Abstract
Voltage-sensitive dye imaging of mouse thalamocortical slices demonstrated that electrical stimulation of the centrolateral intralaminar thalamic nucleus (CL) resulted in the specific activation of thalamic reticular nucleus, striatum/putamen, and cortical layers 5, 6, and 1. By contrast, ventrobasal (VB) thalamic stimulation, while activating the reticular and basal ganglia nuclei, also activated directly layers 4 and deep 5 of the cortex. Conjoined stimulation of the VB and CL nuclei resulted in supralinear summation of the two inputs at cortical output layer 5, demonstrating coincidence detection along the apical dendrites. This supralinear summation was also noticed at gamma band stimulus frequency (≈40 Hz). Direct stimulation of cortical layer 1, after a radial section of the cortex that spared only that layer, was shown to sum supralinearly with the cortical activation triggered by VB stimulation, providing a second demonstration for coincidence detection. Coincidence detection by coactivation of the specific (VB) and nonspecific (CL) thalamic nuclei has been proposed as the basis for the temporal conjunction that supports cognitive binding in the brain.
A fundamental issue concerning global brain function relates to the mechanism responsible for the unity of perception, given the spatially fractured nature of sensory representation over the cortical mantle (1–10). One possibility is that such binding occurs via temporal coincidence of specific and nonspecific thalamocortical synaptic activation onto the apical dendrites of pyramidal cells (6, 7), resulting in the generation of outgoing axonic action potentials. This outgoing activation returns to the thalamus and establishes a resonant thalamocortical reactivation loop. Because many such loops are simultaneously activated, coherent function can be supported in brain areas remote from each other (6, 7). These large sets of isochronous events, supported by the oscillatory properties of thalamic neurons, can conjoin in time all sensory inputs on the basis of temporal coincidence. In this proposal, the specific thalamic inputs would be constantly updating cortical structures concerning external events (content) while the nonspecific inputs would serve to bind such content information on the basis of internal significance (context) (11). According to this hypothesis, the activation of the thalamus via corticothalamic recurrent activity would serve to maintain a self-supporting feedback activity loop continuously modified by the incoming sensory input.
To test this hypothesis, we used the mouse thalamocortical slice preparation developed previously by Agmon and Connors (12). This in vitro preparation includes the centrolateral (CL) and ventrobasal (VB) thalamic nuclei as well as the somatosensory “barrel” cortex.
Three experimental protocols were developed: (i) CL and VB nuclei were electrically stimulated separately, and the voltage responses evoked at different sites in the brain slice were determined via voltage-sensitive dye-imaging technique (VSDI); (ii) VSDI was implemented during repetitive electrical stimulation of these two thalamic sites at gamma band frequency (≈40Hz) and then during paired stimulation of the two thalamic sites to test for coincidence detection; and (iii) a radial section of the cortical mantle that spared layer 1 was followed by direct electrical stimulation of the cortex such that only the layer 1 activity would cross the cortical gap.
The VSDI response to such cortical activation (protocol iii above) was compared with that after paired activation with VB stimuli as a second test for coincidence detection along the apical dendrite of the large pyramidal cells in layers 5 and 6 of the cortex. Beyond coincidence detection, the powerful coactivation of specific and nonspecific inputs resulted in a temporally tuned return output to the thalamus and, thus, in the generation of recursive dynamic loop activity.
This work was presented previously in an abstract form.†,‡
Materials and Methods
Thalamocortical Slices.
Forty-seven slices from 2- to 4-week-old black mice (C57BL/6, either sex; Taconic Farms) were used in this study. Mice were anesthetized with ketamine (30 mg/kg) and decapitated, and the bone and dura mater covering the cortical surface was carefully peeled away. The New York University Medical Center Institutional Animal Care and Use Committee approved the use and care of animals.
The procedure for preparing thalamocortical slices was modified from the one previously described by Agmon and Connors (12). In brief, brain was dissected along the midline, each hemisphere was glued with the midsagittal plane down onto a 45° ramp attached to the Microslicer stage (Microslicer Model 1500E, Dosaka Em, Kyoto, Japan, distributed in U.S. by Ted Pella, Redding, CA), and 400- to 450-μm-thick slices were cut.
