the mean aortic lesion area was significantly reduced by 65% in the hABCA1-Tg mice. Thus, overexpression of ABCA1 markedly reduced diet-induced aortic atherosclerosis in C57BL/6 mice.
Figure 2.
Analyses of aortic atherosclerosis in C57BL/6 (■; n = 11) and hABCA1-Tg (○; n = 18) mice maintained for 15 weeks on an atherogenic diet.
Plasma Lipid and Lipoprotein Profiles in hABCA1-Tg × apoE-KO Mice.
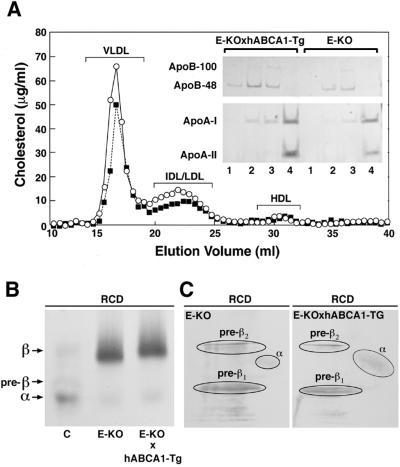
The apoE-KO mouse model has been frequently used to determine the effects of specific genes on the development of spontaneous aortic atherosclerosis. To evaluate the effects of ABCA1 overexpression on aortic lesion formation in apoE-KO mice, we crossed hABCA1-Tg mice with apoE-KO mice. ABCA1 had only a minimum effect on the plasma lipid profile of both male and female hABCA1-Tg × apoE-KO mice (Table 2). A trend for increased TC and non-HDL-C levels that did not reach statistical significance was noted in hABCA1-Tg × apoE-KO of both sexes when compared with age and sex-matched apoE-KO mice (Table 2). FPLC analysis of fasting, pooled mouse plasma also revealed increased cholesterol in the plasma lipoproteins eluting in the VLDL, IDL, and LDL fractions of hABCA1-Tg × apoE-KO mice, compared with age- and sex-matched apoE-KO sibling controls (Fig. 3A). Analyses of the plasma lipoproteins by native agarose gel electrophoresis (Fig. 3B) was consistent with these unexpected findings. However, plasma apoB levels in hABCA1-Tg × apoE-KO mice were not increased when compared with apoE-KO mice (Table 2), indicating an increase in the cholesterol content rather than the number of apoB-containing lipoprotein particles. The plasma concentrations of HDL-C, pre-β HDL, as well as apoA-I, were similar in hABCA1-Tg × apoE-KO and apoE-KO mice (Table 2 and Fig. 3 A, B, and C). These data indicate that, in a mouse model with deficiency of apoE, ABCA1 overexpression leads to only a minimal change in the plasma lipoprotein profile, characterized by a small increase in the plasma levels of apoB-containing lipoprotein cholesterol.
Table 2.
Lipid, lipoprotein, and apolipoprotein analyses of hABCA1/ApoE-KO and ApoE-KO mice
| ApoE-KO (females) n = 12 3 mo | hABCA1 × ApoE-KO (females) n = 11 3 mo | ApoE-KO (males) n = 12 3 mo | hABCA1 × ApoE-KO (males) n = 13 3 mo | |
|---|---|---|---|---|
| TC | 616 ± 27 | 654 ± 32 | 809 ± 45 | 873 ± 55 |
| TG | 113 ± 6 | 117 ± 9 | 185 ± 13 | 187 ± 17 |
| PL | 331 ± 13 | 346 ± 15 | 478 ± 21 | 505 ± 32 |
| CE | 412 ± 20 | 454 ± 21 | 577 ± 33 | 617 ± 42 |
| FC | 204 ± 19 | 200 ± 17 | 231 ± 16 | 256 ± 19 |
| HDL-C | 12 ± 1 | 14 ± 1 | 27 ± 3 | 35 ± 4 |
| Non-HDL-C | 604 ± 27 | 640 ± 39 | 782 ± 45 | 838 ± 52 |
| ApoB* | 219.9 ± 7.4 | 201.9 ± 24.9 | 214.7 ± 12.3 | 211.3 ± 15.1 |
| ApoE* | — | — | — | — |
| Apo-AI* | 13.3 ± 2.2 | 13.9 ± 2.3 | 22.6 ± 6.4 | 26.7 ± 6.4 |
Data expressed as mean (mg/dl) ± SEM; P > 0.05, all.
n = 8.
