Abstract
We have used recombinant wild-type human von Willebrand factor (VWF) and deletion mutants lacking the A1 and A3 domains, as well as specific function-blocking monoclonal antibodies, to demonstrate a functionally relevant self-association at the interface of soluble and surface-bound VWF. Platelets perfused at the wall shear rate of 1,500 s−1 over immobilized VWF lacking A1 domain function failed to become tethered to the surface when they were in a plasma-free suspension with erythrocytes, but adhered promptly if soluble VWF with functional A1 domain was added to the cells. The same results were observed when VWF was immobilized onto collagen through its A3 domain and soluble VWF with deleted A3 domain was added to the cells. Thus, VWF bound to glass or collagen sustains a process of homotypic self-association with soluble VWF multimers that, as a result, can mediate platelet adhesion. The latter finding demonstrates that direct immobilization on a substrate is not a strict requirement for VWF binding to platelet glycoprotein Ibα. The dynamic and reversible interaction of surface-bound and soluble VWF appears to be specifically homotypic, because immobilized BSA, human fibrinogen, and fibronectin cannot substitute for VWF in the process. Our findings highlight a newly recognized role of circulating VWF in the initiation of platelet adhesion. The self-assembly of VWF multimers on an injured vascular surface may provide a relevant contribution to the arrest of flowing platelets opposing hemodynamic forces, thus facilitating subsequent thrombus growth.
Platelet adhesion and aggregation at sites of vascular injury exposed to rapid blood flow, such as in arterioles of the normal circulation (1) or larger arteries with pathological lumen restrictions (2), require von Willebrand factor (VWF) (3–6). Under these hemodynamic conditions, immobilized VWF, either as an intrinsic subendothelial matrix component (7, 8) or bound from plasma onto collagens (6, 9–11) and other extracellular substrates (12), mediates platelet deposition by interacting with the membrane glycoprotein (GP) Ibα receptor. Platelets become activated after the initial tethering, which allows their irreversible adhesion to the surface and the binding of plasma VWF and fibrinogen to the integrin αIIbβ3. These ligands, immobilized on the membrane of adherent platelets, provide the substrate for the recruitment of additional platelets, which in turn become activated and bind the same plasma ligands repeating a cycle that supports continuing platelet-to-platelet cohesion, i.e., aggregation, into the thrombus mass (4).
The domains of VWF involved in matrix and platelet interactions have been identified. Domain A3 plays a predominant role in VWF binding to collagen (13), and domain A1 interacts with platelet GP Ibα (14, 15), a component of the GP Ib–IX–V receptor complex (16). Finally, the C1 domain sequence Arg-Gly-Asp-Ser (RGDS), located near the C terminus of the VWF subunit (17, 18), by binding to activated αIIbβ3 (19), stabilizes the initial platelet adhesion mediated by the A1 domain, which can only support rolling (3). Current concepts suggest that, at elevated shear rates, platelets at the base of a thrombus are initially recruited by VWF bound to the vascular wall. Thus, a functional defect in the A1 domain of immobilized VWF should impair platelet adhesion and, therefore, affect subsequent events of thrombus growth. We have verified this hypothesis by using recombinant multimeric VWF with specific deletions of either domain A1 or A3, or with a single amino acid substitution to replace the integrin recognition motif, RGDS, with the nonfunctional sequence, RGGS. Unexpectedly, we observed considerable platelet adhesion even when blood was exposed to surface-immobilized VWF that lacked the A1 domain. The studies performed to explain this observation, which was in apparent contradiction to accepted knowledge, demonstrated that circulating VWF can interact with VWF directly bound to a surface and provide the A1 domain function necessary for the tethering of fast flowing platelets. Thus, the adhesive function of plasma VWF may be supported by a process of dynamically reversible self-association at the boundary between circulating blood and a reactive surface presenting immobilized VWF.
Materials and Methods
Preparation of Recombinant VWF Mutants.
Recombinant wild-type (WT) human VWF, RGGS-VWF, and deletion mutants that lack the A1 domain (residues 478–716; ΔA1) or the A3 domain (residues 910-1113; ΔA3) were prepared as described by using furin-transfected baby hamster kidney cells (9, 20).
Blood Donors.
Blood was collected through a 19-gauge needle from the antecubital vein of healthy, medication-free adult donors into syringes containing d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride (PPACK; 80 μM final concentration) to inhibit thrombin activity (3).
Preparation of Plasma-Free Blood.
