Abstract
Efficient replication in vivo is essential for a microparasite to colonize its host and the understanding of the molecular mechanisms by which microbial pathogens grow within host tissues can lead to the discovery of novel therapies to treat infection. Here we present evidence that the foodborne bacterial pathogen Listeria monocytogenes, a facultative intracellular parasite, exploits hexose phosphates (HP) from the host cell as a source of carbon and energy to fuel fast intracellular growth. HP uptake is mediated by Hpt, a bacterial homolog of the mammalian translocase that transports glucose-6-phosphate from the cytosol into the endoplasmic reticulum in the final step of gluconeogenesis and glycogenolysis. Expression of the Hpt permease is tightly controlled by the central virulence regulator PrfA, which upon entry into host cells induces a set of virulence factors required for listerial intracellular parasitism. Loss of Hpt resulted in impaired listerial intracytosolic proliferation and attenuated virulence in mice. Hpt is the first virulence factor to be identified as specifically involved in the replication phase of a facultative intracellular pathogen. It is also a clear example of how adaptation to intracellular parasitism by microbial pathogens involves mimicry of physiological mechanisms of their eukaryotic host cells.
The facultative intracellular parasite Listeria monocytogenes is the etiological agent of listeriosis, a highly fatal foodborne infection (1). In the intracellular compartment of the host, this Gram-positive bacterium goes through an infectious cycle involving (i) early escape from the phagocytic vacuole, (ii) rapid cytosolic replication, and (iii) actin-based motility and direct spread to neighboring cells, where the cycle reinitiates. Various virulence factors responsible for steps i and iii have been identified in recent years. Step i involves a pore-forming toxin [listeriolysin O] and two phospholipases C (PlcA and PlcB), which cooperate to disrupt the phagosomal membrane. Step iii is mediated by the surface protein ActA, which induces host cell actin polymerization (2, 3). Nothing is known, however, about the possible virulence mechanisms involved in step ii, a critical phase of the infectious cycle as it allows L. monocytogenes to increase its infective load within the host.
L. monocytogenes pathogenesis requires the coordinated expression of the genes encoding the virulence factors described above. These genes form a regulon under the control of the transcriptional activator protein PrfA (4, 5). PrfA-dependent expression is normally weak or undetectable in normal broth culture and extracellular conditions (6, 7) but is fully induced in the host cell cytosol (7–9). We recently found that the two pathogenic species of the genus Listeria, L. monocytogenes and L. ivanovii, are able to use glucose-1-phosphate (G1P) whereas nonpathogenic Listeria spp. cannot. G1P utilization was found to strictly depend on PrfA and to be coexpressed with PrfA-dependent virulence factors (10). Glucose, in contrast, is used constitutively by all pathogenic and nonpathogenic Listeria spp. Because G1P is a phosphate sugar that is available in the intracellular compartment of the host, these findings led us to suggest that the mechanism responsible for its utilization might play a role in Listeria intracellular parasitism (10).
Materials and Methods
Bacterial Strains, Media, and Culture Conditions.
L. monocytogenes strains P14 (serovar 4b) and EGD (serovar 1/2a) (6, 11) were used in this study. They were grown at 37°C in brain–heart infusion (Difco), which favors growth of L. monocytogenes to a high density (maximal OD600 ≈2.5). LB was used when a carbon source-deficient medium was required (maximal OD600 ≈ 0.5) (10). The PrfA regulon in L. monocytogenes was artificially activated in vitro by either the addition of the adsorbents, activated charcoal (0.2% wt/vol) or Amberlite XAD (0.1% wt/vol) to the culture medium, or by the use of prfA* derivatives, which due to a point mutation in PrfA overexpress all PrfA-dependent genes constitutively (6). Sugars were filter-sterilized and added to a final concentration of 10 mM.
Construction and Complementation of a Δhpt Mutant.
A DNA fragment containing an in-frame Δhpt allele coding for the 21 N-terminal and 17 C-terminal amino acids of Hpt (i.e., ≈8% of the protein) was constructed by ligation of two PCR products covering the corresponding parts of the gene plus its flanking regions. This fragment was inserted into the thermosensitive shuttle vector pLSV1 (12) and the resulting mutagenesis plasmid, pLSΔhpt, was introduced into L. monocytogenes by electroporation. The chromosomal wild-type (wt) hpt allele was replaced by its Δhpt derivative by selecting for double crossover. For trans-complementation experiments, hpt plus its up- and downstream intergenic regions were amplified by PCR and inserted into the Escherichia coli-Bacillus/Listeria shuttle vector pHPS9 (13). General procedures, reagents, and enzymes for recombinant DNA technology were as described (6).
Intracellular Infection Assays.
