Abstract
Purpose
Abdominal wall reconstruction is a common surgical procedure, with a post-operative risk of mesh-associated infection of which management is poorly known. This study aims to comprehensively analyze clinical and microbiological aspects of mesh infection, treatment modalities, and associated outcomes.
Methods
Patients with abdominal mesh infection were included in a retrospective observational cohort (2010-2023). Patients characteristics and management were described, and determinants for failure were assessed by logistic regression and treatment failure-free survival curve analysis (Kaplan-Meier).
Results
Two hundred and nine patients (median age, 62 [IQR, 55-71] years) presented a mesh infection occurring within 15 (IQR, 7-31) days after surgery, mainly as an abdominal wall or deep abscess (n=189, 90.4%). Infection was polymicrobial in 89/166 (79.4%) cases, S. aureus (n=60, 36.1%), Enterobacteriaceae (n=60, 36.1%) and anaerobes (n=40, 24.1%) being the most prevalent pathogens. Surgery was performed in 130 (62.2%) patients, associated with a 13.5 (IQR, 8-21) day course of antimicrobial therapy in 172/207 (83.1%) cases. Sixty-three (30.1%) treatment failures occurred, associated with previous multiple abdominal surgeries (OR, 3.305; 95%CI, 1.297-8.425), complete mesh removal (OR, 0.145; 95%CI, 0.063-0.335) and antimicrobial therapy (OR, 0.328; 95%CI, 0.136-0.787). The higher failure rate of conservative strategies was associated with symptom duration >1 month (OR, 3.378; 95%CI, 1.089-4.005) and retromuscular mesh position (OR, 0.444; 95%CI, 0.199-0.992).
Conclusion
Mesh infection is associated with high treatment failure rates. Complete mesh removal coupled with targeted antibiotic therapy is associated with better outcomes. Conservative treatment strategies must rely on careful patient selection based on symptom duration and mesh placement.
Keywords: Abdominal wall repair, Hernia mesh infection, Microbiology, Treatment outcomes, Conservative treatment
Introduction
Reconstruction of abdominal wall defect can be challenging, especially in comorbid patients and/or with complex hernias, following multiple surgeries or abdominal trauma. The final strategy must be safe and effective, and the use of mesh is now one of the most widely-performed surgical procedures [1, 2]. Post-operative infection represents a notable complication, with reported incidences ranging from 0.3% in clean inguinal hernia repairs to as high as 19.6% in complicated hernia cases [3, 4]. Such infections can lead to severe consequences, including sepsis, need for subsequent surgeries, development of chronic fistulas, persistent pain, or hernia recurrence [5]. Determinants of post-operative mesh infection have been extensively documented in existing literature [3, 6, 7]. They include mainly patient- and surgery-related parameters, such as age, body mass index (BMI), smoking status, length of surgery, open surgery compared with laparoscopic procedures, and hernia localization.
Various management strategies have been described [8–10], involving the following interventions, alone or in combination: systemic antibiotic therapy, percutaneous drainage, complete or partial mesh removal, and negative pressure therapy [11]. However, these strategies have been poorly investigated, and criteria guiding clinicians in the selection of the most appropriate therapeutic approach are not available [12].
The objective of this study is to provide a comprehensive account of the clinical and microbiologic aspects of mesh infection observed in our tertiary care hospital, while describing treatment modalities employed and associated outcomes.
Patients and methods
Ethical statement
The study received the approval of the Scientific and Ethical Committees of Hospices Civils de Lyon, France (reference number 23-5457). In accordance with French legislation regarding retrospective observational studies, all patients received written information about the study and their possibility to decline to participate, but the need for written informed consent was waived.
Study design and data collection
Patients diagnosed with abdominal mesh infection in the four digestive surgery wards of our tertiary care center were identified from records from 2010 to 2023 and included in a retrospective observational cohort study. Patients were identified through electronic medical records by cross-referencing medical documents with keywords related to mesh infection, and using diagnosis data associated with each hospital stay, including a combination of International Classification of Diseases (ICD) codes, and hospital pharmacy registries regarding mesh placement and removal. In patients having experienced several episodes of mesh-related infections, only the first episode was considered.
For each patient, data were collected from medical records into an anonymous standardized case report form. Co-morbidities were summarized using the modified Charlson comorbidity index [13], and the American Society of Anesthesiologists (ASA) score.
Definitions
The diagnosis of mesh infection was based on clinical evaluation by the treating surgeon. Infections involving skin and subcutaneous tissue only were categorized as superficial. All other infections were considered as deep. Assessment of the depth of infection was performed by collecting clinical and surgical data, and imaging reports when available. Patients with the superficial wounds who did not undergo subsequent imaging nor surgery were classified as superficial infection on the basis of clinical findings, only. The time to infection was calculated as the number of days between mesh placement or the last surgery at the site and the onset of symptoms. Antimicrobial therapy was deemed appropriate when all pathogens identified in gold-standard sample cultures were targeted by at least one antimicrobial agent. Treatment was considered conservative in every case where the initial management strategy did not involve complete removal of the mesh. Treatment failure was defined as infection persistence or relapse after treatment on a clinical basis (i.e., wound abnormalities suspected of infection, abscess and/or fistula) and/or the need for additional surgery motivated by septic reasons and not planned in the initial treatment scheme.
Microbiology etiology was described according to the results of cultures from normally sterile site samples, excluding superficial swab. Common contaminants, such as Coagulase-Negative Staphylococci (CNS), Corynebacterium spp. or Cutibacterium spp., were considered only when found in at least two samples.
The mesh material was described according to the manufacturer’s notice, describing both the material used (synthetic, semi-synthetic, biologic) and its resorption characteristics once implanted (absorbable, semi-absorbable, non-absorbable).
Statistical analysis
Variables were presented as percentages for dichotomous variables and as medians with interquartile ranges (IQR) for continuous variables. In percentage calculations, missing values were excluded from the denominator. Groups were compared using non-parametric tests (Chi-square, Fisher exact, and Mann-Whitney U tests), as appropriate. Kaplan-Meier curves were used to compare treatment-failure free survival rates between groups, with statistical significance assessed by the log-rank (Mantel-Cox) test. The same analysis process was applied to the subgroup of patients who did not undergo complete mesh removal (conservative treatment group). After analysis of the whole cohort, and in order to consider a potential recruitment bias, patients primarily managed in our reference center (HCL group), i.e. who were not referred due to a previous mesh infection management failure, were independently analyzed.
