Abstract
On the basis of ex vivo studies using insulin-responsive cells, activation of a Class IA phosphoinositide 3-kinase (PI3K) seems to be required for a wide variety of cellular responses downstream of insulin. The Class IA PI3K enzymes are heterodimers of catalytic and regulatory subunits. In mammals, insulin-responsive tissues express both the p85α and p85β isoforms of the regulatory subunit. Surprisingly, recent studies have revealed that disruption of the p85α gene in the mouse (p85α−/− mice) results in hypoglycemia with decreased plasma insulin, and the p85α+/− mice exhibit significantly increased insulin sensitivity. These results suggest either that p85α negatively regulates insulin signaling, or that p85β, which mediates the major fraction of Class IA PI3K signaling in the absence of p85α, is more efficient than p85α in mediating insulin responses. To address this question, we have generated mice in which the p85β gene is deleted (p85β−/− mice). As with the p85α−/− mice, the p85β−/− mice showed hypoinsulinemia, hypoglycemia, and improved insulin sensitivity. At the molecular level, PI3K activity associated with phosphotyrosine complexes was preserved despite a 20–30% reduction in the total protein level of the regulatory subunits. Moreover, insulin-induced activation of AKT was significantly up-regulated in muscle from the p85β−/− mice. In addition, insulin-dependent tyrosine phosphorylation of insulin receptor substrate-2 was enhanced in the p85β−/− mice, a phenotype not observed in the p85α−/− mice. These results indicate that in addition to their roles in recruiting the catalytic subunit of PI3K to the insulin receptor substrate proteins, both p85α and p85β play negative roles in insulin signaling.
Insulin activates Class IA phosphoinositide 3-kinase (PI3K) to initiate a cascade of events that control cell growth and metabolism (1–5). The activation of PI3K by insulin is mediated by the p85 regulatory subunit binding to tyrosine-phosphorylated insulin receptor substrate (IRS) proteins (e.g., IRS-1 and IRS-2; refs. 6 and 7). The regulatory subunit plays a dual role in regulation of the p110 catalytic subunit of PI3K. Binding of p85 to p110 prevents denaturation of p110 but also maintains p110 in a low activity state (8). Association of the SH2 domains of p85 with tyrosine-phosphorylated IRS proteins recruits PI3K to the membrane and turns up the catalytic activity. The lipid products of PI3K then can activate a variety of intracellular signaling pathways, including the AKT/PKB protein Ser/Thr kinase, which negatively regulates glycogen synthase kinase 3 (GSK3) and thereby controls glycogen synthesis in muscle (9, 10).
Three distinct genes encoding Class IA PI3K regulatory subunits exist in mammals, Pik3r1 (p85α), Pik3r2 (p85β), and p55PIK (11–13). The Pik3r1 gene encodes three spliced variants, p85α, AS53 (also known as p55α; refs. 14 and 15), and p50α (12, 16), whereas the Pik3r2 gene and the p55PIK gene seem to encode single products, p85β and p55PIK, respectively. All isoforms share a highly homologous structure in their carboxyl-terminal regions, composed of two SH2 domains (referred to as nSH2 and cSH2 domains) flanking an inter SH2 (iSH2) domain containing the p110 binding region (5, 17). In the amino-terminal regions, p85α and p85β also share a common structure with an SH3 domain and a rho-GAP homology domain flanked by two proline-rich domains (11). AS53, p50α, and p55PIK lack this structure and in its place have unique sequences (34, 6, and 34 amino acids, respectively). These short isoforms are expressed only in restricted tissues (12, 14–16). On the other hand, p85α is ubiquitously expressed and is thought to be the major response pathway for most stimuli, whereas p85β also is widely expressed but at a lower level than p85α (5, 11).
In addition to a role for the p85α regulatory subunits in recruiting the p110 catalytic subunit to activated receptors or adaptors at the cell membrane, there is growing evidence that p85α can play a negative role in regulation of insulin responses. Indeed, in cultured cells, overexpression of p85α or its splice variants inhibits insulin actions by decreasing the p85-p110 dimer bound to IRS proteins and by directly attenuating the catalytic activity of the p110 subunit (18, 19). This result can in part be explained by an increase in monomeric p85α competing with p85-p110 holoenzyme for binding to IRS proteins. A role for excess p85α in negative regulation of insulin signaling may be physiologically relevant because dexamethasone treatment of cultured muscle cells was shown to cause a 3-fold increase in p85α and a consequent inhibition of insulin-like growth factor-1-dependent recruitment of PI3K to IRS-1 (20). Consistent with the idea that p85α plays a negative role in insulin signaling in vivo, we found that heterozygous loss of all three splice variants of p85α in the mouse resulted in improved sensitivity to insulin (21). In addition, heterozygous loss of p85α provided protection of mice carrying heterozygous null mutations of insulin receptor (IR) and IRS-1 (22) from the development of overt diabetes (21). Moreover, homozygous deletion of p85α also resulted in hypoglycemia and hypoinsulinemia, although it is difficult to assess insulin sensitivity in these animals because they die within a few weeks of birth (23, 24). Improved insulin sensitivity also was detected in mice lacking full-length p85α but still expressing the alternative splice forms of this gene, p55α and p50α (25). These data indicate that although p85α and its splice variants are critical for PI3K-dependent signaling needed for normal development, insulin responses are improved when the levels of these proteins are reduced.