Figure 3.
Analyses of the plasma lipoproteins in hABCA1-Tg × apoE-KO and apoE-KO mice maintained on a regular chow diet. (A) The distribution of cholesterol in different FPLC elution fractions was analyzed after separation of the plasma lipoproteins present in pooled plasma from female apoE-KO (■; n = 5) and hABCA1-Tg × apoE-KO (○; n = 5) mice by FPLC. ApoA-I, apoA-II, and apoB present in FPLC fractions corresponding to VLDL (lanes 1 and 2), IDL/LDL (lanes 3), and HDL (lanes 4) elution volumes (16, 17, 22.5, and 31 ml, respectively) were determined by immunoblot analysis (Inset). (B) Two microliters of plasma from C57BL/6 (C), apoE-KO (E-KO), and hABCA1-Tg × apoE-KO (E-KO × hABCA1-Tg) mice were analyzed by native agarose gel electrophoresis followed by staining with fat red B. (C) Detection of apoA-I by immunoblotting after two-dimensional gel electrophoresis of plasma from apoE-KO and hABCA1-Tg × apoE-KO mice.
ABCA1 Increases Aortic Atherosclerosis in apoE-KO Mice Overexpressing ABCA1.
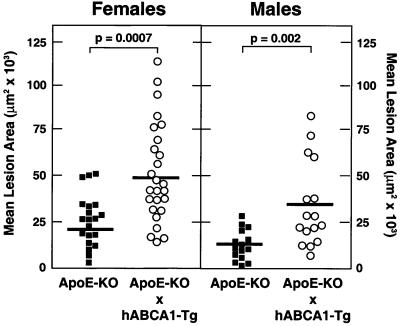
In striking contrast to our results in hABCA1-Tg mice, the mean aortic lesion area in both hABCA1-Tg × apoE-KO female and male mice maintained on a regular chow diet was significantly increased (2.0- and 2.6-fold, respectively), compared with age- and sex-matched apoE-KO sibling controls (Fig. 4). Thus, in the absence of apoE, ABCA1 overexpression does not protect against the development of aortic atherosclerosis.
Figure 4.
Analyses of aortic atherosclerosis in apoE-KO (■; male n = 15 and female n = 20) and hABCA1-Tg × apoE-KO (○; male n = 16 and female n = 26) mice maintained for 15 weeks on an atherogenic diet.
Discussion
The ABCA1 transporter plays a pivotal role in the removal of excess intracellular cholesterol and phospholipids to lipid-poor apolipoprotein acceptors in the initial step of reverse cholesterol transport. Mutations in the ABCA1 gene have been established as the genetic defect in Tangier disease, a condition characterized by extremely low HDL levels and an increased risk of cardiovascular disease. In vitro studies in several laboratories have shown that ABCA1 plays an important role in the removal of excess cholesterol from macrophages (15, 17, 37). The development of foam cells, resulting from the accumulation of excess cholesterol in macrophages, leads to the formation of fatty streaks, complex lesions, and eventually plaque rupture (38). Thus, stimulation of macrophage cholesterol efflux by enhanced ABCA1 expression would be predicted to inhibit foam cell formation and consequently reduce atherosclerosis. However, although there is considerable interest in the development of pharmacological agents that may increase ABCA1 gene expression, the definitive effect of in vivo ABCA1 overexpression on the development of atherosclerosis remains unknown. To directly test the potential importance of modulating ABCA1 gene expression to beneficially alter atherogenic risk, we recently created ABCA1 transgenic mice (28). In the present study, we evaluate the effect of ABCA1 overexpression on the plasma lipid profile and development of aortic atherosclerosis in mice fed an atherogenic diet and in the atherogenic prone apoE-KO mouse model system.
