Abstract
Introduction
The current literature on the prevalence and potential association between disease-modifying therapies (DMTs) and cancer risk in the MS population has yielded mixed findings.
Methods
This study aimed to estimate cancer prevalence and cancer risk in patients with MS (PwMS) under prolonged DMT exposure. Database search include: MEDLINE PUBMED, SCOPUS, and Google Scholar.
Results
A total of 13 studies involving 333,779 PwMS were included, reporting cancer events over periods ranging from 6 to 32 years. The aggregated pooled prevalence of cancer events in MS patients receiving disease-modifying therapies (DMTs) was 3.8% (95% CI 2.6, 5.2%), with substantial heterogeneity (I2 = 99.7%, p = 0). Two studies compared cancer events in MS patients who received DMTs versus those who did not. The relative risk of cancer associated with DMTs was 0.8 (95% CI 0.59–1.31, I2 = 93.6%, p = 0.53), indicating no significant increase in cancer risk due to DMTs. Breast and basal cell carcinomas had a high prevalence (18.4% and 11.3, respectively) in PwMS under DMTs.
Conclusion
This study reports a 3.8% pooled prevalence of cancer in PwMS receiving DMTs. The findings of this study suggest that DMTs alone do not increase cancer risk in PwMS. Breast cancer and basal cell carcinoma had the highest prevalence among the different types of cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12882-4.
Keywords: Multiple sclerosis, Autoimmune diseases, Cancer, Malignancy, Disease-modifying therapies
Introduction
Multiple sclerosis (MS) is a chronic autoimmune inflammatory disease characterized by loss of myelin in the central nervous system [1]. Disease-modifying therapies (DMTs) are employed to curb disease activity, slow the progression of physical disability associated with the disease, and improve its clinical course [2]. DMTs are usually initiated shortly after MS diagnosis, raising the need for accurate evaluation of the long-term health-related implications of these treatments [3].
Although the link between autoimmune diseases and cancer is incompletely understood, several researchers have suggested a potential link between MS treatments and elevated cancer risk [4–7]. According to the current literature, the pathogenesis of MS is thought to involve an improper immune response, which is driven by autoreactive lymphocytes within the central nervous system [1]. Several studies have reported that prolonged exposure to DMTs could induce immunological response alterations, affecting T cell activation, which may potentially increase the cancer risk in patients with MS (PwMS) [4–8]. Conversely, a number of studies have not been able to establish a link between DMTs and cancer occurrence in the MS population, on the basis that autoreactive T cells can also play a part in cancer prevention [9, 10].
Given the contradictory findings in the current literature and the potential influence of DMTs on cancer risk, it is essential to evaluate their long-term effects beyond the scope of short-duration clinical trials. Hence, this systematic review and meta-analysis aims at estimating the prevalence of cancer in PwMS under prolonged DMT exposure.
Methods
Standard protocol approvals, registrations
The pre-specified protocol of this systematic review and meta-analysis is registered in the Open Search Foundation (OSF) (Registration: osf.io/nwdyb). The meta-analysis was reported as per the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11] and was written in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) proposal [12]. The study did not require an ethical board approval or written informed consent.
Data sources, searches and study selection
A systematic literature search was conducted to identify eligible studies reporting on cancer events in PwMS under DMTs. The literature search was performed by two independent reviewers (VG, VS). The MEDLINE, PubMed, and Scopus databases were searched, as well as the first 200 results of Google Scholar. A systematic literature search was conducted to identify eligible studies reporting on cancer events in PwMS who had or had not received DMTs that was performed independently by two reviewers (VG, VS). Search queries included the terms: “multiples sclerosis”, “MS”, “cancer”, “cancer incidence”, “malignancy”, “neoplasm”, “DMTs”, “disease-modifying therapies”, and “disease-modifying drugs”. No language or other restrictions were applied. Our search spanned from inception of each electronic database to July 20th, 2024.
Per study protocol, we excluded studies: (1) including patients with diagnosis of clinically isolated syndrome (CIS); (2) uncertain MS and/or cancer diagnosis; (3) reporting outcomes not aligned with our inclusion criteria; (4) clinical trials, case series, case reports, commentaries, narrative and systematic reviews, non-peer reviewed studies, pre-prints and conference abstracts. In the case of overlapping data between studies, the study with the largest dataset was retained. All retrieved studies were independently assessed by two reviewers (VG, VS), and any disagreements were resolved after discussion with a third tie-breaking evaluator (SG).
