Abstract
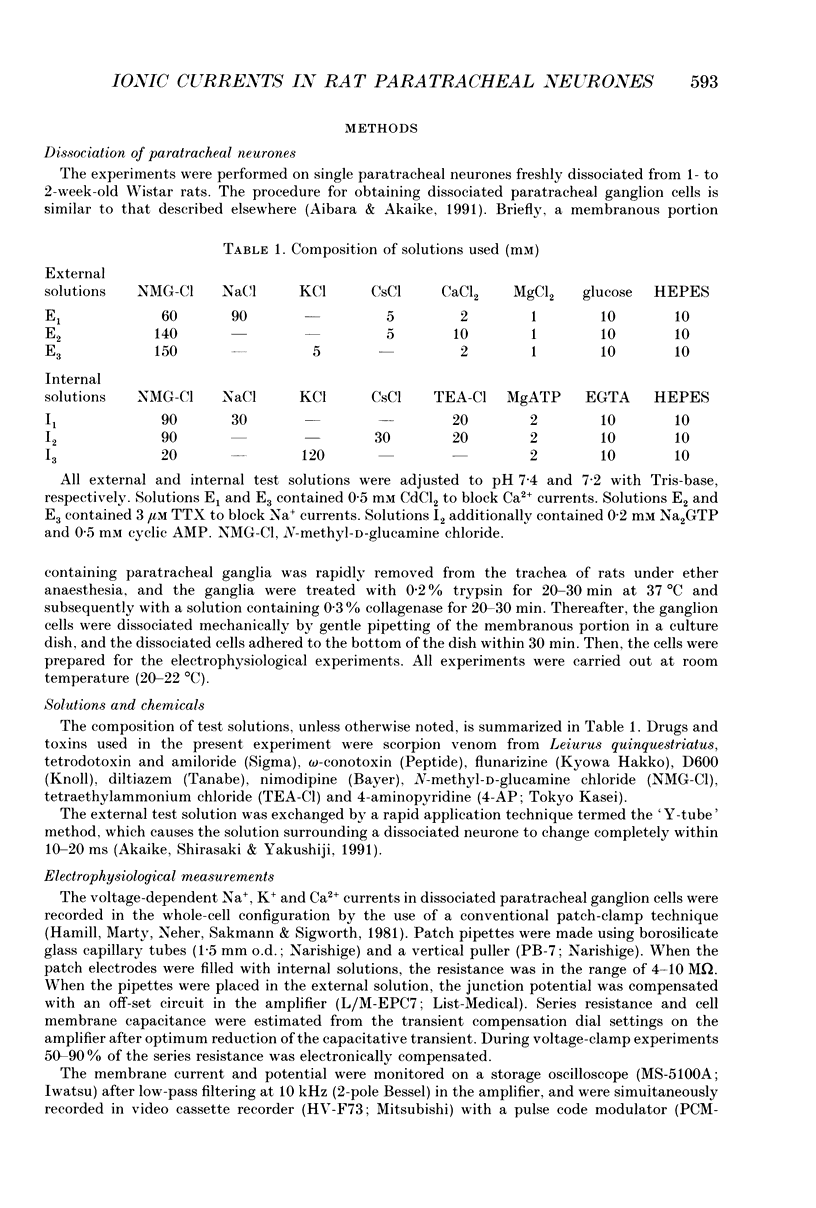
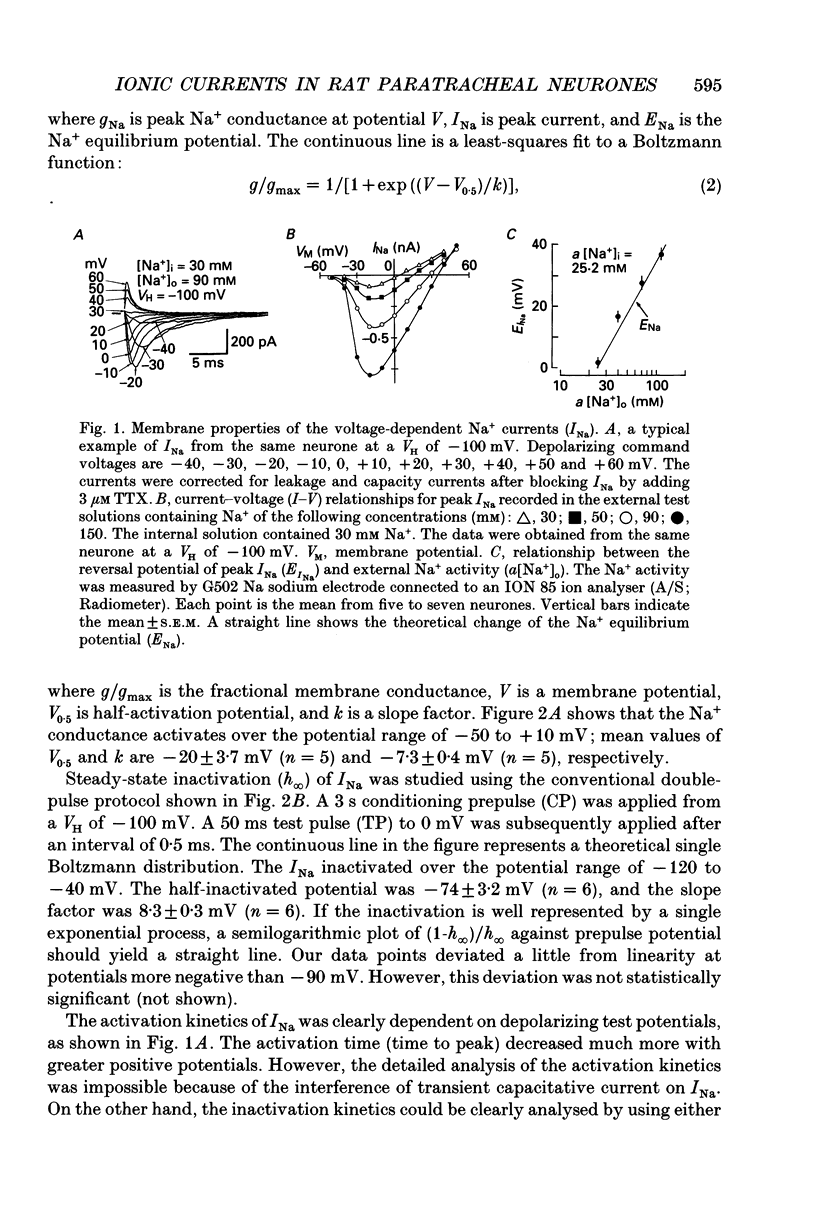
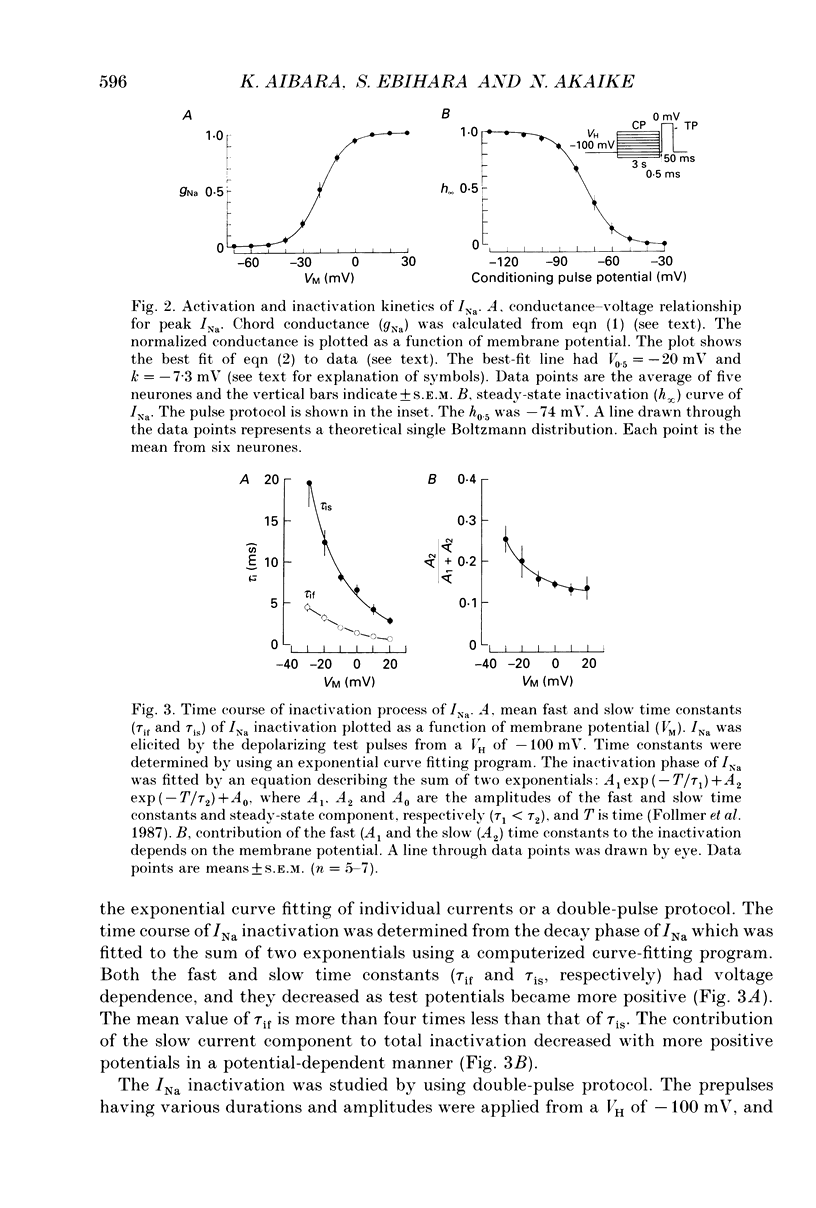
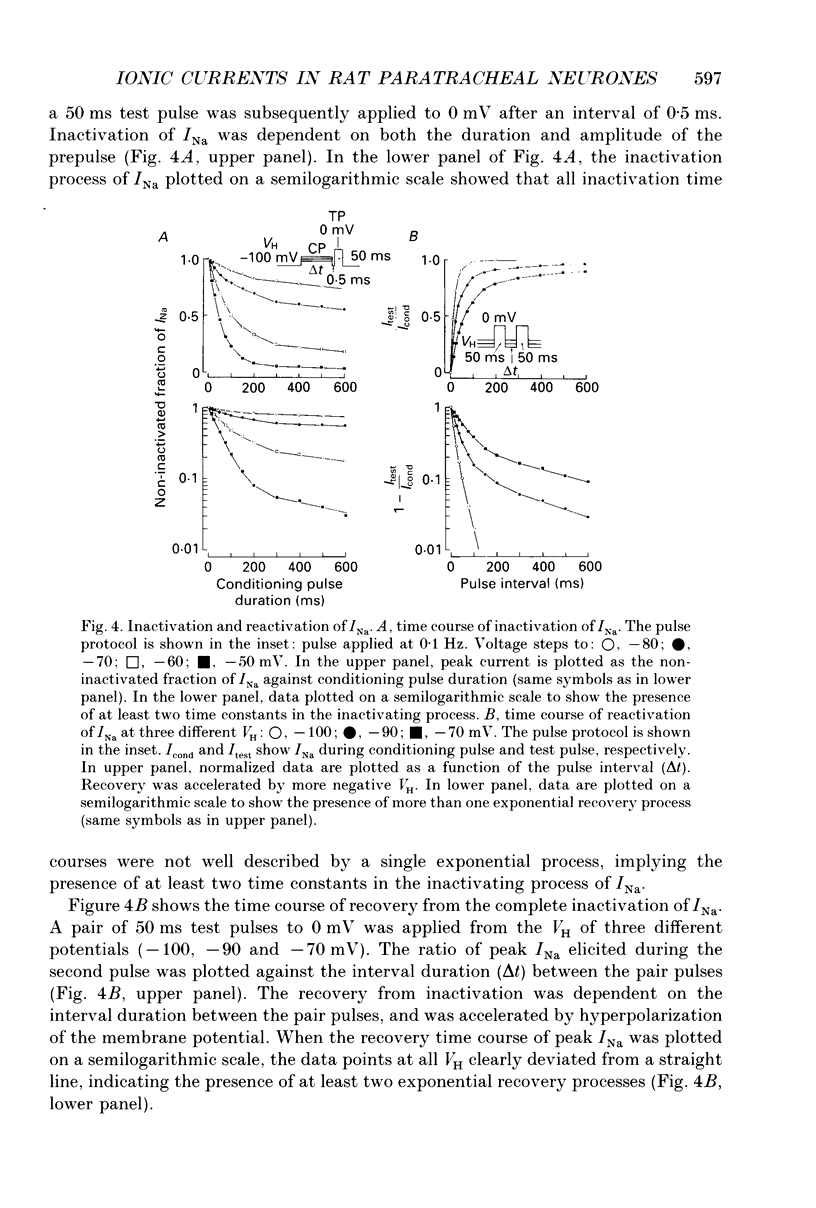
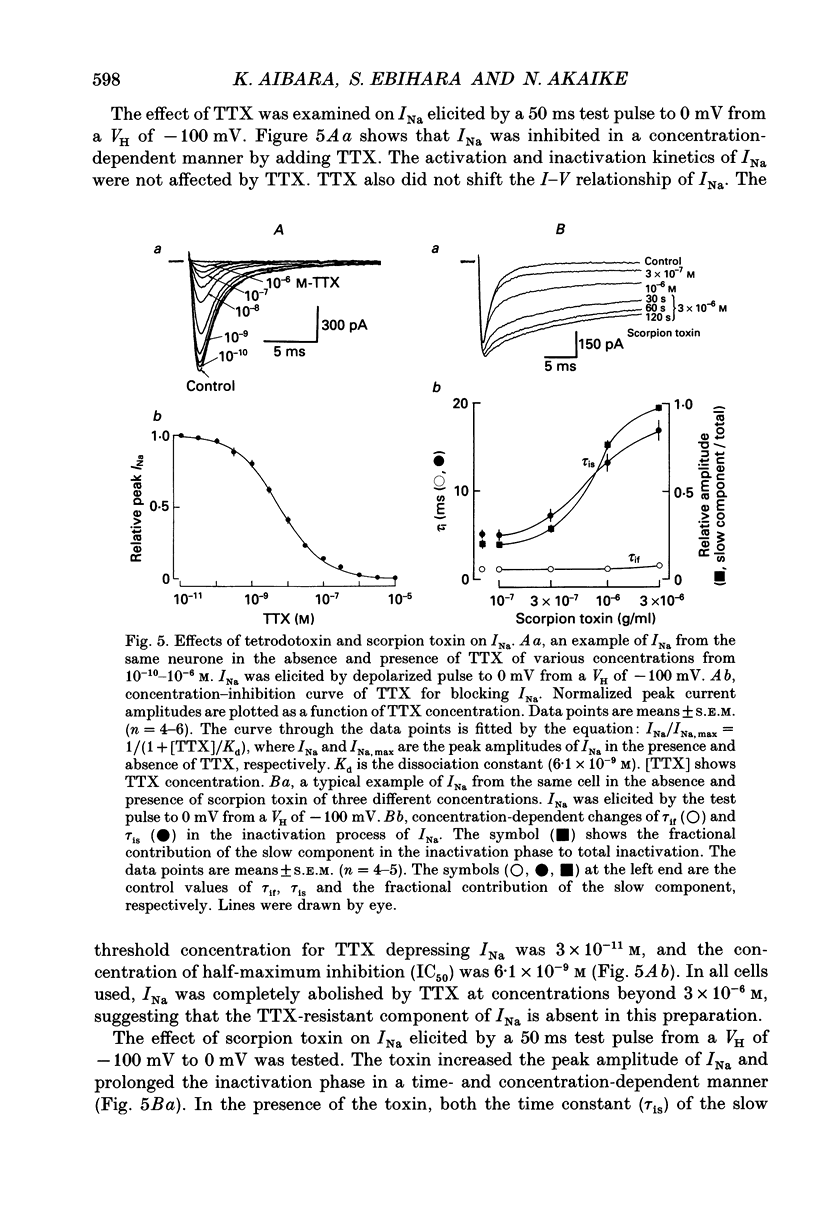
1. Conventional whole-cell voltage-clamp technique was used to study the electrophysiological and pharmacological properties of voltage-dependent Na+, K+ and Ca2+ channels in parasympathetic neurones enzymatically dissociated from the paratracheal ganglia of rat trachea. The voltage-dependent Na+, K+ and Ca2+ currents (INa, IK and ICa) were separated by the use of ion subtraction and pharmacological treatments. 2. INa was activated by a step depolarization more positive than -50 mV and fully activated at positive potentials more than +10 mV. The inactivation phase of INa consisted of fast and slow exponential components (tau if and tau is, respectively). The tau if and tau is were voltage dependent and decreased with a more positive step pulse. 