Abstract
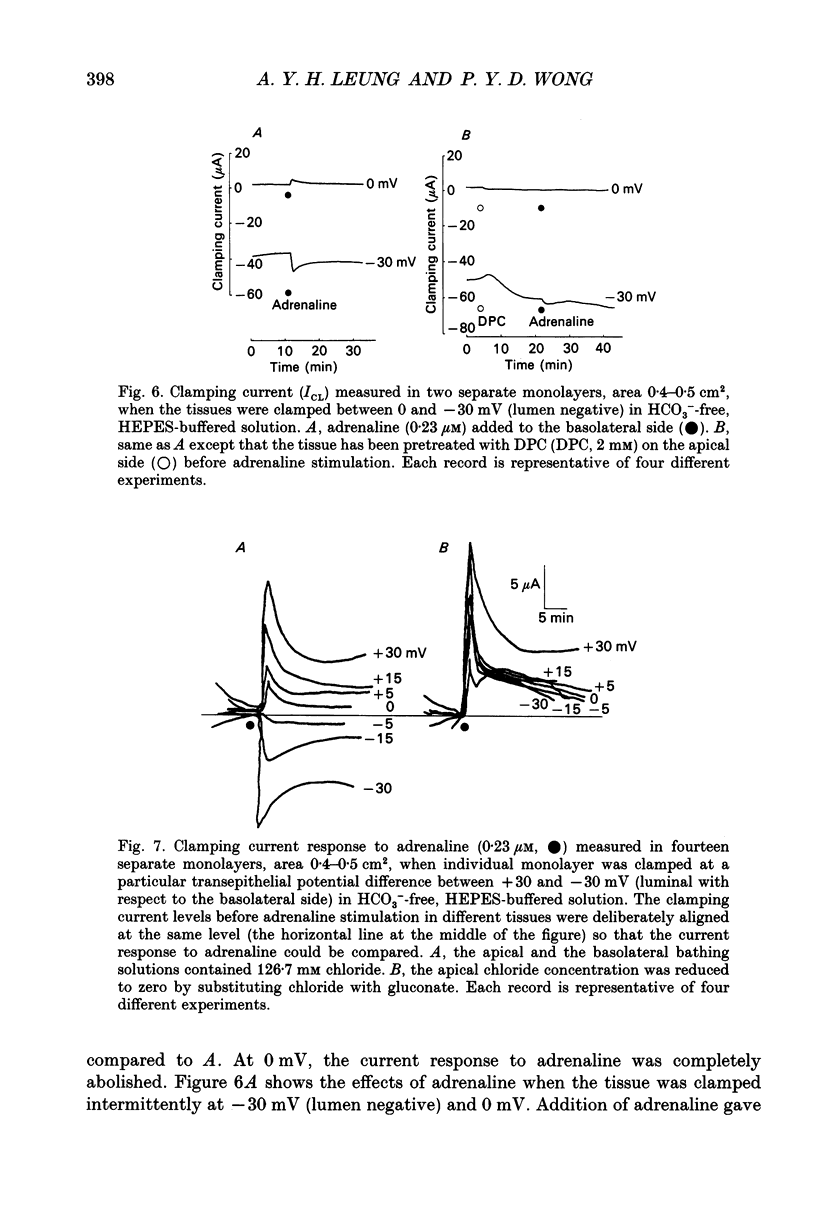
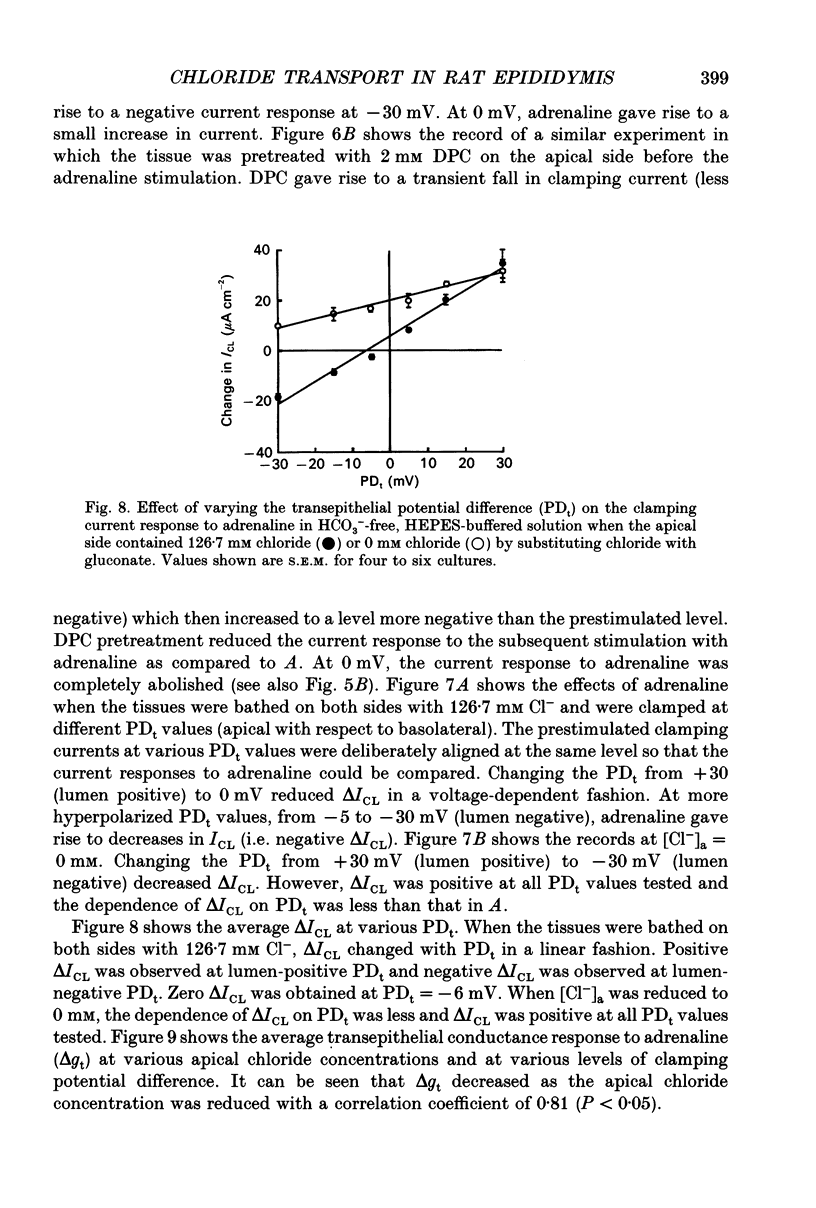
1. Monolayer cultures of cauda epididymides from male Sprague-Dawley rats (210-230 g) were studied by the short-circuit current (ISC) technique to characterize the properties of the transepithelial chloride transport. In HCO(3-)-free, HEPES-buffered solution, adrenaline (0.23 microM) added to the basolateral side led to an increase in ISC and transepithelial conductance (gt). 2. Decreasing apical chloride concentration ([Cl-]a) progressively from 126.7 to 0 mM by substituting chloride with gluconate increased the ISC response to adrenaline (delta ISC) in a linear fashion with a slope of -1.6 x 10(-3) mu equiv h-1 cm-2 per millimolar change in [Cl-]a. Pretreating the tissue with a chloride channel blocker diphenylamine-2-carboxylate (DPC) on the apical side significantly reduced the slope to -4.9 x 10(-4) mu equiv h-1 cm-2 per millimolar change in [Cl-]a. 3. By substituting apical chloride with various anions and measuring the change in ISC upon adrenaline stimulation, the selectivity sequence of the apical anion conductance was found to be NO3- approximately Br- > Cl- > I- > gluconate > isethionate. 4. When the monolayers were bathed with Krebs-Henseleit solution containing 25 mM HCO3- and 5% CO2, the delta ISC at each [Cl-]a as well as the dependence of delta ISC on [Cl-]a (slope = -3.3 x 10(-3) mu equiv h-1 cm-2 per millimolar change in [Cl-]a) were significantly greater than the HCO(3-)-free counterpart. Addition of 0.1 mM acetazolamide or 0.5 mM SITS (4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid) to the basolateral side significantly reduced the effects of HCO3- and CO2. 5. When the tissues were bathed on both sides with HCO(3-)-free, HEPES-buffered solution and were clamped at various transepithelial potential differences (PDt) from +30 mV (lumen positive) to -30 mV (lumen negative), the relationship between the clamping current response to adrenaline (delta ICL) and the PDt applied was linear. Zero clamping current response was found at -6 mV. Decreasing [Cl-]a to 0 mM reduced the dependence of delta ICL on PDt and delta ICL was positive at all PDt tested. The response of the transepithelial conductance to adrenaline (delta gt) did not depend on the PDt applied but was reduced with decreasing apical chloride concentration.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Cheng E. Effect of catecholamines on ion transport in dog tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):397–403. doi: 10.1152/jappl.1979.47.2.397. [DOI] [PubMed] [Google Scholar]