Quality control, bias assessment and data extraction
The risk of bias for relevant domains in each included study was assessed by two reviewers (VG, VS), using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) [13]. Any disagreements were settled by the corresponding author (SG). Data from individual studies, including author names, date of publication, study design, country, and events, were extracted.
Outcomes and statistical analysis
An aggregate data meta-analysis was performed which included the identified population-based studies or registries, and observational cohort studies.
The predefined primary outcome measures were twofold: (i) the pooled prevalence of cancer in PwMS under DMTs; (ii) the pooled relative risk of cancer; and (iii) the pooled prevalence of different cancer types in PwMS under DMTs.
Statistical analysis
For the aggregate meta-analysis, the pooled prevalence with the corresponding 95% confidence interval (95% CI) was calculated by the R-meta meta-prop function using the Freeman–Tukey variance-stabilizing double arcsine transformation and the random-effects model for the calculation of pooled estimates (DerSimonian and Laird) [14]. Heterogeneity was assessed with the I2 index and Cochran Q statistics [15, 16]. The significance level for the Q statistic was set at 0.1 in order to detect statistical trends. Publication bias across individual studies was assessed by Funnel plot inspection and the result of the Egger’s linear regression test [17]. All statistical analyses and figure production were carried out using RStudio for IOS [R studio/R Meta package] [11].
Data availability statement
All data generated or analyzed during this study are included in this article and its supplementary information files.
Results
Literature search
The systematic literature review yielded a total of 4440 results. After duplicate extraction, removal of systematic reviews and meta-analysis, removal of studies that did not include PwMS and implementation of selection criteria 13 studies were included (Fig. 1).
Fig. 1.
PRISMA flow chart
Quality and risk of bias assessment
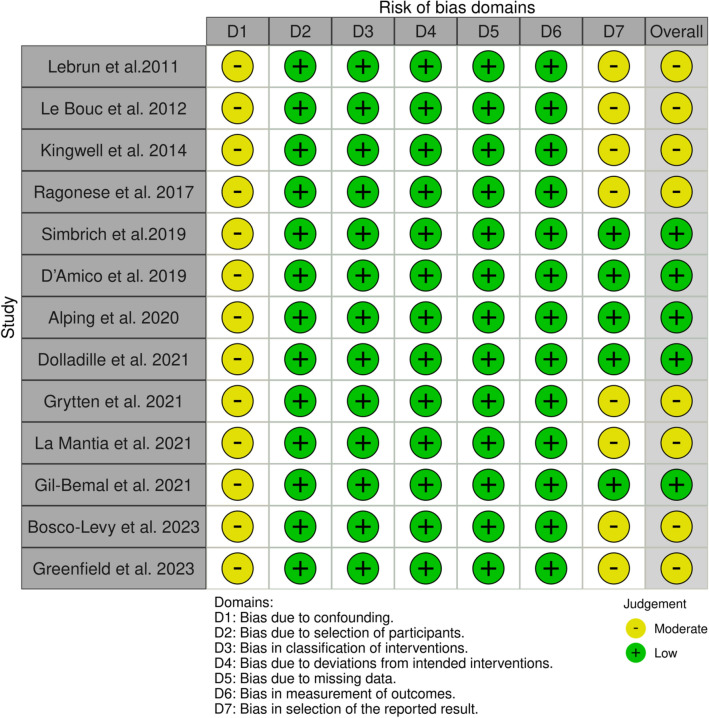
Included studies were assessed using the ROBINS-I with most of them reporting moderate bias in the confounding variables and reported outcomes domains (Figs. 2, 3, 4).
Fig. 2.
ROBINS-I traffic light plot
Fig. 3.
Forest plot of pooled cancer prevalence in PwMS under DMTs versus PwMS not receiving DMTs
Fig. 4.