Blood cells were washed free of plasma constituents by using a modification of a method previously described in detail (21). Six parts of blood containing PPACK were mixed with one part of acid/citrate/dextrose (pH 4.5) to adjust the pH of the mixture to 6.5, and supplemented with 5 units/ml of the ADP scavenger apyrase (grade III, Sigma). Prostaglandin (PG) E1 (final concentration, 10 μM; Sigma) and EDTA (final concentration, 5 mM) were added to block platelet activation and inhibit integrin receptor function, respectively, and the blood was centrifuged at 2,100 × g for 13 min at 23°C. The supernatant plasma was discarded, and the blood cells were resuspended to the original volume with Hepes-Tyrode buffer (pH 6.5; ref. 21) containing apyrase (2.5 units/ml) and EDTA (5 mM). The suspension was again centrifuged, and the cell-free supernatant was removed. This process was repeated twice more, decreasing the amount of apyrase to 1.25 units/ml in the third wash and nil in the final cycle. Blood cells were finally resuspended in Hepes-Tyrode buffer (pH 7.4), containing 5 mM EDTA, 10 μM PGE1, and 50 mg/ml BSA (Sigma). The final cell counts in plasma-free blood were within 5% of the original values in whole blood.
Functional Inhibition of VWF Domains.
All monoclonal antibodies used in these experiments were obtained and characterized as previously described. They were purified by using protein A (Sigma) chromatography, according to published procedures (22). NMC-4 binds to the A1 domain of VWF (23) and blocks the interaction of VWF with platelet GP Ibα (24). The epitope recognized by this antibody has been defined at the atomic level (25). MR-5 binds to a specific sequence (residues 948–998) in the A3 domain of VWF (26) and prevents the interaction between VWF and collagen under a variety of experimental conditions (27). Monovalent Fab fragments of antibodies NMC-4 and MR-5 were prepared according to a published method (28).
Flow Studies and Videomicroscopy.
Recombinant WT-VWF or VWF deletion mutants were immobilized onto glass coverslips by using coating solutions with a concentration of 40 μg/ml. Platelet interaction with these substrates was studied in real time by using a flow chamber mounted on the stage of a Zeiss Axiovert 135 M/LSM 410 invert microscope as described (3, 6, 29). Whole blood or plasma-free blood cells were perfused over the substrate at a wall shear rate of 1,500 s−1. Platelets were visualized with the fluorescent dye mepacrine (quinacrine dihydrochloride; Sigma) at a final concentration of 10 μM, and experiments were recorded in real time on videotapes at a rate of 30 frames per second. Images were captured from tapes at the times indicated and analyzed with the software package METAMORPH (version 4.1, Universal Imaging, Media, PA). The number of platelets interacting with the surface was measured after fixing the contrast and brightness of the images to defined values and applying a constant threshold to distinguish signal from noise.
For flow experiments with immobilized collagen, acid-soluble type I collagen from human placenta (Sigma) was dissolved in 0.1 M acetic acid and coated on glass coverslips at a final concentration of 200 μg/ml in an airtight container for 60 min at 22°C to 25°C. Under these conditions, the surface was fully saturated with collagen (29). Collagen-coated coverslips were rinsed four times with PBS (pH 7.4) before being assembled in the flow chamber. In some experiments, the immobilized collagen was rinsed and incubated with ΔA1-VWF or WT-VWF (both at a final concentration of 20 μg/ml) for 30 min before perfusion.
Results
Real-Time Analysis of Platelet Interaction with Surface-Bound Recombinant VWF Mutants Under Flow Conditions.
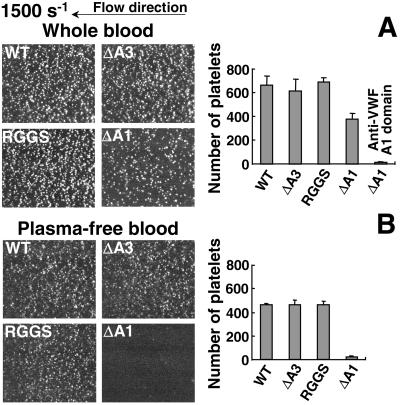
Platelets adhered to a glass surface coated with recombinant WT-VWF when blood was perfused at the wall shear rate of 1,500 s−1 (Fig. 1A). In agreement with results previously obtained with VWF purified from plasma (3), adhesion to recombinant WT-VWF was characterized by translocation of platelets in the direction of flow, which was blocked by a function inhibiting monoclonal antibody directed against platelet GP Ibα (data not shown). Platelet adhesion to immobilized ΔA3-VWF or RGGS-VWF was quantitatively similar to that seen with WT-VWF; surprisingly, however, numerous platelets were also seen rolling on ΔA1-VWF (Fig. 1A), although their number was significantly lower (P < 0.05) than on the other surfaces tested. The latter interaction was unexpected, because the A1 domain is generally viewed as the exclusive site capable of mediating platelet tethering through GP Ibα under high-flow conditions. In contrast, the normal function displayed by ΔA3-VWF and RGGS-VWF was anticipated, because both of these mutants contain a normal A1 domain. Because the blood used for these experiments contained native VWF, we hypothesized that the A1 domain of plasma VWF could be responsible for the observed platelet adhesion. In fact, addition to the blood of a monoclonal antibody that blocks A1 domain binding to GP Ibα completely inhibited platelet adhesion to ΔA1-VWF (Fig. 1A), confirming that plasma VWF may be capable of mediating platelet interaction with the latter substrate.