Determinants of treatment failure were examined using stepwise binary logistic regression and expressed as odds ratios (ORs) along with their corresponding 95% confidence intervals (95%CI). Variables included in the final multivariate model were selected from non-interacting variables with clinical significance and p-values obtained in univariate analysis less than 0.15. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19.0 (SPSS, Chicago, IL, USA) and GraphPad Prism version 5.03 (GraphPad, San Diego, CA, USA) softwares.
Results
Included population and index surgery
A total of 209 patients were included, comprising 119 (56.9%) males with a median age of 62 (IQR, 55–71) years. The median modified Charlson’s comorbidity Index was 3 (IQR, 2–3), main comorbidities being obesity (median body mass index [BMI], 29.7 [IQR, 26.4–33.2]; >30 in 96 [46.8%] patients), diabetes mellitus (n = 60, 28.7%) and solid tumor or hemopathy (n = 55, 26.3%). Patient and infection characteristics are summarized in Table 1. The majority (n = 199, 95.2%) of patients had undergone previous abdominal surgery, with multiple procedures in 166 (79.4%) cases. Abdominal wall repair was planned for incisional hernias in 154 (76.2%) patients, mostly using synthetic (n = 131/175, 74.9%) and non-absorbable (n = 83/173, 50.9%) meshes. The most frequent mesh locations were retromuscular (76/190, 40.0%) and intraperitoneal (56/190, 29.5%). Antibiotics were administered as prophylaxis during surgery to 109/122 (89.3%) patients, with cefazolin being the most frequently used drug.
Table 1.
Description of the included population, comparison of patients without or with treatment failure, and determinants of treatment failure (univariate analysis)
| All patients | Descriptive analysis | Univariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure | Success | p-value | OR (95%CI) | p-value | |||||
| n | 209 | 63 | 146 | ||||||
| Demographics and comorbidities | |||||||||
| Gender (male) | 119/209 (56.9%) | 38/63 (60.3%) | 81/146 (55.5%) | 0.517 | 1.220 (0.669–2.225) | 0.517 | |||
| Age (years) | 62 (55–71) | 61 (55-69.5) | 63 (55–72) | 0.432 | 0.992 (0.969–1.016) | 0.528 | |||
| BMI (kg/m²) | 29.7 (26.4–33.2) | 29.9 (27.3–32.9) | 29.7 (26.1–33.3) | 0.606 | 1. 012 (0.963–1.063) | 0.634 | |||
| ASA score | 2 (2–3) | 2 (2-2.3) | 2 (2–3) | 0.793 (0.490–1.281) | 0.342 | ||||
| Modified Charlson’s comorbidity index | 3 (2–5) | 3 (1.5-4) | 3 (2–5) | 0.972 (0.857–1.103) | 0.663 | ||||
| Active smoking | 42/209 (20.1%) | 14/63 (22.2%) | 28/146 (19.2%) | 0.614 | 1.204 (0.584–2.481) | 0.615 | |||
| Previous abdominal surgery | 199/209 (95.2%) | 59/63 (93.7%) | 140/146 (95.9%) | 0.486 | 0.632 (0.172–2.322) | 0.490 | |||
| > 1 previous abdominal surgeries | 166/209 (79.4%) | 56/63 (88.9%) | 110/146 (75.3%) | 0.026 | 2.618 (1.096–6.257) | 0.030 | |||
| Index surgery | |||||||||
| Operative indication | |||||||||
| Primary hernia | 42/202 (20.8%) | 14/62 (22.6%) | 28/140 (20.0%) | 0.677 | 1.167 (0.565–2.409) | 0.677 | |||
| Incisional hernia | 154/202 (76.2%) | 45/62 (72.6%) | 109/140 (77.9%) | 0.416 | 0.753 (0.379–1.495) | 0.417 | |||
| Acute complication | 11/202 (5.4%) | 4/62 (6.5%) | 7/140 (5.0%) | 0.740 | 1.310 (0.369–4.650) | 0.676 | |||
| Post-surgical parietal closure | 6/202 (3.0%) | 3/62 (4.8%) | 3/140 (2.1%) | 0.374 | 2.322 (0.455–11.841) | 0.311 | |||
| Mesh characteristics | |||||||||
| Size (cm²) | 400 (225-775.5) | 500 (300–900) | 360 (225-667.5) | 0.106 | 1.001 (1.000-1.002) | 0.058 | |||
| Synthetic | 131/175 (74.9%) | 41/58 (70.7%) | 90/117 (76.9%) | 0.371 | 0.724 (0.356–1.472) | 0.372 | |||
| Biosynthetic | 43/175 (24.6%) | 17/58 (29.3%) | 26/117 (22.2%) | 0.305 | 1.451 (0.711–2.963) | 0.307 | |||
| Biological | 1/175 (0.6%) | 0/58 (0%) | 1/117 (0.9%) | 1.000 | 0.991 (0.975–1.008) | ||||
| Absorbable | 34/173 (19.7%) | 15/57 (26.3%) | 19/116 (16.4%) | 0.122 | 1.823 (0.846–3.929) | 0.125 | |||
| Semi-absorbable | 51/173 (29.5%) | 13/57 (22.8%) | 38/116 (32.8%) | 0.177 | 0.606 (0.292–1.259) | 0.179 | |||
| Non-absorbable | 88/173 (50.9%) | 29/57 (50.9%) | 59/116 (50.9%) | 0.999 | 1.001 (0.531–1.887) | 0.999 | |||
| Mesh location | |||||||||
| Subcutaneous | 37/190 (19.5%) | 11/60 (18.3%) | 26/130 (20.0%) | 0.787 | 0.898 (0.411–1.964) | 0.787 | |||
