Abstract
Over the past decade xenotransplantation systems have been used with increasing success to gain a better understanding of human cells that are able to initiate and maintain the hematopoietic system in vivo. The nonobese diabetic/severe combined immunodeficiency (SCID) mouse has been a particularly useful model. Human cells capable of hematopoietic repopulation in this mouse, termed SCID-repopulating cells, have been assumed to represent the most primitive elements of the hematopoietic system, responsible for long-term maintenance of hematopoiesis. However, we demonstrate that SCID-repopulating cells present in the CD34+ cell fraction of cord blood can be segregated into subpopulations with distinct repopulation characteristics. CD34+/CD38+ progenitors can repopulate recipients rapidly, but can only maintain the graft for 12 weeks or less and have no secondary repopulation potential. Conversely, the more primitive CD34+/CD38− subpopulation repopulates recipients more gradually, can maintain the graft for at least 20 weeks, and contains cells with serial repopulation potential throughout the engraftment period. Additionally, a much higher frequency of T cell precursors are found among SCID-repopulating cells in the CD34+/CD38− subpopulation. These findings demonstrate that cells with variable repopulation potential comprise the human CD34+ population and that short- and long-term potential of human precursors can be evaluated in the mouse model.
Our understanding of the hierarchical structure within the early developmental stages of the hematopoietic system have been largely defined by findings from experimental manipulations in the mouse (1, 2). Transplantation studies using retrovirus-marked cells or populations isolated based on specific cell surface molecules have provided compelling evidence for heterogeneity within the subset of precursors with repopulating potential (3–9). Findings from numerous studies indicate that precursors with repopulating potential represent a developmental continuum, ranging from relatively mature cells with short-term repopulating potential to the most primitive cells able to provide sustained, long-term engraftment (3, 10). These primitive cells, referred to as long-term repopulating stem cells (LTRSC), are distinguished from other repopulating cells, by their capacity to self-replicate through a process known as self-renewal (1). Multipotentiality, the ability to generate multiple blood-cell lineages including myeloid, erythroid, and lymphoid was at one time also considered to be a characteristic unique to the LTRSC. Although LTRSC are multipotential, evidence now suggests that not all multipotential cells are LTRSC (8, 10). Rather, some multipotential cells appear to represent a somewhat more advanced population with a limited engraftment potential.
Comparable dissection of the structure of the human hematopoietic system has not been possible in the past, given the lack of appropriate systems for experimental transplantation. Over the last 8 years, however, several different xenotransplantation models have been developed that will facilitate investigation into the relationship of different populations and begin to define the hierarchy of the human hematopoietic system. Transplantation studies using nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice (11) as recipients have identified a population of SCID-repopulating cells (SRC) present in human umbilical cord blood (CB), bone marrow, and growth factor mobilized peripheral blood progenitor cells (PBPC) (12–18). The fact that most SRC express the cell surface molecule CD34 (19), thought to be expressed on the human LTRSC, and that at least a subset of SRC are multipotent (20, 21) suggests that the NOD/SCID assay detects a population of early hematopoietic precursors that could include the most primitive stem cell. Recent retroviral marking experiments (22), as well as cell fractionation experiments (23), have provided evidence that suggests SRC represent a heterogeneous population that contains cells at different developmental stages capable of engrafting mice for varying periods of time. Further evidence for heterogeneity within the human hematopoietic repopulating cell compartment has been provided by studies using a fetal sheep model of transplantation (24). This model is capable of discriminating discreet long-term potentials of CD34+ bone marrow subpopulations based on CD38 expression (25). Collectively, these observations suggest that the human hematopoietic system shares similarities with the mouse and contains cells with the potential to provide hematopoietic repopulation for different periods of time.
Analysis of patients transplanted with the CD34+ fraction of human bone marrow, PBPC, and CB further support the concept that hematopoietic repopulation is mediated by distinct subpopulations of cells with differing potential. Within 7–9 days of transplantation, CD34+ PBPC can generate sufficient numbers of mature neutrophils to raise the absolute neutrophil count above 500/μl in the peripheral circulation of the patient (26). In the allogeneic setting, this fraction has been shown to exhibit durable donor engraftment in transplant patients for several years (27, 28). If the human hematopoietic system maintains the same functional components as are found in the mouse, an obvious explanation for these repopulation patterns would be the presence of both short- and long-term repopulating cells within the CD34+ fraction. At the same time numerous reports have documented the ability of human CD34+ cells to engraft, proliferate, and develop in the NOD/SCID model (13–15, 17). In most studies to date mice were analyzed 5–8 weeks subsequent to transplantation. Although it has been widely assumed that SRC are long-term repopulating cells, a 5–8 week assay can only reflect the cells' capacity to home to the marrow and proliferate, but does not address the question of durable engraftment.
