Abstract
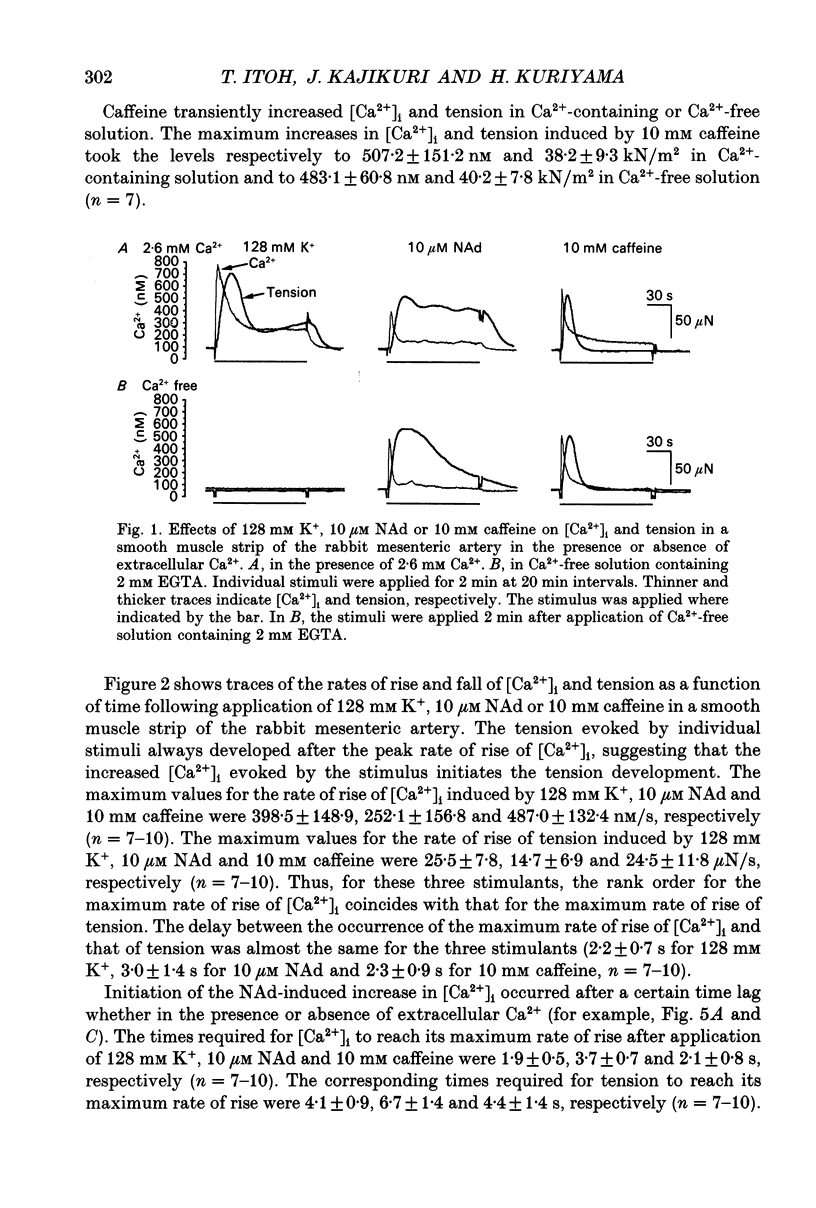
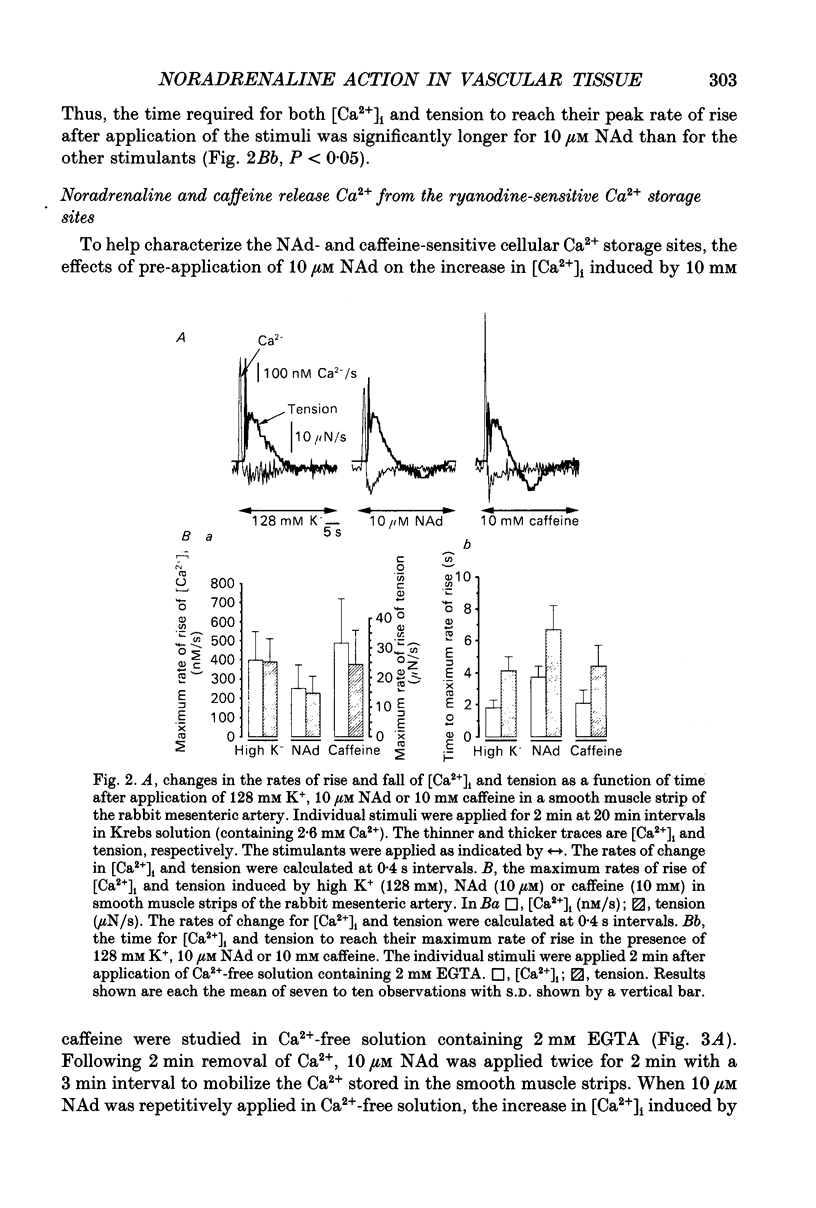
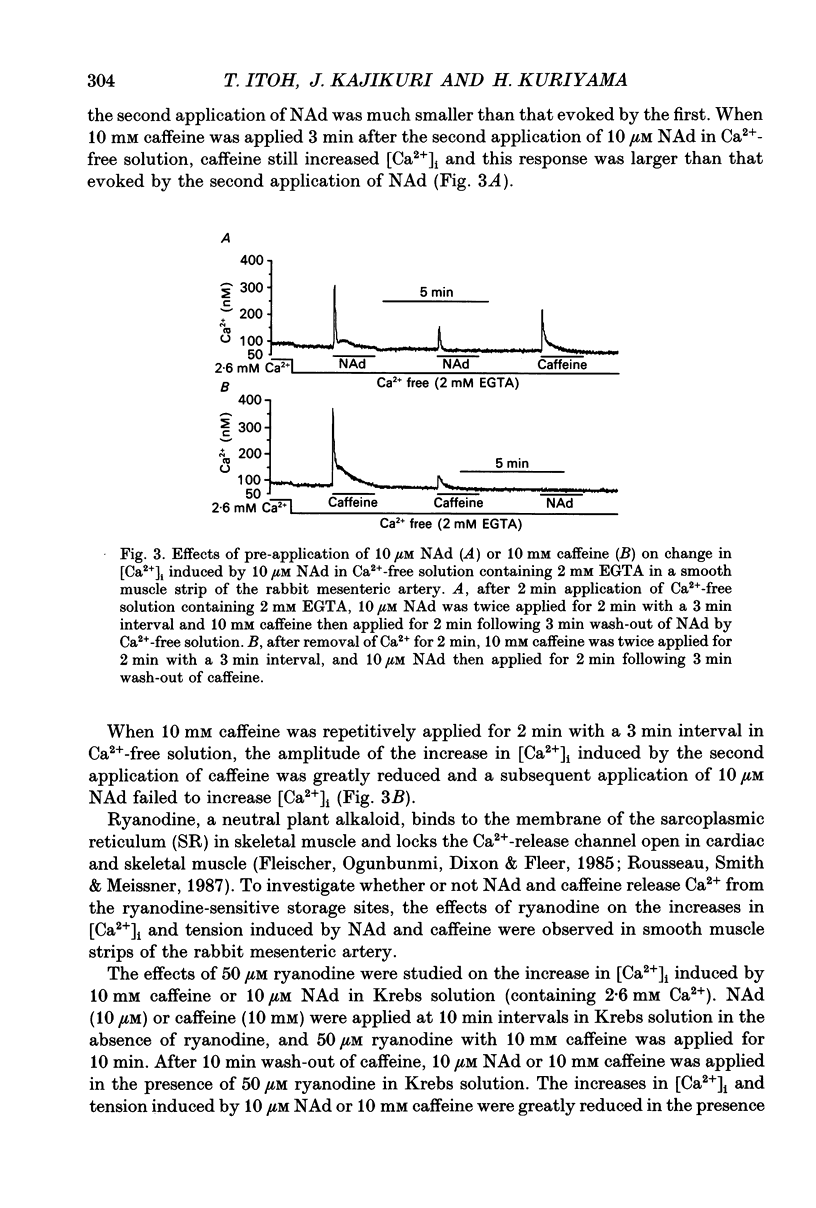
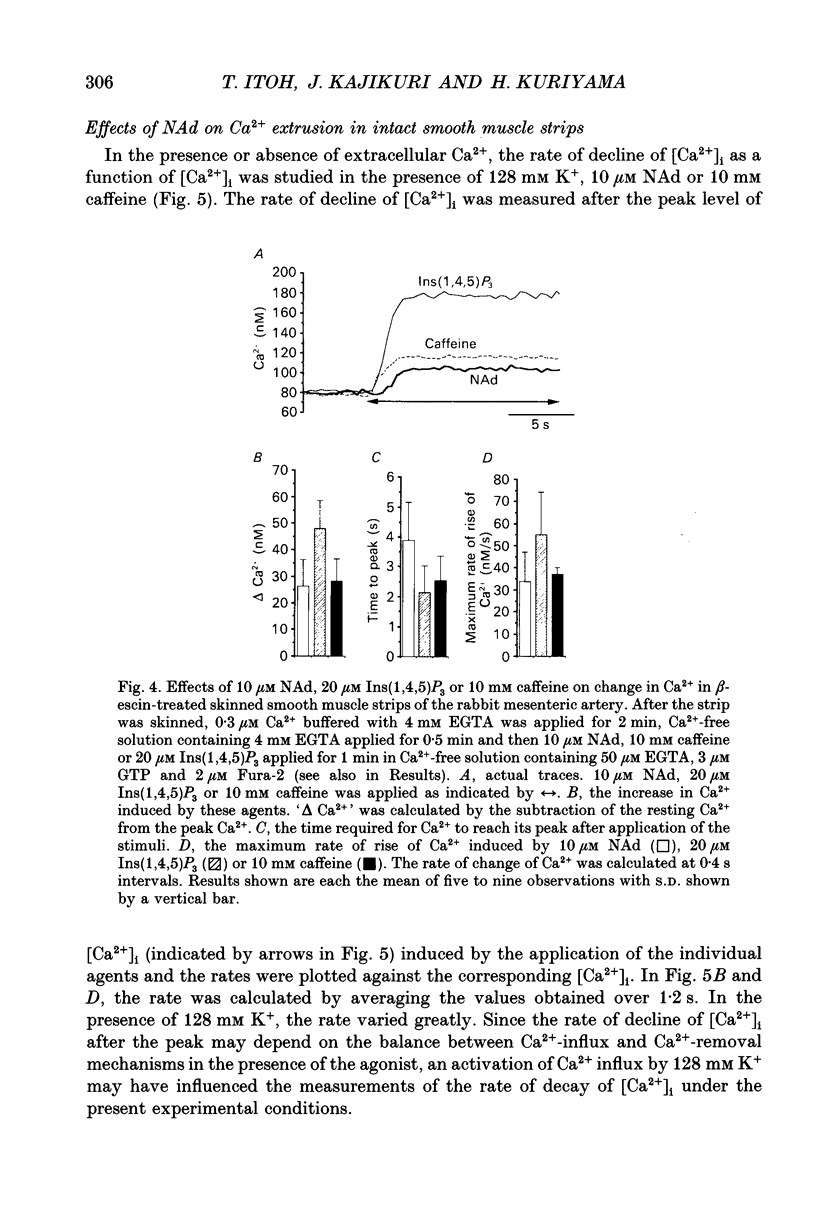
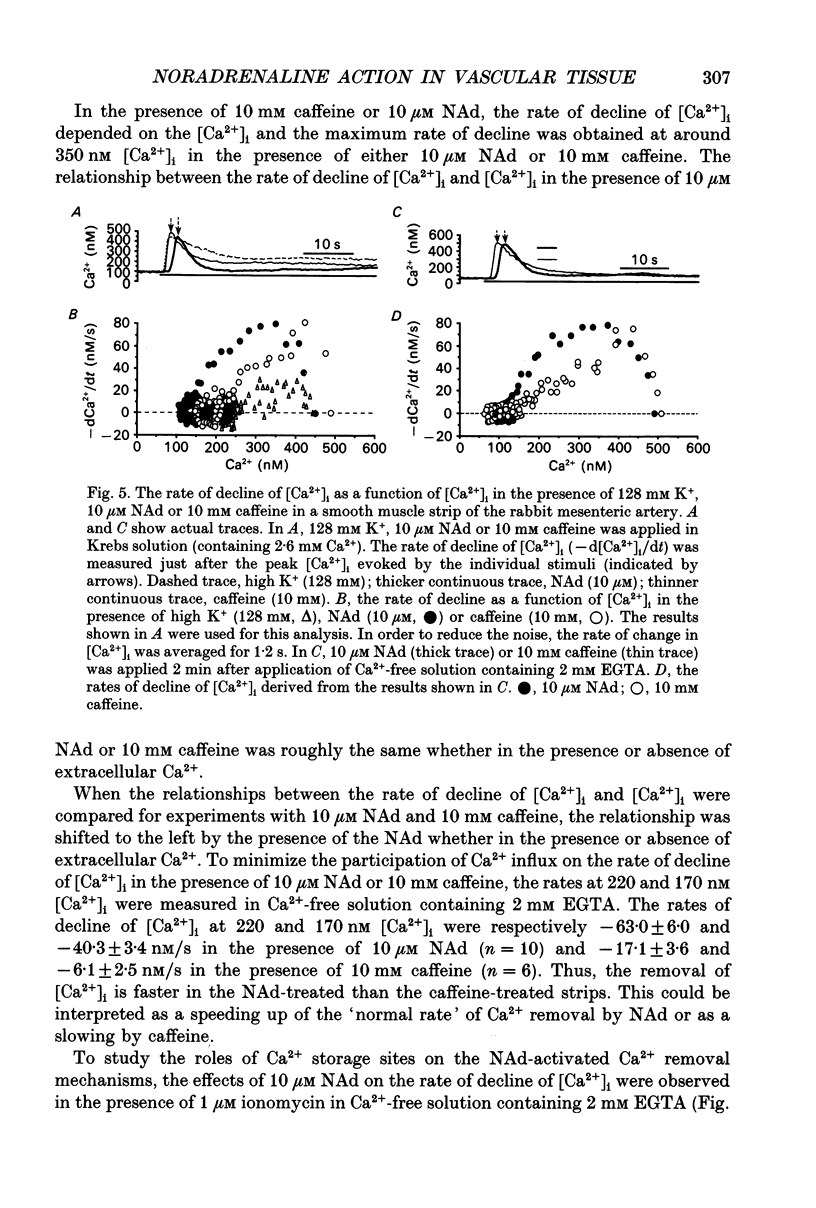
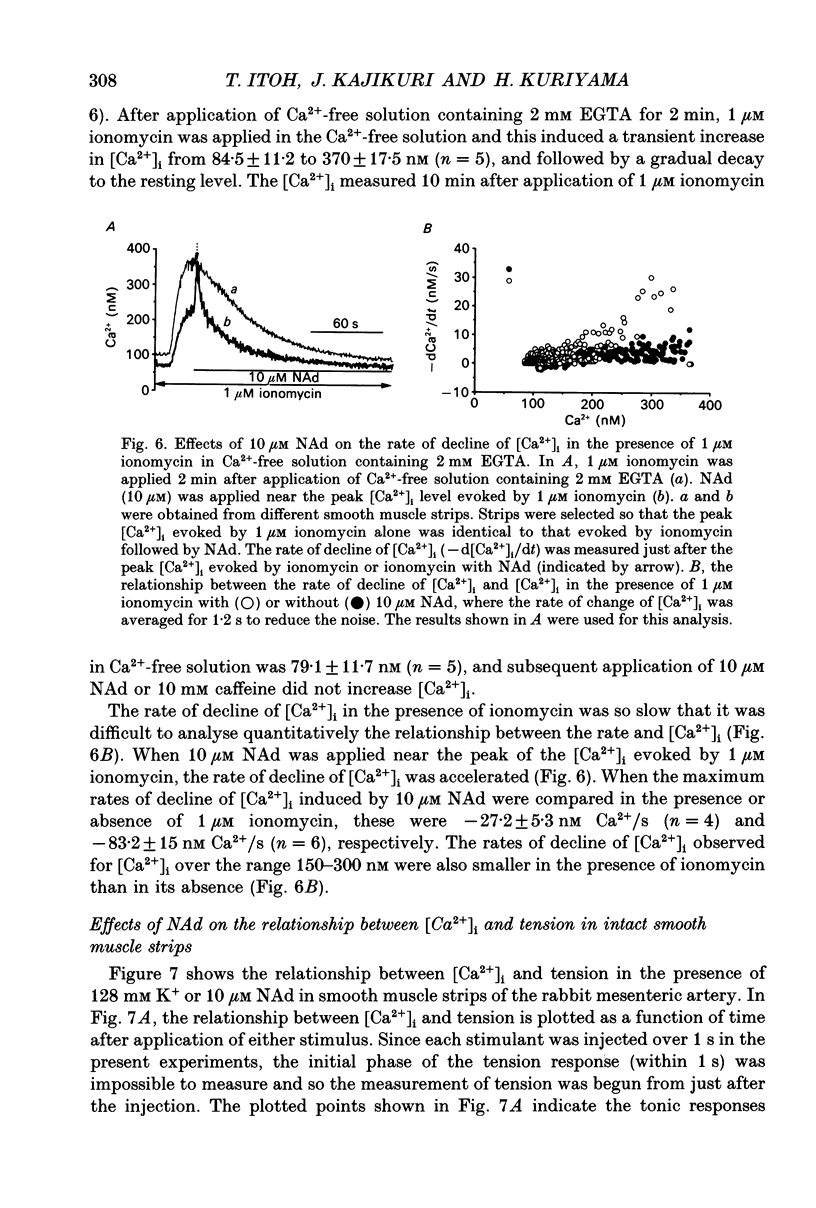
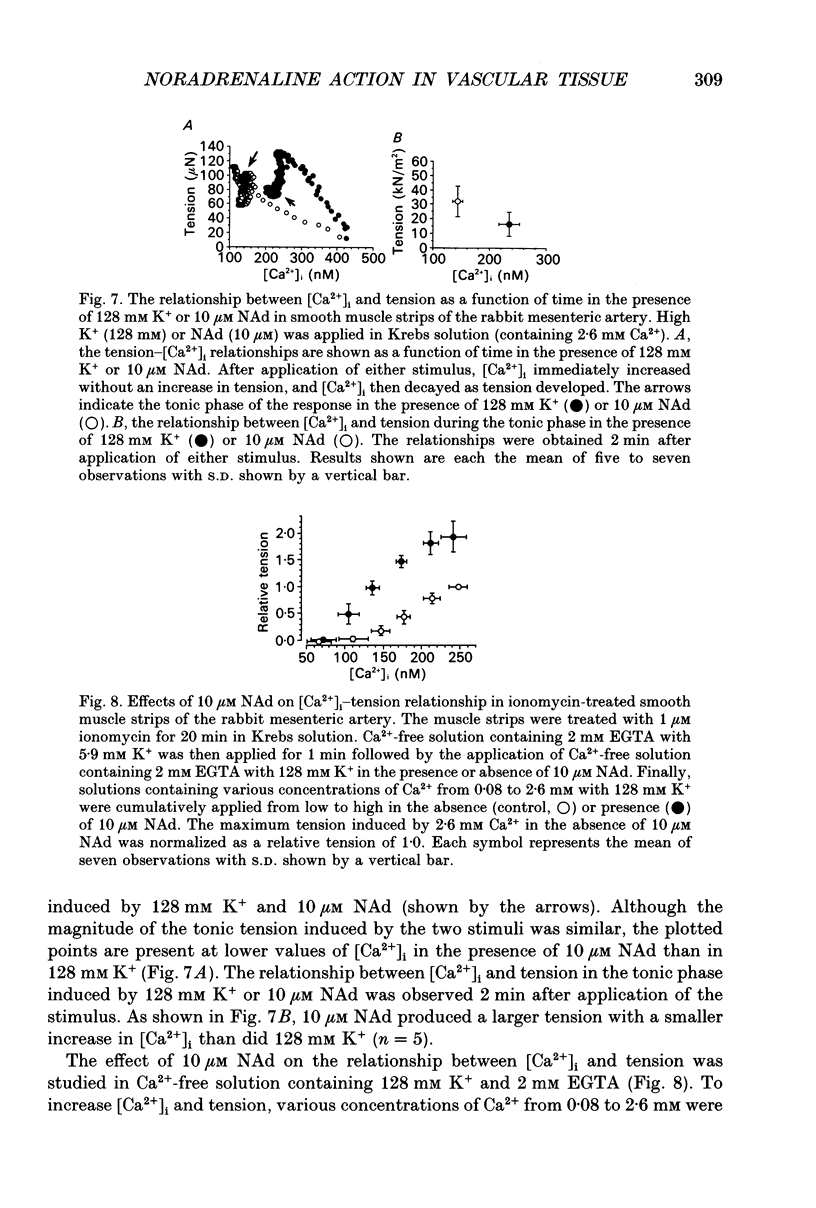
1. Effects of noradrenaline (NAd) on changes in cellular Ca2+ concentration ([Ca2+]i) and tension were investigated, and these effects were compared with those evoked by 128 mM K+ or caffeine in intact smooth muscle strips or by inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) or caffeine in beta-escin-treated chemically skinned smooth muscle strips of the rabbit mesenteric artery. 