Abstract
In Neurospora crassa vegetative hyphae, chitin, β-1,3-glucan (laminarin), and a mixed β-1,3−/β-1,4-glucan (lichenin) are the major cell wall polysaccharides. GH72 enzymes have been shown to function as β-1,3-glucanases and glucanosyltransferases and can function in crosslinking β-1,3-glucans together. To characterize the enzymatic activities of the N. crassa enzymes, we expressed GEL-1 with a HIS6 tag in N. crassa. A chimeric maltose binding protein:GEL-2 was produced in E. coli. Purified GEL-1 and GEL-2 were used to characterize their enzymatic activities. We employed thin-layer chromatography (TLC) and polyacrylamide carbohydrate gel electrophoresis (PACE) assays to visualize GEL-1 and GEL-2 hydrolase and transferase activities on lichenin and laminarin substrates. We determined that GEL-1 functions as a laminarinase (β-1,3-glucanase) and as a laminarin transferase. We found that GEL-2 can function as a laminarinase and as a licheninase (β-1,3−/β-1,4-mixed-glucanase) and can crosslink β-1,3-glucans together. We demonstrated that GEL-2 can form enzyme:lichenin intermediates, providing evidence that GEL-2 functions as a lichenin transferase as well as a β-1,3-glucan transferase and crosslinks both types of polysaccharides into the N. crassa cell wall.
Keywords: GH72 family, Lichenin transferase, Laminarin transferase, Fungal cell wall, Neurospora crassa
Highlights
-
•
GH72 family glucan hydrolases/glucan transferases are essential for the formation of a functional N. crassa cell wall.
-
•
Purified N. crassa GEL-1 (NCU08909) has β-1-3-glucan hydrolase and β-1,3-glucan transferase activities.
-
•
Purified N. crassa GEL-2 (NCU07253) has β-1-3-glucan hydrolase, β-1,3-glucantransferase, lichenin hydrolase, and lichenin transferase activities.
-
•
Purified GEL-2 forms enzyme:lichenin and enzyme:β-1,3-glucan intermediates.
-
•
GEL-2 functions in the incorporation of lichenin, a major cell wall polysaccharide, into the N. crassa cell wall.
1. Introduction
The cell wall is an important structural element for plant, bacteria, and fungal cells. The fungal wall contains a diverse array of polysaccharides and glycoproteins. It is a matrix of crosslinked β-1,3-glucan, chitin, and glycoproteins (Patel and Free, 2019; Klis et al., 2006; Lesage and Bussey, 2006; Latge, 2007; Gow et al., 2017; Gow and Lenardon, 2023). Most fungal cell walls also contain additional polysaccharides that are crosslinked into the cell wall matrix, such as β-1,6-glucan, lichenin (a mixed β-1,3/1,4-glucan), α-1,3-glucan, and chitosan (Ruiz-Herrera and Ortiz-Castellanos, 2019). The synthesis of cell wall polysaccharides and glycoproteins is regulated during the development of asexual spores and fruiting bodies to generate cell type-specific cell wall compositions (Ao and Free, 2017; Mazan et al., 2011). Cell wall composition and structure are also regulated in response to environmental changes. In pathogenic fungi, cell wall components are recognized as PAMPs and can play important roles in virulence, pathogenesis and host interactions (Gow et al., 2017). The cell wall matrix plays a critical role in the generation of cell morphology and is needed for generating turgor pressure. Cell wall polysaccharides are synthesized by polysaccharide synthases located in the plasma membrane and extruded into the cell wall space during their synthesis. The synthesis, secretion, and incorporation of cell wall glycoproteins into the cell wall play a central role in cell wall biogenesis. Cell wall glycoproteins have been shown to function as structural elements, adhesins, receptors for cell signaling pathways, and in nutrient acquisition. Among the most important functions that can be ascribed to cell wall glycoproteins is the enzymatic crosslinking of cell wall polysaccharides and glycoproteins together to generate the matrix structure characteristic of a cell wall.