Our initial studies established that, on a low cholesterol regular chow diet, C57BI/6 mice overexpressing ABCA1 in the liver and macrophages developed a protective lipoprotein profile with increased plasma levels of HDL-C, pre-β HDL, and apoA-I (28). The increased plasma apoA-I and HDL-C levels were due to delayed HDL catabolism; however, the efflux of cholesterol from macrophages and the net delivery of HDL-C to the liver was increased, consistent with increased hepatic reverse cholesterol transport (28). In the present study, hABCA1-Tg mice placed on an atherogenic diet were shown to have significantly increased plasma levels of HDL-C, pre-β HDL, apoA-I, and apoE when compared with control mice. However, in marked contrast to the small increase in the plasma concentrations of apoB observed in hABCA1-Tg mice on the regular chow diet (28), hABCA1-Tg mice had markedly reduced plasma levels of non-HDL-C and apoB when challenged with the atherogenic diet. FPLC analysis and native agarose gel electrophoresis confirmed the significant reduction in the cholesterol content of the apoB-containing lipoproteins in VLDL, IDL, and LDL in hABCA1-Tg mice. These combined findings are consistent with a decrease in the number of atherogenic apoB-containing particles in hABCA1-Tg mice. Analysis of diet-induced atherosclerosis in hABCA1-Tg mice revealed a 65% reduction in aortic lesion formation when compared with control C57BL/6 mice. The decreased aortic atherosclerosis in hABCA1-Tg mice provides support for the concept that it is the small but critical macrophage cholesterol pool that determines the development of atherosclerosis. Thus, modulation of genes such as ABCA1, which increase hepatic reverse cholesterol transport with reduction in the macrophage cholesterol content, can be associated with decreased atherosclerosis. These combined data clearly establish that ABCA1 overexpression in mice on a high cholesterol diet results in an atheroprotective lipoprotein profile and decreased atherosclerosis, and thus provide previously undocumented in vivo evidence of an anti-atherogenic role for the ABCA1 transporter.
To gain further insights into the coordinate role of ABCA1 and apoE in modulating plasma lipoprotein metabolism and atherosclerosis, we crossed the hABCA1-Tg mice with apoE-KO mice. Overexpression of ABCA1 in apoE-KO mice had only a minimal impact on the plasma lipid profile. Unlike our findings in ABCA1-Tg mice on a high cholesterol diet, overexpression of ABCA1 in apoE-KO mice resulted in a small increase in the cholesterol content of the apoB-containing lipoproteins, VLDL, IDL, and LDL. In dramatic contrast to the results in hABCA1-Tg mice, ABCA1 overexpression significantly enhanced rather than reduced aortic atherosclerosis in apoE-KO mice.
The results of the atherosclerosis study in hABCA1-Tg × apoE-KO mice provide interesting new data on the importance of apoE and the ABCA1 transporter in cholesterol metabolism in the macrophage. ApoE is synthesized not only in the liver but also macrophages, the two major sites of ABCA1 overexpression in our ABCA1-Tg mice (28, 39). Extensive clinical and in vitro studies have established the importance of apoE as a ligand that mediates the plasma clearance of chylomicron remnants and intermediate density lipoproteins (39–41). In addition, the cholesterol secreted from cholesterol-loaded macrophages has been correlated with apoE secretion, as well as the functionality of the ABCA1 transporter (39, 42–44). Furthermore, we have recently shown that apoE is as effective as apoA-I as an apolipoprotein acceptor for ABCA1-mediated cholesterol efflux (16). Thus, both apoA-I and apoE may function as acceptors for ABCA1 mediate cholesterol efflux from the macrophage.
