Abstract
1. Smooth muscle cells were enzymatically isolated from arteries dissected from mesenteric fat removed from patients undergoing routine surgery. The whole-cell patch clamp technique was used to characterize the potassium (K+) currents and passive electrical properties of these cells, using high-K(+)-containing pipette solutions with either 0.2 mM EGTA or 10 mM EGTA and 10 mM BAPTA. 2. Cell capacitance, which is proportional to membrane surface area, was normally distributed around a value of 46 pF, and independent of artery size between 0.4 and 3.6 mm. The mean membrane potential measured under current clamp was -44.1 +/- 1.9 mV (n = 52). 3. Cells dialysed with 0.2 mM EGTA in order to weakly buffer intracellular Ca2+ demonstrated a noisy outward current with an apparent threshold near -30 mV, upon which were superimposed spontaneous transient outward currents (STOCs). In the presence, but not the absence, of extracellular Ca2+, this current was potentiated if the holding potential was depolarized into the voltage range between -40 and +50 mV. This potentiation had a bell-shaped potential dependency which reflected the activation of voltage-gated Ca2+ channels in these cells. 4. The noisy current was blocked by externally applied tetraethylammonium (the dissociation constant, Kd = 0.85 mM), as were STOCs. This current was also reduced by about 40% by 8 nM charybdotoxin, and was