The CD34+ fraction of bone marrow, PBPC, and cord blood can be subfractionated into functionally distinct populations based on the expression of the transmembrane glycoprotein CD38. The CD34+/CD38− subpopulation that represents ≈5% of the total CD34+ fraction, appears to contain the most immature elements (29, 30), including the majority of the long-term cell-initiating cells (LTC-IC) and SRC (20, 21, 31, 32). The CD34+/CD38+ population, on the other hand, is generally considered to represent more mature cells and contains most of the in vitro colony-forming cells (CFC) (31, 32). Although the majority of SRC are found in the CD34+/CD38− fraction, a small subpopulation of these precursors can be detected in the CD34+/CD38+ population (17, 20, 21). One possible explanation of this observation is that the CD34+/CD38+ SRC represents a more advanced stage of development compared with SRC found in the CD34+/CD38− fraction. To address this issue, we have isolated CD34+/CD38− and CD34+/CD38+ populations from cord blood and compared their developmental potential in NOD/SCID recipients. In this report, we demonstrate the SRC found in these populations can be distinguished by their engraftment kinetics, lineage potential, duration, and ability to repopulate secondary recipients in the NOD/SCID mouse model.
Methods
CB Manipulations and CD34+ Cell Enrichment.
CB units were obtained from those rejected by the University of Colorado Cord Blood Bank (UCCBB) on the basis of low cell count or volume. All units used in this study were donated following signed informed consent under Institutional Review Board-approved research protocols from the University of Colorado with explicit approval for research use granted. CB from placental cord blood veins was collected into blood bags containing anticoagulant immediately following normal vaginal deliveries at local area hospitals participating in collections for the UCCBB. CB samples were further processed for immunomagnetic isolation of CD34+ cells as described (13) within 24 h of collection.
Fluorescence-Activated Cell Sorter (FACS).
Freshly enriched CD34+ cells were incubated with the appropriate amount of antibodies to both CD34 and CD38 conjugated to allophycocyanin (APC) and R-phycoerythrin (PE; Becton Dickenson). Cells were washed and sorted aseptically into CD34+/CD38+ and CD34+/CD38− or into CD34+/CD38+, CD34+/CD38low, and CD34+/CD38− fractions. Sorting was performed on a MoFlo cell sorter (Cytomation, Ft. Collins, CO), using helium/neon (633 nm for APC) and argon (488 nm for PE) lasers for fluorochrome excitation and 675 nm (APC) and 575 nm (PE) band pass filters for discrimination of APC and PE signals.
NOD/SCID Mouse Manipulations.
NOD/SCID mice were obtained from breeding colonies that we have maintained in a restricted barrier facility at the National Jewish Hospital and Research Center (NJHRC, Denver, CO). Breeding pairs have been generously and periodically supplied to us by Leonard D. Shultz (The Jackson Laboratory). All animal experiments were approved by the Animal Care Committee of the NJHRC. Mouse conditioning, transplantation, and harvesting of hematopoietic tissues was performed as described (13). For secondary transplantations bone marrow aspirates from a primary recipient's femurs and tibia were used to transplant two secondary recipients or the bone marrows from two primary recipients were combined and used to transplant three secondary recipients.
Flow Cytometric Analysis.
At least 5 × 104 nucleated cells from mouse hematopoietic tissues were used for each two- or three-color set up. Staining and flow analysis were performed exactly as described (13). For human T cell analysis mouse thymocytes were first analyzed with anti-human CD45 and CD2, and on finding a significant population of positive cells were further analyzed with anti-human CD45/CD3/CD4/CD8 tetraChrome antibodies. Finally, Vβ analysis was performed on human T cell fractions that exhibited high levels of surface CD3, using various combinations of PerCP- and FITC-conjugated anti-human CD3, CD4, and CD8 along with biotinylated antibodies against 16 different human Vβs. Vβ staining was detected with streptavidin-PE (Becton Dickinson PharMingen). Vβ antibodies were kindly supplied by Brian Kotzin (University of Colorado Health Sciences Center) and used as described (33). All other antibodies were from Becton Dickinson, except for anti-CD13, anti-glycophorin A, and the anti-CD45,3,4,8 tetraChrome antibodies (Beckman Coulter), anti-mouse CD45 (Becton Dickinson PharMingen), and anti-human IgM (Caltag, South San Francisco, CA).
Results
Kinetics of CD34+ Cell Repopulation.
As a first approach to characterizing the heterogeneity of the CB CD34+/lin− fraction with respect to repopulating potential, we analyzed the kinetics of engraftment following transplantation of these cells into NOD/SCID mice. For this analysis, a group of 20 mice were transplanted with CD34+/lin− cells (2 × 105 cells per recipient; lin− = lineage depleted by FACS for CD3, -4, -13, -14, and -19) isolated from a single CB. Groups of four mice were killed and analyzed on days 4, 7, 14, 28, and 56 posttransplantation. At each time point bone marrow, spleen, thymus, and peripheral blood were analyzed from each mouse for human myeloid (CD33, CD13, CD14), lymphoid (CD19, IgM, CD2, CD3, CD4, CD8), and precursor (erythroid: glycophorin A, CD71; multilineage: CD34) populations. Fig. 1 illustrates the ranges exhibited by the groups of animals at each time point for the proportion of human cells in each hematopoietic organ (Fig. 1a), for the percent of cells expressing lineage-specific markers within the human population from bone marrow and thymus (Fig. 1b), and the median number of total human cells expressing a particular marker (Fig. 1c).