| Retromuscular | 76/190 (40.0%) | 23/60 (38.3%) | 53/130 (40.8%) | 0.750 | 0.903 (0.482–1.691) | 0.750 | |||
| Preperitoneal | 24/190 (12.6%) | 6/60 (10.0%) | 18/130 (13.8%) | 0.458 | 0.691 (0.260–1.841) | 0.460 | |||
| Intraperitoneal | 56/190 (29.5%) | 22/60 (36.7%) | 34/130 (26.2%) | 0.140 | 1.635 (0.849–3.146) | 0.141 | |||
| Altemeier class 1 | 165/190 (86.8%) | 51/60 (85%) | 114/130 (87.7%) | 0.610 | 0.795 (0.330–1.919) | 0.610 | |||
| Infection presentation | |||||||||
| Time from surgery to onset of symptoms (days) | 15 (7–31) | 15 (7.3–31) | 17 (7–31) | 0.662 | 0.999 (0.999-1.000) | 0.110 | |||
| Highest CRP level (mg/L) | 124 (50–200) | 130 (60–250) | 114 (40.8-183.5) | 0.238 | 1.002 (0.999–1.005) | 0.179 | |||
| Infection type | |||||||||
| Inflammatory wound dehiscence | 9/209 (4.3%) | 0/63 (0%) | 9/146 (6.2%) | 0.060 | - | ||||
| Abscess | 189/209 (90.4%) | 61/63 (96.8%) | 128/146 (87.7%) | 0.042 | 4.289 (0.964–19.076) | 0.056 | |||
| Abdominal wall abscess | 177/209 (84.7%) | 56/63 (88.9%) | 121/146 (82.9%) | 0.268 | 1.653 (0.675–4.049) | 0.272 | |||
| Deep abscess | 25/209 (12.0%) | 8/63 (12.7%) | 17/146 (11.6%) | 0.829 | 1.104 (0.450–2.708) | 0.829 | |||
| Sinus tract | 127/209 (60.8%) | 42/63 (66.7%) | 85/146 (58.2%) | 0.251 | 1.435 (0.773–2.664) | 0.252 | |||
| Enterocutaneous sinus tract | 20/209 (9.6%) | 4/63 (6.3%) | 16/146 (11.0%) | 0.443 | 0.551 (0.177–1.719) | 0.304 | |||
| Early post-operative peritonitis | 4/209 (1.9%) | 1/63 (1.6%) | 3/146 (2.1%) | 1.000 | 0.769 (0.078–7.537) | 0.821 | |||
| Microbiology results | 166/209 (79.4%) | 51/63 (81.0%) | 115/146 (78.8%) | 0.720 | 1.146 (0.545–2.410) | 0.720 | |||
| Sterile | 10/166 (6.0%) | 4/51 (7.8%) | 6/115 (5.2%) | 0.498 | 1.546 (0.417–5.733) | 0.515 | |||
| Polymicrobial | 89/166 (53.6%) | 28/51 (54.9%) | 61/115 (53.0%) | 0.825 | 1.078 (0.556–2.089) | 0.825 | |||
| S.aureus | 60/166 (36.1%) | 17/51 (33.3%) | 43/115 (37.4%) | 0.616 | 0.837 (0.418–1.676) | 0.616 | |||
| CoNS | 11/166 (6.6%) | 4/51 (7.8%) | 7/115 (6.1%) | 0.738 | 1.313 (0.367–4.701) | 0.676 | |||
| Streptococcus spp. | 21/166 (12.7%) | 4/51 (7.8%) | 17/115 (14.8%) | 0.312 | 0.491 (0.156–1.539) | 0.222 | |||
| Enterococcus spp. | 33/166 (19.9%) | 7/51 (13.7%) | 26/115 (22.6%) | 0.186 | 0.545 (0.219–1.352) | 0.190 | |||
| Corynebacterium spp. | 3/166 (1.8%) | 2/51 (3.9%) | 1/115 (0.9%) | 0.224 | 4.653 (0.412–52.521) | 0.214 | |||
| Cutibacterium spp | 5/166 (3.0%) | 3/51 (5.9%) | 2/115 (1.7%) | 0.170 | 3.531 (0.572–21.811) | 0.174 | |||
| Enterobacteriaceae | 60/166 (36.1%) | 19/51 (37.3%) | 41/115 (35.7%) | 0.843 | 1.072 (0.541–2.124) | 0.843 | |||
| Pseudomonas spp. | 10/166 (6.0%) | 3/51 (5.9%) | 7/115 (6.1%) | 1.000 | 0.964 (0.239–3.889) | 0.959 | |||
| Anaerobes | 40/166 (24.1%) | 11/51 (21.6%) | 29/115 (25.2%) | 0.612 | 0.816 (0.371–1.795) | 0.612 | |||
| Actinomyces spp. | 9/166 (5.4%) | 2/51 (3.9%) | 7/115 (6.1%) | 0.723 | 0.630 (0.126–3.142) | 0.573 | |||
| Fungi | 12/166 (7.2%) | 4/51 (7.8%) | 8/115 (7.0%) | 0.533 | 1.138 (0.327–3.966) | 0.839 | |||
| Infection management | |||||||||
| Local treatment only | 46/209 (22.0%) | 22/63 (34.9%) | 24/146 (16.4%) | 0.003 | 2.728 (1.384–5.374) | 0.004 | |||
| Radiological drainage | 19/209 (9.1%) | 6/63 (9.5%) | 13/146 (8.9%) | 1.000 | 1.077 (0.390–2.974) | 0.886 | |||
| Surgery | 130/209 (62.2%) | 29/63 (46%) | 101/146 (69.2%) | 0.002 | 0.380 (0.207–0.698) | 0.002 | |||
| Complete mesh removal | 78/209 (37.3%) | 9/63 (14.3%) | 69/146 (47.3%) | < 10− 3 | 0.186 (0.86 − 0.404) | < 10− 3 | |||
| Partial mesh removal | 11/209 (5.3%) | 3/63 (4.8%) | 8/146 (5.5%) | 1.000 | 0.863 (0.221–3.364) | 0.831 | |||
| Single-stage abdominal wall reconstruction | 16/129 (12.4%) | 3/29 (10.3%) | 13/100 (13.0%) | 1.000 | 0.772 (0.204–2.919) | 0.703 | |||
| Post-operative negative pressure therapy | 38/209 (18.2%) | 10/63 (15.9%) | 28/146 (19.2%) | 0.570 | 0.795 (0 0.360-1.755) | 0.570 | |||
| Antibiotic treatment | 172/207 (83.1%) | 47/62 (75.8%) | 125/145 (86.2%) | 0.067 | 0.501 (0.237–1.060) | 0.071 | |||
| Appropriate empiric antibiotic therapy | 99/131 (75.6%) | 25/33 (75.8%) | 74/98 (75.5%) | 0.977 | 1.014 (0.404–2.542) | 0.977 | |||