Immediately before infection, cell monolayers (≈5 × 105 cells/well, 24-well tissue culture plates) were washed with fresh cell culture medium. Bacteria grown to midlog phase were washed, resuspended in cell culture medium, and added to the cell monolayer. After a 45-min incubation, the wells were washed twice with PBS and overlaid with fresh cell culture medium containing 50 μg/ml of gentamicin to kill extracellular bacteria. Thirty minutes after gentamicin addition was defined as t = 0. At the specified times, the gentamicin-containing medium was removed, the infected cell monolayer was washed, lysed and homogenized, and the number of colony-forming units (CFUs) of intracellular bacteria was determined by plate counting.
Monitoring Intracytosolic Bacteria.
The plasmid pPactA-gfp (14), carrying the gfp cDNA encoding the green fluorescent protein (GFP) under the control of the promoter of the listerial actA gene, was used to monitor the bacterial population present in the host cell cytosol. The actA promoter, strictly dependent on PrfA (see Fig. 1C), is inactive outside host cells and in the vacuolar compartment but is fully induced when the bacterium enters the cytosol (7, 8, 15). Fluorescence emitted by GFP-expressing bacteria in live infected cells was measured by flow cytometry. Infected mammalian cells were collected by trypsinization, washed in PBS, and analyzed by using an Epics XL cell sorter (Coulter). GFP fluorescence was quantified in the green light channel (525/10-nm bandpass filter), whereas the red light channel (630/20-nm bandpass filter) was used to exclude dead cells after staining with propidium iodide. Infected cell monolayers also were analyzed by epifluorescence microscopy by using a Leica DM IRB inverted microscope (Leica, Deerfield, IL). Photographs were taken by using a MicroMAX charge-coupled device camera (Princeton Instruments, Trenton, NJ) and assembled and edited by using the metamorph imaging software (Universal Imaging, Downingtown, PA).
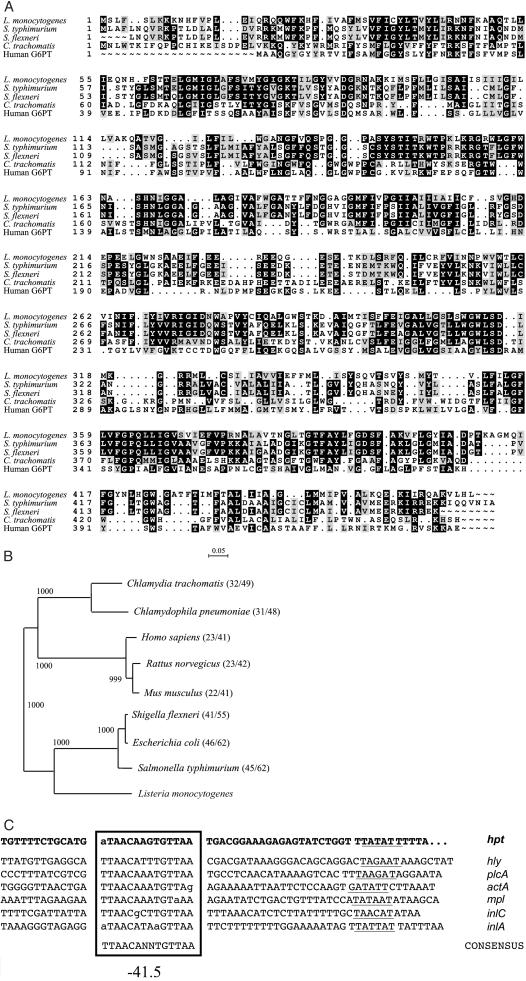
Figure 1.
(A) Alignment of the sequence of Hpt from L. monocytogenes P14 (EMBL database accession no. AJ315765) with that of the UhpT-related transporters from S. enterica serovar Typhimurium (SwissProt database accession no. P27670), S. flexneri (S. flexneri 2a genome sequence in progress, University of Wisconsin-Madison, http://www.genome.wisc.edu/html/sflex.html), C. trachomatis (SwissProt database accession no. O84548), and human G6PT (SwissProt database accession no. O43826). (B) Phylogenetic tree of bacterial and mammalian HP transporters. The dendrogram was generated with the neighbor-joining algorythm by using the clustalx package (20). The bifurcations are supported by the indicated bootstrap values. The segment above the tree indicates the genetic distance. SwissProt database accession nos.: C. pneumoniae, Q9Z7N9; E. coli, P13408; Rattus norvegicus, Q9Z296; and Mus musculus, Q9D1F9. In parentheses after each species name, the percentage identity/similarity with respect to the L. monocytogenes Hpt (C). Promoter regions of PrfA-dependent virulence genes of L. monocytogenes. PrfA binding sites, centered on position −41.5 relative to the transcription start site, are boxed and −10 motifs are underlined. Promoters with a perfectly symmetric PrfA box (i.e., hly and plcA) are very sensitive to PrfA whereas those with mismatches in their PrfA box require larger amounts of the regulatory protein to become fully activated. The hpt gene has a PrfA box with one terminal mismatch, similar to actA, the promoter of which has a high threshold for PrfA-mediated activation and is therefore inactive extracellularly but fully induced in the host cell cytosol (see text). Regulation by PrfA is less stringent in promoters with PrfA boxes with more than one mismatch (e.g., inlA) (4, 5).