Figure 1.
Kinetics of CD34+ cell repopulation of NOD/SCID mice. (a) Time course of mouse repopulation of various tissues with human CD45+ cells, including bone marrow (■), spleen (□), thymus (▤), and peripheral blood (PB; ▨). The bone marrow shows the first signs of human repopulation, followed closely by the spleen and peripheral blood. Finally, the thymus is repopulated, beginning 28 days posttransplantation. (b) Time course of lineage-specific development of human CD45+ cells in the bone marrow and thymus. Lineages assessed in the bone marrow included early myeloid cells (CD33+; ■), developing myeloid (CD13+/CD33+; □), erythroid progenitors (glycophorin A+/CD71+/CD45−; ), B cell precursors (CD19+; ▩), and developing B cells (CD19+/IgM+; ▤). All lineages, except erythroid, were assessed from cells within the human CD45+ gate. Lineages assessed in mouse thymi included T cell precursors (CD45+; ▧) and developing, double-positive T cells (CD3+/CD4+/CD8+; ▨). A wave of myeloid development, followed by erythroid and B cell development, is evident. T cell development occurred last in the thymus. Bars in a and b represent the range of values obtained from four mice that were killed at each time point. (c) Median of the absolute number of human CD45+ cells (●), as well as human myeloid (CD33+; ■), B lymphoid (CD19+; ▴), and erythroid (glycophorin A+/CD71+/CD45−; ▾) compartments obtained from the femurs and tibia of mice during the 8-week engraftment period.
Engraftment was evident first in the bone marrow and was detected as early as 7 days posttransplantation, where 3–5% of leukocytes were human CD45+. Peripheral blood and splenic repopulation were observed by day 14 followed by thymic repopulation by day 28. The proportion of human cells in all four organs increased significantly throughout the duration of the experiment. With respect to lineage development in the bone marrow (Fig. 1b), the myeloid compartment was repopulated first (day 7) with cells expressing early myeloid markers (CD33). Cells displaying a more mature myeloid phenotype as defined by the coexpression of CD13 and CD33 were detected in the bone marrow by the second week of repopulation. Myeloid cells were the predominant population in the human graft throughout the first month following transplantation. Beyond this point, the frequency of these cells diminished as the proportion of B cell precursors increased. Erythroid precursors developed by day 14 of repopulation and were maintained as a decreasing proportion of the human cell population throughout the experiment. B cell precursors as defined by CD19+/IgM− expression were present as early as day 14, and by day 28 these cells represented the second largest population of human cells. Further maturation within the B cell compartment, defined by surface IgM expression, was not evident until day 28 posttransplantation. At day 56 B cells were the predominant human cell population, although low levels of myeloid cells and erythroid precursors persisted in the graft. Although the proportion of specific lineages within the human graft changed significantly throughout the 56-day experimental period, the total number of cells within each of the lineages either remained relatively constant (erythroid) or continued to increase throughout the course of the experiment (Fig. 1c). The growth curve illustrates that the number of human CD45+ cells increased ≈4,000-fold between the first and eighth week of the study, initially because of myeloid cell increases and later (after day 28) of increases in the B cell compartment (Fig. 1c).
As was the case with B cell repopulation, thymic reconstitution with human cells did not occur until day 28, at which time only CD45+CD3−/CD4−/CD8− cells were present. It was not until day 56 that human CD3+/CD4+/CD8+ cells comprised the bulk of the human cell population in NOD/SCID thymi. Collectively, the findings from this kinetic study suggest that the precursor population within the CD34+lin− fraction of cord blood is heterogeneous and contains cells that function at early stages following transplantation as well as those that can sustain significant engraftment for at least 8 weeks.
Kinetics of CD34+/CD38+ and CD34+/CD38− Repopulation.
To dissect the broad repopulation potential of CD34+ CB cells and to evaluate the ability of the NOD/SCID model to discriminate these potentials in vivo, this population was fractionated into CD38− and CD38+ or into CD38−, CD38low, and CD38+ subpopulations (see Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org). The isolated cell populations were then transplanted into equal numbers of recipient animals. Mice each received 4.5 × 103-6 × 104 CD34+/CD38− cells (representing 5–8% of the CD34+ fraction, Fig. 4), and 4 × 104-5 × 105 CD34+/CD38+, and in some experiments, CD34+/CD38low cells (that represented the remainder of the CD34+ fraction, Fig. 4). The fractions were consistently at least 97% pure (data not shown). Mice were killed at serial time points posttransplantation and their hematopoietic tissues were analyzed for engraftment and development of human hematopoietic cells.