3. The time course for recovery of INa from the complete inactivation exhibited two exponential processes. 4. The reversal potential of INa was equal to the Na+ equilibrium potential (ENa) and resembled a simple Na+ electrode depending only on external Na+ concentration. 5. Tetrodotoxin (TTX) reduced INa without affecting the current kinetics in a concentration-dependent manner, and the concentration of half-maximal inhibition (IC50) was 6 x 10(-9) M. There was no TTX-resistant component of INa in any of the cells tested. 6. Scorpion toxin increased the peak amplitude of INa and prolonged the inactivation phase in a time- and concentration-dependent manner. In the presence of toxin, both tau is and the fractional contribution of the slow current component to total INa increased concentration dependently. 7. High-threshold (L-type) ICa was activated by a step depolarization more positive than -30 mV and reached a peak at near 0 mV in the external solution with 2.5 mM Ca2+. The current was inactivated to only a small extent (< 10%) during 100 ms of depolarizing step pulse. There was no low-threshold (T-type) ICa in this preparation. 8. The maximum ICa in individual current-voltage (I-V) relationships was saturated by an increase in extracellular Ca2+ concentration ([Ca2+]o). The I-V relationships were also shifted along the voltage axis to the more positive potential with increasing [Ca2+]o. 9. The inactivation process of the L-type ICa was dependent on Ca2+ influxes (ICa-dependent inactivation). 10. Relative maximum peak currents of divalent cations passing through the L-type Ca2+ channels were in the order of IBa > ICa > ISr. 11. Organic and inorganic Ca2+ antagonists blocked the ICa in a concentration-dependent manner.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Galvan M. Voltage-dependent currents of vertebrate neurons and their role in membrane excitability. Adv Neurol. 1986;44:137–170. [PubMed] [Google Scholar]