Mouse Virulence Assays.
Female ICR mice weighing 18–22 g (Harlan, Ibérica, Spain) were kept in isolators and supplied with autoclaved water and feed ad libitum. For LD50 determinations, groups of five mice were inoculated i.v. with one of a series of doses differing by factor 10 and mortality was scored during a 7-day observation period. LD50 values were calculated as described by Reed and Muench (16). For bacterial survival in organs, infected mice were killed at various times, their livers and spleens were removed and homogenized, and the CFUs were determined by plate counting.
Results
Identification of the hpt Gene of L. monocytogenes.
We determined that L. monocytogenes efficiently utilizes, in a PrfA-dependent manner, not only G1P but also other HPs, including glucose-6-phosphate (G6P), fructose-6-phosphate (F6P), and mannose-6-phosphate (M6P) (Table 1). The use of readily metabolizable carbon sources that are normally transported by the phosphoenolpyruvate:sugar phosphotransferase system (PTS), such as glucose, strongly represses PrfA-dependent genes in L. monocytogenes (10, 17). The use of HPs, in contrast, has no such effect. We therefore thought it likely that HPs are taken up by L. monocytogenes by means of a PrfA-dependent, non-PTS permease (10). To identify this permease, we screened in silico the L. monocytogenes genome sequence (19) for genes preceded by a PrfA box (the binding site of PrfA in target promoters) (Fig. 1C). Nine genes were identified with an appropriately located consensus PrfA-box with no or one mismatch. They included the known PrfA-regulated genes hly, plcA, mpl, actA, and inlC (Fig. 1C), plus four new putative members of the PrfA regulon. One gene encoded a protein similar to UhpT, a hexose phosphate permease found in enteric bacteria (18) (Fig. 1A). This gene was designated hpt, for hexose phosphate transporter.
Table 1.
Utilization of hexoses and HP by wt L. monocytogenes (wt) and ΔprfA and Δhpt derivatives
| Glc | G1P | G6P | Fru | F6P | Man | M6P | |
|---|---|---|---|---|---|---|---|
| wt | + | + | + | + | + | + | + |
| ΔprfA | + | − | − | + | − | + | − |
| ΔprfA + prfA | + | + | + | + | + | + | + |
| Δhpt | + | − | − | + | − | + | − |
| Δhpt + hpt | + | + | + | + | + | + | + |
The assays were performed at 37°C in PrfA-activating conditions (see Materials and Methods) in phenol red broth supplemented with the test sugar as the sole carbon source. Use of the sugar is indicated by acidification of the medium. +, positive reaction at 24 h; −, negative reaction after prolonged (>4 wk) incubation. Hexoses: glucose, Glc; fructose, Fru; and mannose, Man. In-frame deletion of prfA, the gene coding for PrfA, was carried out by using the same strategy as that used for Δhpt (229 of the 237 codons of prfA were removed). For transcomplementation of ΔprfA bacteria, previously described plasmid constructs were used (6).
Interestingly, the L. monocytogenes hpt gene product, Hpt, also showed significant similarity to the translocase component of the mammalian glucose-6-phosphatase (G6Pase) enzyme complex (Fig. 1A), which catalyzes the final step of gluconeogenesis and glycogenolysis (21). Glucose-6-phosphate translocase (G6PT) is an integral membrane protein that mediates the transport of G6P from the cytosol to the lumen of the endoplasmic reticulum, where the phosphate sugar is converted into glucose by the catalytic component of G6Pase (22). Thus, Hpt in intracytosolic listeriae might play a role analogous to that of G6PT in the endoplasmic reticulum, mediating the import into the bacterial cell of G6P and other HPs, such as the glycogen precursor, G1P, and the glycolytic intermediate, F6P. These metabolites from the host cell cytosol could then be used as exogenous carbon and energy sources for intracellular bacterial replication.
Characterization of a Δhpt Mutant.