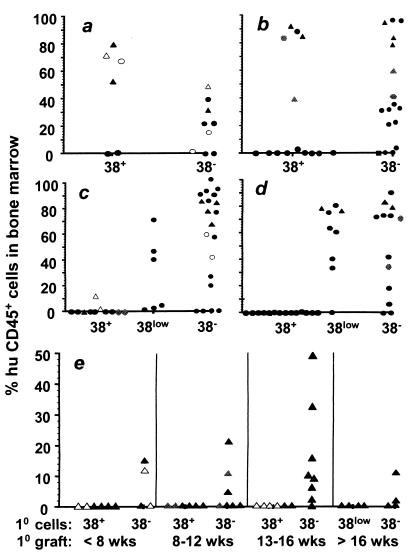
Mice that received CD34+/CD38+ cells exhibited high levels of human cell engraftment within 4–7 weeks of transplantation. Between 8 and 12 weeks some of the animals in this group continued to display high levels of human cell repopulation (Fig. 2 a and b). However, more than half the animals transplanted with the CD34+/CD38+ fraction showed no evidence of human cell engraftment at these analysis times. Beyond 12 weeks posttransplantation, the level of human cells in the mouse marrow decreased dramatically and the majority of CD34+/CD38+ recipients exhibited no human hematopoietic cells (Fig. 2 c and d). The multilineage developmental potential of the CD34+/CD38+ fraction was similar to that of the total CD34+ population (13). Repopulated mice were found to contain cells of the myeloid (CD33+, CD13+, and CD14+), B lymphoid (CD19+), and erythroid (glycophorin A+/CD71+/CD45−) lineages in the bone marrow and the myeloid and B lymphoid lineages in the peripheral tissues. In addition repopulated animals also maintained a significant CD34+ precursor population in the bone marrow (see Fig. 5a, stippled bars, which is published as supporting information on the PNAS web site, www.pnas.org).
Figure 2.
Human CD45+ bone marrow engraftment of primary (a–d) and secondary (e) NOD/SCID recipients following transplantation of CD34+ subfractions for varying lengths of time. Engraftment was evaluated following periods of 4–7 weeks (a), 8–12 weeks (b), 12–16 weeks (c), and 16–20 weeks (d) posttransplantation. Bone marrows from selected mice at each time point (triangles in a–d) were transplanted into irradiated secondary recipients. Results from those transplants, 8–16 weeks postserial transplantation, are presented in e. The data represents animals from a number of different experiments. Two of the experiments are coded so that tracking across time intervals is possible. In these cases all mice represented by open symbols or by gray symbols were transplanted with CD34+ subfractions from the same CB, respectively, or in the case of secondary recipients, with bone marrow from those recipients.
Mice transplanted with CD34+/CD38− cells exhibited somewhat different engraftment kinetics than those that received CD34+/CD38+ cells. At the 4–8 week time point, animals with the CD34+/CD38− graft contained lower numbers of human cells than those transplanted with the corresponding CD34+/CD38+ fraction (Fig. 2a). Between 8 and 12 weeks, some animals from the CD34+/CD38− group showed comparable levels of human cells to those with the CD34+/CD38+ graft. However, beyond week 12, when most recipients of the CD34+/CD38+ fraction showed little or no repopulation, the majority of animals transplanted with CD34+/CD38− cells contained a significant human graft (Fig. 2 b–d). The multilineage nature of engraftment in the bone marrow and peripheral tissues in these animals was similar to that observed in mice transplanted with the CD34+/CD38+ fraction (Fig. 5a, open bars) with the notable exception that they displayed more frequent thymic repopulation (see below).
In experiments where CD34+ cells were fractionated into three subpopulations (Fig. 2 c and d, and Fig. 4), animals transplanted with the CD38low fraction were not harvested before 12 weeks posttransplantation. Animals transplanted with these cells displayed robust repopulation, similar to those that received CD38− cells, when analyzed between 12 and 20 weeks following transplantation. Distribution of lineage markers on human cells in the mouse bone marrow and peripheral tissues was typical of output cells found after transplantation of the other two fractions (data not shown).
Taken together, the findings from these cell fractionation experiments are consistent with the interpretation that the CD34+/CD38+ fraction contains relatively mature precursors that provide an early, but transient wave of repopulation, whereas the CD34+/CD38− fraction contains a more primitive subset of cells with long-term repopulating potential.
Long-Term SRC Content of CD34+ Subfractions.
A benchmark for determining the long-term reconstituting potential of hematopoietic cell populations in the mouse has been the ability to serially repopulate secondary recipients with bone marrow from primary recipients engrafted with the experimental fraction (2, 4–6, 34). To further evaluate the repopulating potential of the different CD34+ fractions, bone marrow from primary recipients of each was assessed for SRC with secondary repopulation capacity. The majority of the bone marrow cells (except for 2–5% that were used for multilineage analysis) from selected mice at each time point (indicated by triangles in Fig. 2 a–d) were transplanted into irradiated secondary recipients. Donor mice in secondary transplantation studies had originally been engrafted with 1.8 × 105-3 × 105 CD34+/CD38+, 1 × 105-3 × 105 CD34+/CD38low, or 4.5 × 103-3.2 × 104 CD34+/CD38− fractionated human cells. Secondary recipients were then analyzed for multilineage engraftment 8–16 weeks posttransplantation (Fig. 2e). None of the secondary recipients of bone marrow from mice originally transplanted with either CD34+/CD38+ or CD34+/CD38low fractions (Fig. 2e) showed any evidence of human cell engraftment. This finding was apparent even when secondary transplants were performed at early time points, when human cells comprised the major proportion of bone marrow of the primary recipient. By contrast, at every primary engraftment time interval analyzed, mice transplanted with the CD34+/CD38− fraction contained SRC able to repopulate secondary recipients (Fig. 2e). Three color analysis confirmed that secondary engraftment was multilineage. Human cells that stained positive for myeloid (CD33, CD14), lymphoid (CD19), erythroid (glycophorin A+/CD71+/CD45−), and progenitor (CD34) cell markers were detected in the mouse bone marrow by flow cytometry (Fig. 5b). The findings from this serial transplant analysis further support the notion that the CD34+/CD38+ and CD34+/CD38− fractions contain short- and long-term repopulating cells, respectively.