Forest plot: relative risk of cancer in PwMS under DMTs
Quantitative results
13 studies [3–5, 18–27] with 333,779 PwMS were included. Studies reported data on cancer events over a period of 6–32 years (Table 1). The aggregated pooled prevalence of cancer events in PwMS receiving DMTs was 3.8% (95% CI [2.56, 5.16], I2 = 99.7%, pQ = 0) (Fig. 3). Two studies reported cancer events in MS patients, which compared individuals who had received DMTs to those who had not. The relative risk of cancer due to DMTs was 0.88 (95% CI [0.59, 1.31], I2 = 93.6%, pQ < 0.001, pz = 0.53) (Fig. 4) indicating that DMTs do not increase cancer risk. Subsequent subset analyses regarding the prevalence of different types of cancers in PwMS were performed and are presented in Table 2 (Fig. 5, and Supplementary Figs. S1–S10).
Table 1.
Included studies
| Author | Sample | Cancer events | Disease duration | Age at cancer diagnosis (years) | EDSS |
|---|---|---|---|---|---|
| Lebrun et al. 2011 [27] | 22,563 | 182 | – | 48.1 | – |
| Le Bouc et al. 2012 [18] | 354 | 15 | – | 59.8 | – |
| Kingwell et al. 2014 [19] | 5146 | 227 | 24.2 | 60.9 | – |
| Ragonese et al. 2017 [20] | 531 | 13 | 19.2 | – | – |
| Simbrich et al. 2019 [21] | 15,377 | 630 | 4.5 | – | – |
| D’Amico et al. 2019 [22] | 1180 | 36 | 10 | 49.3 | 4.2 |
| Alping et al. 2020 [23] | 6136 | 168 | 8.85 | 39.2 | 2.5 |
| Dolladille et al. 2021 [4] | 240.993 | 5.966 | 19 | – | – |
| Grytten et al. 2021 [24] | 34,352 | 6228 | - | 50.4 | – |
| La Mantia et al. 2021 [5] | 500 | 22 | 21.7 | – | 3.7 |
| Gil-Bemal et al. 2021 [25] | 250 | 17 | 38 | 39.8 | – |
| Bosco-Levy et al. 2023 [26] | 28,720 | 407 | 6 | – | – |
| Greenfield et al. 2023 [3] | 5801 | 145 | 8.4 | – | – |
Table 2.
Pooled prevalence of different types of cancer
| Cancer type | Pooled sample size | Prevalence (%, CI) |
|---|---|---|
| Pancreatic | 196 | 2.3% [0, 10.6] |
| Prostate | 1239 | 3.1% [0, 6.1] |
| Lung | 1274 | 3.8% [0.8, 7.5] |
| CNS | 1021 | 3.8% [0, 8] |
| Oropharyngeal | 1067 | 4.0% [0.1, 11.2] |
| Vesico-urethral | 1036 | 4.8% [1.3, 9.9] |
| Hematological | 1073 | 5.4% [1.3, 11.4] |
| Gynecological | 1047 | 6.9% [2.7, 12.5] |
| Colorectal | 662 | 7.3% [5.6, 10.0] |
| Basal cell carcinoma | 1034 | 11.3% [4.3, 20.7] |
| Breast | 1046 | 18.4% [7.6, 32.0] |
Fig. 5.
Pooled prevalence of different types of cancer in PwMS under DMTs
Finally, in a set of 10 studies that reported data on MS disease duration, meta-regression revealed a statistically significant association between MS disease duration and cancer prevalence (β = 0.003, p = 0.01) which translates to 0.3% increase in cancer prevalence every 1-year post-MS diagnosis. There was no statistically significant relationship between cancer prevalence and age at cancer diagnosis (β = 0.0006, p = 0.87) or cancer prevalence and EDSS (β = 0.01, p = 0.23).
Publication bias
Publication bias was assessed for studies reporting cancer events. Funnel plot inspection revealed asymmetries with a non-significant Egger’s linear regression test (β = −8.35, p = 0.22) which are indicative of moderate publication bias (Supplementary Fig. 12).