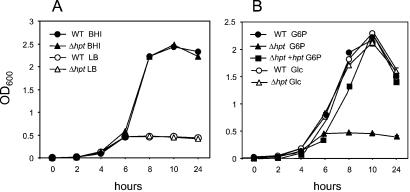
The Δhpt mutation had no effect on the growth of L. monocytogenes in broth culture (Fig. 2A) and did not affect the utilization of nonphosphate sugars such as glucose. However, it totally prevented the utilization of G1P, G6P, F6P, and M6P (Table 1; Fig. 2B). When the PrfA system is activated, supplementation of a poor growth medium (LB) with HP stimulates rapid, strong growth of L. monocytogenes, to levels similar to those constitutively reached in rich culture medium (brain–heart infusion) or in the presence of glucose (10). With the PrfA system turned on, the Δhpt mutant grew only poorly in LB supplemented with HP, and trans-complementation with hpt on a plasmid restored the ability to grow on HP (Fig. 2B). These data demonstrated that the product of the hpt gene is responsible for the PrfA-dependent utilization of HP by L. monocytogenes.
Figure 2.
(A) Growth curves of the L. monocytogenes wt strain P14 (WT) and its Δhpt derivative in brain–heart infusion and LB at 37°C. (B) Growth curves of wt, Δhpt and its hpt-complemented derivative (Δhpt +hpt) in LB-Amberlite (PrfA-activating conditions; see Materials and Methods) supplemented with 10 mM of G6P or Glc. Identical results were obtained in LB without Amberlite by using bacteria of prfA* genotype, in which hpt is constitutively overexpressed (6). When the PrfA system is inactive (bacteria of wt prfA background in normal LB), the growth curves of wt and Δhpt +hpt in G6P (not in Glc) are identical to that of the Δhpt mutant (i.e., 0.5 OD600 units maximum growth).
Role of hpt in Intracellular Proliferation.
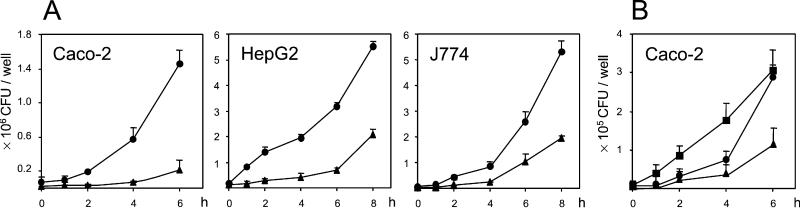
The Δhpt mutant was clearly impaired in intracellular growth in various mammalian cell types (Fig. 3A). The Δhpt mutation caused a reduction of the bacterial growth rate, consistent with a deficit in the assimilation of a fueling metabolite. Mean intracellular doubling times of Hpt-proficient bacteria (between 58.3 and 66.3 min) were in accordance with the values previously reported for L. monocytogenes (23). In Caco-2 intestinal epithelial cells and HepG2 hepatocytes, the doubling times of Δhpt bacteria during the first 4 h of intracellular growth were more than double those of Hpt-proficient bacteria (between 125.0 and 142.8 min). In J774 macrophages, the decrease in the intracellular growth rate was less pronounced than in Caco-2 and HepG2 cells (mean doubling time, 106.6 min). Reintroduction of the plasmid carrying hpt resulted in the rescue of the mutant (Fig. 3B).
Figure 3.
(A) Effect of the Δhpt mutation on the intracellular replication of L. monocytogenes P14 in mammalian cells. Cell monolayers were infected with the following multiplicities of infection: Caco-2, 5:1; HepG2, 10:1; and J774.A1, 1:1. (B) Trans-complementation of L. monocytogenes Δhpt with a plasmid carrying hpt restores rapid intracellular bacterial replication. The experiment shown was performed with strain EGD (multiplicity of infection 10:1) to show that the Δhpt mutation has the same effect independently of the strain used. Results are the mean of at least three independent experiments (each performed in duplicate) ± SE. wt (●); Δhpt mutant (▴), and Δhpt transcomplemented with hpt (■).
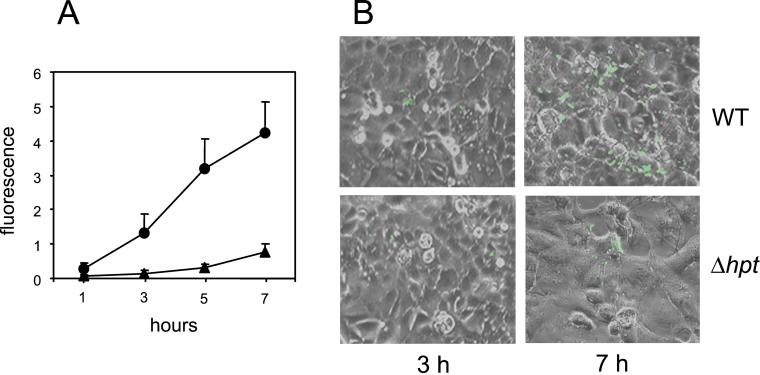
To initiate intracellular growth, L. monocytogenes must escape the phagosome and reach the cytoplasm (2, 3, 24). The hpt mutation did not affect the expression of the phagosome-disrupting virulence factors listeriolysin O, PlcA and PlcB (not shown), so it was unlikely that the reduced intracellular growth capacity of Δhpt bacteria was caused by a delay in phagosomal escape. We checked this by following the kinetics of the cytosolic bacterial population by using a GFP-based reporter system (see Materials and Methods). We infected cell cultures with wt L. monocytogenes and its isogenic Δhpt derivative, each containing the pPactA-gfp reporter plasmid. Infected cells were analyzed by fluorescence microscopy and, for precise quantification of the cytoplasmic bacterial population, by flow cytometry (Fig. 4). One hour after gentamicin addition (i.e., ≈2 h after infection), by which time most intracellular Listeria have generally escaped from the phagocytic vacuole (25, 26), fluorescence intensity was similar for the wt and the Δhpt mutant. This finding indicated that the two strains did not differ in phagosomal escape. The fluorescence intensities of the two strains subsequently followed kinetics concordant with their corresponding intracellular populations determined by plate counting (Figs. 3 and 4A). Overall, these data provide strong evidence that Hpt-mediated utilization of HP is necessary for the efficient cytosolic replication of L. monocytogenes.