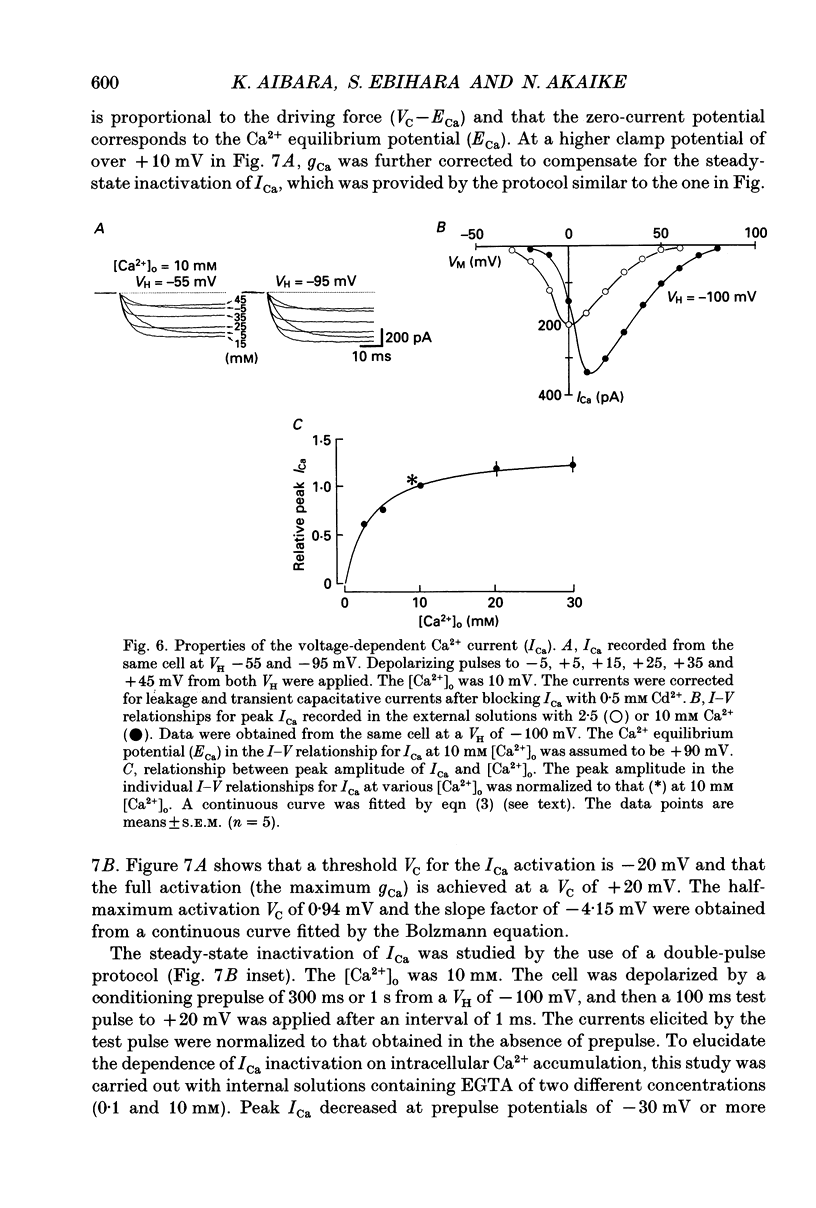
- Aibara K., Akaike N. Acetylcholine-activated ionic currents in isolated paratracheal ganglion cells of the rat. Brain Res. 1991 Aug 30;558(1):20–26. doi: 10.1016/0006-8993(91)90709-5. [DOI] [PubMed] [Google Scholar]
- Akaike N., Shirasaki T., Yakushiji T. Quinolones and fenbufen interact with GABAA receptor in dissociated hippocampal cells of rat. J Neurophysiol. 1991 Aug;66(2):497–504. doi: 10.1152/jn.1991.66.2.497. [DOI] [PubMed] [Google Scholar]
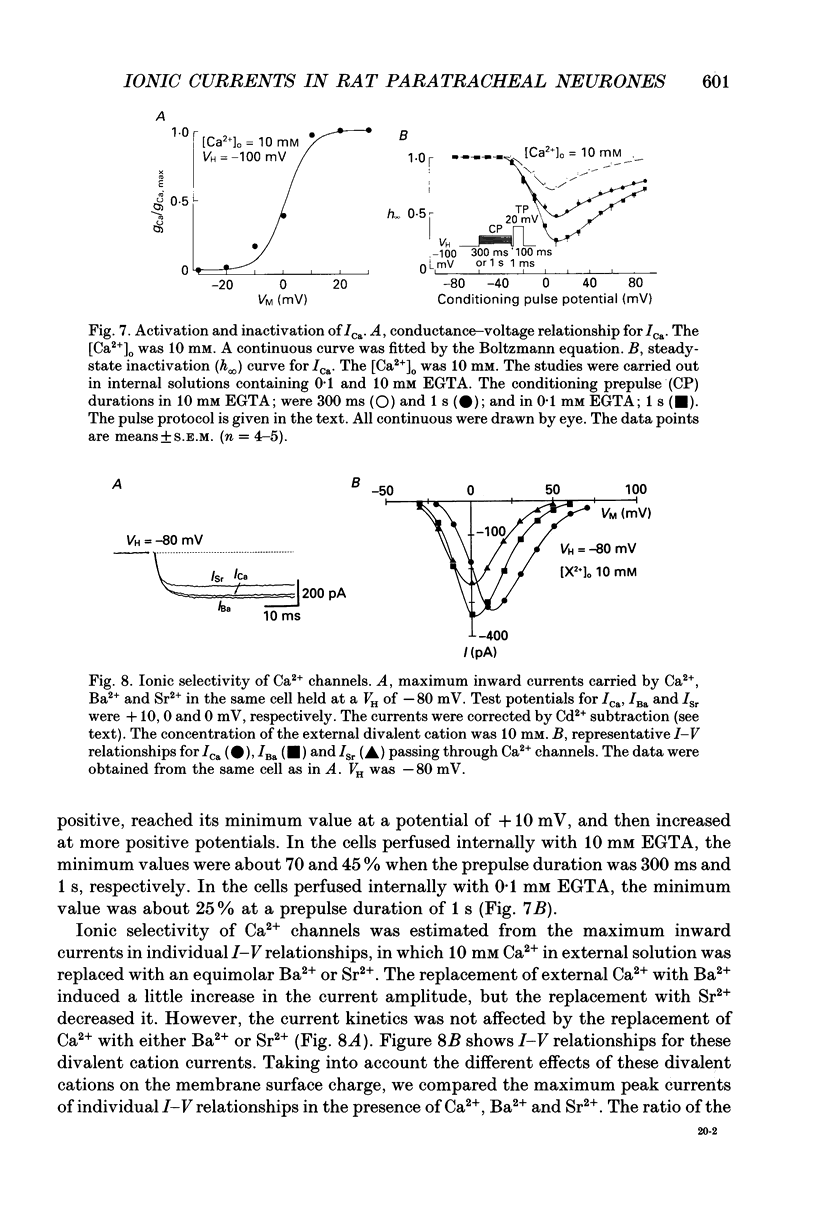
- Akaike N., Tsuda Y., Oyama Y. Separation of current- and voltage-dependent inactivation of calcium current in frog sensory neuron. Neurosci Lett. 1988 Jan 11;84(1):46–50. doi: 10.1016/0304-3940(88)90335-7. [DOI] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. A voltage-clamp study of the electrophysiological characteristics of the intramural neurones of the rat trachea. J Physiol. 