2. In physiological solution containing 2.6 mM Ca2+, application of 128 mM K+ or 10 microM NAd produced a phasic, followed by a tonic increase in [Ca2+]i and tension. NAd (10 microM) produced a larger tonic tension than did 128 mM K+ but a smaller increase in [Ca2+]i. When the [Ca2+]i-tension relationship was observed in ionomycin- and 128 mM K(+)-treated muscle strips, 10 microM NAs shifted the relationship to the left and enhanced the maximum amplitude of contraction. These results suggest that NAd increases the sensitivity of contractile proteins to Ca2+ in smooth muscle of the rabbit mesenteric artery. 3. Noradrenaline (10 microM) or caffeine (10 mM), but not 128 mM K+, produced a phasic increase in both [Ca2+]i and tension in Ca(2+)-free solution containing 2 mM EGTA. When 10 mM caffeine had been applied in Ca(2+)-free solution, subsequent application of 10 microM NAd did not increase [Ca2+]i. By contrast, when 10 microM NAd had been applied in Ca(2+)-free solution, subsequent application of 10 mM caffeine still increased [Ca2+]i. Ryanodine (50 microM) abolished the increase in [Ca2+]i induced by 10 mM caffeine or 10 microM NAd in intact and in skinned smooth muscle strips. These results suggest that NAd releases Ca2+ from the ryanodine-sensitive Ca2+ storage sites. 4. Noradrenaline (10 microM) synthesized Ins(1,4,5)P3 in Ca(2+)-free solution in intact smooth muscle strips. Following application of 10 microM NAd, a relatively long time lag (around 1 s) was always observed before the initiation of