Figure 4.
Kinetics of L. monocytogenes populations in the cytoplasmic compartment of host cells by using the reporter plasmid pPactA-gfp. Caco-2 cells were infected (multiplicity of infection 10:1) with pPactA-gfp-containing wt and Δhpt bacteria, washed, and incubated in the presence of gentamicin for 2 h (t = 0). (A) Flow cytofluorimetry experiments: (●), wt, (▴) Δhpt mutant. Fluorescence intensity is expressed in arbitrary units. Data are mean values of three experiments ± SE. (B) Representative fluorescence micrographs. With this technique, less sensitive than flow cytofluorimetry, fluorescent bacteria were first detected 2 h after gentamicin addition (i.e., ≈3 h after infection).
Hpt Is Required for Full Virulence in Mice.
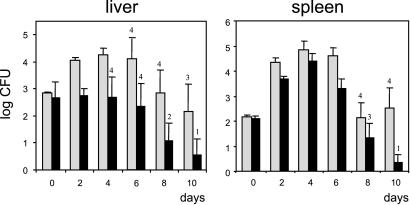
The i.v. LD50 of the Δhpt mutant was 1 log higher than that of the wt (4.17 ± 1.10 × 105 vs. 3.05 ± 2.50 × 104, mean values of three independent determinations), indicating that Hpt contributes to infection in vivo. We also determined the kinetics of the bacterial populations in the liver and spleen of mice infected i.v. with a sublethal dose. wt L. monocytogenes followed the expected pattern (3), with an increase in the bacterial numbers in the liver and spleen until day 4 after infection, when the establishment of the immune response restricts listerial proliferation and progressively eliminates the bacteria from the organs (Fig. 5). The loads of Δhpt bacteria in the liver and spleen were lower than those of Hpt-proficient bacteria at all time points tested. This was especially evident during the resolution phase of the infection (days 8 and 10): here more animals infected with Δhpt had totally cleared the inoculum. At day 10, the difference between the bacterial burdens of the Δhpt mutant and the wt was −1.48 log in the liver and −2.18 log in the spleen (Fig. 5). These data indicate that loss of Hpt impairs L. monocytogenes proliferation and survival in vivo in host tissues.
Figure 5.
L. monocytogenes bacteria lacking hpt are less virulent. Mice were injected by i.v. route with 5 × 103 bacteria for each strain, and bacterial counts in organs were determined 1 h after infection (t = 0) and at the specified days. Data represent the mean bacterial counts of five animals ± SE; the number of animals with positive cultures is indicated. Gray bars, wt L. monocytogenes; black bars, Δhpt mutant. Similar results were observed after an intragastric inoculation. Note that during the acute phase of infection (days 0–4), no net increase in the load of Δhpt bacteria was observed in the liver, whereas in the spleen there was substantial proliferation of the Δhpt mutant. This finding suggests a more specific involvement of Hpt in colonization of the liver than the spleen by L. monocytogenes. It is tempting to correlate this observation with the fact that hepatocytes are among the mammalian cells that have the highest content of glycogen (up to 8–10% wet weight). Turnover of glycogen is high in hepatocytes to ensure glucose homeostasis, and its breakdown releases G1P, rapidly converted via phosphoglucomutase into G6P, which in turn enters glycolysis after conversion into F6P (21). Thus, the liver constitutes an unlimited source of Hpt-transported substrates, which may account for the more pronounced effect of the hpt mutation in this organ.