Thymic Repopulation Following Transplantation with CD34+/CD38− and CD34+/CD38+/low Fractions.
The above observations suggest that the CD34+/CD38− fraction of CD34+ cells is more primitive than the CD34+/CD38+ or CD34+/CD38low subfractions with respect to long-term repopulation potential. To determine whether these fractions differed with respect to other aspects of developmental potential, we next evaluated their capacity to generate T lymphocytes in the thymus of the transplanted recipients. Our earlier studies demonstrated that mice transplanted with CD34+lin− cells occasionally exhibit thymic repopulation, indicating that this potential does exist in this population and that the NOD/SCID thymic environment is able to support human T cell development (Fig. 1 a and b; ref. 13).
Thymic repopulation was compared between mice engrafted with each of the CD34+ subpopulations. To compare the potential of fractions at early engraftment time points when engraftment by CD34+/CD38+ cells was most prevalent, but likely before the expression of T cell-specific markers (cf. Fig. 1b), we assayed cells isolated from mouse thymi for expression of human CD45 by using parameters illustrated in the first two histograms of Fig. 3. As indicated in Table 1 the frequency, both of input fractions that produced thymic repopulation and of animals displaying thymic repopulation, was significantly higher in the group transplanted with the CD34+/CD38− fraction (0.75 and 0.61, respectively) than in the group that received CD34+/CD38+ cells (0.25 and 0.22, respectively). CD34+ cells appeared to lose T cell potential as expression of CD38 progressed. This finding was indicated where the thymic repopulating potential of the CD34+/CD38low fraction appeared to be intermediate between the CD34+/CD38+ and CD34+/CD38− subpopulations (Table 1). Although T cell potential is not altogether absent in the CD38+ fraction, the frequency of cells with this potential is far lower than that found in the CD38− fraction, given that each recipient received 10–20 times more CD34+/CD38+ than CD34+/CD38− cells in each experiment.
Figure 3.
Development of human thymocytes in the NOD/SCID mouse thymus. Cells isolated from the thymus were initially gated on a low forward and side scatter population. Cells in this gate were then assessed for human vs. mouse CD45 staining. If present, cells within the human CD45+ gate were then assessed for CD2 as well as CD3/CD4/CD8 staining. Histograms are presented for the entire CD3+ population (A), the CD3low subpopulation (B), and the CD3high subpopulation (C). This example is representative of mice engrafted for periods of 12 weeks or longer. In mice engrafted for shorter periods of time typically only the CD3low subpopulation of cells was present that contained >95% CD4+/CD8+ double positive cells.
Table 1.
Repopulation of NOD/SCID thymi with human progenitor fractions
| Input cell fraction | Input cell no. | No. input fractions yielding repopulation*/No. different input fractions | % Input fractions yielding thymic repopulation* | No. mice with thymic repopulation†/No. mice transplanted | % Mice with thymic repopulation† | No. of human T cells |
|---|---|---|---|---|---|---|
| CD34+/CD38+ | 0.4–4.3 × 105 | 4/16 | 25 | 8/37 | 22 | 1.9 × 104 –4.2 × 105 |
| CD34+/CD38low | 0.3–5 × 105 | 3/7 | 43 | 6/15 | 40 | 7 × 104 –3.7 × 106 |
| CD34+/CD38− | 0.45–6 × 104 | 12/16 | 75 | 22/36 | 61 | 5 × 104 –7.6 × 106 |
Where any mouse that received the input fraction had >1% human CD45+ cells in the thymus.
Where the mouse thymus contained >1% human CD45+ cells.
Development of Human CD45+ Cells in NOD/SCID Mouse Thymi.
In many recipients repopulated for more than 7 weeks, the human CD45+ cell component in the mouse thymus ranged from 30–99% (data not shown). The vast majority of CD45+ cells in these thymi also expressed CD2, CD3, CD4, and CD8 (Fig. 3). Interestingly, mice with thymic engraftment that were repopulated for 12 or more weeks, displayed a bimodal distribution of human thymocyte CD3 expression (Fig. 3). In contrast, those analyzed before this time exhibited a single population, expressing low levels of CD3, similar to that illustrated in gate B of Fig. 3. Invariably, low CD3 expression (i.e., TCR expression) corresponded to almost all cells being CD4+/CD8+ double positive (histogram from gate B in Fig. 3). Cells expressing high levels of CD3, on the other hand, contained substantial proportions of CD4+ and CD8+ single positive T cells (histogram from gate C in Fig. 3).