Figure 1.
Interaction of flowing platelets with immobilized recombinant VWF. (A) Whole blood, containing 80 μM PPACK as an anticoagulant, 10 μM PGE1 to block platelet activation, 5 mM EDTA to prevent the function of integrin receptors, and 10 μM mepacrine to render platelets fluorescent, was perfused at the wall shear rate of 1,500 s−1 over recombinant WT-VWF and VWF mutants, as indicated, immobilized on a glass surface. In one set of experiments, the blood also contained 40 μg/ml NMC4 Fab to block the GP Ibα-binding A1 domain of plasma VWF. (B) Plasma-free washed blood cells—containing PGE1, EDTA, and mepacrine, but not PPACK—were perfused instead of whole blood. (Left) Images represent an area of 65,536 μm2, and are single frames from real time recordings taken after 2 min of perfusion on the different surfaces. All interacting platelets exhibited translocation in the direction of flow. The images are representative of four separate experiments for each condition studied, by using blood from different donors. (Right) Bar graphs show the number of platelets interacting with each surface, expressed as mean ± SEM of the four separate experiments.
To demonstrate that the A1 domain of soluble VWF in blood can compensate for the functional defect of immobilized ΔA1-VWF, plasma was replaced with a buffer after centrifugation of the blood. When the plasma-free cell suspension, with platelet and red cell counts comparable to whole blood, was perfused over a ΔA1-VWF coated surface, virtually no platelet adhesion was observed (Fig. 1B). In contrast, platelet rolling was readily observed on WT-VWF, ΔA3-VWF, and RGGS-VWF even when blood cells were perfused without plasma proteins, although the number of interacting platelets on the surface was ≈30% lower than that seen with normal whole blood (Fig. 1). These results are in agreement with the concept that soluble VWF present in the perfusing blood participates with surface-bound VWF in the initiation of platelet adhesion.
Functional Association of Soluble and Immobilized VWF.
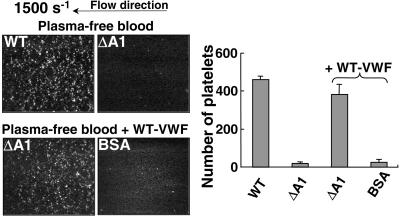
To evaluate the functional role of soluble VWF in the absence of other plasma proteins, plasma-free blood cells were supplemented with recombinant VWF and perfused over ΔA1-VWF bound to glass. Platelet adhesion to the surface coated with ΔA1-VWF was fully restored when plasma-free blood contained WT-VWF (20 μg/ml), demonstrating that soluble VWF is sufficient to support this process without the requirement for other plasma components (Fig. 2). Of note, the effect of soluble VWF in supporting platelet adhesion was seen on surfaces coated with ΔA1-VWF, but not with BSA (Fig. 2), fibrinogen, or fibronectin (3); moreover, no platelet interaction was ever observed when blood was perfused on uncoated glass surfaces (data not shown).
Figure 2.
Soluble VWF interacts with immobilized VWF and mediates platelet adhesion under flow. Plasma-free washed blood cells (see Fig. 1B), without or with the addition of WT-VWF (20 μg/ml), as indicated, were perfused at the wall shear rate of 1,500 s−1 over immobilized WT-VWF, ΔA1- VWF, or BSA as a control. The results of four separate experiments are presented as described in the legend to Fig. 1.