In some experiments, a radial section of the cortical mantle that spared layer 1, was performed in a holding chamber at room temperature that contained artificial cerebrospinal fluid (ACSF), aerated with 95% O2 and 5% CO2. ACSF was composed of 124 mM NaCl/26 mM NaHCO3/1.25 mM NaH2PO4/5 mM KCl/1.2 mM MgSO4/2.4 mM CaCl2/10 mM glucose, pH 7.3; 332 mOsM. Slices were kept for at least 1 h before use at room temperature in ACSF.
Recordings of VSDI.
Individual slices were stained with the voltage-sensitive dye RH 795 (1 mg/ml in ACSF; Molecular Probes). The dye was placed in a static bath continuously aerated with 95% O2 and 5% CO2 for 7 min. The stained slice then was transferred to an interface-type slice chamber at 33°C and continuously perfused with ACSF. Constant current pulses were delivered through stimulus isolator driven by a digital pulse generator (Master 8; Armon Micro-Processors Instruments, Jerusalem). The same pulse generator was used to trigger image acquisition. Bipolar electrodes were used to deliver extracellular stimuli (100–150 μs; 100–200 μA). Intensity of stimulation was adjusted to obtain visible responses and left constant during the experiment.
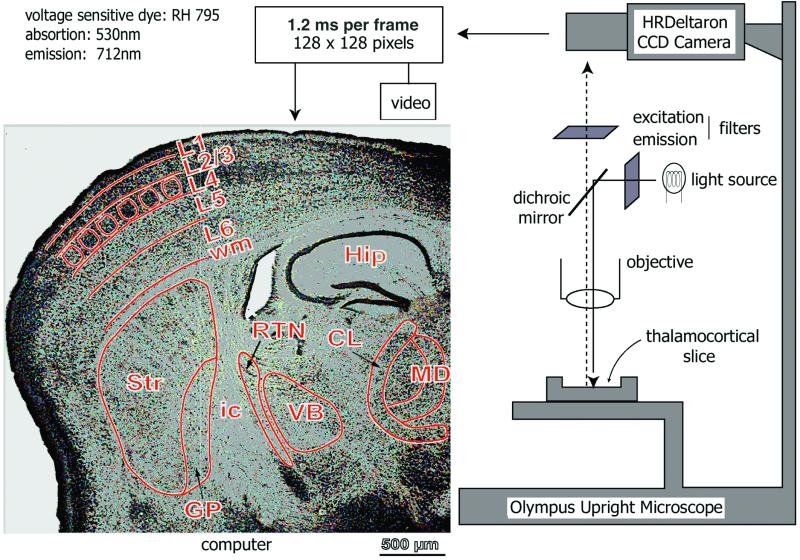
A schematic diagram of the imaging setup is shown in Fig. 1. In brief, the recording chamber, attached to an upright microscope (Olympus BX50WI), was illuminated with a halogen lamp (12 W; light source) driven by a stable power supply (Kepco, Flushing, NY). The microscope was mounted onto a (X-Y) table (Warner Instruments, Hamden, CT). The excitation light reaches the preparation through a band-pass filter (515 ± 35 nm) via a dichroic mirror, and the emitted light returned through a long-pass filter (>590 nm). Optical signals were monitored with a fast, charge-coupled device camera (HR Deltaron 1700; FUJIX, Tokyo) with a 128 × 128-pixel spatial resolution. Images were sampled every 1.2 ms. The total area imaged was 5 mm × 5 mm, and each pixel collected light from a surface of about 39 μm × 39 μm. Optical recordings were monitored with a personal computer. Optical recordings were averaged over either eight or 16 trails, and, for each train, the base fluorescence level (Fo) was initially calculated. Changes in membrane potentials were evaluated as DF/F by using matlab software (Mathworks, Natick, MA). Bleaching and irregularities of staining and illumination were corrected off-line by using matlab-based software. In brief, the optical signals were first detrended to compensate for bleaching of the dye and slow responses from glial cells (13, 14). The signals then were filtered with three-dimensional moving average (3 × 3 × 3) and with a Gaussian low-pass filter. Finally, the optical signals were displayed on the RGB 256 color scale in such a way that their maximum amplitude equaled the maximum red color intensity of the scale.