Discussion
Little is known about the mechanism used by microparasites for the acquisition of nutrients in vivo or the biochemistry of microbial growth in infected host tissues (27, 28). Although it is obvious that the host cell supplies intermediates for the mutiplication of intracellular parasites (29), there is also growing indirect evidence that the ability to replicate within host cells requires specific adaptations in the microbe (24, 30). Here we report one such adaptation. We show that L. monocytogenes possesses a HP transporter that mediates rapid intracellular replication and that is required for normal proliferation in mouse organs. This transporter, Hpt, is a structural and functional homolog of the microsomal G6PT, a key element of glucose homeostasis in eukaryotes. The mammalian G6PT is responsible for the uptake of G6P from the cytosol into the endoplasmic reticulum for its conversion into the central fueling metabolite, glucose (21). Loss of function of the G6PT caused by mutations in its coding gene causes a severe condition in humans, glycogen storage disease type 1b, characterized by hypoglycemia, hepatomegaly, growth retardation, lactic acidemia, hyperlipidemia, and hyperuricemia, symptoms clearly related to a defect in glucose metabolism (31). The evidence presented here indicates that its bacterial homologous protein, the Hpt permease, with an identical function in HP transport, is used by intracytosolic Listeria parasites to steal fueling metabolites from the host cell cytosol for the benefit of the microbe. Hpt therefore represents a clear example of an emerging concept in the biology of host-pathogen interaction: adaptation to parasitic life often involves the mimicry of eukaryotic host functions by microbial virulence factors (32). Indeed, intracytosolic Listeria bacteria would behave very much like pieces of the endoplasmic reticulum in terms of HP uptake and possibly compete with that eukaryotic organelle for the capture of G6P during intracellular parasitism.
Hpt and the mammalian G6PT belong to the organophosphate:inorganic phosphate antiporter (OPA) family (33), of the major facilitator superfamily of permeases (34). The OPA family also includes the extensively characterized HP transporter UhpT from E. coli (18, 35, 36). We in fact identified the listerial hpt gene by the structural similarities of its encoded product with the enterobacterial UhpT permease. Interestingly, the gene encoding the mammalian G6PT was recently identified in a similar way, by screening an expressed sequence tag database from human liver for homology with the enterobacterial HP transporter system (37). E. coli (EIEC) can live within mammalian host cells, and UhpT is also present in Salmonella enterica and Shigella flexneri (Fig. 1 A and B), which are also facultative intracellular pathogens. There is experimental evidence suggesting that Chlamydia trachomatis imports G6P from the host cell (38), and a gene for an UhpT homolog is also found in this obligate intracellular pathogen and the related species, Chlamydophila pneumoniae (Fig. 1 A and B). Although they mediate the uptake of nutrients that are found in large amounts in the intracellular compartment of animal host tissues, no role in virulence had previously been allocated to these bacterial HP transporters. Our data on the listerial Hpt demonstrate that UhpT-related HP transporters may play a major role in the adaptation of bacterial pathogens to life in the intracellular niche.
The substantial structural homology between the bacterial HP transporters and the mammalian G6PT (Fig. 1A) indicates that both have a common origin (32). The dentrogram in Fig. 1B shows that the chlamydial transporters seem to be evolutionarily more closely related to their eukaryotic orthologs. The listerial Hpt, in contrast, is phylogenetically more related to the enterobacterial UhpT transporters, suggesting a relatively recent horizontal exchange of the HP permease gene between these two groups of eubacteria. One can thus envisage an evolutionary dynamics in which an ancestral parasitic prokaryote obtained, via horizontal gene transfer from its eukaryotic host, a HP transporter, which was subsequently transferred laterally among bacteria at different moments of evolution. Alternatively, the mammalian G6PT may represent conservation of an ancestral prokaryotic HP permease, which was horizontally transferred to early eukaryotes. It cannot be excluded, however, that HP transporters arose in bacteria and eukaryotes by vertical transmission and convergent evolution from a primordial gene for the OPA family of permeases (34).
It has been reported that Bacillus subtilis can replicate in the cytosol of J774 macrophages if released from the phagosome by heterologous overexpression of the hly gene encoding listeriolysin O from L. monocytogenes (23). However, B. subtilis does not have a UhpT-related HP transporter. Albeit at a reduced rate, the L. monocytogenes hpt mutant was still able to replicate within host cells (especially in J774 cells; see above), indicating that the cytosolic compartment provides alternative carbon sources, other than HPs, for bacterial growth. Glucose is clearly a potential growth substrate for intracytosolic bacteria. However, in L. monocytogenes the uptake of that sugar triggers down-regulation of the PrfA regulon (see above). HP uptake, in contrast, does not provoke the repressor effect triggered by glucose (10), making the use of a carbon source compatible with the induction of prfA and the PrfA-dependent virulence genes necessary for the completion of the intracellular life cycle (including hpt itself). L. monocytogenes does not manifest any preference for glucose or HP if Hpt is expressed (unpublished data). Thus, the regulatory effect on PrfA caused by glucose may be exploited by L. monocytogenes to fine-tune the levels of intracellular virulence gene expression. This mechanism may be important for the maintenance of the intracellular replication niche of L. monocytogenes, as it could help preventing cytotoxicity caused by an excess production by the cytosolically growing bacteria of the PrfA-dependent membrane-damaging factors listeriolysin O, PlcA, and PlcB.