1990 Apr;423:593–614. doi: 10.1113/jphysiol.1990.sp018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
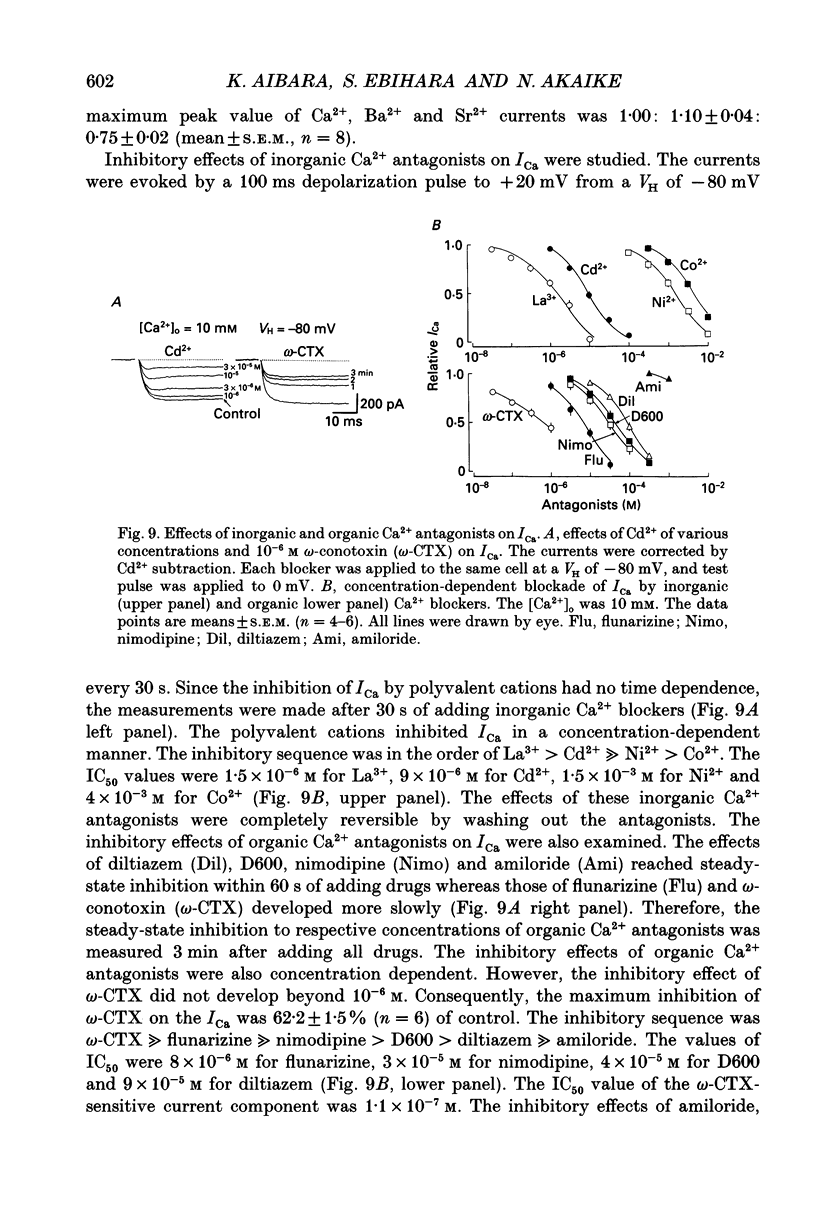
- Allen T. G., Burnstock G. GABAA receptor-mediated increase in membrane chloride conductance in rat paratracheal neurones. Br J Pharmacol. 1990 Jun;100(2):261–268. doi: 10.1111/j.1476-5381.1990.tb15793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni H., Böcker D., Eickhorn R. Sodium current kinetics in intact rat papillary muscle: measurements with the loose-patch-clamp technique. J Physiol. 1988 Dec;406:199–213. doi: 10.1113/jphysiol.1988.sp017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit E., Dubois J. M. Properties of maintained sodium current induced by a toxin from Androctonus scorpion in frog node of Ranvier. J Physiol. 