The improved insulin sensitivity in mice lacking p85α and its splice variants raises the possibility that p85β, which is up-regulated in tissues from p85α−/− mice (19), may be more efficient, or even essential, for transmitting insulin signals. Although p85β has structural homology with p85α, it is unclear whether there are functional differences between p85α and p85β in vivo, or how much each regulatory subunit contributes to Class IA PI3K-dependent signaling. To assess these issues in this study, we have generated mice lacking p85β and investigated glucose metabolism and insulin signaling in vivo. We find that disruption of the p85β gene results in a modest reduction of the total regulatory subunits of PI3K in muscle and liver. Interestingly, the p85β−/− mice, like the p85α+/− mice, have increased insulin sensitivity, suggesting that both of these regulatory subunits play negative roles in insulin signaling.
Materials and Methods
Generation of Mice Lacking the Pik3r2 Gene.
We generated mice lacking p85β (p85β−/− mice) with disruption of the first exon of the Pik3r2 gene by homologous recombination. The p85β−/− mice are viable and indistinguishable from their littermates, although they tend to be smaller than wild-type mice. The detailed strategy for the knockout will be described elsewhere. All animals were housed on a 12-h light/12-h dark cycle and were fed a standard rodent chow (Purina). All protocols for animal use and euthanasia were reviewed and approved by the Animal Care Use Committee of Harvard Medical School and were in accordance with National Institutes of Health guidelines.
Metabolic Studies.
All blood samples were taken from mouse tails; to measure insulin concentrations, we used heparinized microcapillaries to extract the plasma by spinning. For the glucose tolerance test, blood samples were obtained at 0, 15, 30, 60, and 120 min after i.p. injection of 2 g/kg dextrose. For the insulin tolerance test, blood samples were obtained at 0, 15, 30 and 60 min after i.p. injection of 0.75 units/kg regular human insulin (Lilly Research Laboratories, Indianapolis). For the glucose-stimulated insulin secretion test, blood samples were obtained at 0 and 2 min after i.p. injection of 3 g/kg dextrose. Blood glucose values were determined from whole venous blood taken by using an automatic glucose monitor (One Touch II, Lifescan, Mountain View, CA). Insulin levels were measured in plasma by ELISA by using mouse insulin as a standard (Crystal Chem, Chicago).
In Vivo Insulin Stimulation and Analysis of Insulin Signaling Proteins.
Two-month old male mice were starved overnight, anesthetized with pentobarbital, and injected with 5 units of regular human insulin (Lilly Research Laboratories) into the inferior vena cava. Liver and muscle were removed at 5 min and instantly frozen in liquid nitrogen. Immunoprecipitation and immunoblot analysis of insulin-signaling molecules were performed on tissue homogenates extracted with buffer A containing 25 mM Tris⋅HCl (pH 7.4), 10 mM Na3VO4, 100 mM NaF, 50 mM Na4P2O7, 10 mM EGTA, 10 mM EDTA, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 2 mM PMSF, and 1% (vol/vol) Nonidet P-40, as described (22).
Antibodies.
Rabbit polyclonal anti-p85α antibody (αp85pan) and mouse monoclonal anti-p85α antibody (αp85α) were purchased from Upstate Biotechnology, Lake Placid, NY. Rabbit polyclonal anti-p85β (αp85β) antibody was generated as described (23). Rabbit polyclonal anti-p110α antibody (αp110α) and anti-p110β antibody (αp110β) were purchased from Santa Cruz Biotechnology. Goat polyclonal anti-AKT antibody (αAKT) and rabbit polyclonal anti-GSK3α antibody were purchased from Santa Cruz Biotechnology, and rabbit polyclonal anti-phospho-AKT antibody (αp-AKT) recognizing phosphorylated Ser-473 of AKT1 and rabbit anti-phsopho-GSK3 antibody were purchased from Cell Signaling Technology, Beverly, MA. Rabbit polyclonal anti-IRS-1 antibody (αIRS-1) and anti-IRS-2 antibody (αIRS-2) were generated as described (22). Mouse monoclonal anti-phosphotyrosine antibody (4G10) was purchased from Upstate Biotechnology.
Affinity Purification of Regulatory Subunits of PI3K by Using a pYMXM Column.