Of additional interest are recent studies, which have identified liver X receptor response elements in the promoters of the genes for both ABCA1 and apoE, suggesting coordinate regulation of ABCA1 and apoE gene expression (22–24, 45). Cholesterol and/or oxysterol loading of macrophages would be anticipated to increase the expression of both apoE as well as ABCA1 by stimulation of the liver X receptor/retinoid X receptor pathway. The secreted apoE could then act as an apolipoprotein acceptor for cholesterol and phospholipid efflux mediated by ABCA1, which would further enhance the removal of cholesterol from macrophages. Thus, the combined effects of increased expression of apoE and ABCA1 would decrease the cholesterol content of the macrophage and protect against the development of atherosclerosis.
The lack of reduction in aortic atherosclerosis observed in the hABCA1-Tg × apoE-KO mice may be the result of several changes in lipoprotein and cholesterol metabolism. The absence of apoE results in delayed clearance of the apoB-containing lipoproteins, and would also be anticipated to decrease both the secretion of cholesterol from macrophages, as well as prevent apoE from acting as an apolipoprotein acceptor for ABCA1 mediated efflux. Thus, the absence of apoE in the apoE-KO mice would limit the effectiveness of overexpression of ABCA1 in reducing atherosclerosis in the hABCA1-Tg × apoE-KO mice. In addition, the low plasma apoA-I levels present in the apoE-KO mice may also limit the ability of apoA-I to function as an effective apolipoprotein acceptor in the vessel wall. Nevertheless, the increased atherosclerosis in the hABCA1-Tg × apoE-KO mice was an unexpected finding. This latter result suggests that other changes either in the plasma lipoproteins not readily apparent in the plasma lipoprotein profile or in macrophage cholesterol metabolism may be responsible for the increased atherosclerosis. It is important to note that ABCA1 overexpression in apoE-KO mice resulted in increased plasma levels of cholesterol in the apoB-containing lipoproteins, which may reflect a marked difference in hepatic cholesterol metabolism. It is also of interest that increased atherosclerosis was observed in hLCAT-Tg × apoE-KO mice, which has been proposed to be the result of accumulation of a dysfunctional HDL that did not function effectively in reverse cholesterol transport.¶ Additional studies on the hABCA1-Tg × apoE-KO mice will undoubtedly provide new and important insights into the pathophysiology of experimental atherosclerosis.
In summary, overexpression of ABCA1 in mice with a C57BL/6 background results in a lipoprotein profile characterized by markedly increased plasma levels of HDL, pre-β HDL, and apoA-I, as well as decreased apoB-containing lipoproteins, which protects against the development of diet-induced aortic lesions. In contrast, overexpression of ABCA1 in an apoE-KO background increased atherosclerosis, further substantiating the importance of apoE in macrophage cholesterol metabolism and the development of cardiovascular disease. The major conclusion that can be drawn from our combined studies is that increased expression of ABCA1 can reduce atherosclerosis. These data suggest that treatment with pharmacological agents that increase ABCA1 expression may be an effective approach for reducing atherogenic risk in humans.
Acknowledgments
We thank Ms. Cindy McFarland for expert technical assistance and Ms. Donna James for excellent secretarial assistance in the preparation of the manuscript.
Abbreviations
- ABCA1
ATP binding cassette transporter A1
- TC
total cholesterol
- FC
free cholesterol
- CE
cholesteryl esters
- PL
phospholipids
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- VLDL
very low density lipoprotein
- apo
apolipoprotein
- KO
knockout
- Tg
transgenic
- IDL
intermediate density lipoprotein
Footnotes
Vaisman, B. L., Berard, A. M., Paigen, B., Marcovina, S., Eckhaus, M., Maeda, N., Brewer, H. B., Jr., & Santamarina-Fojo, S. (1996) Circulation 94, I-633 (abstr.).