Figure 1.
Schematic diagram of the imaging set-up. Light from a 12-V halogen source was passed through an excitation filter (515 ± 35 nm), dichroic mirror, and microscope objective (×5) before reaching the slice stained with the voltage-sensitive dye RH-795. Emitted fluorescent light was projected onto a charge-coupled device (CCD) camera after passing through the objective, dichroic mirror, and cut-off filter (>590 nm). The CCD camera (HR Deltaron 1700; FUJIX) consisted of 128 × 128 pixels, and each pixel collected light from a surface of about 39 μm × 39 μm. Images were sampled every 1.2 ms. The optical data were analyzed off-line with matlab-based software. A low-magnification, Nissl-stained image of a thalamocortical slice (50-μm thickness) is shown at left. Nuclei and layer subdivisions were demarcated in green lines. Dorsal is up. Hip, hippocampus; ic, internal capsule; Str, neostriatum; VB, ventrobasal nucleus; CL, centrolateral; MD, mediodorsal; wm, white matter; L1, 2/3, 4, 5, 6, different cortical layers. Borders of the cortical “barrels” are demarcated.
Nissl Staining.
Three-week-old mice were anaesthetized with ketamine (30 mg/kg) and perfused transaortically using 0.2 M phosphate buffer (50 ml) to wash out the circulatory system. The tissue was then fixed by using 4% paraformaldehyde and 0.2% glutaraldehyde in 0.09 M phosphate buffer (for 10 min). The brain was removed, each hemisphere was sectioned with a vibroslicer and resectioned on a cryostat at 30–50 μm. Sections were collected and air-dried overnight on slides and then were stained with cresyl violet, dehydrated in graded alcohols, cleared, and coverslipped.
Nissl-stained slices were photographed with a Nikon digital camera (Coolpix 950) and computer acquired as .jpeg extension files.
Results
Spatial Distribution of Voltage-Dependent Dye Imaging from VB and CL Nuclei.
During the present study, we combined the use of an in vitro thalamocortical slice preparation modified by Agmon and Connors (12) with a fast VSDI recording system (Fig. 1) during the stimulation of specific (VB) and nonspecific (CL) thalamocortical pathways. This technique allowed us to study, with high temporal resolution, the spread of activity after either single or repetitive stimulation of both nuclei, as well as their mutual interaction. Furthermore, after the experiments, we used Nissl-stained thalamocortical slices for a better spatial identification of the multiple nuclei boundaries and the cortical layers present in the thalamocortical slices (Fig. 1).
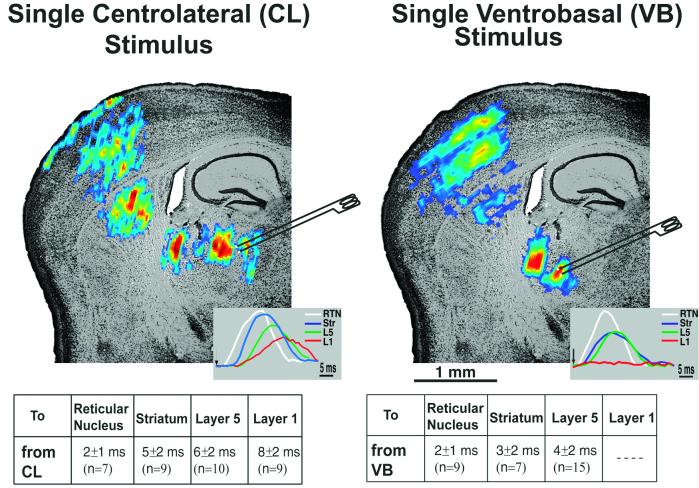
Fig. 2 shows the activation pattern after VB and CL stimulation on top of a Nissl-stained thalamocortical slice. VB stimulation was followed by activation of reticular nucleus, striatum/putamen, and the somatosensory cortex. On the cortex, layer 4 activation was continued by the radial spread of activity to layers 2/3 and 5, as reported (15, §, ¶). The same results were obtained in 17 slices. CL stimulation also was followed by the activation of reticular nucleus and striatum/putamen, but different layers of the cortex (n = 10 slices) were activated. Moreover, in the cortex, larger spreads within cortical layers 5, 6, and 1 were observed. All these optical signals recorded were blocked in nominally Ca2+-free ACSF (data not shown; n = 3 slices), suggesting the postsynaptic nature of these VSDIs.