To determine whether normal polyclonal development is taking place within these repopulated thymi, cells from them were analyzed for Vβ diversity by using a panel of 16 different antibodies directed against specific human Vβ epitopes. The Vβs recognized by this panel of antibodies represent ≈70% of the human repertoire. All Vβs analyzed were found to be present in these thymi at variable levels (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). In addition, there was no evidence of predominant clones or abnormal skewing to certain Vβs, suggesting that normal human T cell development was occurring in these mice.
Discussion
The findings presented in this report provide strong evidence that the CD34+/CD38− and CD34+/CD38+ fractions of cord blood represent distinct populations with respect to NOD/SCID engraftment potential. Specifically, the following observations are consistent with the interpretation that the CD34+/CD38− cells represent the more primitive component of the population commonly referred to as SRC. First, CD34+/CD38+ cells repopulate early, whereas CD34+/CD38− repopulation is more robust at later stages. Second, only mice repopulated with CD34+/CD38− cells contained SRC that were able to repopulate secondary recipients. Third, T cell potential was apparent in the majority of CD34+/CD38− fractions, but diminished as levels of CD38 expression increased. These findings are consistent with previous in vitro and in vivo studies that have indicated that cells expressing no or low levels of CD38 represent the most primitive fraction of the CD34+ population with respect to colony potential and SRC content, respectively (20, 21, 29–32). These data support recent findings from an elegant set of experiments where retrovirally marked CB was transplanted into NOD/SCID recipients and identifiable clones exhibited differential periods of longevity (22). Those clones that persisted may have come from transduced CD34+/CD38− cells, whereas those that appear to decline may have been derived from transduced CD34+/CD38+ cells. Our data also supports early work in sheep, as well as more recent experiments in NOD/SCID β2 microglobulin null mice that showed that longevity of grafts varies depending on CD38 expression on input CD34+ cells (23, 25). However, in contrast to results reported by Glimm and coworkers (23) we found that the NOD/SCID mouse readily detects short-term (6–12 week), multilineage reconstitution provided by CD34+/CD38+ and CD34+/CD38low cell populations. This may be explained by the fact that we treat mice with anti-asialo GM1 during the early engraftment period to further inhibit NK cell activity. The β2 microglobulin knockout on the NOD/SCID background confers a complete loss of NK cell activity on mice. Thus if short-term precursors are sensitive to host NK activity, use of the antibody in NOD/SCID mice may render the models somewhat equivalent in detecting this fraction of repopulating cells. Together, these findings suggest that one must exercise caution when using the NOD/SCID assay to define a repopulating cell and that one must look at both duration and potential of the graft. This situation is similar to that observed in the mouse, where early, intermediate, and long-term repopulation are mediated by distinct subsets of precursors (1, 35).
As discussed above, along with the longer-term component of the CD34+ fraction, this study demonstrates that a short-term component can be readily distinguished in the NOD/SCID model. The earliest stages of human hematopoietic reconstitution from CD34+ CB cells in NOD/SCID mice, that occur within 7 days of transplantation, are marked exclusively by myeloid repopulation of the bone marrow (ref. 36; Fig. 1b). This finding is significant because it parallels the rapid granulocyte recovery observed in humans following myeloablative treatment and hematopoietic rescue with CD34+ cells (26). These observations suggests that both species may be repopulated initially by equivalent cells. By correlating neutrophil recovery in humans with the level of myeloid progenitors in the mouse bone marrow, the mouse model could be a useful predictor of early myeloid engraftment in humans. Establishing such a correlative relationship would then make it possible to predict the performance in humans of various rare cell fractions, or of hematopoietic precursors manipulated ex vivo, based on their reconstitutive capacity in mice.
This study highlights the differences in T cell potential in vivo between the CD38+ and CD38− subpopulations of CD34+ CB cells. An indication of progenitor cell differentiation is the progressive limiting of multilineage potential as the differentiation program proceeds. This phenomenon was clearly illustrated by showing that most precursors capable of giving rise to T cells in the NOD/SCID thymus were found in the CD38− fraction, and that T cell potential is lost amongst CD34+ SRC as CD38 expression increases and cells progressively differentiate into more mature progenitors. In vitro it has been demonstrated that CD34+/CD38+ and CD34+/CD38− CB precursors can give rise to T cell development in fetal thymic organ culture (FTOC) (37–40). Blom et al. (37) present evidence that these subpopulations of CD34+ CB cells have the ability to differentiate into T cells in the thymic environment, yet none show a priori commitment to T cell development before reaching the thymus (with respect to TCR-δ or TCR-β rearrangements or RAG1 expression). That work implies that both the CD38+ and CD38− populations are equally capable of T cell development in FTOC. This work suggests that although both the CD38+ and CD38− populations of CD34+ CB contain cells capable of thymic seeding and T cell development, such cells occur at a much higher frequency in the CD38− population when these fractions are subjected to transplantation conditions in vivo.