One mg of 16-mer peptide (Lys-Lys-His-Thr-Asp-Asp-Gly-Tyr-Met-Pro-Met-Ser-Pro-Gly-Val-Ala) surrounding Tyr-608 of rat IRS-1 protein (Biomol, Plymouth Meeting, PA) was phosphorylated by the purified cytoplasmic domain of β-subunit of human IR (Biomol) by using ATPγS, as described (26). The phosphorylated peptide was immobilized on Affi-Gel 10 beads (Bio-Rad) and packed in a column. Lysates (10 mg) of each genotype of tissue lysates were applied to the column and washed extensively with buffer A with 500 mM NaCl. The proteins bound to pYMXM peptide were eluted with the elution buffer composed of 2.5 M glycine (pH 4.5) and 2 M NaCl and dialyzed with PBS containing 1% (vol/vol) glycerol. The purified proteins were subjected to SDS/PAGE and visualized by silver staining.
PI3K Assay.
The immunoprecipitates with αp85pan, αp85α, αp85β, 4G10, αIRS-1, or αIRS-2 were washed three times with buffer A and twice with PI3K reaction buffer (20 mM Tris⋅HCl, pH 7.4/100 mM NaCl/0.5 mM EGTA) and suspended in 50 μl of PI3K reaction buffer containing 0.1 mg/ml of phosphoinositide (PI; Avanti Polar Lipids). The reactions were performed, and the phosphorylated lipids were separated by TLC as described (19).
In Vitro Kinase Assays.
Tissue homogenates were subjected to immunoprecipitation with αAKT followed by AKT kinase assay with crosstide or immunoprecipitation with αGSK3α followed by GSK3 kinase assay, as described (19). Briefly, the immunoprecipitates were washed and resuspended in 50 mM Tris⋅HCl, pH 7.5/10 mM MgCl2/1 mM DTT to which 20 μM ATP, 5 μCi [γ-32P]ATP (1 Ci = 37 GBq), and 5 μg of crosstide for AKT assay or 1 μg of phospho-glycogen synthase peptide 2 (Upstate Biotechnology) for GSK3 assay had been added. After 20 min at 30°C, the reaction was stopped, and the aliquots were spotted on squares of P-81 paper, washed with 0.5% of phosphoric acid, and counted for radioactivity.
Results
Effects of Disrupting the Pik3r2 Gene on Insulin Sensitivity in Vivo.
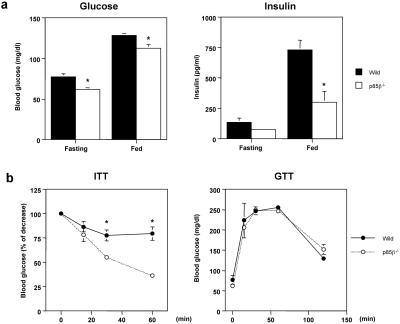
To assess the effects of deletion of p85β regulatory subunit on insulin signaling in vivo, we performed physiological studies on glucose metabolism by using 2-month-old wild-type and p85β−/− mice. The p85β−/− mice showed significantly lower glucose levels compared with the wild-type mice in both fasting and fed states (Fig. 1a). These decreased glucose levels were not associated with an increase in plasma insulin concentrations. Plasma insulin levels in the p85β−/− mice also were significantly lower in the fed state and tended to be lower in the fasting state than those in wild-type (Fig. 1a), suggesting that the p85β−/− mice have improved insulin sensitivity. Indeed, the glucose-lowering effect after i.p. insulin injection in the p85β−/− mice was significantly greater than that in the wild-type mice (Fig. 1b). On the other hand, there was no significant difference in blood glucose concentrations between the p85β−/− and wild-type mice during glucose tolerance tests (Fig. 1b). We also evaluated pancreatic β-cell function by measuring acute-phase glucose-stimulated insulin secretion that seems to be modulated by insulin signaling in β-cells (27) and found that there was no manifest change in the pattern of acute-phase insulin secretion by glucose stimulation (data not shown).
Figure 1.
Increased in vivo insulin sensitivity in the p85β−/− mice. (a) Lower glucose and insulin concentrations in p85β−/− mice. Blood-glucose concentrations in fasting and fed state (Left) as well as insulin concentrations (Right) were determined by tail bleeding in 2-month-old mice. Values of glucose and insulin represent the mean ± SEM of eight mice (*, P < 0.05 wild type vs. p85β−/−). (b) Changes in glucose levels in insulin tolerance test and glucose tolerance test. Insulin tolerance test was performed by giving 0.75 units/kg of body weight of insulin to 2-month-old mice (Left). Values are expressed as the % of the glucose levels at 0 min point and represent the mean ± SEM of eight mice (*, P < 0.05 wild type vs. p85β−/−). Glucose tolerance test was performed by giving 2 g/kg of body weight of dextrose to 2-month-old mice (Right). Values represent the mean ± SEM of eight mice.