transiently potentiated by 10 mM caffeine. The characteristics of this current therefore suggested that it was carried by large-conductance Ca(2+)-activated K+ channels. 5. Dialysis of human mesenteric arterial cells with 10 mM EGTA and 10 mM BATPA was not able to completely suppress the Ca(2+)-activated current, and reduced by approximately 50% the amplitude of the outward current recorded at positive potentials. 6. Depolarization of strongly Ca(2+)-buffered cells in the presence of 30 mM TEA to block Ca(2+)-activated K+ channels revealed a residual outward current which had both transient and sustained components. These were blocked by 4-aminopyridine (4-AP) with a similar efficiency (Kd was 1.04 and 1.16 mM at +60 mV for transient and sustained current, respectively), but the voltage ranges over which they inactivated, and their rates of recovery from inactivation, were significantly different. 7. The transient and sustained currents had different sensitivities to external Ca2+ and Cd2+ ions. Ca2+ (5 mM) significantly reduced the amplitude and shifted the voltage dependency of inactivation of the transient but not the sustained component of the outward current. Cd2+ (0.2 mM) reduced the transient current by about 30% without affecting the sustained component amplitude.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
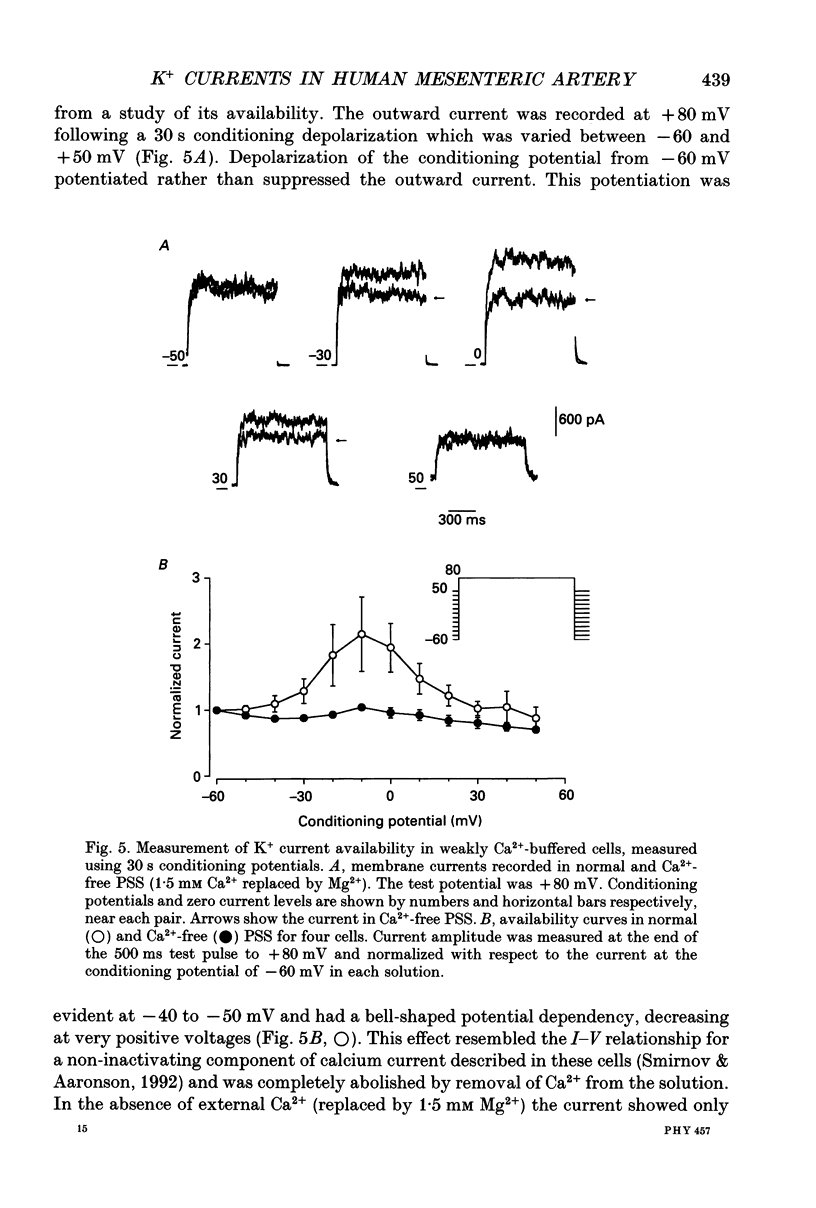
PDF