The observed sequence of development in the NOD/SCID thymus appears to be similar to what occurs in the human thymus. In the kinetic study described above at 4 weeks CD45+ cells were present in the thymus, but T-specific markers were not expressed until 8 weeks (Fig. 1). Thus the T cell lineage is the last to recover, much as the case in patients following myeloablative treatments and hematopoietic rescue. In mice analyzed 8 weeks subsequent to engraftment >90% of the cells were CD45+/CD2+/CD3+/CD4+/CD8+. Single positive CD4 or CD8 cells were not detected. Interestingly, in experiments where mice were killed at 12 weeks and beyond T cell development proceeded to yield significant CD3+/CD4+ and CD3+/CD8+ populations within the thymus. Moreover, the profile of cell surface expression of these markers appears developmentally normal: low CD3 (TCR) expression corresponds to >95% of cells being CD4+/CD8+, whereas CD4+ and CD8+ single positive cells comprise a larger proportion of cells expressing high levels of CD3. This is a somewhat surprising result because it has been found that human coreceptors, such as CD8, do not interact well with mouse ligands (MHC class I; ref. 41). Additionally, we have demonstrated, through the use of Vβ antibody staining, that the T cells that develop are at least oligoclonal, and probably polyclonal, with respect to β chain rearrangements of their TCRs. The antibodies used in this study collectively stain TCRs that comprise ≈70% of the human repertoire. In both samples analyzed, every Vβ was represented with no gross skewing toward any particular Vβ that might indicate proliferation of a particular clone. Taken together these data describe events consistent with normal human thymic T cell development. These findings suggest that a NOD/SCID mouse assay may be a reliable assay for discerning the in vivo T cell potential of various cell fractions considered for transplantation. Additionally the model may prove to be an optimal system for evaluating various aspects of human immune reconstitution in vivo, such as factors and accessory cells required for peripheral maintenance of T cells or that influence the determination of T cell repertoire.
This report, along with other recent findings (22, 23, 25), demonstrates that different classes of human SRC exist that possess differing potentials with respect to graft longevity and lineage restrictions. At least two of these classes are isolatable from the CD34+ population of CB and are readily and differentially detected in the NOD/SCID mouse model. Our findings lead us to speculate that the CD34+/CD38− fraction may contain LTRSCs, whereas the CD34+/CD38+ fraction may contain those cells capable of providing short-term and intermediate stage engraftment in humans. It will be interesting and informative to discover how these findings relate to human repopulation.
Supplementary Material
Acknowledgments
We thank the staff of the clinical Stem Cell Lab at the University of Colorado Hospital for collection and timely provision of cord blood units; as well as the staff at the National Jewish Hospital and Research Center for excellent care of mice. We are grateful to Karen Helm of the University of Colorado Cancer Center Flow Cytometry Core for cell sorting, which was supported in part by a Cancer Center Core Grant CA46934 from the National Cancer Institute/National Institutes of Health. This work was supported by Grant ROI CA61508 from the NCI/NIH to E.J.S. and by Grant ROI HL61382 from the NHLBI/NIH to C.J.H.
Abbreviations
- NOD
nonobese diabetic
- SCID
severe combined immunodeficiency
- LTRSC
long-term repopulating stem cell
- CB
umbilical cord blood
- SRC
SCID-repopulating cell
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Keller G. Curr Opin Immunol. 1992;4:133–139. doi: 10.1016/0952-7915(92)90002-v. [DOI] [PubMed] [Google Scholar]
- 2.Morrison S J, Uchida N, Weissman I L. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 3.Jones R J, Wagner J E, Celano P, Zicha M S, Sharkis S J. Nature (London) 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 4.Jones R J, Collector M I, Barber J P, Vala M S, Fackler M J, May W S, Griffin C A, Hawkins A L, Zehnbauer B A, Hilton J, et al. Blood. 1996;88:487–491. [PubMed] [Google Scholar]