Figure 2.
Single-pulse stimulation of CL and VB thalamic nuclei. The spread of activity after a single CL (Left) and VB (Right) stimulus is shown superimposed on a Nissl-stained slice. Both CL and VB stimulation activated the reticular nucleus, followed by the striatum. However, at the cortex, different patterns of activation were observed for both stimulations. VB stimulation activated layer 4 followed by layers 2/3 and layer 5; CL stimulation activated layer 6, layer 4–5, and, finally, layer 1. Insets Left and Right correspond to individual pixel profiles at RTN (white lines), striatum/putamen (blue lines), layer 5 (green lines), and layer 1 (red lines) after CL and VB stimulation, respectively. The average delays to different areas of the slice (measured as time between stimulus and the beginning of the individual pixel responses) for both stimulation conditions are shown as a table under each slice.
In conclusion, these results show the presence of a specific and nonspecific connectivity from VB and CL thalamic nuclei to the somatosensory primary cortex, as reported in morphological studies (16–21).
Stimulation of the CL and VB Nuclei at Gamma Band Frequency.
The temporal binding of the specific and nonspecific thalamic nuclei activity in the range of gamma band (≈40 Hz) oscillatory activity has been proposed to be at the basis of the cognitive events (8, 11, 22, 23). Therefore, we studied the spatial spread of activity when either VB or CL nuclei, or both, were stimulated with 40-Hz trains.
Fig. 3 shows the spatial spread of activity of the response to the first and fifth shock of a 40-Hz train of 10 shocks, delivered at CL, VB, and both simultaneously (VB + CL). On the right, voltage-sensitive dye pixel values taken from a spot in layer 5 (Fig. 3A Right, black asterisk) are represented for each stimulation conditions. CL stimulation (Fig. 3A, CL) was able to activate reticular nucleus, striatum/putamen, cortical layers 5 and 6, and layer 1 from the 1st through the 10th pulses (Fig. 3, see pixel values at right) and, at the same time, incrementing the magnitude and spatial spread of activity on the cortex during the successive shocks of the train (to a steady state around fifth stimulus). Also, an increment of activity on reticular nucleus, striatum/putamen, and layers 2/3 and 5/6 was observed when VB was activated individually with the same train of stimuli (Fig. 3A, VB).
Figure 3.
Stimulation of the CL and VB thalamic nuclei at gamma band frequency. (A) Propagation of optically recorded responses in the somatosensory cortex in response to first and fifth stimuli from CL, VB, and both VB and CL thalamic nuclei. Both thalamic nuclei were stimulated at 40 Hz. Optical signals were superimposed on top of a Nissl-stained slice for a better spatial reference. (Right) Profiles of a single pixel taken from layer 5 are shown for the three different stimulation conditions. Pixel profiles are the averages of 16 trains of 10 stimuli at 40 Hz. Numbers in brackets point at the pixel amplitude corresponding to both first and fifth stimuli. (B) Slope estimation of the rising phase of the first and fifth shock optical responses from CL (red line), VB (blue line), and both CL and VB (black line) is shown with discontinuous lines. Values of the slopes are shown on the right.