Effects of Disrupting the Pik3r2 Gene on Insulin Signaling in Insulin-Sensitive Tissues.
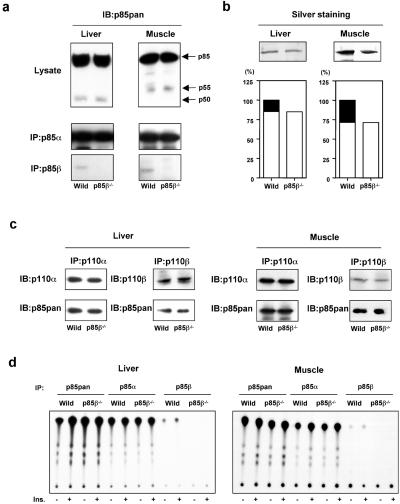
To assess the mechanism by which deletion of p85β increased insulin sensitivity in vivo, we investigated insulin-dependent signaling events involved in glucose metabolism in two major insulin-sensitive tissues, liver and skeletal muscle, of these mice. Total p85 proteins in liver and muscle of the p85β−/− mouse seemed to be only slightly decreased, as estimated by anti-p85pan antibody (Fig. 2a), which recognizes equally all isoforms of the regulatory subunit derived from the Pik3r1 gene and, to a lesser extent, also p85β and p55PIK. p85β protein in the immunoprecipitates using p85β-specific antibody was abolished in the tissues of the knockout mouse (Fig. 2a), whereas there were no changes in the levels of p85α or shorter isoforms of this regulatory subunit (p50α in liver and AS53 in muscle; Fig. 2a).
Figure 2.
Changes in Class IA PI3K complex by disruption of the Pik3r2 gene. (a) Expression of each regulatory subunit isoform. After the homogenization of the tissues, the lysates form liver (Left) or muscle (Right) were subjected to immunoblotting with anti-p85pan antibody (Top) and to immunoprecipitation with p85α specific antibody (Middle) or p85β specific antibody (Bottom) followed by immunoblotting with anti-p85pan antibody. (b) Affinity purification of the regulatory subunits by using a phospho-peptide column. The lysates from liver (Left) or muscle (Right) were applied to the column coupled with the phosphorylated p85-binding domain peptide of IRS-1 as described in Materials and Methods. The collected proteins were visualized by silver staining (Upper). In the graphs (Lower), each bar represents the level of eluted protein, and the solid area represents the theoretical level of p85β. The value is the mean of two independent experiments and is expressed as a ratio to the total p85 protein level in wild-type tissues. (c) Estimation of Class IA PI3K complex. The lysates from liver (Left) or muscle (Right) were subjected to immunoprecipitation with p110α or p110β specific antibody followed by immunoblotting with the same antibody (Upper) or anti-p85pan antibody (Lower). (d) PI3K activity associated with each regulatory subunit. Mice were starved and injected with insulin intravenously. The livers were removed 5 min after and muscles were removed 7.5 min after injection. The lysates from liver (Left) or muscle (Right) were subjected to immunoprecipitation with anti-p85pan, anti-p85α, or anti-p85β antibody followed by PI3K assay.
To evaluate more precisely the reduction of the p85 proteins by deletion of p85β, we purified the SH2 domain-containing proteins that can bind to the consensus-binding motif for PI3K (28) in liver and muscle of the wild-type and p85β−/− mouse using an affinity column coupled with a phospho-YMPM peptide corresponding to a region around Tyr-608 of IRS-1 (29). As shown in Fig. 2b, the total p85 proteins bound to pYMPM motif in the p85β−/− mouse were decreased by ≈20% in liver and ≈30% in muscle, respectively, indicating that these are the contribution of p85β to the total regulatory subunit pool.
We have reported previously that under normal conditions, the regulatory subunits of PI3K are more abundant than p110 catalytic subunits (19), and that more than 30% of p85 protein exists as a monomer (21, 26). We also demonstrated that a partial reduction of p85, such as what occurs with heterozygous disruption of p85α, does not affect the amount of p85-p110 dimer (21, 26). Consistent with these observations, despite the 20–30% decrease in total p85 protein, there was no significant difference in the amount of p110 proteins or p85 proteins bound to p110 subunits in liver and muscle between the wild-type and p85β−/− mouse (Fig. 2c). Likewise, PI3K activity associated with total p85 proteins and that associated with p85α were not changed by deletion of p85β, whereas PI3K activity associated with p85β was abolished in the p85β−/− mouse both in liver and muscle (Fig. 2d).