the increase in [Ca2+]i whether in the presence or absence of Ca2+. The maximum rate of rise of [Ca2+]i induced by 10 mM caffeine was much larger than that induced by 10 microM NAd in Ca(2+)-containing or Ca(2+)-free solution (containing 2 mM EGTA). Both [Ca2+]i and tension reached their peak in a shorter time with caffeine (10 mM) than with 10 microM NAd. In Beta-escin-treated skinned smooth muscle strips, 20 microM Ins(1,4,5)P3 10 mM caffeine or 10 microM NAd increased Ca2+ in Ca(2+)-free solution following brief application of 0.3 microM Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Vrolix M., Raeymaekers L., Wuytack F., Casteels R. Ca2+-transport ATPases of vascular smooth muscle. Circ Res. 1988 Feb;62(2):266–278. doi: 10.1161/01.res.62.2.266. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tawada Y., Shigekawa M. Protein kinase C activation stimulates plasma membrane Ca2+ pump in cultured vascular smooth muscle cells. J Biol Chem. 1989 Mar 25;264(9):4844–4849. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. P. Desensitization to cytoplasmic Ca2+ and Ca2+ sensitivities of guinea-pig ileum and rabbit pulmonary artery smooth muscle. J Physiol. 1989 Jun;413:489–503. doi: 10.1113/jphysiol.1989.sp017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. Inorganic phosphate regulates the contraction-relaxation cycle in skinned muscles of the rabbit mesenteric artery. J Physiol. 