Two families of glycosyl hydrolases, GH17 enzymes and GH72 enzymes, have been shown to have β-1,3-glucanase and glucanosyltransferase activities (Gastebois et al., 2010a; Mouyna et al., 1998; Goldman et al., 1995). The GH72 enzymes function in crosslinking β-1,3-glucans together (Aimanianda et al., 2017; Blatzer et al., 2020; Mazan et al., 2011; Mouyna et al., 2000). In N. crassa the GH72 enzymes have been found to be critical for cell wall formation (Ao and Free, 2017). These enzymes cleave a β-1,3-glucan (laminarin) at a location near the reducing end and form an enzyme:glucan intermediate. As transferases, they have been shown to transfer the attached glucan to another β-1,3-glucan. During the transfer reaction they can form new β-1,3-bonds to elongate a glucan and β-1,6-bonds to form branched glucans (Aimanianda et al., 2017). These enzymes are unique to fungi, consistent with their highly specialized role in fungal cell wall biogenesis. Because of their importance in crosslinking the fungal cell wall together, characterization of GH72 enzymes could be helpful in designing anti-fungal agents.
The GH72 glucanosyltransferases can be placed into two sub-families. Sub-family 1 enzymes contain the transferase catalytic domain followed by a carbohydrate binding domain (a CBM43 domain) at the carboxyl end of the protein. The CBM43 domain plays a role in the recognition and binding of the β-1,3-glucan for hydrolysis and transfer to a second β-1,3-glucan (Aimanianda et al., 2017). The enzyme activities of S. cerevisiae Gas1p and A. fumigatus Gel4p, two glucanosyltransferases from sub-family 1, have been found to be dependent upon the presence of both domains (Aimanianda et al., 2017). Sub-family 2 enzymes contain the glucanosyltransferase catalytic domain but lack the CBM43 domain. In N. crassa, the GEL-1 transferase encoded by NCU08909 is a sub-family 1 enzyme with a C-terminus CBM43 domain. The other N. crassa GH72 transferases are sub-family 2 enzymes.
The N. crassa genome encodes five GH72 enzymes (Ao and Free, 2017; Kamei et al., 2013). The annotated sequence from FungiDB for one of these genes, NCU06850 (GEL-4), shows that it is expressed during the sexual stage of the life cycle. A second GH72 enzyme, NCU01162 (GEL-3), is highly expressed during asexual and sexual development, and deletion mutants have a conidiation mutant phenotype (Ao and Free, 2017). The other three enzymes (NCU08909/GEL-1, NCU07253/GEL-2, and NCU06781/GEL-5) are expressed during vegetative growth (Ao and Free, 2017). The deletion of a single one of these three GH72 genes in N. crassa does not affect the growth and morphology of the fungus, but if all three of these vegetatively expressed GH72 genes are deleted the fungus has a dramatic growth and morphology phenotype (Ao and Free, 2017; Patel and Free, 2022). Instead of growing as a filamentous fungus, the mutant grows with a tight colonial morphology. This demonstrates that the glucanosyltransferases encoded by GEL-1, GEL-2 and GEL-5 are functionally redundant. The dramatic growth and morphology defect associated with the loss of these glucanosyltransferases highlights the importance of the GH72 enzymes in generating the cell wall matrix.
In the present study, we further characterize the enzymatic activities of two N. crassa GH72 enzymes on laminarin (β-1,3-glucan) and lichenin (mixed β-1,3−/β-1,4-glucan). We show that purified N. crassa GEL-1, a sub-family 1 enzyme with a carboxyl terminus CBM43 domain, functions specifically as a β-1,3-glucan hydrolase and transferase and does not function as a lichenin hydrolase and transferase. We show that purified N. crassa GEL-2, a sub-family 2 enzyme lacking the CBM43 domain, hydrolyzes β-1,3-glucan and lichenin. We also demonstrate that GEL-2 can form enzyme:β-1,3-glucan and enzyme:lichenin intermediates, providing evidence that GEL-2 functions to crosslink β-1,3-glucan and lichenin polysaccharides into the N. crassa cell wall.