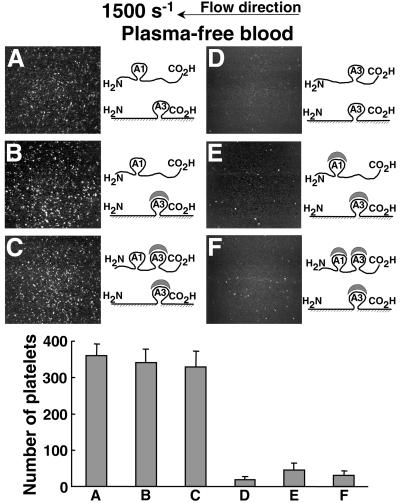
The collagen-binding A3 domain of VWF (13, 30) was not involved in the functional association of soluble and immobilized VWF. In fact, when plasma-free blood cells supplemented with soluble ΔA3-VWF were perfused over surface-bound ΔA1-VWF, platelet adhesion was similar to that observed when soluble WT-VWF was used (Fig. 3A). The same result was observed regardless of whether the immobilized VWF A3 domain was blocked with a function-inhibiting antibody (Fig. 3B), or the A3 domains in both soluble WT-VWF and immobilized ΔA1-VWF were concurrently blocked (Fig. 3C). In contrast, when soluble ΔA1-VWF was added to plasma-free blood cells and perfused over immobilized ΔA1-VWF, no rolling platelets were seen (Fig. 3D). In like manner, when the A1 domain of added soluble ΔA3-VWF was blocked (Fig. 3E), or the A1 and A3 domains of WT-VWF were concurrently blocked (Fig. 3F), perfusion of plasma-free blood cells over immobilized ΔA1-VWF with antibody-blocked A3 domain resulted in extremely rare and short-lasting platelet contacts with the surface. Whether the A1 domain is required for the interaction of soluble VWF with immobilized VWF cannot be discerned from these experiments, because its deletion or functional inhibition also prevents binding to platelet GP Ibα. It is, however, apparent that the A3 domain of both soluble and immobilized VWF has no functional effect in these assays.
Figure 3.
Role of soluble VWF A1 domain in mediating platelet interaction with surface-bound VWF. (Upper) Plasma-free washed blood cells (see Fig. 1B), with the additions indicated below, were perfused over immobilized ΔA1-VWF treated as indicated. (A) ΔA3-VWF (20 μg/ml) was added to the cell suspension. (B) Immobilized ΔA1-VWF was treated before perfusion with 40 μg/ml MR5 Fab to block the collagen-binding A3 domain, and ΔA3-VWF was added to the cells as in A. (C) Immobilized ΔA1-VWF was treated as in B, and WT-VWF (20 μg/ml) mixed with MR5 Fab was added to the cells. (D) ΔA1-VWF (20 μg/ml) was added to the cells perfused over immobilized ΔA1-VWF. (E) Immobilized ΔA1-VWF was treated as in B, and ΔA3-VWF was added to the cells as in A but with the addition of 40 μg/ml NMC4 Fab to block the GP Ibα-binding A1 domain. (F) Immobilized ΔA1-VWF was treated as in B, and the cells contained WT-VWF as in C but with the addition of both NMC4 Fab and MR5 Fab. The scheme at the right of each image depicts the soluble and immobilized VWF used in the experiments with respect to A1 and A3 domain presence and function. The images are single frames from a real-time recording representing an area of 65,536 μm2. (Lower) Bar graph showing the number of surface-interacting platelets after 2 min of perfusion under the corresponding conditions described above, expressed as mean ± SEM of two separate experiments.
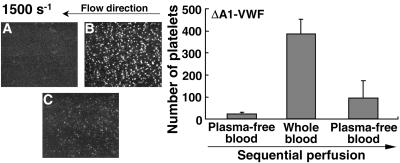
To determine the extent to which the interaction of soluble VWF with immobilized VWF is a reversible process, we examined the functional consequence of exposing immobilized ΔA1-VWF sequentially to plasma-free blood, followed without interruption by whole blood, followed finally by continued perfusion of plasma-free blood (Fig. 4). In the first phase, minimal platelet–surface contacts were seen with plasma-free blood. In contrast, numerous platelets were rapidly seen rolling on the surface when plasma-free blood was replaced by whole blood; the situation quickly reverted to occasional and transient platelet-surface contacts when whole blood was replaced by plasma-free blood (Fig. 4). Thus, soluble VWF in the perfusing blood can rapidly interact with immobilized VWF and mediate the surface capture of platelets, but this association is also rapidly reversible.
Figure 4.
Reversible interaction of plasma VWF with immobilized VWF. (A) Plasma-free washed blood cells (see Fig. 1B) were perfused over ΔA1-VWF immobilized on glass, and the number of platelets tethered to the surface was measured after 2 min. (B) Perfusion was then continued without interruption with whole blood for a further 2 min, and the number of platelets tethered to the surface was measured. (C) Whole blood was finally replaced with plasma-free washed blood cells without interrupting the perfusion, and the number of platelets tethered to the surface was measured after a further 2 min. Each single frame from a real-time recording represents an area of 65,536 μm2. (Right) Bar graph showing the number of surface interacting platelets measured at the times indicated above, expressed as mean ± SEM of three separate experiments.