Selected References
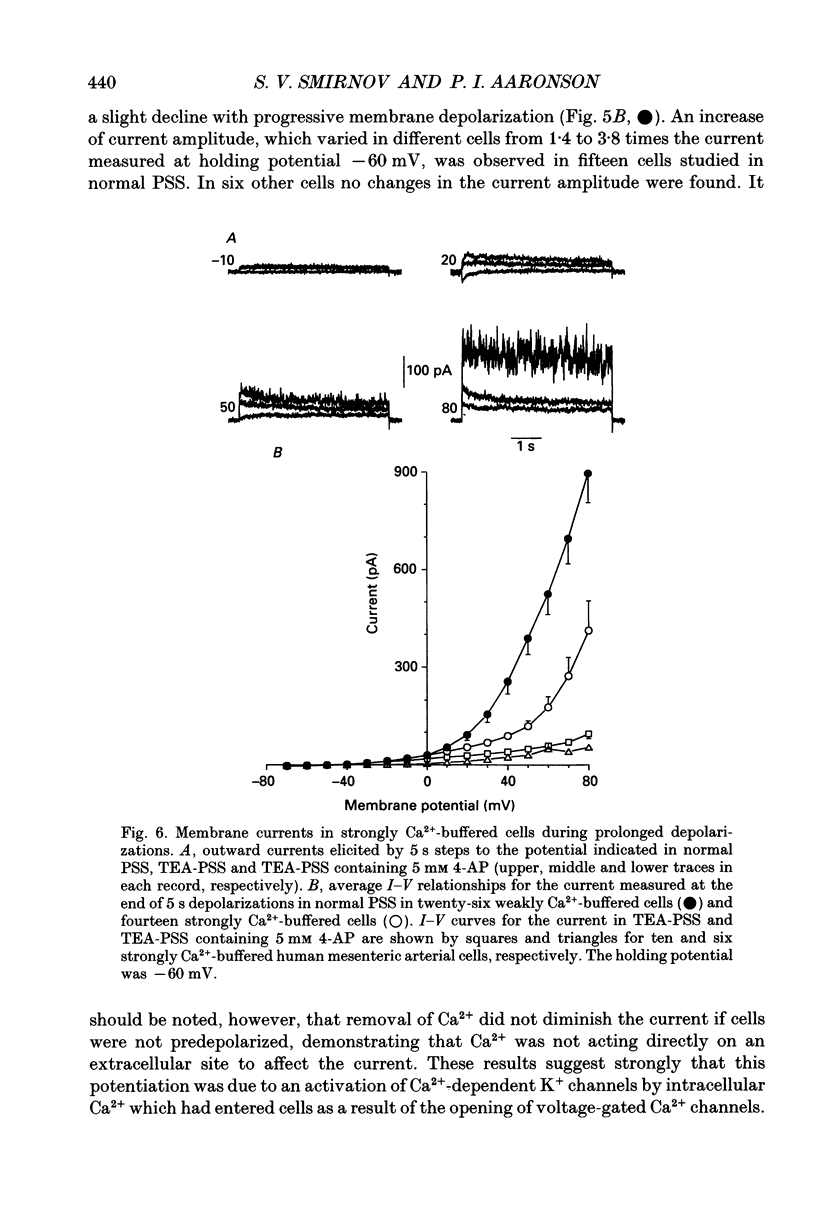
These references are in PubMed. This may not be the complete list of references from this article.
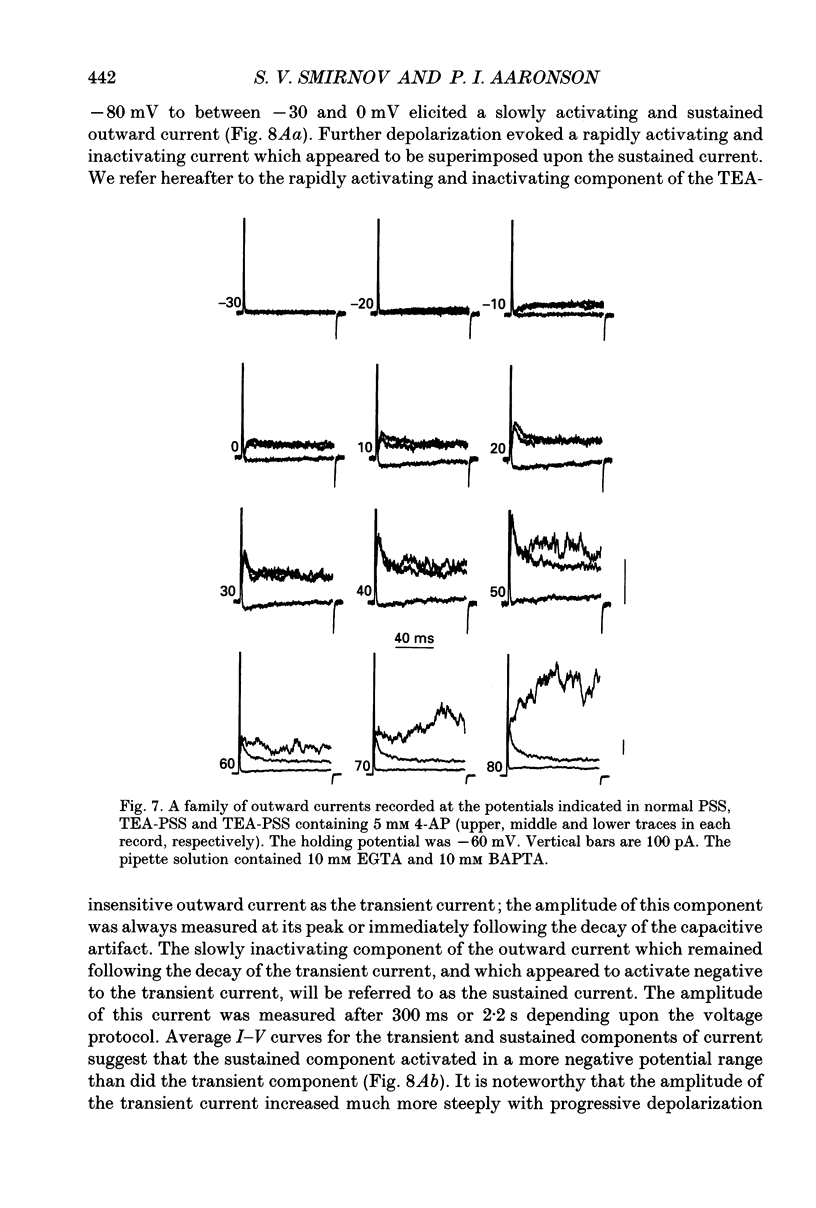
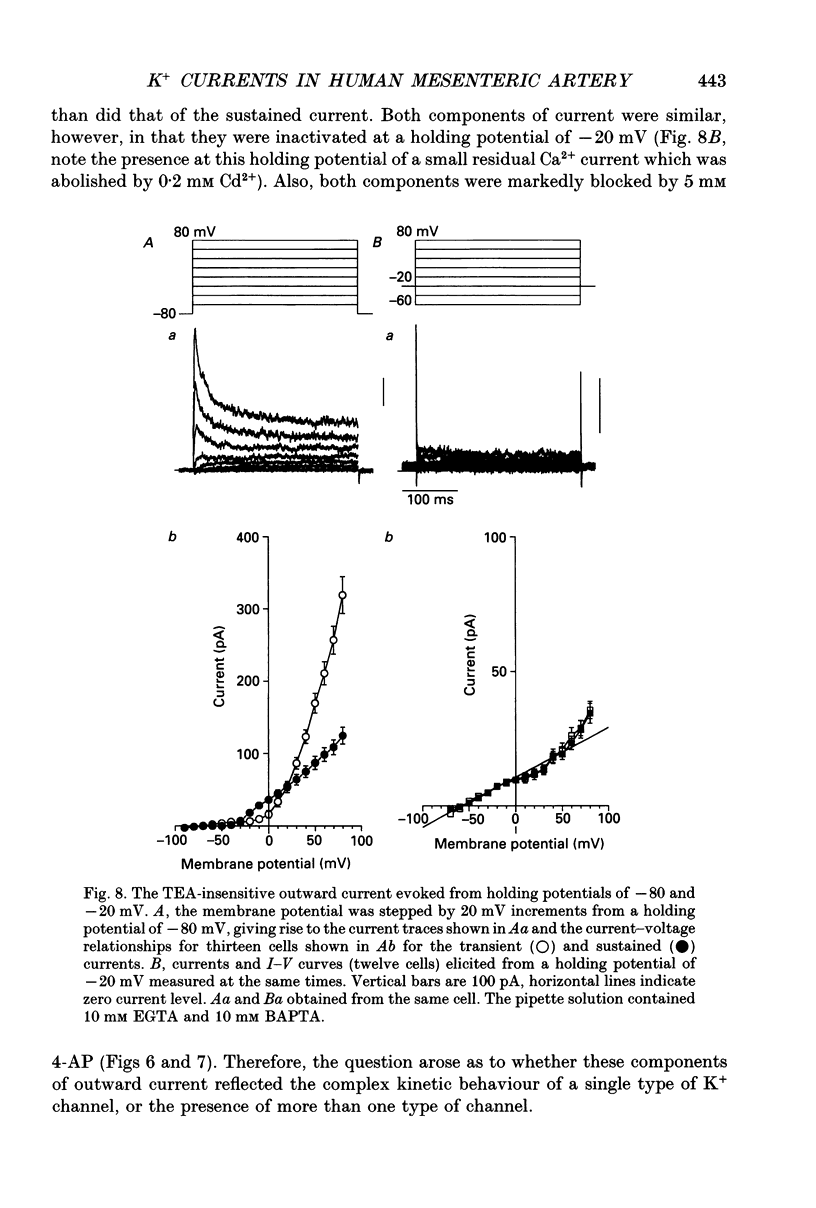
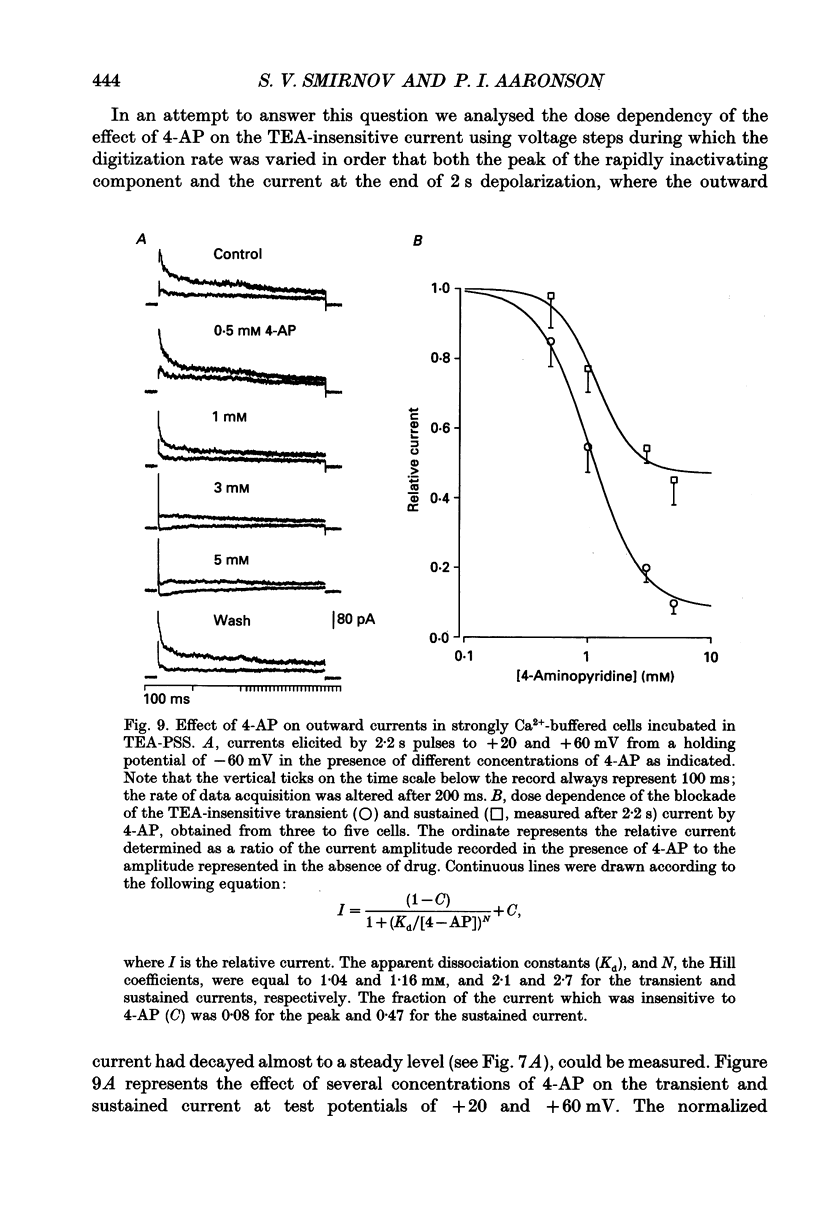
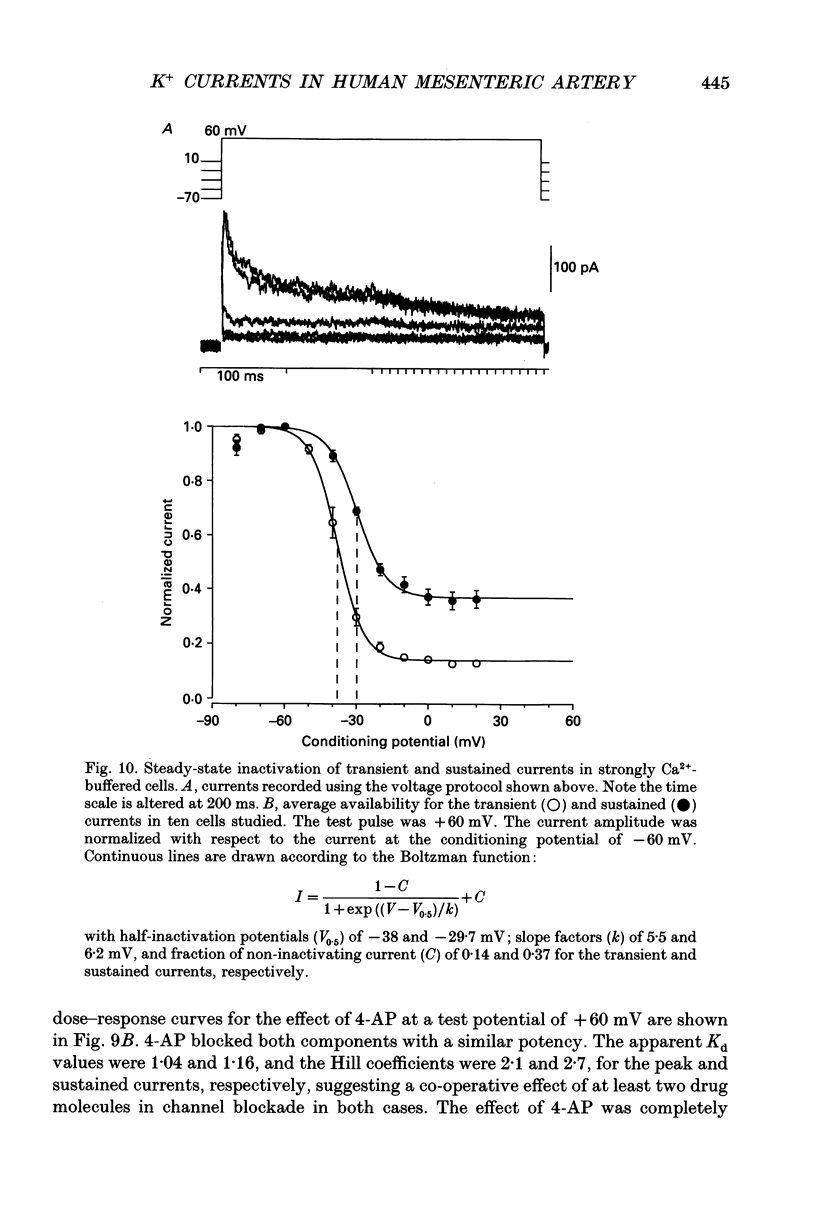
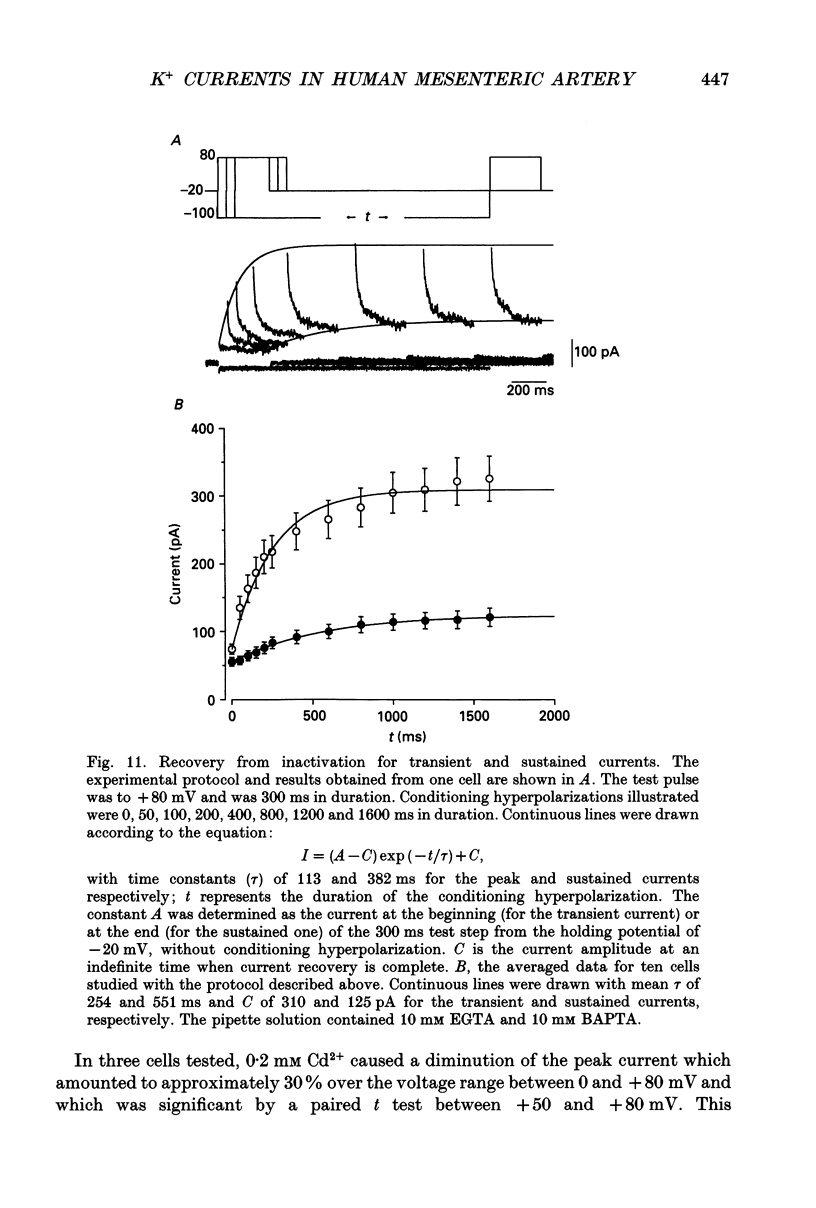
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
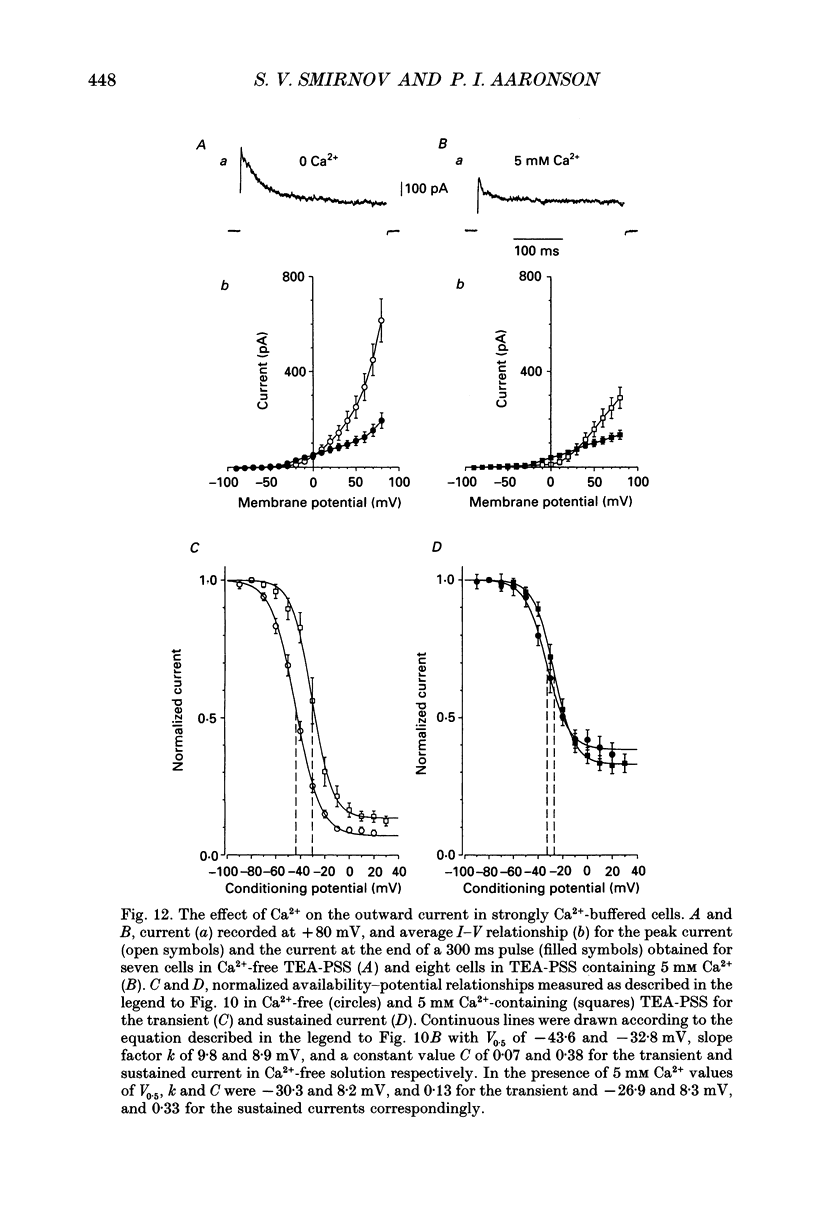
- Amédée T., Mironneau C., Mironneau J. The calcium channel current of pregnant rat single myometrial cells in short-term primary culture. J Physiol. 