| Secondary adaptation | 79/123 (64.2%) | 15/29 (51.7%) | 64/94 (68.1%) | 0.108 | 0.502 (0.215–1.172) | 0.111 | |||
| Treatment duration (days) | 13.5 (8–21) | 10 (7–19) | 14 (9–21) | 0.239 | 0.998 (0.975–1.021) | 0.835 | |||
| Outcomes | |||||||||
| Failure | 63/209 (30.1%) | 63/63 (100%) | NA | NA | NA | NA | |||
| Time to failure (days) | 38 (16–183) | 38 (16–183) | NA | NA | NA | NA | |||
| Hernia recurrence | 67/209 (32.1%) | 20/63 (31.7%) | 47/146 (32.2%) | 0.949 | 0.980 (0.520–1.847) | 0.949 | |||
| Subsequent surgery for infectious purpose | 48/209 (23.0%) | 48/63 (76.2%) | NA | NA | NA | NA | |||
| Follow-up time (weeks) | 73 (26.4-191.1) | 74.9 (32.7-211.8) | 72 (24.5-189.8) | 0.316 | 1.001 (0.999–1.004) | 0.199 | |||
95%CI, 95% confidence interval; ASA, American Society of Anesthesiologists, BMI, Body mass index; CoNS, Coagulase negative staphylococci; CRP, C-reactive protein; NA, Not applicable; OR, Odd ratio
Forty patients (19.1%) were referred to our center after initial management elsewhere. Of note, baseline characteristics of this subset of patients were not statistically different from patients primarily managed in our center (data not shown).
Infection presentation
Median time between index surgery and symptoms was 15 (IQR, 7–31) days. Fever was present in 88 (42.1%) patients, only. A CT-scan was performed in 167 (76.9%) patients. Abdominal wall and deep abscesses were observed in 177 (84.7%) and 25 (12.0%) patients, respectively. Sinus tracts were identified in 127 (60.8%) patients. Less common presentations included enterocutaneous fistula (n = 20, 9.6%) and isolated inflammatory wound dehiscence (n = 9, 4.3%).
Microbiology findings
Reliable samples for microbiological examination were obtained from 166 (79.4%) patients. Ten (6%) samples were culture-sterile. Cultures revealed polymicrobial infections in 86 (53.6%) cases. Staphylococcus aureus (n = 60, 36.1%) and Enterobacteriaceae (n = 60, 36.1%) were the most frequently isolated organisms. Multidrug-resistant (MDR) bacteria were uncommon, with 3/60 (5%) methicillin-resistant S. aureus, and 9/60 (15%) Enterobacteriaceae resistant to 3rd generation cephalosporins. Surgeries involving progressive preoperative pneumoperitoneum were associated with higher occurrence of MDR bacterial infection (3/13 versus 9/196, OR, 6.964 [1.509–32.142]; p = 0.013).
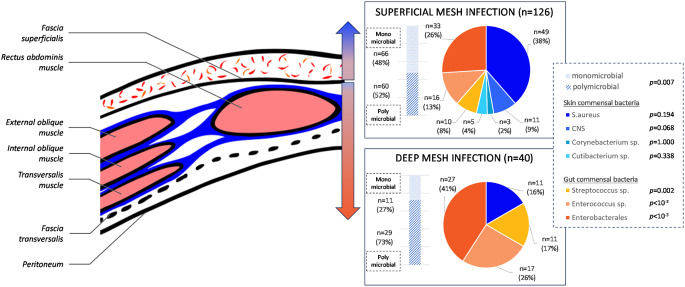
Microbiology data highlights significant differences toward clinical presentations (Fig. 1).
Fig. 1.
Microbial distribution according to infection site
Infection management
Surgical intervention was undertaken in 130 (62.2%) cases, with total or partial mesh removal in 78 (37.3%) and 11 (5.3%) cases, respectively. Single stage abdominal wall reconstruction with a new mesh was performed in 16/129 (12.4%) patients, predominantly using absorbable meshes. Post-operative negative pressure therapy was utilized in 38 (18.2%) patients, while radiological drainage was performed in 19 (9.1%) patients. Antibiotic treatment was prescribed to 172/207 (83.1%) patients for a median duration of 13.5 (IQR, 8–21) days, with therapy deemed appropriate in 99/131 (75.6%) cases.
Outcomes
After a median follow-up of 73 (IQR, 26.4-191.1) weeks, treatment failure was observed in 63 (30.1%) patients, with a median time to failure of 38 (IQR, 16–183) days after the end of initial infection care. Most patients experiencing failure (n = 48/63, 76.2%) underwent subsequent surgery for infectious purposes. Baseline characteristics of patients with treatment failure and success were similar (Table 1). In univariate analysis, the only comorbidity associated with failure was multiple previous abdominal surgeries (OR, 2.618; 95%CI, 1.096–6.257; p = 0.030). Index surgery parameters, clinical presentation and implicated pathogens were not significantly related with outcomes. Surgical treatment (OR, 0.380; 95%CI, 0.207–0.698; p = 0.002), and especially total mesh removal (OR, 0.186; 95%CI, 0.860 − 0.404; p < 10− 3), were protective for treatment failure (Fig. 2). Of note, the evaluation of outcome predictors of the subset of patients primarily managed in our center did not allow to highlight specific risk factors compared to the whole population (data not shown).
Fig. 2.