2. Materials and methods
2.1. Materials, strains, and growth conditions
Single mutant isolates for GH72 family enzymes (ΔNCU08909, ΔNCU07253 or ΔNCU06781) were procured from the Fungal Genetic Stock Center (Colot et al., 2006). All the strains were maintained in Vogel's 2 % sucrose agar medium (Davis and DeSerres, 1970). A triple mutant isolate with deletions for all three GH72 members was generated previously in the laboratory (Ao and Free, 2017). Polysaccharides released into a Vogel's 2 % sucrose liquid medium from the triple mutant were isolated by precipitation with 70 % ethanol and used as substrates for the glucanosyltransferase assays. Laminaribiose, laminaritriose, laminaritetraose, laminaripentaose, and laminarihexaose were obtained from Neogen (Lansing, MI, USA). Lichenin was purchased from InvivoGen (San Diego, CA, USA). General lab chemicals, maltodextrin, and laminarin were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Cloning, expressing and purifying GH72 enzymes
Codon-optimized versions of NCU08909 (GEL-1) and NCU07253 (GEL-2) for expression in E. coli were designed and produced by Genewiz (Waltham, MA, USA). The genes were cloned into the pMAL-p2 vector and transformed into E. coli for production of maltose binding protein chimeric proteins (MBP:GEL-1 and MBP:GEL-2 fusion proteins) (Patel and Free, 2022). The expressed MBP:GEL-2 fusion protein was purified with an amylose resin column according to the manufacturer's specification (New England BioLabs, Ipswich, MA, USA). The expression and purification of MBP:GEL-2 has been previously reported (Patel and Free, 2022).
We cloned a HIS6-tagged version of GEL-1 for expression in N. crassa (Figs. S1 and S2). The N. crassa gene was PCR amplified from wildtype genomic DNA and inserted into the pMF272 vector (Bowman et al., 2009). The forward and reverse primers were designed to amplify the coding region from the N-terminus up to a position just before the omega site in the GPI anchor target sequence used for the attachment of the GPI anchors to the proteins. The reverse primer included a HIS6 tag sequence to place the HIS6 sequence at the carboxyl terminus of the protein. The amplified NCU08909 gene was digested by XbaI and XmaI and inserted into the pMF272 vector (Bowman et al., 2009). The resultant plasmid contains the ccg-1/grg-1 promoter upstream of the coding regions for expressing the proteins at high levels in N. crassa. This vector also contains coding sequences from the his-3 gene and a region of the his-3 gene 3′ utr. These sequences allow for the homologous insertion of the plasmid into a site within the his-3 gene 3′ utr region (Bowman et al., 2009). When transforming a his-3 mutant strain, the plasmid allows the targeted insertion of the gene of interest at his-3 gene locus in the genome and transforms the mutant from a histidine auxotroph into a prototroph to facilitate the isolation of transformants. The vector for expression of GEL-1 in N. crassa was sequenced at Azenta (Waltham, MA, USA) to verify that it contained the desired sequences. The vector was used to transform N. crassa using the protocol described by Margolin et al. (1997) and N. crassa transformants were isolated on Vogel's 2 % sorbose plates (Davis and DeSerres, 1970). The synthesis of a HIS6-tagged GH72 enzyme by the transformants was evaluated by western blot experiments using an anti-HIS6 antibody from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). The HIS6-tagged GEL-1 was purified from transformant cell extracts using ProBond resin from Thermo Fisher (Carlsbad, CA, USA) according to the manufacturer's protocol. The proteins were eluted from the ProBond resin column with a linear gradient of imidazole from 25 mM to 250 mM. Peak elution fractions, as determined by a western blot, were then used for characterizing the enzymes as glycosylhydrolases and glycosyltransferases. Proteomic analysis of the purified GEL-1 was carried out at the Complex Carbohydrate Research Center at the University of Georgia (Athens, GA, USA). The identification of proteins within the purified GEL-1 sample was carried out by trypsin digestion followed by a LC-ESI-MS-MS analysis.