- Brown P. D., Elliott A. C., Lau K. R. Indirect evidence for the presence of non-specific anion channels in rabbit mandibular salivary gland acinar cells. J Physiol. 1989 Jul;414:415–431. doi: 10.1113/jphysiol.1989.sp017696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton C. U., Boucher R. C., Gatzy J. T. Paths of ion transport across canine fetal tracheal epithelium. J Appl Physiol (1985) 1988 Dec;65(6):2376–2382. doi: 10.1152/jappl.1988.65.6.2376. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Wong P. Y. Electrogenic anion secretion in cultured rat epididymal epithelium. J Physiol. 1986 Sep;378:335–345. doi: 10.1113/jphysiol.1986.sp016222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolf I., Van Driessche W., Nagel W. Forskolin activates gated Cl- channels in frog skin. Am J Physiol. 1989 Jun;256(6 Pt 1):C1239–C1249. doi: 10.1152/ajpcell.1989.256.6.C1239. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Harris A., Coleman L., Greenwell J. R., Argent B. E. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989 Aug;257(2 Pt 1):C240–C251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Hanrahan J. W., Tabcharani J. A. Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J Membr Biol. 1990 Jun;116(1):65–77. doi: 10.1007/BF01871673. [DOI] [PubMed] [Google Scholar]
- Hviid Larsen E., Kristensen P. Properties of a conductive cellular chloride pathway in the skin of the toad (Bufo bufo). Acta Physiol Scand. 1978 Jan;102(1):1–21. doi: 10.1111/j.1748-1716.1978.tb06041.x. [DOI] [PubMed] [Google Scholar]
- Jefferson D. M., Valentich J. D., Marini F. C., Grubman S. A., Iannuzzi M. C., Dorkin H. L., Li M., Klinger K. W., Welsh M. J. Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am J Physiol. 1990 Dec;259(6 Pt 1):L496–L505. doi: 10.1152/ajplung.1990.259.6.L496. [DOI] [PubMed] [Google Scholar]
- Jenkins A. D., Lechene C. P., Howards S. S. Concentrations of seven elements in the intraluminal fluids of the rat seminiferous tubules, rate testis, and epididymis. Biol Reprod. 1980 Dec;23(5):981–987. doi: 10.1095/biolreprod23.5.981. [DOI] [PubMed] [Google Scholar]
- Klyce S. D., Wong R. K. Site and mode of adrenaline action on chloride transport across the rabbit corneal epithelium. J Physiol. 1977 Apr;266(3):777–799. doi: 10.1113/jphysiol.1977.sp011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E. H., Ussing H. H., Spring K. R. Ion transport by mitochondria-rich cells in toad skin. J Membr Biol. 1987;99(1):25–40. doi: 10.1007/BF01870619. [DOI] [PubMed] [Google Scholar]
- Levine N., Marsh D. J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971 Mar;213(3):557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W., Reinach P. Mechanism of stimulation by epinephrine of active transepithelial Cl transport in isolated frog cornea. J Membr Biol. 1980 Aug 21;56(1):73–79. doi: 10.1007/BF01869354. [DOI] [PubMed] [Google Scholar]
- Novak I., Young J. A. Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch. 1986 Dec;407(6):649–656. doi: 10.1007/BF00582647. [DOI] [PubMed] [Google Scholar]