A key to the adaptation of Hpt as a mediator of Listeria intracellular parasitism is its PrfA dependency. By placing hpt under the control of the virulence regulator PrfA, L. monocytogenes ensures that Hpt operates in the cytosolic compartment of the host and not in the extracellular environment, where the substrates transported by Hpt are not likely to be abundant. The loss of Hpt had no effect on bacterial growth in vitro in standard broth culture (see Fig. 2) but affected pathogenicity. This finding indicates that Hpt is a “true” virulence factor that has selectively evolved to fulfill a role in host tissue colonization. Together with the isocitrate lyase of Mycobacterium tuberculosis, involved in fatty acid utilization and recently shown to be required for persistence in vivo (39), Hpt defines a class of bacterial pathogenicity determinants that could be termed “metabolic” virulence factors.
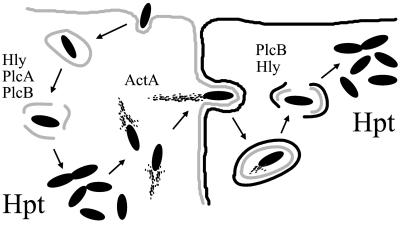
In summary, we have identified a new virulence mechanism contributing to the intracellular infectious cycle of Listeria (Fig. 6). The role of this mechanism is to optimize the bacterial proliferation rate in vivo by widening the range of carbon sources available for growth within host cells. An adequate rate of in vivo growth is important for the outcome of an infection as it allows the microparasite to counteract its destruction by host defense mechanisms. Homologs of G6PT are encoded by other microbial pathogens, in which they may also play a role in fueling bacterial intracellular proliferation. Inhibitors of the mammalian G6PT have been described (41), and the development of drugs with a specific inhibitory effect on the bacterial homologs of G6PT may lead to novel therapeutic strategies for combating intracellular parasites.
Figure 6.
Hpt, a novel virulence factor involved in the intracellular infectious cycle of L. monocytogenes. PrfA-dependent virulence factors acting at each of the steps of the cycle are indicated (reviewed in refs. 2 and 3). Scheme adapted from ref. 40, based on an original drawing in ref. 26.
Acknowledgments
We thank M. T. Ripio for her initial contributions to this study, Y. Vega and A. Tierrez for help with prfA and hpt constructs, and C. Cozar for excellent technical assistance. This work was supported by the European Commission (Grants BMH4-CT96-0659 and QLRT-1999-932), and the Spanish Ministry for Science and Technology Grants PB97-0327 and BMC2000-0553. I.C.-C. holds a predoctoral fellowship from the Spanish Ministry for Science and Technology.
Abbreviations
- HP
hexose phosphate
- G1P
glucose-1-phosphate
- GFP
green fluorescent protein
- G6P
glucose-6-phosphate
- F6P
fructose-6-phosphate
- M6P
mannose-6-phosphate
- G6Pase
glucose-6-phosphatase
- G6PT
glucose-6-phosphate translocase
- CFU
colony-forming unit
- wt
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the EMBL database (accession no. AJ315765).