Discussion
This SR-MA included 13 studies with a total of 333,779 PwMS receiving DMTs, with a pooled cancer prevalence of 3.8%. Included studies reported non-stratified data for cancer events in PwMS under different DMTs that included Interferon beta, acetate, Dimethyl fumarate, Teriflunomide, Fingolimod, Natalizumab, Alemtuzumab, Ocrelizumab, Rituximab, Azathioprine, Mitoxantrone, Methotrexate, and Cyclophosphamide. The findings of this study are in close proximity to the percentage yielded by Marrie et al., who reported 4.39% prevalence of any type of cancer in PwMS [28]. Compared to the data from the last GLOBACAN report where cancer incidence ranged from 0.1 to 12.4% (based on cancer type and location), the pooled cancer prevalence in PwMS produced by our study places the prevalence of cancer in PwMS under DMT exposure in the 8th place (out of the 33), while breast cancer and basal cell carcinomas in PwMS under prolonged DMT exposure are placed even higher [29]. Moreover, in the subsequent subset analysis of studies that included MS patients with and without DMTs, the pooled risk ratio of cancer under DMT exposure was estimated to be 0.81 (95% CI [0.59, 1.31]), indicating that exposure to DMTs does not increase the risk of cancer development. It is worth noting that based on the limited data regarding MS specific characteristics, disease duration has a statistically significant association with cancer prevalence (0.3% increase every year), whereas disability level (as measured by the EDSS) has no significant association with cancer prevalence.
With regards to the prevalence of the various cancer types among patients under DMTs, our findings are in accordance to previous studies which have reported an elevated risk of breast and skin cancers in PwMS and is correlated to exposure to either ocrelizumab or S1P receptor modulators [30, 31]. Especially in the case of breast cancer in MS, these findings are not surprising since breast cancer and MS are both female-dominant conditions. Nevertheless, it is worth noting that although both conditions are female dominant, previous research which analyzed data from observational and Mendelian randomization studies has found no statistically significant correlation between specific genetic variants for breast cancer and MS [32].
On the other hand, several studies have explored the proposed anti-cancer properties of different DMTs. Sphingosine 1-Phosphate Receptor (S1PR) modulators such as Siponimod and fingolimod have been shown to inhibit sphingosine kinase 1 (SK1). This enzyme is upregulated in various forms cancers, including CNS, gastrointestinal tract, genitourinary system, lungs, and breast cancers [33, 34]. Previous studies have reported a statistically significant association between long-term use of S1P receptor modulators and the development of basal cell carcinoma and melanoma, suggesting a heightened risk of skin cancers in the MS population. This could be potentially attributed to safety signaling and reduction of circulating lymphocytes which are responsible for the identification and elimination of malignant cells [31]. On the contrary, recent evidence has demonstrated the anti-cancer properties of anti-CD20 B-cell therapies (including rituximab, ocrelizumab, and ofatumumab) against B-cell lymphomas. The main hypothesis poses that anti-CD20 B-cell therapies target the CD20 cell surface marker on malignant B cells. Lastly, despite the aforementioned findings, it is important to also acknowledge the effect of the interference of B-cell therapies with the tumor’s microenvironment, which could explain the observed cancer predisposition among patients receiving B-cell therapies [35, 36].
Finally, the anti-cancer role of DNA synthesis inhibitors (DSIs), such as Dimethyl Fumarate, should not be overlooked, as they reduce inflammatory T cells via the nuclear factor-erythroid 2-related factor 2(Nrf2) and the upregulation of glutathione involved in the antioxidant response [37]. Additional evidence suggests that DSIs may downregulate the Nrf2-DJ-1 antioxidant pathway, subsequently causing cancer cell induction. More specifically, in several patients with melanoma treated with DSIs, they were shown to inhibit cell proliferation, induce apoptosis and arrest of the cell cycle, consequently reducing the cancer risk in these patients [37].
It is worth noting that even though DMTs do not seem to increase the risk of cancer in PwMS, research poses that escalating or de-escalating by switching DMTs can lead to higher cancer risk [22].
Limitations
This study is not without its limitations. To begin with, there is increased heterogeneity between the included studies, with moderate evidence of publication bias. Second, most of the studies did not provide data on the participants’ age and disability level (EDSS) at the time of cancer diagnosis, thus not allowing for sensitivity analysis to be performed, which would allow us to examine of the role of neuroinflammation and/or neurodegeneration in cancer prevalence. Third, the majority of the included studies did not report the number of DMT switches and the corresponding cancer events, nor did they report the duration of exposure for every included DMT which prevented us from performing sensitivity analyses to further assess the role of DMT exposure in cancer prevalence.