However, when both VB and CL were stimulated at the same time, a facilitated response was observed in the reticular nucleus and the cortex (Fig. 3A, VB + CL). As Fig. 3B shows, these facilitated responses (solid, black line) resulted from a supralinear summation of the individual VB (solid, red line) and CL (solid, blue line).
Accordingly, the slopes of the rising phase of the responses to the first and fifth shocks from each train, measured from cortical layers 5 and 6, were more than two times higher during the stimulation of VB + CL (1.1% DF/F per ms) compared with either VB (0.4% DF/F per ms) or CL (0.3% DF/F per ms) individually (Fig. 3B). Importantly, the algebraic summation of VB and CL responses had a smaller slope (0.5% DF/F per ms) than the actual response when both were stimulated at the same time (1.1% DF/F per ms). The average slopes calculated as percent DF/F per ms were (mean ± SD): VB, 0.5 ± 0.2 (n = 7); CL, 0.4 ± 0.3 (n = 7); and VB + CL, 0.8 ± 0.2 (n = 7).
These results demonstrate that, at gamma band frequency, the focus of cortical activity increases for the duration of the train, as demonstrated for subcortical white matter stimulation (24). This is clearly observed here for layer 5 and 6 cells, where specific and nonspecific thalamic input coincidence stimulus generates supralinear summation at gamma band frequency that increases during train stimulation.
Single-Pulse Stimulation to Layer 1 and VB at Radially Sectioned Cortex.
A second test to the interactions properties of the specific and nonspecific thalamic inputs to the cortex was obtained when VB and layer 1 stimulation was studied on a somatosensory cortex in which a radial incision was made in the cortical mantle spanning from layers 2/3 to white matter such that only layer 1 activity crossed the cortical gap (n = 20).
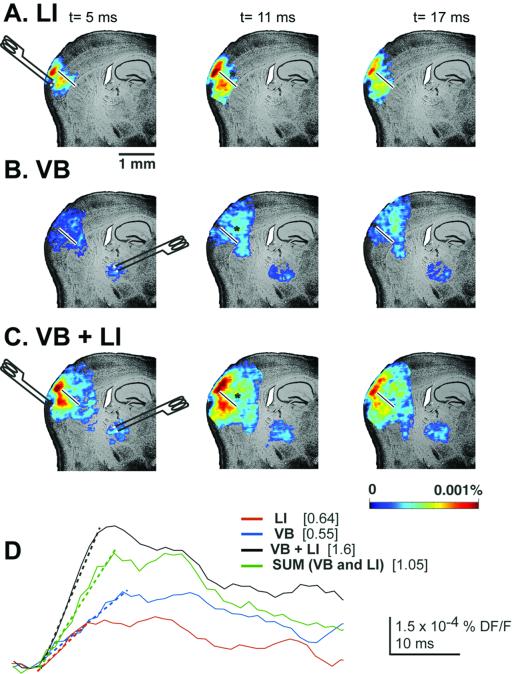
Fig. 4A shows the typical spread of activity in the cut cortex (see white bar) at 5, 11, and 17 ms after single-shock stimulation of VB. In the case of layer 1 (LI) stimulation, a spread of activity through layer 1 was also clearly observable at 11 and 17 ms after stimulus (Fig. 4B). However, when both stimuli were delivered together, a much higher activation of deep layers (Fig. 4C) was observed at different intervals after the stimulus.
Figure 4.
Single-pulse stimulation of layer 1 and VB at radially sectioned cortex. Optically recorded responses are shown in the somatosensory cortex 5, 11, and 17 ms after single stimulation of layer 1 (A), VB (B), and both layer 1 and VB (C). Note how layer 1 stimulation on the other side of the cut allowed the spread of activity only through the upper layers of the cortex. (D) Layer 5 pixel profile for the three different stimulation conditions. The slopes of all of them are shown overlapping the pixel profiles (discontinuous lines).
Fig. 4D shows the voltage-sensitive dye responses taken from a layer 5 spot (see black asterisk) for the three conditions. Again, both VB and LI stimuli elicited a supralinear response (i.e., higher than algebraic summation of the individuals VB and LI) compared with either VB or LI individually. Furthermore, a more than 2-fold increase in the slope of the rising part of the response was observed.