Kaplan-Meier survival curve for probability of survival without treatment failure in all patients with mesh infection after abdominal wall reconstruction, according to the main determinants of outcome
In multivariate analysis, multiple previous abdominal surgeries (OR, 3.305; 95%CI, 1.297–8.425; p = 0.012), complete mesh removal (OR, 0.145; 95%CI, 0.063–0.335; p < 10− 3) and antibiotic therapy (OR, 0.328; 95%CI, 0.136–0.787; p = 0.013) were independent predictors of treatment outcome. Of note, outcome of patients referred to our center after a previous failure of infection management was similar.
Conservative treatment group
The 131 patients who underwent conservative strategy are presented in Table 2. After a median follow-up of 76 (IQR, 25.9-201.1) weeks, treatment failure was observed in 54 (41.2%) patients, with a median time to failure of 36.5 (IQR, 16–183) days after the end of initial infection care. In this subgroup, factors associated with treatment failure were multiple previous abdominal surgeries (OR, 3.846; 95%CI, 1.452–10.182; p = 0.006), intraperitoneal mesh placement (OR, 2.723; 95%CI, 1.216–6.096; p = 0.014), symptoms duration superior to one month (OR, 3.051; 95%CI, 1.175–7.919; p = 0.021), and presence of a fistula (OR, 2.162; 95%CI, 0.965–6.556; p = 0.036). Retromuscular mesh placement (OR, 0.428; 95%CI, 0.207–0.886; p = 0.022) and antibiotic treatment (OR, 0.381; 95%CI, 0.137–1.060; p = 0.064) emerged as protective factors against treatment failure (Fig. 3).
Table 2.
Description of the conservative treatment subgroup, comparison of patients without or with treatment failure, and determinants of treatment failure (univariate analysis)
| Conservative treatment | Descriptive analysis | Univariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure | Success | p-value | OR (95% CI) | p-value | |||||
| n | 131 | 54 | 77 | ||||||
| Demographics and comorbidities | |||||||||
| Gender (male) | 74/131 (56.5%) | 32/54 (59.3%) | 42/77 (54.5%) | 0.592 | 1.212 (0.599–2.451) | 0.592 | |||
| Age (years) | 62 (55–69) | 61.5 (55.3–69.8) | 62 (54.5–69) | 0.924 | 1.006 (0.976–1.036) | 0.681 | |||
| BMI (kg/m²) | 29.7 (26.9–33.2) | 29.1 (26.8–32.6) | 30.1 (26-33.6) | 0.680 | 0.993 (0.937–1.052) | 0.825 | |||
| ASA score | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.825 | 0.965 (0.547–1.704) | 0.904 | |||
| Modified Charlson’s Comorbidity index | 3 (2-4.5) | 3 (2–4) | 3 (2–5) | 0.945 | 1.003 (0.868–1.157) | 0.966 | |||
| Active smoking | 25/131 (19.1%) | 11/54 (20.4%) | 14/77 (18.2%) | 0.754 | 1.151 (0.477–2.774) | 0.753 | |||
| Previous abdominal surgery | 126/131 (96.2%) | 51/54 (94.4%) | 75/77 (97.4%) | 0.403 | 0.453 (0.073–2.809) | 0.395 | |||
| > 1 previous abdominal surgeries | 100/131 (76.3%) | 48/54 (88.9%) | 52/77 (67.5%) | 0.005 | 3.846 (1.452–10.182) | 0.006 | |||
| Index surgery | |||||||||
| Operative indication | |||||||||
| Primary hernia | 23/129 (17.8%) | 11/53 (20.8%) | 12/76 (15.8%) | 0.469 | 1.396 (0.564–3.456) | 0.469 | |||
| Incisional hernia | 102/129 (79.1%) | 39/53 (73.6%) | 63/76 (82.9%) | 0.201 | 0.574 (0.244–1.350) | 0.203 | |||
| Acute complication | 6/129 (4.7%) | 3/53 (5.7%) | 3/76 (3.9%) | 0.689 | 1.459 (0.283–7.528) | 0.651 | |||
| Post-surgical parietal closure | 4/129 (3.1%) | 3/53 (5.7%) | 1/76 (1.3%) | 0.305 | 4.499 (0.455–44.494) | 0.198 | |||
| Mesh characteristics | |||||||||
| Size (cm²) | 500 (288–900) | 500 (300–900) | 450 (0-900) | 0.301 | 1.000 (0.999–1.001) | 0.178 | |||
| Synthetic | 82/115 (71.3%) | 32/49 (65.3%) | 50/66 (75.8%) | 0.220 | 0.602 (0.266–1.359) | 0.222 | |||
| Biosynthetic | 32/115 (27.8%) | 17/49 (34.7%) | 15/66 (22.7%) | 0.157 | 1.806 (0.793–4.113) | 0.159 | |||
| Biological | 1/115 (0.9%) | 0/49 (0%) | 1/66 (1.5%) | 1.000 | |||||
| Absorbable | 29/116 (25%) | 14/49 (28.6%) | 15/67 (22.4%) | 0.447 | 1.386 (0.595–3.228) | 0.448 | |||
| Semi-absorbable | 35/116 (30.2%) | 11/49 (22.4%) | 24/67 (35.8%) | 0.121 | 0.518 (0.224–1.197) | 0.123 | |||
| Non-absorbable | 52/116 (44.8%) | 24/49 (49.0%) | 28/67 (41.8%) | 0.442 | 1.337 (0.637–2.805) | 0.442 | |||
| Mesh location | |||||||||
| Subcutaneous | 25/127 (19.7%) | 11/52 (21.2%) | 14/75 (18.7%) | 0.729 | 1.168 (0.483–2.827) | 0.729 | |||
| Retromuscular | 62/127 (48.8%) | 19/52 (36.5%) | 43/75 (57.3%) | 0.021 | 0.428 (0.207–0.886) | 0.022 | |||
| Preperitoneal | 8/127 (6.3%) | 4/52 (7.7%) | 4/75 (5.3%) | 0.715 | 1.479 (0.352–6.202) | 0.592 | |||
| Intraperitoneal | 34/127 (26.8%) | 20/52 (38.5%) | 14/75 (18.7%) | 0.013 | 2.723 (1.216–6.096) | 0.014 | |||