1986 Jul;376:231–252. doi: 10.1113/jphysiol.1986.sp016151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kubota Y., Kuriyama H. Effects of a phorbol ester on acetylcholine-induced Ca2+ mobilization and contraction in the porcine coronary artery. J Physiol. 1988 Mar;397:401–419. doi: 10.1113/jphysiol.1988.sp017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Suzuki S., Kuriyama H. Effects of pinacidil on contractile proteins in high K(+)-treated intact, and in beta-escin-treated skinned smooth muscle of the rabbit mesenteric artery. Br J Pharmacol. 1991 Jul;103(3):1697–1702. doi: 10.1111/j.1476-5381.1991.tb09849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sage S. O. Stimulated calcium efflux from fura-2-loaded human platelets. J Physiol. 1987 Dec;393:513–524. doi: 10.1113/jphysiol.1987.sp016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E., Smith J. S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987 Sep;253(3 Pt 1):C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Flash photolysis studies of excitation-contraction coupling, regulation, and contraction in smooth muscle. Annu Rev Physiol. 1990;52:857–874. doi: 10.1146/annurev.ph.52.030190.004233. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Walker J. W., Goldman Y. E., Trentham D. R., Kobayashi S., Kitazawa T., Somlyo A. V. Inositol trisphosphate, calcium and muscle contraction. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):399–414. doi: 10.1098/rstb.1988.0084. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]