- Pedersen P. S. Chloride permeability regulation via a cyclic AMP pathway in cultured human sweat duct cells. J Physiol. 1990 Feb;421:379–397. doi: 10.1113/jphysiol.1990.sp017950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. M., Quinton P. M. Localization of Cl- conductance in normal and Cl- impermeability in cystic fibrosis sweat duct epithelium. Am J Physiol. 1989 Oct;257(4 Pt 1):C727–C735. doi: 10.1152/ajpcell.1989.257.4.C727. [DOI] [PubMed] [Google Scholar]
- Saito Y., Wright E. M. Regulation of bicarbonate transport across the brush border membrane of the bull-frog choroid plexus. J Physiol. 1984 May;350:327–342. doi: 10.1113/jphysiol.1984.sp015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorofsky S. R., Field M., Fozzard H. A. Electrophysiology of Cl secretion in canine trachea. J Membr Biol. 1983;72(1-2):105–115. doi: 10.1007/BF01870318. [DOI] [PubMed] [Google Scholar]
- Simmons N. L. Chloride secretion stimulated by prostaglandin E1 and by forskolin in a canine renal epithelial cell line. J Physiol. 1991 Jan;432:459–472. doi: 10.1113/jphysiol.1991.sp018394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. Abnormal regulation of ion channels in cystic fibrosis epithelia. FASEB J. 1990 Jul;4(10):2718–2725. doi: 10.1096/fasebj.4.10.1695593. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Basolateral membrane potassium conductance is independent of sodium pump activity and membrane voltage in canine tracheal epithelium. J Membr Biol. 1985;84(1):25–33. doi: 10.1007/BF01871645. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: III. Membrane resistances and electromotive forces. J Membr Biol. 1983;71(3):209–218. doi: 10.1007/BF01875462. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989 Feb;256(2 Pt 1):C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Huang S. J. Intracellular pH measurement in primary monolayer cultures of rat epididymal cells. Pflugers Arch. 1989 Feb;413(4):414–421. doi: 10.1007/BF00584492. [DOI] [PubMed] [Google Scholar]
- Wong P. Y., Huang S. J. Secretory agonists stimulate a rise in intracellular cyclic AMP but not Ca2+ and inositol phosphates in cultured rat epididymal epithelium. Exp Physiol. 1990 May;75(3):321–337. doi: 10.1113/expphysiol.1990.sp003407. [DOI] [PubMed] [Google Scholar]
- Wong P. Y. Inhibition by chloride channel blockers of anion secretion in cultured epididymal epithelium and intact epididymis of rats. Br J Pharmacol. 1988 May;94(1):155–163. doi: 10.1111/j.1476-5381.1988.tb11510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. Y. Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium. Am J Physiol. 1988 Jan;254(1 Pt 2):F121–F133. doi: 10.1152/ajprenal.1988.254.1.F121. [DOI] [PubMed] [Google Scholar]