1987 Nov;392:253–272. doi: 10.1113/jphysiol.1987.sp016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld D. R., Hume J. R., Krier J. Action potentials and membrane currents of isolated single smooth muscle cells of cat and rabbit colon. Pflugers Arch. 1990 Mar;415(6):678–687. doi: 10.1007/BF02584005. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Characteristics of transient outward currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1990 Aug;427:301–324. doi: 10.1113/jphysiol.1990.sp018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N., Shigenobu K., Kasuya Y. Contractile response and electrophysiological properties in enzymatically dispersed smooth muscle cells of rat vas deferens. Pflugers Arch. 1987 Feb;408(2):112–119. doi: 10.1007/BF00581338. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Pak M. D., Baker K., Covarrubias M., Butler A., Ratcliffe A., Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Russell S. N., Smirnov S. V., Aaronson P. I. Effects of BRL 38227 on potassium currents in smooth muscle cells isolated from rabbit portal vein and human mesenteric artery. Br J Pharmacol. 1992 Mar;105(3):549–556. doi: 10.1111/j.1476-5381.1992.tb09017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov S. V., Aaronson P. I. Ca2+ currents in single myocytes from human mesenteric arteries: evidence for a physiological role of L-type channels. J Physiol. 1992 Nov;457:455–475. doi: 10.1113/jphysiol.1992.sp019387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov S. V., Zholos A. V., Shuba M. F. A potential-dependent fast outward current in single smooth muscle cells isolated from the newborn rat ileum. J Physiol. 1992 Aug;454:573–589. doi: 10.1113/jphysiol.1992.sp019280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Volk K. A., Matsuda J. J., Shibata E. F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J Physiol. 1991 Aug;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos A. V., Baidan L. V., Shuba M. F. Properties of the late transient outward current in isolated intestinal smooth muscle cells of the guinea-pig. J Physiol. 1991 Nov;443:555–574. doi: 10.1113/jphysiol.1991.sp018851. [DOI] [PMC free article] [PubMed] [Google Scholar]