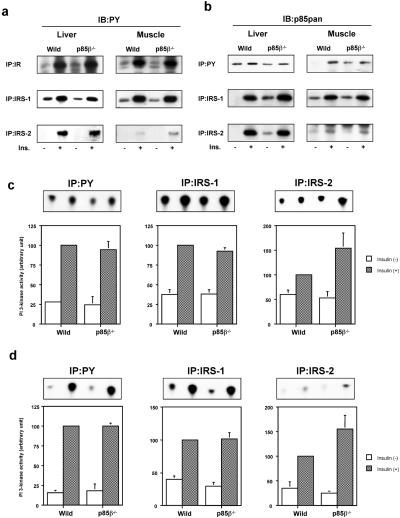
Activation of PI3K induced by insulin can be estimated by the amount of p85-p110 dimer bound to tyrosine-phosphorylated proteins. The levels of tyrosine phosphorylation of IR and IRS-1 in liver and muscle of the p85β−/− mouse were comparable to those in the wild-type mouse (Fig. 3a). p85 protein interacting with IRS-1 was not reduced by deletion of p85β compared with wild-type (Fig. 3b). Thus, PI3K activity associated with IRS-1 in the p85β−/− mouse was preserved both in liver and muscle (Fig. 3 c and d). Somewhat unexpectedly, phosphorylation of IRS-2 was up-regulated in the p85β−/− mouse, especially in muscle where the phosphorylation levels are very low (Fig. 3a). Although no obvious increase in p85 protein interacting with IRS-2 could be identified in the p85β−/− mouse (Fig. 3b), PI3K activity associated with IRS-2 tended to be increased, consistent with the increase in phosphorylation (Fig. 3 c and d). Finally, p85 proteins and PI3K activity associated with phosphotyrosine complexes in liver and muscle of the p85β−/− mouse were unchanged (Fig. 3 b–d), despite the 20–30% decrease in the regulatory subunit.
Figure 3.
Effects of disruption of the Pik3r2 gene on insulin-induced tyrosine phosphorylation and PI3K activation. (a) Insulin-induced tyrosine phosphorylation of IR and its substrates. Mice were starved and injected with insulin intravenously. The livers were removed 5 min after and muscles were removed 7.5 min after injection. The lysates from liver (Left) or muscle (Right) were subjected to immunoprecipitation with anti-IR (Top), anti-IRS-1 (Middle), or anti-IRS-2 (Bottom) antibody followed by immunoblotting with 4G10 (PY). (b) Insulin-induced interaction between tyrosine-phosphorylated proteins and the regulatory subunits. The lysates from liver (Left) or muscle (Right) were subjected to immunoprecipitation with 4G10 (Top), anti-IRS-1 (Middle), or anti-IRS-2 (Bottom) antibody followed by immunoblotting with anti-p85pan antibody. (c) PI3K activities associated with tyrosine-phosphorylated proteins in liver. (d) PI3K activities associated with tyrosine-phosphorylated proteins in muscle. The lysates were subjected to immunoprecipitation with 4G10 (Left), anti-IRS-1 (Center), or anti-IRS-2 (Right) followed by PI3K assay. Panels (Upper) show representative results; in graphs (Lower), each bar represents the mean ± SEM of the relative PI3K activity calculated from the results of three independent experiments.
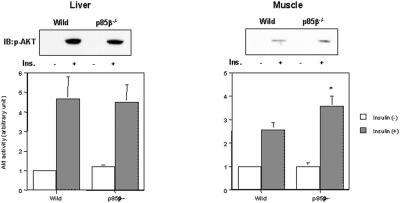
AKT is a key enzyme that lies downstream of PI3K and modulates multiple biological functions of insulin, including activation of glycogen synthesis and inhibition of hepatic-glucose production (9, 10, 30, 31), although its role in glucose transport is still controversial (32–34). In the p85β−/− mice, AKT activity in muscle was significantly up-regulated, whereas AKT activity was unchanged in liver (Fig. 4). This finding stands in contrast to the finding that in the p85α+/− mice that also exhibit improved insulin sensitivity, the increased Akt activity in liver seems to play an important role (21). In muscle, AKT has been shown to regulate glycogen synthase (GS) activity through deactivation of GSK3α and β (9, 10). Protein phosphatase 1 (PP1) controlled by the regulatory subunits (GM and PTG) also modulates GS activity, although the regulatory mechanism of PP1 is still unclear (35). Indeed, in muscle of the p85β−/− mice, phosphorylation of GSK3α, an immediately downstream effector of AKT, was up-regulated and the activity was down-regulated; GS activity in muscle also tended to be up-regulated (data not shown).
Figure 4.
Insulin-induced AKT activity is up-regulated in muscle of p85β knockout mouse. Mice were starved and injected with insulin intravenously. The livers were removed 5 min after and muscles were removed 7.5 min after injection. The lysates from liver (Left) or muscle (Right) were subjected to immunoblotting with anti-phospho-AKT (Ser-473) antibody or immunoprecipitation with anti-Akt antibody, followed by immune complex kinase assay. Panels (Upper) show representative results of immunoblot analysis; in graphs (Lower), each bar represents the mean ± SEM of the in vitro AKT kinase activity calculated from the results of three independent experiments (*, P < 0.05 wild-type vs. p85β−/−).