In conclusion, independently of the protocol used, specific and nonspecific inputs to the somatosensory cortex were able to work in a coincidence-detection mode.
Discussion
The present findings indicate that specific and nonspecific thalamocortical inputs sum supralinearly at the cortical level in a time-sensitive fashion. Indeed, coincidence of layer 1 and layer 4 activation is integrated at the cortex most probably over the apical dendrite of the pyramidal cells in layers 5 and 6. This is a central issue because these two layers represent the return output to thalamus (via layer 6) and the main output to the rest of the nervous system (via layer 5). Concerning the thalamocortical projections, the fact that the nonspecific system activates cortical layer 1 is in agreement with the anatomical distributions of such terminal axons (17, 19–21) and addresses the importance of synaptic inputs to the tuft of pyramidal cells, apical dendrites, which reside in that layer (25).
This tuft has been shown experimentally (26), and by computer modeling (27), to be an important site for neuronal integration as it generates because of its particular electrical characteristics, partial dendritic spikes that may sum with proximal synaptic inputs. That VB stimulation generates a well-organized activation of cortical layer 4 has been amply and elegantly demonstrated by using VSDI in the past (15, §, ¶).
We report here that temporal conjunction of layer 1 and layer 4 inputs results in coincidence detection as it had been proposed on theoretical ground (8, 28) and recently reported at the single-cell level (29–31). That such integration occurred by the interaction of specific and nonspecific thalamic coactivation of pyramidal cells, however, had not been demonstrated directly before. This conjunction is significant because it represents a possible mechanism for the global temporal binding required to generate single, cognitive events from the large number of sensory inputs arriving at the brain at any particular time.
Coincidence Activation and Thalamocortical Resonance.
The possibility that coherent electrical activity in the cortex represents the functional basis for cognitive binding has been addressed by several authors (1–7, 9, 10, 23). In particular, it has been proposed that coherent cortical activation is the primary binding substrate (1, 9). Others have proposed that the binding event must not be cortical, but rather thalamocortical (5, 6, 8, 22, 23, 32). The main arguments for the latter reside on neurological grounds. Thus, lesions of the specific thalamic nuclei affect specific brain functions [lateral geniculate nucleus lesion or visual cortex lesion produces local blindness (scotomas) but does not affect other senses]. Midline lesions of the thalamus affect general cognition, resulting in lethargy or coma (33, 34) if the lesion is unilateral hemineglect (35), despite the fact that the specific sensory stimuli are relayed to the cortex. Thus, none of the two thalamocortical circuits alone can support cognition. On the other hand, that the nonspecific thalamic system can support coincidence detection is in agreement with the view that such coincidence is of importance in cognition. It may do so by supporting dynamic thalamocortical recurrent activity specially, because nonspecific thalamic neurons are capable of oscillating at gamma band frequency (36) as are the specific thalamic system neurons (37, 38).
The results also suggest that the basic coinage for cognition is the existence of thalamocortical-resonant columns that can support the complex thalamocortical synchronization and coherence required for global cognitive binding. In this view, the specific system thus would provide the content that relates to the external world and the nonspecific system would give rise to the temporal conjunction, or the context (on the basis of a more interoceptive context concerned with alertness) (23), that would together generate a single cognitive experience.
Conjuction of Specific and Nonspecific Gamma Range-Resonant Activity and Cognitive Binding.