1987 Feb;383:93–114. doi: 10.1113/jphysiol.1987.sp016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci Lett. 1984 Oct 12;51(2):241–246. doi: 10.1016/0304-3940(84)90558-5. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988 Mar;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F. Peripheral airway ganglia. Annu Rev Physiol. 1987;49:573–582. doi: 10.1146/annurev.ph.49.030187.003041. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Bean B. P., Colatsky T. J., Tsien R. W. Tetrodotoxin block of sodium channels in rabbit Purkinje fibers. Interactions between toxin binding and channel gating. J Gen Physiol. 1981 Oct;78(4):383–411. doi: 10.1085/jgp.78.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER A. W. THE INTRINSIC INNERVATION OF THE TRACHEA. J Anat. 1964 Jan;98:117–124. [PMC free article] [PubMed] [Google Scholar]
- Follmer C. H., ten Eick R. E., Yeh J. Z. Sodium current kinetics in cat atrial myocytes. J Physiol. 1987 Mar;384:169–197. doi: 10.1113/jphysiol.1987.sp016449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J Physiol. 1983 Sep;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Hille B., Catterall W. A. Voltage clamp analysis of sodium channels in normal and scorpion toxin-resistant neuroblastoma cells. J Neurosci. 1984 Nov;4(11):2836–2842. doi: 10.1523/JNEUROSCI.04-11-02836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G., Weight F. F. Na+ and Ca2+ currents of acutely isolated adult rat nodose ganglion cells. J Neurophysiol. 1986 Mar;55(3):527–539. doi: 10.1152/jn.1986.55.3.527. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M., Oyama Y., Ikemoto Y., Akaike N. Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine. Brain Res. 1989 Apr 10;484(1-2):348–351. doi: 10.1016/0006-8993(89)90379-x. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Oyama Y., Ikemoto Y., Akaike N. Scorpion toxin prolongs an inactivation phase of the voltage-dependent sodium current in rat isolated single hippocampal neurons. Brain Res. 1989 May 15;487(1):192–195. doi: 10.1016/0006-8993(89)90958-x. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G. Intracellular perfusion of nerve cells and its effects on membrane currents. Physiol Rev. 1984 Apr;64(2):435–454. doi: 10.1152/physrev.1984.64.2.435. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B. Calcium current inactivation in identified neurones of Helix aspersa. J Physiol. 1981 Dec;321:273–285. doi: 10.1113/jphysiol.1981.sp013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Lux H. D. Do calcium channel classifications account for neuronal calcium channel diversity? Trends Neurosci. 1991 Feb;14(2):46–51. doi: 10.1016/0166-2236(91)90018-p. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke E., Ferroni A., Malgaroli A., Ambrosini A., Pozzan T., Meldolesi J. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]