| Altemeier class 1 | 110/128 (85.9%) | 44/52 (84.6%) | 66/76 (86.8%) | 0.722 | 0.833 (0.305–2.276) | 0.722 | |||
| Infection presentation | |||||||||
| Time from surgery to onset of symptoms (days) | 14 (8–26) | 13 (7–50) | 14 (8-23.3) | 0.801 | 1.000 (0.999-1.000) | 0.533 | |||
| Highest CRP level (mg/L) | 130 (60–205) | 142 (60–250) | 124.5 (0-180.8) | 0.234 | 1.002 (0.998–1.005) | 0.213 | |||
| Infection type | |||||||||
| Inflammatory wound dehiscence | 9/131 (6.9%) | 0/54 (0%) | 9/77 (11.7%) | 0.009 | NC | NC | |||
| Abscess | 120/131 (91.6%) | 53/54 (98.1%) | 67/77 (87.0%) | 0.026 | 7.910 (0.981–63.761) | 0.052 | |||
| Abdominal wall abscess | 114/131 (87.0%) | 49/54 (90.7%) | 65/77 (84.4%) | 0.289 | 1.809 (0.597–5.474) | 0.293 | |||
| Deep abscess | 13/131 (9.9%) | 7/54 (13.0%) | 6/77 (7.8%) | 0.330 | 1.762 (0.557–5.571) | 0.334 | |||
| Sinus tract | 73/131 (55.7%) | 36/54 (66.7%) | 37/77 (48.1%) | 0.035 | 2.162 (1.051–4.446) | 0.036 | |||
| Enterocutaneous sinus tract | 6/131 (4.6%) | 4/54 (7.4%) | 2/77 (2.6%) | 0.229 | 2.999 (0.529–17.001) | 0.214 | |||
| Early post-operative peritonitis | 0/131 (0%) | 0/54 (0%) | 0/77 (0%) | NC | NC | NC | |||
| Microbiology results | 94/131 (71.8%) | 43/54 (79.6%) | 51/77 (66.2%) | 0.094 | 1.992 (0.883–4.495) | 0.096 | |||
| Sterile | 6/94 (6.4%) | 3/43 (7.0%) | 3/51 (5.9%) | 1.000 | 1.199 (0.229–6.275) | 0.828 | |||
| Polymicrobial | 47/94 (50.0%) | 24/43 (55.8%) | 23/51 (45.1%) | 0.301 | 1.537 (0.679–3.478) | 0.301 | |||
| S.aureus | 36/94 (38.3%) | 14/43 (32.6%) | 22/51 (43.1%) | 0.293 | 0.636 (0.273–1.481) | 0.294 | |||
| CoNS | 6/94 (6.4%) | 3/43 (7.0%) | 3/51 (5.9%) | 1.000 | 1.199 (0.229–6.275) | 0.828 | |||
| Streptococcus spp. | 8/94 (8.5%) | 4/43 (9.3%) | 4/51 (7.8%) | 1.000 | 1.205 (0.282–5.134) | 0.800 | |||
| Enterococcus spp. | 16/94 (17.0%) | 6/43 (14.0%) | 10/51 (19.6%) | 0.467 | 0.664 (0.220–2.008) | 0.469 | |||
| Corynebacterium spp. | 3/94 (3.2%) | 2/43 (4.7%) | 1/51 (2.0%) | 0.591 | 2.439 (0.213–27.863) | 0.473 | |||
| Cutibacterium spp. | 2/94 (2.1%) | 2/43 (4.7%) | 0/51 (0%) | 0.207 | NC | NC | |||
| Enterobacteriaceae | 29/94 (30.9%) | 14/43 (32.6%) | 15/51 (29.4%) | 0.742 | 1.158 (0.481–2.785) | 0.742 | |||
| Pseudomonas spp. | 5/94 (5.3%) | 2/43 (4.7%) | 3/51 (5.9%) | 1.000 | 0.780 (0.124-4.900) | 0.791 | |||
| Anaerobes | 22/94 (23.4%) | 11/43 (25.6%) | 11/51 (21.6%) | 0.647 | 1.249 (0.480–3.252) | 0.647 | |||
| Actinomyces spp. | 4/94 (4.3%) | 2/43 (4.7%) | 2/51 (3.9%) | 1.000 | 1.195 (0.161–8.860) | 0.861 | |||
| Fungi | 3/94 (3.2%) | 3/43 (7.0%) | 0/51 (0%) | 0.092 | NC | NC | |||
| Infection management | |||||||||
| Local treatment only | 45/131 (34.4%) | 22/54 (40.7%) | 23/77 (29.9%) | 0.197 | 1.614 (0.777–3.349) | 0.198 | |||
| Radiological drainage | 18/131 (13.7%) | 6/54 (11.1%) | 12/77 (15.6%) | 0.608 | 0.677 (0.237–1.932) | 0.466 | |||
| Surgery | 41/131 (31.3%) | 17/54 (31.5%) | 24/77 (31.2%) | 0.970 | 1.014 (0.479–2.147) | 0.969 | |||
| Partial mesh removal | 11/131 (8.4%) | 3/54 (5.6%) | 8/77 (10.4%) | 0.524 | 0.507 (0.128–2.007) | 0.333 | |||
| Single-stage abdominal wall reconstruction | 0/51 (0%) | 0/20 (0%) | 0/31 (0%) | NC | NC | NC | |||
| Negative pressure therapy alone | 4/131 (3.1%) | 1/54 (1.9%) | 3/77 (3.9%) | 0.643 | 0.465 (0.047–4.598) | 0.512 | |||
| Post-operative negative pressure therapy | 29/131 (22.1%) | 8/54 (14.8%) | 21/77 (27.3%) | 0.134 | 0.463 (0.188–1.143) | 0.095 | |||
| Antibiotic treatment | 112/130 (86.2%) | 42/53 (79.2%) | 70/77 (90.9%) | 0.058 | 0.381 (0.137–1.060) | 0.064 | |||
| Adapted empiric antibiotic therapy | 58/75 (77.3%) | 22/29 (75.9%) | 36/46 (78.3%) | 0.809 | 0.873 (0.290–2.627) | 0.809 | |||
| Secondary adaptation | 41/68 (60.3%) | 13/25 (52.0%) | 28/43 (65.1%) | 0.286 | 0.580 (0.212–1.584) | 0.288 | |||
| Treatment duration (days) | 14 (9–21) | 11 (7–19) | 14 (0–21) | 0.086 | 0.996 (0.972–1.021) | 0.782 | |||
| Outcomes | |||||||||
| Failure | 54/131 (41.2%) | 54/54 (100%) | NA | NA | NA | NA | |||
| Time to failure (days) | 36.5 (16–183) | 36.5 (16–183) | NA | NA | NA | NA | |||
| Hernia recurrence | 32/131 (24.4%) | 16/54 (29.6%) | 16/77 (20.8%) | 0.246 | 1.605 (0.719–3.582) | 0.247 | |||
| Subsequent surgery for infectious purpose | 42/131 (32.1%) | 42/54 (77.8%) | NA | NA | NA | NA | |||
| Follow-up time (weeks) | 76 (25.9-201.1) | 73.2 (32.3-180.3) | 77.9 (26-203.3) | 0.861 | 1.000 (0.997–1.002) | 0.846 | |||
95%CI, 95% confidence interval; ASA, American Society of Anesthesiologists, BMI, Body mass index; CoNS, Coagulase negative staphylococci; CRP, C-reactive protein; NA, Not applicable; NC, Not calculable; OR, Odd ratio
Fig. 3.