References
- 1.Gordon D J, Rifkind B M. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Miller N E. Am Heart J. 1987;113:589–597. doi: 10.1016/0002-8703(87)90638-7. [DOI] [PubMed] [Google Scholar]
- 3.Gordon D J, Probstfield J L, Garrison R J, Neaton J D, Castelli W P, Knoke J D, Jr, Jacobs D R, Jr, Bangdiwala S, Tyroler H A. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Glomset J A. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 5.Fielding C J, Fielding P E. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 6.Brooks-Wilson A, Marcil M, Clee S M, Zhang L-H, Roomp K, van Dam M, Yui L, Brewer C, Collins J A, Molhuizen H O F, et al. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 7.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, et al. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 8.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette J C, Deleuze J F, Brewer H B, Jr, Duverger N, Denefle P, Assmann G. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 9.Remaley A T, Rust S, Rosier M, Knapper C, Naudin L, Broccardo C, Peterson K M, Koch C, Arnould I, Prades C, et al. Proc Natl Acad Sci USA. 1999;96:12685–12690. doi: 10.1073/pnas.96.22.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mott S, Yu L, Marcil M, Boucher B, Rondeau C, Genest J. Atherosclerosis. 2000;152:457–468. doi: 10.1016/s0021-9150(99)00498-0. [DOI] [PubMed] [Google Scholar]
- 11.Lawn R M, Wade D P, Garvin M R, Wang X, Schwartz K, Porter J G, Seilhamer J J, Vaughan A M, Oram J F. J Clin Invest. 1999;104:R25–R31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brousseau M E, Schaefer E J, Dupuis J, Eustace B, Van Eerdewegh P, Goldkamp A L, Thurston L M, Fitzgerald M G, Yasek-McKenna D, O'Neill G, et al. J Lipid Res. 2000;41:433–441. [PubMed] [Google Scholar]
- 13.Fredrickson D S, Attrocchi P H, Avioli L V, Goodman D S, Goodman H C. Ann Intern Med. 1961;55:1016–1031. [Google Scholar]
- 14.Assmann G, von Eckardstein A, Brewer H B., Jr . In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 2000. pp. 2937–2980. [Google Scholar]
- 15.Bortnick A E, Rothblat G H, Stoudt G, Hoppe K L, Royer L J, McNeish J, Francone O L. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- 16.Remaley A T, Stonik J A, Demosky S J, Neufeld E B, Bocharov A V, Vishnyakova T G, Eggerman T L, Patterson A P, Duverger N J, Santamarina-Fojo S, Brewer H B., Jr Biochem Biophys Res Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 17.Orso E, Broccardo C, Kiminski W E, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T, et al. Nat Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 18.Wang N, Silver D L, Thiele C, Tall A R. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- 19.Neufeld E B, Remaley A T, Demosky S J, Jr, Stonik J A, Cooney A M, Comly M, Dwyer N K, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, Brewer H B., Jr J Biol Chem. 2001;276:27584–27590. doi: 10.1074/jbc.M103264200. [DOI] [PubMed] [Google Scholar]
- 20.Santamarina-Fojo S, Peterson K, Knapper C, Qiu Y, Freeman L, Cheng J-F, Osorio J, Remaley A, Yang X P, Haudenschild C, et al. Proc Natl Acad Sci USA. 2000;97:7987–7992. doi: 10.1073/pnas.97.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullinger C R, Hakamata H, Duchateau P N, Eng C, Aouizerat B E, Cho M H, Fielding C J, Kane J P. Biochem Biophys Res Commun. 2000;271:451–455. doi: 10.1006/bbrc.2000.2652. [DOI] [PubMed] [Google Scholar]