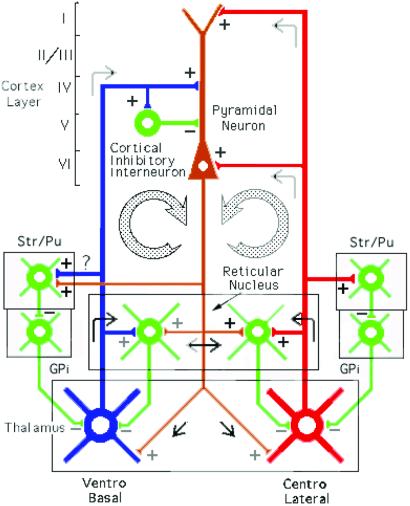
A schematic of a neuronal circuit that may subserve temporal binding is presented in Fig. 5 Left. Gamma oscillations in neurons in VB-specific thalamic nucleus (in blue) establish cortical resonance through direct activation of pyramidal cells (in brown) and feed forward inhibition through activation of 40-Hz inhibitory interneurons in layer 5 (6). These oscillations reenter the thalamus via layer VI pyramidal–cell axon collaterals, producing thalamic-feedback inhibition via the reticular nucleus (39). A second system is illustrated in Fig. 5 Right (in red). Here, the CL intralaminar-nonspecific thalamic nucleus projection to cortical layers 1 and 5 and to the reticular nucleus is illustrated. Layer 5 pyramidal cells return oscillations to the reticular and intralaminar nuclei. The cells in this complex have been shown to oscillate at gamma band frequency (36) and to be capable of recursive activation.
Figure 5.
Thalamocortical circuits proposed to subserve temporal binding; diagram of the two thalamocortical systems. The first loop shows the specific ventrobasal nucleus projecting to layer IV of the cortex and to inhibitory interneurons and collaterally to reticular nucleus and striatum/putamen. The second loop shows the nonspecific centrolateral intralaminar nucleus projecting to layers I and VI and also giving collaterals to reticular nucleus and striatum/putamen. Collaterals of these two thalamocortical projections also produce thalamic feedback inhibition via the reticular nucleus and globus pallidus (GPi). The return pathway (in brown) from deep layers V–VI returns the oscillation to the thalamic reticular, ventrobasal, and centrolateral nuclei.
In conclusion, coincidence activation based on active dendritic conduction along the apical dendrites of pyramidal cells can support thalamocortical coherence. In this fashion, the specific and nonspecific oscillatory inputs, by synchronously summing distal and proximal activity in given pyramidal dendritic elements along the full extent of the cortex, would provide one possible mechanism for temporal global binding.
Acknowledgments
We thank Dr. Diego Contreras for reading the paper and providing his software for the analyses of voltage-sensitive dye imaging. Grant NS13742 from the National Institutes of Health, National Institute of Neurological Disorders and Stroke, supported this work.
Abbreviations
- CL
centrolateral intralaminar thalamic nucleus
- VB
ventrobasal
- VSDI
voltage-sensitive dye imaging
- ACSF
artificial cerebrospinal fluid
Footnotes
Leznik, E., Urbano, F. J. & Llinás, R. (2001) Soc. Neurosci. Abstr. 27, 48.8.
Urbano, F. J., Leznik, E. & Llinás, R. (2001) Soc. Neurosci. Abstr. 27, 48.10.
Contreras, D., Pedroarena, C. M. & Llinás, R. (1998) Soc. Neurosci. Abstr. 24, 55.12.
Pedroarena, C. M., Contreras, D. & Llinás, R. (1998) Soc. Neurosci. Abstr. 24, 55.11.