Discussion
PI3K activity is required for a wide variety of insulin responses, including stimulation of glucose transport and glycogen synthesis (3, 4, 36, 37). Upon insulin stimulation, an interaction between tyrosine-phosphorylated IRS proteins and Class IA PI3K initiates various biological responses (6, 7, 38). Class IA PI3Ks are composed of a p110 catalytic subunit and a regulatory subunit (usually known as p85 subunit), of which p85α and p85β proteins represent the large majority (5, 17).
We have reported previously that mice lacking p85α and its splice variants (p85α−/− mice) die within a few weeks after birth with abnormalities in multiple organs (24) and immunodeficiency caused by B cell dysfunction (23), presumably caused by a marked reduction of PI3K-dependent signaling, indicating the indispensable role of these gene products. By contrast, a specific knockout of only the full-length p85α isoform (leaving the p55α and p50α isoforms) in mice results in viable animals that exhibit increased insulin sensitivity (25). A similar increase in insulin sensitivity is noted in the p85α+/− mice (21), and even the p85α−/− mice exhibit hypoglycemia with significantly lower plasma insulin concentrations (24). Although Terauchi et al. (25) suggested that a molecular switch from p85α to p50α in the full-length p85α specific knockout resulted in improved insulin sensitivity, our results with the p85α+/− and p85α−/− mice suggested either that a reduction in total regulatory subunit eliminates an inhibitory effect on insulin signaling or that the p85β isoform, which mediates the major fraction of Class IA PI3K signaling in the absence of p85α, is a more efficient or even essential signal transmitter in insulin signaling than p85α.
The physiological role of p85β in vivo is poorly understood. Although p85α and p85β isoforms have similar functions in vitro (5), there are reports of proteins that preferentially bind p85α or p85β (39), suggesting the existence of isoform specific role. Thus, to assess two hypotheses raised by the Pik3r1 gene knockout and clarify the physiological role of p85β in insulin signaling, we have generated mice lacking p85β and investigated the effect on glucose metabolism and insulin sensitivity in vivo.
The p85β−/− mice exhibit significantly lower blood-glucose concentrations in both fasting and fed states with lower insulin concentrations compared with the wild-type mice. They also exhibit a significantly greater response to insulin during the insulin tolerance test, indicating that the p85β−/− mice have improved systemic insulin sensitivity, thereby maintaining lower glucose and insulin levels. These data suggest that a reduction of p85 by deleting the p85β subunit results in increased insulin sensitivity, as observed in the p85α+/− mice, and support the hypothesis that a reduction in p85 regulatory subunit (either p85α or p85β) can enhance insulin signaling. Although the p85β−/− mice do not show the improved glucose tolerance as was observed in the p85α+/− mice that exhibit significantly enhanced insulin signaling in liver, this result may be because the improvement of insulin sensitivity occurs primarily in muscle (as described below) and is associated with lower insulin levels.
At the molecular level, we show that p85α represents 70–80% of the total regulatory subunit proteins, and p85β seems to represent most of the rest in insulin-sensitive tissues. Thus, disruption of the Pik3r2 gene decreases 20–30% of the total regulatory subunit proteins but does not affect the amount of p85-p110 dimer. This finding is consistent with the findings in our previous study that under normal conditions, regulatory subunits are more abundant than p110 catalytic subunits, and heterozygous disruption of the Pik3r1 gene with an ≈40% reduction in the total regulatory subunits does not manifestly decrease the p85-p110 dimer (19, 21, 26). Hence, both of these modest reductions of p85 protein decrease p85 monomer preferentially and result in only a very small decrease in p85-p110 dimer (21, 26). Parallel with the level of the p85-p110 dimer, total p85 associated-PI3K activity is unchanged in the p85β−/− mice. PI3K signaling activated by insulin reflects the amount of p85-p110 dimer interacting with tyrosine-phosphorylated IRS proteins. PI3K activity and p85 protein associated with phosphotyrosine complex are preserved in the p85β−/− mice, suggesting that the 20–30% reduction in p85 protein by disruption of the Pik3r2 gene mainly decreases p85 monomer and maintains the amount of IRS/p85/p110 complex. In addition, as we showed previously, the decrease in p85 proteins can be associated with a reduction in the degradation of phosphatidylinositol (3,4,5)-triphosphate PIP3), thereby enhancing PI3K signaling (26). Indeed, insulin-induced AKT activation is up-regulated in muscle, whereas the PI3K activity associated with phosphotyrosine complex is unchanged. Although it is unclear why AKT activity is enhanced only in muscle but not in liver, this fact may be associated with the fact that the ratio of p85β to p85α in muscle is higher than that in liver.