Kaplan-Meier survival curve for probability of survival without treatment failure after conservative management of patients with mesh infection after abdominal wall reconstruction, according to the main determinants of outcome
In multivariate analysis, multiple previous abdominal surgeries (OR, 4.335; 95%CI, 1.534–12.252; p = 0.006), symptoms duration superior to one month (OR, 3.378; 95%CI, 1.089–10.476; p = 0.035), and retromuscular mesh placement (OR, 0.444; 95%CI, 0.199–0.992; p = 0.048) remained independent predictors for failure.
Discussion
Our study represents one of the largest series examining characteristics, management strategies and outcome determinants of abdominal wall mesh infection [6, 8, 14]. However, some limitations must be acknowledged. First, the retrospective monocentric design of our study introduces inherent biases. Our tertiary care center tends to handle more complex cases with greater comorbidity and surgical complexity, potentially influencing treatment outcomes. Nevertheless, the diversity of practices across the four different visceral surgery wards within our center provides a comprehensive perspective. Notably, results from the subgroup analysis of patients not referred to our center (HCL group) were consistent with those of the total population, mitigating concerns regarding referral bias. A key limitation stems from the retrospective nature of patient recruitment, relying on keyword searches within medical records. This method may have overlooked minor wound events deemed insignificant by surgeons, resulting in underreporting of less severe infections such as inflammatory wound dehiscence. Consequently, our conclusions may not be generalized to cases presenting solely as wound dehiscence. Also, because of the retrospective design and the clinical definition of infection, it is difficult to state whether patients experiencing a complex healing course of the surgical wounds and who did not undergo subsequent surgical procedures had actually an infection involving the mesh. This doubt is in line with the observation that 24/146 (16.4%) patients were cured without local treatment only (i.e., wound care), arguing that they might have experienced a superficial surgical site infection or a wound hazard, but no deep infection. However, we decided to keep them in our analysis because they were considered as suspect of mesh infection by the treating team. Future works using a prospective design and standardized approach of wound management after abdominal wall repair could address this question. Nonetheless, our study offers valuable insights into the management and predictive factors for treatment success in more challenging presentations, such as abscesses and fistulas.
Microbiological analysis provided precise data, mostly consistent with the literature although the rate of S. aureus infections (36.1%) appeared lower than usually described (46–66%) [8, 14–16], but higher than in a recent French cohort from another tertiary care center (23%) [17]. Our findings regarding microbial distribution according to infection depth revealed a gradient in bacterial types, reflecting distinct pathophysiological mechanisms between deep infections where gut commensal bacteria are prevailing, and superficial infections where skin commensal bacteria are predominant. Regarding empiric antibiotic treatment, combinations of amoxicillin plus clavulanate, or cefotaxime plus metronidazole, represent good options as they offer coverage regarding most strains of S. aureus, Enterobacteriaceae, and streptococci. Empiric use of broad-spectrum antimicrobials is not warranted in our setting. While differences in bacterial distribution were observed, it seems appropriate to use the same antibiotics for these different infection locations and types. Finally, pre-surgical pneumoperitoneum was associated with higher occurrence of MDR bacterial infection. As this technic is usually proposed for the most complicated abdominal wall repairs, pre-surgical invasive procedure and prolonged hospital stay in more comorbid patients might facilitate surgical site colonization and infection by nosocomial flora.
Management of hernia mesh infection is not codified and remains challenging, as evidenced by the 30.1% treatment failure rate in our study. This rate is congruent with 32.5% (39/120) patients presenting an infection after treatment in the cohort of Zou et al. [8]. Our study confirms the crucial impact of complete mesh removal for infection resolution, while partial mesh removal showed no significant impact on outcomes. Antibiotic therapy appears to be the second cornerstone of hernia mesh infection management.
In cases where complete surgical removal poses significant risks or challenges, conservative treatment may be considered. However, success rate appears to be lower, particularly in patients with chronic infection (symptom duration > 1 month), non-retromuscular mesh placement, and a history of multiple surgeries. These factors exerted a cumulative effect on treatment success, underscoring the importance of careful patient selection and stratification before adopting a conservative strategy.
While previous studies have highlighted certain predictors of treatment outcomes in conservative strategies, such as the early onset of infection [18], this finding regarding symptom duration as a prognostic factor is original. The association between symptom duration and treatment failure aligns with biofilm-related complications observed in orthopedic prosthetic material infections and the existing proof regarding biofilm development on hernia mesh [19, 20].
In conclusion, hernia mesh infection management remains challenging with high risk of treatment failure. The strategy of combining complete mesh removal with antibiotic therapy is the most promising approach for achieving successful outcomes. When conservative treatment is being considered, the physician must evaluate symptom duration and mesh placement, as the association of symptom duration > 1 month with non retromuscular located mesh in a multi operated abdomen is associated with > 75% failure risk.