References
- 1.Farber J M, Peterkin P I. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossart P, Portnoy D A. In: Gram-Positive Pathogens. Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 507–515. [Google Scholar]
- 3.Vázquez-Boland J A, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goebel W, Kreft J, Böckmann R. In: Gram-Positive Pathogens. Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 499–506. [Google Scholar]
- 5.Kreft J, Vázquez-Boland J A. Int J Med Microbiol Infect Dis. 2001;291:145–157. doi: 10.1078/1438-4221-00111. [DOI] [PubMed] [Google Scholar]
- 6.Ripio M T, Domínguez-Bernal G, Lara M, Suárez M, Vázquez-Boland J A. J Bacteriol. 1997;179:1533–1540. doi: 10.1128/jb.179.5.1533-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moors M A, Levitt B, Youngman P, Portnoy D A. Infect Immun. 199;67:131–139. doi: 10.1128/iai.67.1.131-139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitag N, Jacobs K E. Infect Immun. 1999;67:1844–1852. doi: 10.1128/iai.67.4.1844-1852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renzoni A, Cossart P, Dramsi S. Mol Microbiol. 1999;34:552–561. doi: 10.1046/j.1365-2958.1999.01621.x. [DOI] [PubMed] [Google Scholar]
- 10.Ripio M T, Brehm K, Lara M, Suárez M, Vázquez-Boland J A. J Bacteriol. 1997;179:7174–7180. doi: 10.1128/jb.179.22.7174-7180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripio M T, Domínguez-Bernal G, Suárez M, Brehm K, Berche P, Vázquez-Boland J A. Res Microbiol. 1996;147:311–384. doi: 10.1016/0923-2508(96)84712-7. [DOI] [PubMed] [Google Scholar]
- 12.Wuenscher M D, Köhler S, Goebel W, Chakraborty T. Mol Gen Genet. 1991;228:177–182. doi: 10.1007/BF00282463. [DOI] [PubMed] [Google Scholar]
- 13.Haima P, van Sinderen D, Schotting H, Bron S, Venema G. Gene. 1990;86:63–69. doi: 10.1016/0378-1119(90)90114-7. [DOI] [PubMed] [Google Scholar]
- 14.Bubert A, Sokolovic Z, Chun S-K, Papatheodorou L, Simm A, Goebel W. Mol Gen Genet. 1999;261:323–336. doi: 10.1007/pl00008633. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich G, Bubert A, Gentschev I, Sokolovic Z, Simm A, Catic A, Kaufmann S H E, Hess J, Szalay A A, Goebel W. Nat Biotechnol. 1998;16:181–185. doi: 10.1038/nbt0298-181. [DOI] [PubMed] [Google Scholar]
- 16.Reed L J, Muench H. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 17.Milenbachs A, Brown D P, Moors M, Youngman P. Mol Microbiol. 1997;23:1075–1085. doi: 10.1046/j.1365-2958.1997.2711634.x. [DOI] [PubMed] [Google Scholar]
- 18.Island M D, Wei B-Y, Kadner R J. J Bacteriol. 1992;174:2754–2762. doi: 10.1128/jb.174.9.2754-2762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, et al. Science. 2001;294:849–852. doi: 10.1126/science.1063447. [DOI] [PubMed] [Google Scholar]
- 20.Thomson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordlie R C, Foster J D, Lange A J. Annu Rev Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 22.Pan C-J, Lin B, Chou J Y. J Biol Chem. 1999;274:13865–13869. doi: 10.1074/jbc.274.20.13865. [DOI] [PubMed] [Google Scholar]
- 23.Bielecki J, Youngman P, Connelly P, Portnoy D A. Nature (London) 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 24.Goebel W, Kuhn M. Curr Opin Microbiol. 2000;3:49–53. doi: 10.1016/s1369-5274(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilney L G, Portnoy D A. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith H. Trends Microbiol. 1998;6:239–243. doi: 10.1016/s0966-842x(98)01250-5. [DOI] [PubMed] [Google Scholar]
- 28.Finlay B B, Falkow S. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulder J W. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler S, Layssac M, Naroeni A, Gentschev I, Rittig M, Liautard J-P. Infect Immun. 2001;69:2753–2756. doi: 10.1128/IAI.69.4.2753-2756.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou J Y, Mansfield B C. Trends Endocrinol Metab. 1999;10:104–113. doi: 10.1016/s1043-2760(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 32.Stebbins C E, Galán J. Nature (London) 2001;412:701–705. doi: 10.1038/35089000. [DOI] [PubMed] [Google Scholar]
- 33.Kadner R J, Weber C A, Island M D. J Bioenerg Biomembr. 1993;25:637–645. doi: 10.1007/BF00770251. [DOI] [PubMed] [Google Scholar]
- 34.Pao S S, Paulsen I T, Saier M H. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich M J, Kadner R J. J Bacteriol. 1987;169:3556–3563. doi: 10.1128/jb.169.8.3556-3563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merkel T J, Dahl J L, Ebright R H, Kadner R J. J Bacteriol. 1995;177:1712–1718. doi: 10.1128/jb.177.7.1712-1718.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerin I, Veiga-da-Cunha M, Achouri Y, Collet J F. FEBS Lett. 1997;419:235–238. doi: 10.1016/s0014-5793(97)01463-4. [DOI] [PubMed] [Google Scholar]
- 38.Moulder J W. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinney J D, zu Bentrup K H, Muñoz-Elías E J, Miczak A, Chen B, Chan W-T, Swenson D, Sacchettini J C, Jacobs W R, Russell D G. Nature (London) 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 40.Vázquez-Boland J A, Domínguez-Bernal G, González-Zorn B, Kreft J, Goebel W. Microbes Infect. 2001;3:571–584. doi: 10.1016/s1286-4579(01)01413-7. [DOI] [PubMed] [Google Scholar]
- 41.Simon C, Herling A W, Preibish G, Burger H-J. Arch Biochem Biophys. 2000;373:418–428. doi: 10.1006/abbi.1999.1560. [DOI] [PubMed] [Google Scholar]