Dual Role of Plasma VWF in Mediating Platelet Adhesion to Collagen Under Flow.
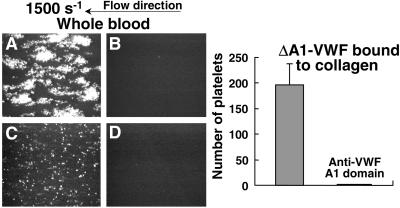
When type I collagen fibrils were exposed to flowing blood at 1,500 s−1, stable platelet attachment and thrombus formation ensued within 2 min (Fig. 5A). This process was inhibited when the blood was treated with PGE1 to block platelet activation, EDTA to block platelet α2β1 and αIIbβ3 function, and the anti-VWF A3 domain monoclonal antibody (MR5) to prevent plasma VWF binding to collagen (Fig. 5B). When blood is perfused at high shear rates, in fact, MR5 alone is sufficient to inhibit not only platelet adhesion but also subsequent thrombus formation (6). Nevertheless, when collagen was precoated with ΔA1-VWF and exposed to flowing blood under the same conditions as shown in Fig. 5B, adhesion of single rolling platelets occurred (Fig. 5C), but was abolished when the GP Ibα binding function of plasma VWF was blocked with the monoclonal antibody NMC4 (Fig. 5D). Thus, plasma-derived VWF has a dual role in supporting platelet adhesion under flow. One role, already known, depends on direct binding to collagen through the A3 domain; the other, demonstrated here, is independent of A3 domain function and depends on a direct interaction with VWF already immobilized on a surface, including collagen fibrils. Remarkably, this latter function of plasma VWF can overcome a functional defect of the A1 domain in the VWF directly bound to collagen (Fig. 5).
Figure 5.
Plasma VWF A1 domain mediates adhesion of flowing platelets to ΔA1- VWF bound to collagen. Whole blood containing PPACK and with the additions indicated below, was perfused at the wall shear rate of 1,500 s−1 over immobilized type I collagen fibrils (A and B), or the same collagen incubated for 30 min with 20 μg/ml of ΔA1-VWF before perfusion (C and D). Blood with PPACK only (A); blood with PPACK, 10 μM PGE1, 5 mM EDTA, and 40 μg/ml MR5 Fab to block the collagen-binding A3 domain of plasma VWF (B and C); blood treated as in B and C, but with the further addition of 40 μg/ml of NMC4 Fab to block the GP Ibα-binding A1 domain of plasma VWF (D). The images, each representing an area of 65,536 μm2 after 2 min of perfusion, are single frames from a real-time recording representative of four separate experiments with blood from different donors. All interacting single platelets seen in C exhibited translocation in the direction of flow; thrombi could not form as in A because the perfused blood contained PGE1 and EDTA (see above). (Right) Bar graph showing the number of platelets interacting with the surface under conditions C and D described above, expressed as mean ± SEM of the four separate experiments.
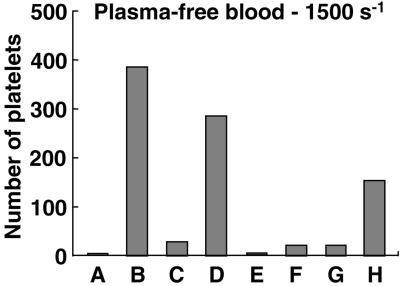
To validate the latter conclusion, which expands our understanding of VWF function at the vessel wall, we performed additional experiments with function-blocking antibodies and recombinant VWF. Platelets in a plasma protein-free blood cell suspension containing EDTA and PGE1 adhered to collagen type I fibrils only if the latter had been precoated with WT-VWF, but not directly, if the perfusion was conducted at a wall shear rate of 1,500 s−1 (Fig. 6 columns A and B). This result is in agreement with the notion that platelet tethering to a collagen-containing surface requires previous immobilization of VWF when the shear rate is above a critical threshold (6). Accordingly, platelet adhesion was markedly reduced when an inhibitory antibody blocked the A1 domain of immobilized VWF (Fig. 6 column C). The inhibition was reversed, however, if the perfused cell suspension contained soluble WT-VWF with antibody-blocked A3 domain, which is not capable of direct binding to collagen (Fig. 6 column D), as demonstrated when the surface was coated only with collagen fibrils (Fig. 6 column E). Such a finding demonstrates that soluble VWF with impaired collagen binding function can still interact with immobilized VWF, itself bound to collagen, and contribute A1 domain function for platelet adhesion. Experimental data with recombinant VWF were in complete agreement with this conclusion. Indeed, collagen fibrils coated with ΔA1-VWF did not support adhesion of plasma-free platelets (Fig. 6 column F). The defect was not corrected by soluble ΔA1-VWF (Fig. 6 column G), but disappeared with addition to the perfusing fluid of ΔA3-VWF (Fig. 6 column H), even though the latter cannot bind to collagen.