References
- 1.Crick F, Koch C. Cold Spring Harbor Symp Quant Biol. 1990;55:953–962. doi: 10.1101/sqb.1990.055.01.089. [DOI] [PubMed] [Google Scholar]
- 2.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitbock H J. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 3.Gray C M, Konig P, Singer W. Nature (London) 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 4.Gray C M, Singer W. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llinás R. In: Fidia Research Foundation Neuroscience Award Lectures. Changeux J-P, Llinás R R, Purves D, Bloom F, editors. Vol. 4. New York: Raven; 1990. pp. 1–10. [Google Scholar]
- 6.Llinás R, Pare D. Neuroscience. 1991;51:245–258. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 7.Llinás R, Ribary U. In: Induced Rhythms in the Brain. Basar E, Bullock T, editors. Basel: Birkhauser; 1992. pp. 147–154. [Google Scholar]
- 8.Llinás R, Ribary U, Contreras D, Pedroarena C. Philos Trans R Soc London B. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer W. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 10.von der Malsburg C. In: Models of Neural Networks II. Domany E, van Hemmen J L, Schulten K, editors. Berlin: Springer; 1994. pp. 95–119. [Google Scholar]
- 11.Llinás R R, Ribary U, Joliot M, Wang X-D. In: Temporal Coding in the Brain. Buzaki G, Llinás R, Singer W, Berthoz A, Christen Y, editors. Berlin: Springer; 1994. pp. 251–272. [Google Scholar]
- 12.Agmon A, Connors B W. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 13.Konnerth A, Obaid A L, Salzberg B M. J Physiol (London) 1987;393:681–702. doi: 10.1113/jphysiol.1987.sp016848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lev-Ram V, Grinvald A. Proc Natl Acad Sci USA. 1986;83:6651–6655. doi: 10.1073/pnas.83.17.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laaris N, Carlson G C, Keller A. J Neurosci. 2000;4:1529–1537. doi: 10.1523/JNEUROSCI.20-04-01529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agmon A, Yang L T, O'Dowd D, Jones E G. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berendse H W, Groenewegen H J. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- 18.Deschênes M, Veinante P, Zhang Z-W. Brain Res Rev. 1998;28:286–308. doi: 10.1016/s0165-0173(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 19.Herkenham M. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- 20.Jones E G, editor. The Thalamus. New York: Plenum; 1985. pp. 85–150. [Google Scholar]
- 21.Lorente de Nó R. In: Physiology of the Nervous System. Fulton J F, editor. New York: Oxford Univ. Press; 1938. pp. 291–325. [Google Scholar]
- 22.Llinás R, Ribary U. Proc Natl Acad Sci USA. 1993;90:2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llinás R, Pare D. In: The Mind-Brain Continuum. Llinás R, Churchland P S, editors. Cambridge, MA: MIT Press; 1996. pp. 1–18. [Google Scholar]
- 24.Contreras D, Llinás R R. J Neurosci. 2001;21:9403–9413. doi: 10.1523/JNEUROSCI.21-23-09403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cajal S-R Consejo Superior de Investigaciones Científicas, editors. Histologie du système nerveux de l'homme et des vertebrés. 2nd Ed. Madrid: Instituto Ramon y Cajal; 1972. pp. 519–598. [Google Scholar]
- 26.Cauller L J, Connors B W. J Neurosci. 1994;14:751–762. doi: 10.1523/JNEUROSCI.14-02-00751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes P A, Llinás R R. J Physiol (London) 2001;1:167–187. doi: 10.1111/j.1469-7793.2001.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llinás R R, Ribary U. Adv Neurol. 1998;77:95–103. [PubMed] [Google Scholar]
- 29.Berger T, Larkum M E, Lüscher H-R. J Neurophysiol. 2001;83:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- 30.Gil Z, Amitai Y. J Neurosci. 1996;16:6567–6578. doi: 10.1523/JNEUROSCI.16-20-06567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkum M E, Zhu J J, Sakmann B. Nature (London) 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- 32.Joliot M, Ribary U, Llinás R. Proc Natl Acad Sci USA. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Façon E, Steriade M, Wertheim N. Rev Neurol. 1958;98:117–133. [PubMed] [Google Scholar]
- 34.Castaigne P, Buge A, Escourolle R, Masson M. Rev Neurol. 1962;106:357–367. [PubMed] [Google Scholar]
- 35.Heilman K M, Balenstein E. In: Clinical Neuropsychology. Heilman K M, Valenstein E, editors. London: Oxford Univ. Press; 1993. pp. 279–336. [Google Scholar]
- 36.Steriade M, Curró-Dossi R, Contreras D. Neuroscience. 1993;56:1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- 37.Pedroarena C M, Llinás R. Proc Natl Acad Sci USA. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedroarena C M, Llinás R. Thalamus Rel Syst. 2001;1:3–14. [Google Scholar]
- 39.Steriade M, Parent A, Hada J. J Comp Neurol. 1984;229:531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]