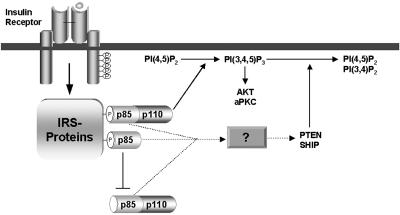
Fig. 5 illustrates a hypothesis explaining how a modest reduction in p85 regulatory subunit improves PI3K-dependent signaling. In this model, p85-p110 dimer transmits the signal to increase PIP3, whereas p85 monomer inhibits PIP3 production by competing with p85-p110 dimer for binding to IRS proteins. In addition, p85 protein might stimulate degradation of PIP3 by lipid phosphatases through an unknown mechanism, independent of PI3K activity. A moderate decrease in the amount of p85 protein (either p85α or p85β) may reduce these inhibitory effects and leaves the amount of p85-p110 dimer bound to tyrosine-phosphorylated IRS proteins almost unchanged. As a result, a reduction of p85 may decrease its negative effects and improve PI3K-dependent signaling. The final effects of the reduction in p85 would be affected by the balance between p85, p110, and phosphorylated IRS proteins in each tissue by the distribution of the deleted isoform and by the intensity of insulin stimulation.
Figure 5.
A hypothetical mechanism by which the p85 regulatory subunits attenuate insulin signaling. The p85 monomer interferes with p85-p110 dimer for binding to phosphorylated IRS proteins. In addition, p85 proteins promote degradation of PIP3, presumably through the activation of lipid phosphatases in a PI3K-independent fashion. Solid lines represent known signaling pathways, and broken lines represent putative pathways.
As a downstream effector of PIP3, AKT regulates GS activity in muscle through the inhibition of GSK3 activity (9, 10). In the p85β−/− mice, GSK3 activity seems to be decreased. Because decreased GS phosphorylation regulated by GSK3 and protein phosphatase 1 is associated with increased GS activity (35), the decrease in activity of GSK3 and/or an increase in activity of PPI in the p85β−/− mice would result in increased GS activity, as compared with the wild-type mice. As a consequence, GS activity in muscle of the p85β−/− mice tends to be increased by bolus insulin injection. This activity may contribute to the increased systemic insulin sensitivity in p85β−/− mice. Consistent with this hypothesis, cultured brown adipocytes derived from the p85β−/− mice show significantly increased insulin-induced GS activity (K.U. and C.R.K., unpublished data).
The other factor that may affect insulin sensitivity is up-regulation of tyrosine phosphorylation of IRS-2. This up-regulation occurs to a different degree in various tissues and is more distinct in muscle than in liver in the present study, whereas this result is even more prominent in the cultured brown adipocytes (K.U. and C.R.K., unpublished data). Although the mechanism and the physiological relevance of this finding need further study, this result is an interesting finding that does not occur with the Pik3r1gene knockout mice (24) or the full-length p85α knockout mice (25), suggesting that p85β specifically mediates the signal to activate a tyrosine phosphatase for IRS-2 or a Ser/Thr kinase for IRS-2 that might decrease tyrosine phosphorylation of IRS-2.
In summary, a reduction of 20–30% in total p85 regulatory subunits produced by disrupting the Pik3r2 gene improves systemic insulin sensitivity through enhanced signaling downstream of PI3K in muscle. This result is due to the decrease in p85 monomer, thereby increasing the stoichiometry of p85/p110/IRS complex, and also is presumably due to amelioration of the inhibitory effect of the p85 regulatory subunit on restoration of lipid products by PI3K. Furthermore, the absence of p85β subunit up-regulates tyrosine-phosphorylation of IRS-2, presumably leading to the enhancement of some part of insulin signaling. These data suggest a potential therapy for insulin resistance by a selective reduction of p85β protein expression.
Acknowledgments
We thank D. Fruman for helpful suggestions and comments. This work was supported by National Institutes of Health Grants DK33201 and DK55545 (to C.R.K.) and GM41890 (to L.C.C.) and Joslin DERC Grant DK34834 (to C.R.K.). S.M.B. was supported by a scholarship from Boehringer Ingelheim Funds.