Figure 6.
Role of soluble VWF A1 domain in mediating platelet interaction with collagen-bound ΔA1-VWF. The bar graph shows the number of surface-interacting platelets after perfusion of plasma-free blood cells containing PGE1 and EDTA (see Fig. 1B) for 2 min at the wall shear rate of 1,500 s−1, under the conditions indicated below. Column A, surface coated with collagen type I fibrils; perfusion with plasma-free blood cells. Column B, surface coated with collagen incubated for 30 min with 20 μg/ml of WT-VWF; perfusion as in A. Column C, surface coated with collagen and incubated for 30 min with 20 μg/ml of WT-VWF mixed with 40 μg/ml of NMC4 Fab to block the GP Ibα-binding A1 domain; perfusion as in A. Column D, surface as in C, perfused with washed blood cells containing 20 μg/ml WT-VWF and 40 μg/ml MR5 Fab to block the collagen-binding A3 domain. Column E, surface coated with collagen, perfused with washed blood cells containing 20 μg/ml WT-VWF and 40 μg/ml MR5 Fab to block the collagen-binding A3 domain. Column F, surface coated with collagen and incubated for 30 min with 20 μg/ml of ΔA1-VWF; perfusion with plasma-free blood cells. Column G, surface as in F, perfused with plasma-free blood cells containing 20 μg/ml ΔA1-VWF. Column H, surface as in F, perfused with plasma-free blood cells containing 20 μg/ml ΔA3-VWF.
Discussion
The results of these studies challenge the current understanding of a mechanism that initiates platelet thrombus formation on the vessel wall, and demonstrate the occurrence of homotypic interactions between VWF molecules bound to a surface and those in the plasma milieu. To date, it was generally thought that platelet adhesion under high wall shear stress is mediated by GP Ibα binding to the A1 domain of VWF presented to flowing blood immobilized onto components of extracellular matrices, notably collagen. The findings reported here, however, indicate that platelets not only adhere to VWF directly bound to a surface, but also interact to a considerable degree with a layer of VWF molecules that are in a state of reversible association with an immobilized substrate. Thus, a direct linkage between VWF and a surface such as collagen is not an absolute requisite for A1 domain expression of GP Ibα binding function. Conversely, it is also apparent that VWF bound to collagen, and possibly any other insoluble substrate as exemplified here by glass, provides the surface onto which VWF in the fluid phase may become transiently adsorbed displaying the A1 domain that mediates platelet adhesion. It has been demonstrated by atomic force microscopy that VWF multimers unfold into extended linear molecules under the influence of shear stress (31). Such a process may take place when circulating VWF first binds to collagen, and then continue through transient homotypic interactions that assemble additional layers of extended plasma VWF polymers over the collagen-bound VWF. In this paradigm, more multimers may achieve a functionally appropriate orientation at the interface with flowing blood, providing a higher density A1 domain surface for the tethering of platelets and, thus, accelerating the hemostatic response to vascular injury.
Several lines of evidence support the conclusions outlined above. Whether the A1 domain was removed by deletion mutagenesis or functionally blocked with an antibody in the WT molecule, the defect in platelet adhesion was apparent only when A1 domain function was absent in both immobilized substrate and flowing blood. The addition of soluble VWF with functional A1 domain to a blood cell suspension perfused over immobilized VWF defective in A1 domain function was always sufficient to restore a considerable level of surface adhesion in a manner that was independent of the collagen-binding activity of the fluid-phase VWF. The latter effect, however, was strictly dependent on the presence of surface-bound VWF molecules, even if functionally defective, and was not observed by using immobilized albumin, fibrinogen, or fibronectin as examples of nonspecific protein substrates, or on uncoated glass surfaces. Although surface-bound VWF appears to initiate a process of self-assembly, we cannot exclude that other proteins, not yet identified, may display a similar function through heterotypic interactions with VWF molecules in the fluid phase. It is also clear that neither the A1 nor the A3 domain is involved in a strictly homotypic association, because ΔA1-VWF on a surface and ΔA3-VWF in the fluid phase could lead to the functional self-assembly required to support platelet adhesion through GP Ibα (Fig. 3A). It is, nevertheless, tempting to speculate that the three homologous type A domains of the VWF subunit are involved in the process described here, perhaps through features that are common to all of them, and are therefore interchangeable. In this context, the modular VWF type A repeat has been shown to possess the property of self-assembly in matrix proteins (32).