Conclusions
This study found a 3.8% pooled prevalence of cancer in PwMS receiving DMTs. The findings of this study suggest that DMTs alone do not increase cancer risk in PwMS. However, this study found that among the different types of cancer seen in PwMS, there is an increased prevalence of breast cancer and basal cell carcinoma in these patients, which may be associated with specific prolonged DMT exposure. These observations are further supported by previous studies which have suggested that switching DMTs may be linked to higher cancer prevalence in PwMS. This study highlights the need for individualized treatment decisions, balancing benefits against potential cancer risks, patients’ education and systematic testing. Studies that systematically record DMT exposure (including type of DMT, duration of use and number of switches) in PwMS who later develop cancer are crucial to advance our understanding on the potential cancer risk resulting from DMT use.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open access funding provided by HEAL-Link Greece.
Data availability
Data are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The author reports no conflict of interest for this work.
Ethical approval
The study did not require an ethical board approval or written informed consent.
References
- 1.Dighriri IM, Aldalbahi AA, Albeladi F, Tahiri AA, Kinani EM, Almohsen RA et al (2023) An overview of the history, pathophysiology, and pharmacological interventions of multiple sclerosis. Cureus 15(1):e33242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingwell E, Tremlett H (2009) Interferons and multiple sclerosis: is it plausible that β-IFN treatment could influence the risk of cancer among MS patients? Expert Rev Neurother 9(9):1263–1265 [DOI] [PubMed] [Google Scholar]
- 3.Greenfield J, Metz LM, Khakban A, Llorian ER, Michaux KD, Traboulsee A et al (2023) Cancer risk, disease-modifying therapy, and age in multiple sclerosis: a retrospective population-based cohort study. Mult Scler Relat Disord 80:105091 [DOI] [PubMed] [Google Scholar]
- 4.Dolladille C, Chrétien B, Peyro-Saint-Paul L, Alexandre J, Dejardin O, Fedrizzi S et al (2021) Association between disease-modifying therapies prescribed to persons with multiple sclerosis and cancer: a WHO pharmacovigilance database analysis. Neurotherapeutics 18(3):1657–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Mantia L, Benedetti MD, Sant M, d’Arma A, Di Tella S, Lillini R et al (2021) Cancer risk for multiple sclerosis patients treated with azathioprine and disease-modifying therapies: an Italian observational study. Neurol Sci 42(12):5157–5163 [DOI] [PubMed] [Google Scholar]
- 6.Prosperini L, Haggiag S, Tortorella C, Galgani S, Gasperini C (2021) Age-related adverse events of disease-modifying treatments for multiple sclerosis: a meta-regression. Mult Scler 27(9):1391–1402 [DOI] [PubMed] [Google Scholar]
- 7.Stamatellos V, Siafis S, Papazisis G (2021) Disease-modifying agents for multiple sclerosis and the risk for reporting cancer: A disproportionality analysis using the US Food and Drug Administration Adverse Event Reporting System database. Br J Clin Pharmacol 87(12):4769–4779 [DOI] [PubMed] [Google Scholar]
- 8.Melamed E, Lee MW (2020) Multiple sclerosis and cancer: the ying-yang effect of disease modifying therapies. Front Immunol 10:2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nørgaard M, Veres K, Didden E, Wormser D, Magyari M (2019) Multiple sclerosis and cancer incidence: a Danish nationwide cohort study. Mult Scler Relat Disord 28:81–85 [DOI] [PubMed] [Google Scholar]
- 10.Hauser SL, Kappos L, Montalban X, Craveiro L, Chognot C, Hughes R et al (2021) Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology 97(16):e1546–e1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannopapas V, Stefanou M, Smyrni V, Kitsos DK, Kosmidou M, Stasi S et al (2024) Waist circumference and body mass index as predictors of disability progression in multiple sclerosis: a systematic review and meta-analysis. JCM 13(6):1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannopapas V, Palaiodimou L, Kitsos D, Papagiannopoulou G, Stavrogianni K, Chasiotis A et al (2023) The prevalence of diabetes mellitus type II (DMII) in the multiple sclerosis population: a systematic review and meta-analysis. JCM 12(15):4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Ann Math Statist 21(4):607–611 [Google Scholar]