- 22.Venkateswaran A, Laffitte B A, Joseph S B, Mak P A, Wilpitz D C, Edwards P A, Tontonoz P. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. . (First Published October 17, 2000; 10.1073/pnas.200367697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz K, Lawn R M, Wade D P. Biochem Biophys Res Commun. 2000;274:794–802. doi: 10.1006/bbrc.2000.3243. [DOI] [PubMed] [Google Scholar]
- 24.Costet P, Luo Y, Wang N, Tall A R. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 25.Repa J J, Turley S D, Lobaccaro J A, Medina J, Li L, Lustig K, Shan B, Heyman R A, Dietschy J M, Mangelsdorf D J. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 26.McNeish J, Aiello R J, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe K L, Roach M L, Royer L J, deWet J, et al. Proc Natl Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christiansen-Weber T A, Voland J R, Wu Y, Ngo K, Roland B L, Nguyen S, Peterson P A, Fung-Leung W P. Am J Pathol. 2000;157:1017–1029. doi: 10.1016/S0002-9440(10)64614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaisman B L, Lambert G, Amar M, Joyce C, Ito T, Shamburek R D, Cain W J, Fruchart-Najib J, Neufeld E B, Remaley A T, Brewer H B, Jr, Santamarina-Fojo S. J Clin Invest. 2001;108:303–309. doi: 10.1172/JCI12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singaraja R R, Bocher V, James E R, Clee S M, Zhang L H, Leavitt B R, Tan B, Brooks-Wilson A, Kwok A, Bissada N, et al. J Biol Chem. 2001;276:33969–33979. doi: 10.1074/jbc.M102503200. [DOI] [PubMed] [Google Scholar]
- 30.Cavelier L B, Qui Y, Bielicki J K, Afzal V, Cheng J-F, Rubin E M. J Biol Chem. 2001;276:18046–18051. doi: 10.1074/jbc.M100565200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 32.Vaisman B L, Klein H-G, Rouis M, Berard A M, Kindt M R, Talley G D, Meyn S M, Hoyt R F, Jr, Marcovina S M, Albers J J, et al. J Biol Chem. 1995;270:12269–12275. doi: 10.1074/jbc.270.20.12269. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan P M, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick R L, Quarfordt S H, Maeda N. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 34.Sprecher D L, Taam L, Brewer H B., Jr Clin Chem. 1984;30:2084–2092. [PubMed] [Google Scholar]
- 35.Foger B, Chase M, Amar M J, Vaisman B L, Shamburek R D, Paigen B, Fruchart-Najib J, Paiz J A, Koch C A, Hoyt R F, Jr, et al. J Biol Chem. 1999;274:36912–36920. doi: 10.1074/jbc.274.52.36912. [DOI] [PubMed] [Google Scholar]
- 36.Paigen B, Morrow A, Holmes P A, Mitchell D, Williams R A. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 37.Oram J F, Lawn R M, Garvin M R, Wade D P. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 38.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 39.Curtiss L K, Boisvert W A. Curr Opin Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Linton M F, Hasty A H, Babaev V R, Fazio S. J Clin Invest. 1998;101:1726–1736. doi: 10.1172/JCI2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregg R E, Zech L A, Schaefer E J, Brewer H B., Jr J Lipid Res. 1984;25:1167–1176. [PubMed] [Google Scholar]
- 42.Zhang W Y, Gaynor P M, Kruth H S. J Biol Chem. 1996;271:28641–28646. doi: 10.1074/jbc.271.45.28641. [DOI] [PubMed] [Google Scholar]
- 43.Lin C Y, Duan H, Mazzone T. J Lipid Res. 1999;40:1618–1627. [PubMed] [Google Scholar]
- 44.Langer C, Huang Y, Cullen P, Wiesenhutter B, Mahley R W, Assmann G, von Eckardstein A. J Mol Med. 2000;78:217–227. doi: 10.1007/s001090000096. [DOI] [PubMed] [Google Scholar]
- 45.Laffitte B A, Repa J J, Joseph S B, Wilpitz D C, Kast H R, Mangelsdorf D J, Tontonoz P. Proc Natl Acad Sci USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. . (First Published January 9, 2001; 10.1073/pnas.021488798) [DOI] [PMC free article] [PubMed] [Google Scholar]