A feature that clearly distinguishes collagen-bound from self-assembled VWF multimers is the stability of interaction with the surface, because homotypic VWF interactions are reversible in a few minutes whereas VWF binding to collagen is apparently irreversible on a comparable time scale. This difference may not be relevant from the standpoint of thrombogenesis, because the initial platelet tethering to VWF is intrinsically transient and is rapidly followed, on a time scale of seconds at the most, by the formation of stable bonds to other adhesive substrates (6). The reversibility of the self-association described here may also explain why previous studies conducted with postperfusion examination of a slightly washed surface reported a severely defective platelet adhesion to ΔA1-VWF adsorbed to glass or bound to collagen and exposed to whole blood (33). In fact, examination in real time appears to be crucial for demonstrating the function of soluble VWF transiently interacting with immobilized VWF in support of platelet adhesion. It is also worth noting that, in experiments using surface plasmon resonance not reported here, one of us failed to detect any measurable self-interaction between surface-bound and soluble purified VWF multimers (P. Lenting and J.J.S., unpublished results). As a possible explanation for this observation, fluid shear forces were considerably higher in the platelet perfusion experiments as compared with those achieved during the measurement of surface plasmon resonance, and the effect of shear on the conformation of VWF multimers (31) may be crucial to expose the molecular domains required for self-association. In addition, or in alternative, the presence of platelets at the interface between immobilized and soluble VWF may be an important contributory factor to the process described here. In fact, large multimers may become more easily unfolded while bound, albeit reversibly, to GP Ibα on the platelet membrane, and then associate more easily with the VWF immobilized on the thrombogenic surface. Functional evidence for the occurrence of transient interactions between VWF in solution and platelet GP Ibα has been provided in the setting of shear-induced aggregation experiments (21). Moreover, transient interactions with the platelet membrane may increase the local concentration of soluble VWF multimers at the boundary between flowing fluid and immobile surface, a condition that may favor the noncovalent self-association of VWF molecules, as directly demonstrated by a physical method (34).
In conclusion, the present studies highlight a newly recognized role played by fluid-phase circulating VWF in the initiation of platelet adhesion, demonstrating that direct immobilization on extracellular matrix structures is not a strict requirement for binding to the GP Ibα receptor. The dynamic self-assembly of VWF on a reactive surface at the onset of the response to vascular injury may contribute in a relevant manner to the arrest of flowing platelets opposing hemodynamic forces, thus facilitating their progression to activation and the subsequent recruitment of additional platelets into larger aggregates.
Acknowledgments
We are indebted to Marion Schiphorst for her invaluable help with the production and purification of recombinant von Willebrand factor. This work was supported by National Institutes of Health Grants HL-31950, HL-42846, and HL-48728, and by The Netherlands Organization for Scientific Research Program Grant PGN 902-26-193. Additional support was provided by National Institutes of Health Grant RR0833 to the General Clinical Research Center of The Scripps Research Institute, and by the Stein Endowment Fund.
Abbreviations
- VWF
von Willebrand factor
- GP
glycoprotein
- WT
wild type
- ΔA1-VWF
VWF lacking the A1 domain
- ΔA3-VWF
VWF lacking the A3 domain
- PPACK
d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride
- PG
prostaglandin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tangelder G J, Slaaf D W, Arts T, Reneman R S. Am J Physiol. 1988;254:H1059–H1064. doi: 10.1152/ajpheart.1988.254.6.H1059. [DOI] [PubMed] [Google Scholar]
- 2.Back C H, Radbill J R, Crawford D W. J Biomech. 1977;10:339–353. doi: 10.1016/0021-9290(77)90006-9. [DOI] [PubMed] [Google Scholar]