- 5.Jordan C T, Lemischka I R. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 6.Keller G, Snodgrass R. J Exp Med. 1990;171:1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith L G, Weissman I L, Heimfeld S. Proc Natl Acad Sci USA. 1991;88:2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snodgrass R, Keller G. EMBO J. 1987;6:3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevisan M, Iscove N N. J Exp Med. 1995;181:93–103. doi: 10.1084/jem.181.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 11.Shultz L D, Schweitzer P A, Christianson S W, Gott B, Schweitzer I B, Tennent B, McKenna S, Mobraaten L, Rajan T V, Greiner D L, et al. J Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 12.Hesselton R M, Greiner D L, Mordes J P, Rajan T V, Sullivan J L, Shultz L D. J Infect Dis. 1995;172:974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 13.Hogan C J, Shpall E J, McNulty O, McNiece I, Dick J E, Shultz L D, Keller G. Blood. 1997;90:85–96. [PubMed] [Google Scholar]
- 14.Hogan C J, Shpall E J, McNiece I, Keller G. Biol Blood Marrow Transplant. 1997;3:236–246. [PubMed] [Google Scholar]
- 15.Holyoake T L, Nicolini F E, Eaves C J. Exp Hematol. 1999;27:1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 16.Larochelle A, Vormoor J, Lapidot T, Sher G, Furukawa T, Li Q, Shultz L D, Olivieri N F, Stamatoyannopoulos G, Dick J E. Hum Mol Genet. 1995;4:163–172. doi: 10.1093/hmg/4.2.163. [DOI] [PubMed] [Google Scholar]
- 17.Larochelle A, Vormoor J, Hanenberg H, Wang J C, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, et al. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 18.Lowry P A, Shultz L D, Greiner D L, Hesselton R M, Kittler E L, Tiarks C Y, Rao S S, Reilly J, Leif J H, Ramshaw H, et al. Biol Blood Marrow Transplant. 1996;2:15–23. [PubMed] [Google Scholar]
- 19.Bhatia M, Bonnet D, Murdoch B, Gan O I, Dick J E. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia M, Wang J C Y, Kapp U, Bonnet D, Dick J E. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conneally E, Cashman J, Petzer A, Eaves C. Proc Natl Acad Sci USA. 1997;94:9836–9841. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guenechea G, Gan O I, Dorrell C, Dick J E. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 23.Glimm H, Eisterer W, Lee K, Cashman J, Holyoake T L, Nicolini F, Shultz L D, von Kalle C, Eaves C J. J Clin Invest. 2001;107:199–206. doi: 10.1172/JCI11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanjani E D, Almeida-Porada G, Flake A W. Int J Hematol. 1996;63:179–192. doi: 10.1016/0925-5710(96)00445-8. [DOI] [PubMed] [Google Scholar]
- 25.Civin C I, Almeida-Porada G, Lee M J, Olweus J, Terstappen L W, Zanjani E D. Blood. 1996;88:4102–4109. [PubMed] [Google Scholar]
- 26.Shpall E J, Jones R B, Bearman S I, Franklin W A, Archer P G, Curiel T, Bitter M, Claman H N, Stemmer S M, Purdy M, et al. J Clin Oncol. 1994;12:28–36. doi: 10.1200/JCO.1994.12.1.28. [DOI] [PubMed] [Google Scholar]
- 27.Finke J, Brugger W, Bertz H, Behringer D, Kunzmann R, Weber-Nordt R M, Kanz L, Mertelsmann R. Bone Marrow Transplant. 1996;18:1081–1086. [PubMed] [Google Scholar]
- 28.Link H, Arseniev L, Bahre O, Kadar J G, Diedrich H, Poliwoda H. Blood. 1996;87:4903–4909. [PubMed] [Google Scholar]
- 29.Craig W, Poppema S, Little M T, Dragowska W, Lansdorp P M. Br J Haematol. 1994;88:24–30. doi: 10.1111/j.1365-2141.1994.tb04972.x. [DOI] [PubMed] [Google Scholar]
- 30.Terstappen L W, Huang S, Safford M, Lansdorp P M, Loken M R. Blood. 1991;77:1218–1227. [PubMed] [Google Scholar]
- 31.Hao Q L, Shah A J, Thiemann F T, Smogorzewska E M, Crooks G M. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 32.Petzer A L, Zandstra P W, Piret J M, Eaves C J. J Exp Med. 1996;183:2551–2558. doi: 10.1084/jem.183.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricalton N S, Roberton C, Norris J M, Rewers M, Hamman R F, Kotzin B L. J Gerontol A Biol Sci Med Sci. 1998;53:B196–B203. doi: 10.1093/gerona/53a.3.b196. [DOI] [PubMed] [Google Scholar]
- 34.Iscove N N, Nawa K. Curr Biol. 1997;7:805–808. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 35.Weissman I L. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 36.Demaison C, Brouns G, Blundell M P, Goldman J P, Levinsky R J, Grez M, Kinnon C, Thrasher A J. Hum Gene Ther. 2000;11:91–100. doi: 10.1089/10430340050016184. [DOI] [PubMed] [Google Scholar]
- 37.Blom B, Res P, Noteboom E, Weijer K, Spits H. J Immunol. 1997;158:3571–3577. [PubMed] [Google Scholar]
- 38.Verhasselt B, De Smedt M, Verhelst R, Naessens E, Plum J. Blood. 1998;91:431–440. [PubMed] [Google Scholar]
- 39.Robin C, Bennaceur-Griscelli A, Louache F, Vainchenker W, Coulombel L. Br J Haematol. 1999;104:809–819. doi: 10.1046/j.1365-2141.1999.01266.x. [DOI] [PubMed] [Google Scholar]
- 40.Weekx S F, Snoeck H W, Offner F, De Smedt M, Van, Bockstaele D R, Nijs G, Lenjou M, Moulijn A, Rodrigus I, et al. Blood. 2000;95:2806–2812. [PubMed] [Google Scholar]
- 41.LaFace D M, Vestberg M, Yang Y, Srivastava R, DiSanto J, Flomenberg N, Brown S, Sherman L A, Peterson P A. J Exp Med. 1995;182:1315–1325. doi: 10.1084/jem.182.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.