- 15.Tsivgoulis G, Katsanos AH, Köhrmann M et al (2019) Duration of implantable cardiac monitoring and detection of atrial fibrillation in ischemic stroke patients: a systematic review and meta-analysis. J Stroke 21:302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borenstein M, Higgins JP (2013) Meta-analysis and subgroups. Prev Sci 14:134–143 [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bouc R, Zéphir H, Majed B, Vérier A, Marcel M, Vermersch P (2012) No increase in cancer incidence detected after cyclophosphamide in a French cohort of patients with progressive multiple sclerosis. Mult Scler 18(1):55–63 [DOI] [PubMed] [Google Scholar]
- 19.Kingwell E, Evans C, Zhu F, Oger J, Hashimoto S, Tremlett H (2014) Assessment of cancer risk with β-interferon treatment for multiple sclerosis. J Neurol Neurosurg Psychiatry 85(10):1096–1102 [DOI] [PubMed] [Google Scholar]
- 20.Ragonese P, Aridon P, Vazzoler G, Mazzola MA, Lo Re V, Lo Re M, Realmuto S, Alessi S, D’Amelio M, Savettieri G, Salemi G (2017) Association between multiple sclerosis, cancer risk, and immunosuppressant treatment: a cohort study. BMC Neurol 17(1):155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simbrich A, Thibaut J, Khil L, Berger K, Riedel O, Schmedt N (2019) Drug-use patterns and severe adverse events with disease-modifying drugs in patients with multiple sclerosis: a cohort study based on German claims data. NDT 15:1439–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Amico E, Chisari CG, Arena S, Zanghì A, Toscano S, Lo Fermo S, Maimone D, Castaing M, Sciacca S, Zappia M, Patti F (2019) Cancer risk and multiple sclerosis: evidence from a large Italian cohort. Front Neurol 10:337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M et al (2020) Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol 87(5):688–699 [DOI] [PubMed] [Google Scholar]
- 24.Grytten N, Myhr K, Celius EG, Benjaminsen E, Kampman MT, Midgard R et al (2021) Incidence of cancer in multiple sclerosis before and after the treatment era—a registry-based cohort study. Mult Scler Relat Disord 55:103209 [DOI] [PubMed] [Google Scholar]
- 25.Gil-Bernal R, González-Caballero JL, Espinosa-Rosso R, Gómez-Gómez C (2021) Potential risk of disease modifying therapies on neoplasm development and coadjutant factors in multiple sclerosis outpatients. Sci Rep 11(1):12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosco-Lévy P, Boutmy E, Guiard E, Foch C, Lassalle R, Favary C et al (2023) Risk of cancer with immunosuppressants compared to immunomodulators in multiple sclerosis: a nested case–control study within the French nationwide claims database. Pharmacoepidemiol Drug 32(12):1421–1430 [DOI] [PubMed] [Google Scholar]
- 27.Lebrun C, Rocher F (2018) Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs 32(10):939–949 [DOI] [PubMed] [Google Scholar]
- 28.Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Sorensen PS et al (2015) A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler 21(3):294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263 [DOI] [PubMed] [Google Scholar]
- 30.Kelsey A, Casinelli G, Tandon M, Sriwastava S (2021) Breast carcinoma after ocrelizumab therapy in multiple sclerosis patients: a case series and literature review. J Cent Nerv Syst Dis 13:11795735211037784 (ecollection) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatellos V, Rigas A, Stamoula E, Lallas A, Papadopoulou A, Papazisis G (2022) S1P receptor modulators in multiple sclerosis: detecting a potential skin cancer safety signal. Mult Scler Relat Disord 59:103681 [DOI] [PubMed] [Google Scholar]
- 32.Fang T, Zhang Z, Zhou H, Wu W, Zou L (2023) Multiple sclerosis and breast cancer risk: a meta-analysis of observational and Mendelian randomization studies. Front Neuroinform 17:1154916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cristobal I, Manso R, Rincon R, Carames C, Senin C, Borrero A et al (2014) PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol Cancer Ther 13:938–947 [DOI] [PubMed] [Google Scholar]
- 34.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL et al (2003) Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res 63:5962–5969 [PubMed] [Google Scholar]
- 35.Salles G, Barrett M, Foà R, Maurer J, O’Brien S, Valente N et al (2017) Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther 34:2232–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt M, Böhm D, Von Törne C, Steiner E, Puhl A, Pilch H et al (2008) The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 68:5405–5413 [DOI] [PubMed] [Google Scholar]
- 37.Bennett Saidu NE, Bretagne M, Mansuet AL, Just PA, Leroy K, Cerles O et al (2018) Dimethyl fumarate is highly cytotoxic in KRAS mutated cancer cells but spares non-tumorigenic cells. Oncotarget 9:9088–9099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files.
Data are available from the corresponding author upon reasonable request.