- 3.Savage B, Saldivar E, Ruggeri Z M. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 4.Ruggeri Z M, Dent J A, Saldivar E. Blood. 1999;94:172–178. [PubMed] [Google Scholar]
- 5.Sakariassen K S, Bolhuis P A, Sixma J J. Nature (London) 1979;279:636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- 6.Savage B, Almus-Jacobs F, Ruggeri Z M. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 7.Wagner D D. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 8.Stel H V, Sakariassen K S, de Groot P G, van Mourik J A, Sixma J J. Blood. 1985;65:85–90. [PubMed] [Google Scholar]
- 9.Lankhof H, van Hoeij M, Schiphorst M E, Bracke M, Wu Y P, Ijsseldijk M J, Vink T, de Groot P G, Sixma J J. Thromb Haemostasis. 1996;75:950–958. [PubMed] [Google Scholar]
- 10.Houdijk W P M, Sakariassen K S, Nievelstein P F E M, Sixma J J. J Clin Invest. 1985;75:531–540. doi: 10.1172/JCI111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzucato M, Spessotto P, Masotti A, De Appollonia L, Cozzi M R, Yoshioka A, Perris R, Colombatti A, De Marco L. J Biol Chem. 1999;274:3033–3041. doi: 10.1074/jbc.274.5.3033. [DOI] [PubMed] [Google Scholar]
- 12.de Groot P G, Ottenhof-Rovers M, van Mourik J A, Sixma J J. J Clin Invest. 1988;82:65–73. doi: 10.1172/JCI113602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizinga E G, van der Plas R M, Kroon J, Sixma J J, Gros P. Structure. 1997;5:1147–1156. doi: 10.1016/s0969-2126(97)00266-9. [DOI] [PubMed] [Google Scholar]
- 14.Miyata S, Ruggeri Z M. J Biol Chem. 1999;274:6586–6593. doi: 10.1074/jbc.274.10.6586. [DOI] [PubMed] [Google Scholar]
- 15.Celikel R, Ruggeri Z M, Varughese K I. Nat Struct Biol. 2000;7:881–884. doi: 10.1038/79639. [DOI] [PubMed] [Google Scholar]
- 16.Ware J. Thromb Haemostasis. 1998;79:466–478. [PubMed] [Google Scholar]
- 17.Titani K, Kumar S, Takio K, Ericsson L H, Wade R D, Ashida K, Walsh K A, Chopek M W, Sadler J E, Fujikawa K. Biochemistry. 1986;25:3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- 18.Shelton-Inloes B B, Titani K, Sadler J E. Biochemistry. 1986;25:3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri Z M, Bader R, De Marco L. Proc Natl Acad Sci USA. 1982;79:6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sixma J J, Schiphorst M E, Verweij C L, Pannekoek H. Eur J Biochem. 1991;196:369–375. doi: 10.1111/j.1432-1033.1991.tb15826.x. [DOI] [PubMed] [Google Scholar]
- 21.Goto S, Salomon D R, Ikeda Y, Ruggeri Z M. J Biol Chem. 1995;270:23352–23361. doi: 10.1074/jbc.270.40.23352. [DOI] [PubMed] [Google Scholar]
- 22.Ey P L, Prowse S J, Jenkin C R. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 23.Fujimura Y, Usami Y, Titani K, Niinomi K, Nishio K, Takase T, Yoshioka A, Fukui H. Blood. 1991;77:113–120. [PubMed] [Google Scholar]
- 24.Mohri H, Yoshioka A, Zimmerman T S, Ruggeri Z M. J Biol Chem. 1989;264:17361–17367. [PubMed] [Google Scholar]
- 25.Celikel R, Varughese K I, Madhusudan, Yoshioka A, Ware J, Ruggeri Z M. Nat Struct Biol. 1998;5:189–194. doi: 10.1038/nsb0398-189. [DOI] [PubMed] [Google Scholar]
- 26.Roth G J, Titani K, Hoyer L W, Hickey M J. Biochemistry. 1986;25:8357–8361. doi: 10.1021/bi00374a004. [DOI] [PubMed] [Google Scholar]
- 27.Pareti F I, Niiya K, McPherson J M, Ruggeri Z M. J Biol Chem. 1987;262:13835–13841. [PubMed] [Google Scholar]
- 28.Celikel R, Madhusudan, Varughese K I, Shima M, Yoshioka A, Ware J, Ruggeri Z M. Blood Cells Mol Dis. 1997;23:124–134. doi: 10.1006/bcmd.1997.0128. [DOI] [PubMed] [Google Scholar]
- 29.Savage B, Ginsberg M H, Ruggeri Z M. Blood. 1999;94:2704–2715. [PubMed] [Google Scholar]
- 30.Bienkowska J, Cruz M A, Handin R I, Liddington R C. J Biol Chem. 1997;272:25162–25167. doi: 10.1074/jbc.272.40.25162. [DOI] [PubMed] [Google Scholar]
- 31.Siediecki C A, Lestini B J, Kottke-Marchant K, Eppell S J, Wilson D L, Marchant R E. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 32.Colombatti A, Bonaldo P. Blood. 1991;77:2305–2315. [PubMed] [Google Scholar]
- 33.Lankhof H, Wu Y-P, Vink T, Schiphorst M E, Zerwes H G, de Groot P G, Sixma J J. Blood. 1995;86:1035–1042. [PubMed] [Google Scholar]
- 34.Loscalzo J, Fisch M, Handin R I. Biochemistry. 1985;24:4468–4475. doi: 10.1021/bi00337a031. [DOI] [PubMed] [Google Scholar]