Authors contribution
All authors participated in patients’ management and inclusion, and in writing and editing the manuscript, and have read and approved the final version of the manuscript. VF collected data. VF, CTF and FV performed the statistical analysis. FV conceived and supervised the study.
Funding
Open access funding provided by Hospices Civils de Lyon.
The study was performed as part of our routine work and did not receive any financial support.
Declarations
Ethical approval
This study received the approval of the Scientific and Ethical Committee of Hospices Civils de Lyon (reference n°23-457). All patients received written information about the study. No written informed consent was required for inclusion.
Competing interest
The authors have no competing interests to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ma Q, Jing W, Liu X et al (2023) The global, regional, and national burden and its trends of inguinal, femoral, and abdominal hernia from 1990 to 2019: findings from the 2019 global burden of Disease Study - a cross-sectional study. Int J Surg Lond Engl 109:333–342. 10.1097/JS9.0000000000000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HerniaSurge Group (2018) International guidelines for groin hernia management. Hernia J Hernias Abdom Wall Surg 22:1–165. 10.1007/s10029-017-1668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mavros MN, Athanasiou S, Alexiou VG et al (2011) Risk factors for mesh-related infections after hernia repair surgery: a meta-analysis of cohort studies. World J Surg 35:2389–2398. 10.1007/s00268-011-1266-5 [DOI] [PubMed] [Google Scholar]
- 4.Cobb WS, Warren JA, Ewing JA et al (2015) Open retromuscular mesh repair of complex incisional hernia: predictors of wound events and recurrence. J Am Coll Surg 220:606–613. 10.1016/j.jamcollsurg.2014.12.055 [DOI] [PubMed] [Google Scholar]
- 5.Akyol C, Kocaay F, Orozakunov E et al (2013) Outcome of the patients with chronic mesh infection following open inguinal hernia repair. J Korean Surg Soc 84:287–291. 10.4174/jkss.2013.84.5.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dipp Ramos R, O’Brien WJ, Gupta K, Itani KMF (2021) Incidence and risk factors for long-term Mesh Explantation due to infection in more than 100,000 hernia operation patients. J Am Coll Surg 232:872–880e2. 10.1016/j.jamcollsurg.2020.12.064 [DOI] [PubMed] [Google Scholar]
- 7.Bueno-Lledó J, Torregrosa-Gallud A, Sala-Hernandez A et al (2017) Predictors of mesh infection and explantation after abdominal wall hernia repair. Am J Surg 213:50–57. 10.1016/j.amjsurg.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 8.Zou Z, Cao J, Zhu Y et al (2023) Treatment of mesh infection after inguinal hernia repair: 3-year experience with 120 patients. Hernia J Hernias Abdom Wall Surg 27:927–933. 10.1007/s10029-022-02702-x [DOI] [PubMed] [Google Scholar]
- 9.Shubinets V, Carney MJ, Colen DL et al (2018) Management of infected Mesh after Abdominal Hernia Repair: systematic review and single-Institution experience. Ann Plast Surg 80:145–153. 10.1097/SAP.0000000000001189 [DOI] [PubMed] [Google Scholar]
- 10.Stremitzer S, Bachleitner-Hofmann T, Gradl B et al (2010) Mesh graft infection following abdominal hernia repair: risk factor evaluation and strategies of mesh graft preservation. A retrospective analysis of 476 operations. World J Surg 34:1702–1709. 10.1007/s00268-010-0543-z [DOI] [PubMed] [Google Scholar]
- 11.Nobaek S, Rogmark P, Petersson U (2017) Negative pressure wound therapy for treatment of mesh infection after abdominal surgery: long-term results and patient-reported outcome. Scand J Surg SJS off Organ Finn Surg Soc Scand Surg Soc 106:285–293. 10.1177/1457496917690966 [DOI] [PubMed] [Google Scholar]
- 12.Kao AM, Arnold MR, Augenstein VA, Heniford BT (2018) Prevention and Treatment strategies for mesh infection in Abdominal Wall Reconstruction. Plast Reconstr Surg 142:149S–155S. 10.1097/PRS.0000000000004871 [DOI] [PubMed] [Google Scholar]
- 13.Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251 [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Xiong Y, Chen J, Shen Y (2020) Study of mesh infection management following inguinal hernioplasty with an analysis of risk factors: a 10-year experience. Hernia J Hernias Abdom Wall Surg 24:301–305. 10.1007/s10029-019-01986-w [DOI] [PubMed] [Google Scholar]
- 15.Birolini C, Faro Junior MP, Terhoch CB et al (2023) Microbiology of chronic mesh infection. Hernia J Hernias Abdom Wall Surg 27:1017–1023. 10.1007/s10029-023-02747-6 [DOI] [PubMed] [Google Scholar]
- 16.Dipp Ramos R, O’Brien WJ, Gupta K, Itani KMF (2021) Re-infection after Explantation of infected hernia mesh: are the same micro-organisms involved? Surg Infect 22:1077–1080. 10.1089/sur.2021.142 [DOI] [PubMed] [Google Scholar]
- 17.Siebert M, Lhomme C, Carbonnelle E et al (2023) Microbiological epidemiology and antibiotic susceptibility of infected meshes after prosthetic abdominal wall repair. J Visc Surg 160:85–89. 10.1016/j.jviscsurg.2023.02.007 [DOI] [PubMed] [Google Scholar]
- 18.O’Brien WJ, Dipp Ramos R, Gupta K, Itani KMF (2021) Risk of Hernia Mesh Explantation following early Versus Late Onset skin and soft tissue infection. Ann Surg Open Perspect Surg Hist Educ Clin Approaches 2:e098. 10.1097/AS9.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydinuraz K, Ağalar C, Ağalar F et al (2009) In vitro S. epidermidis and S. Aureus adherence to composite and lightweight polypropylene grafts. J Surg Res 157:e79–86. 10.1016/j.jss.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Patiniott P, Jacombs A, Kaul L et al (2022) Are late hernia mesh complications linked to Staphylococci biofilms? Hernia J Hernias Abdom Wall Surg 26:1293–1299. 10.1007/s10029-022-02583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]