Abbreviations
- PI
phosphoinositide
- PI3K
phosphoinositide 3-kinase
- IR
insulin receptor
- IRS
insulin receptor substrate
- GS
glycogen synthase
- GSK3
glycogen synthase kinase 3
- PIP3
phosphatidylinositol triphosphate
References
- 1.Ruderman N B, Kapeller R, White M F, Cantley L C. Proc Natl Acad Sci USA. 1990;87:1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endemann G, Yonezawa K, Roth R A. J Biol Chem. 1990;265:396–400. [PubMed] [Google Scholar]
- 3.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 5.Shepherd P R, Withers D J, Siddle K. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backer J M, Myers M G, Jr, Shoelson S E, Chin D J, Sun X J, Miralpeix M, Hu P, Margolis B, Skolnik E Y, Schlessinger J, White M F. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers M G, Jr, Backer J M, Sun X J, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White M F. Proc Natl Acad Sci USA. 1992;89:10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr G A, Backer J M. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 10.Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 11.Otsu M, Hiles I, Gout I, Fry M J, Ruiz-Larrea F, Panayotou G, Thompson A, Dhand R, Hsuan J, Totty N. Cell. 1991;65:91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- 12.Fruman D A, Cantley L C, Carpenter C L. Genomics. 1996;37:113–121. doi: 10.1006/geno.1996.0527. [DOI] [PubMed] [Google Scholar]
- 13.Pons S, Asano T, Glasheen E, Miralpeix M, Zhang Y, Fisher T L, Myers M G, Jr, Sun X J, White M F. Mol Cell Biol. 1995;15:4453–4465. doi: 10.1128/mcb.15.8.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonetti D A, Algenstaedt P, Kahn C R. Mol Cell Biol. 1996;16:2195–2203. doi: 10.1128/mcb.16.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inukai K, Anai M, Van Breda E, Hosaka T, Katagiri H, Funaki M, Fukushima Y, Ogihara T, Yazaki Y, Kikuchi M, et al. J Biol Chem. 1996;271:5317–5320. doi: 10.1074/jbc.271.10.5317. [DOI] [PubMed] [Google Scholar]
- 16.Inukai K, Funaki M, Ogihara T, Katagiri H, Kanda A, Anai M, Fukushima Y, Hosaka T, Suzuki M, Shin B C, et al. J Biol Chem. 1997;272:7873–7882. doi: 10.1074/jbc.272.12.7873. [DOI] [PubMed] [Google Scholar]
- 17.Fruman D A, Meyers R E, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 18.Rameh L E, Chen C S, Cantley L C. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 19.Ueki K, Algenstaedt P, Mauvais-Jarvis F, Kahn C R. Mol Cell Biol. 2000;20:8035–8046. doi: 10.1128/mcb.20.21.8035-8046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgino F, Pedrini M T, Matera L, Smith R J. J Biol Chem. 1997;272:7455–7463. doi: 10.1074/jbc.272.11.7455. [DOI] [PubMed] [Google Scholar]
- 21.Mauvais-Jarvis, F., Ueki, K., Fruman, D. A., Hirshman, M. F., Sakamoto, K., Goodyear, L. J., Iannacone, M., Accili, D., Cantley, L. C. & Kahn, C. R. (2002) J. Clin. Invest., in press. [DOI] [PMC free article] [PubMed]
- 22.Bruning J C, Winnay J, Bonner-Weir S, Taylor S I, Accili D, Kahn C R. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 23.Fruman D A, Snapper S B, Yballe C M, Davidson L, Yu J Y, Alt F W, Cantley L C. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 24.Fruman D A, Mauvais-Jarvis F, Pollard D A, Yballe C M, Brazil D, Bronson R T, Kahn C R, Cantley L C. Nat Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- 25.Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, et al. Nat Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- 26.Ueki, K., Fruman, D. A., Brachmann, S. M., Tseng, Y., Cantley, L. C. & Kahn, C. R. (2002) Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 27.Kulkarni R N, Bruning J C, Winnay J N, Postic C, Magnuson M A, Kahn C R. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 28.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, et al. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 29.Sun X J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Nature (London) 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 30.Coffer P J, Jin J, Woodgett J R. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho H, Mu J, Kim J K, Thorvaldsen J L, Chu Q, Crenshaw E B, 3rd, Kaestner K H, Bartolomei M S, Shulman G I, Birnbaum M J. Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 32.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newgard C B, Brady M J, O'Doherty R M, Saltiel A R. Diabetes. 2000;49:1967–1977. doi: 10.2337/diabetes.49.12.1967. [DOI] [PubMed] [Google Scholar]
- 36.Sakaue H, Hara K, Noguchi T, Matozaki T, Kotani K, Ogawa W, Yonezawa K, Waterfield M D, Kasuga M. J Biol Chem. 1995;270:11304–11309. doi: 10.1074/jbc.270.19.11304. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto-Honda R, Tobe K, Kaburagi Y, Ueki K, Asai S, Yachi M, Shirouzu M, Yodoi J, Akanuma Y, Yokoyama S, et al. J Biol Chem. 1995;270:2729–2734. doi: 10.1074/jbc.270.6.2729. [DOI] [PubMed] [Google Scholar]
- 38.Hara K, Yonezawa K, Sakaue H, Ando A, Kotani K, Kitamura T, Kitamura Y, Ueda H, Stephens L, Jackson T R, et al. Proc Natl Acad Sci USA. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartley D, Meisner H, Corvera S. J Biol Chem. 1995;270:18260–18263. doi: 10.1074/jbc.270.31.18260. [DOI] [PubMed] [Google Scholar]