2.3. Assays for GH72 glycosylhydrolase activity
The hydrolase activity of GH72 enzymes were analyzed on Thin Layer Chromatography (TLC) and by polyacrylamide carbohydrate gel electrophoresis (PACE). To assay for hydrolase activity, an enzyme reaction was set up containing 22 μl of polysaccharide or oligosaccharide substrate, 3 μl of 1 M sodium-acetate (pH 5.6) and 5 μl of purified enzyme in a microfuge tube. The reaction was incubated for 24 h at room temperature. Polysaccharide and oligosaccharide substrates included 10 mg/ml laminarin, 10 mg/ml lichenin, 1 mg/ml laminaribiose, 1 mg/ml laminaritriose, 1 mg/ml laminaritetraose, 1 mg/ml laminaripentaose, 1 mg/ml laminarihexaose, and polysaccharides released into the medium by a ∆gel-1, ∆gel-2, ∆gel-5 triple mutant. After incubation, the enzyme reaction mixture was spotted onto a Silica 60 TLC plate (Merck, Damstadt, Germany) and subjected to chromatography with n-butanol:acetic acid:H2O solvent (2::1::1) for 3.5 h. After drying the plate, the presence of sugars was determined by spraying the plate with a solution of 1 % orcinol in ethanol:H2SO4 (70:3). The plate was heated at 100o C in an oven for 15 min to develop the stain for sugars.
To look at hydrolase activity using PACE, the protocol from Pidatala et al. was followed (Pidatala et al., 2017). Briefly, the hydrolase assay was performed as described above, and the assay samples were dried in a speed vac and derivatized by adding 5 μl of 0.2 M ANTS (8-aminonaphthalene-1,3,6-trisulfonic acid)(Invitrogen, Eugene, OR, USA) in a H2O:acetic acid:DMSO buffer (17:3:20), 5 μl of 0.2 M 2-picoline borane in DMSO, and 10 μl of H2O:acetic acid:DMSO buffer (17:3:20) to the sample. The sample was incubated at 37o C for 16 h in the dark to allow for derivatization. The derivatization attaches the negatively charged ANTS to the reducing end of the polysaccharide and renders the polysaccharide fluorescent. The sample was then dried again in the speed vac and resuspended in 100 μl of 3 M urea. An aliquot of the sample was loaded onto a 15 % polyacrylamide gel and subjected to electrophoresis in the dark at 100 V with a gel and running buffer of 100 mM Tris-Borate, pH 8.2. The derivatized oligosaccharides and polysaccharides were visualized using UV light. A derivatized maltodextrin ladder sample was used to estimate the sizes of the derivatized oligosaccharides.
2.4. Assay for GH72 glycosyltransferase activity
To assay for the ability of the enzymes to transfer a polysaccharide donor to a polysaccharide acceptor we used the TLC and PACE assays described above for observing glycosylhydrolase activity. In assays using laminaritetraose, laminaripentaose, and laminarihexaose as substrates, the formation of an oligosaccharide larger than the substrate being evaluated was used as evidence that a transferase reaction had occurred to generate the larger oligosaccharide.
2.5. Assay for the formation of enzyme:Polysaccharide intermediates
Glycosyltransferases function by cleaving a donor polysaccharide substrate, forming a covalent bond between the donor polysaccharide and the enzyme in an enzyme:polysaccharide intermediate, and then transferring the donor polysaccharide to an acceptor polysaccharide. The formation on an enzyme:polysaccharide intermediate implies that an enzyme is a glycosyltransferase. To assay for the formation of an enzyme:polysaccharide intermediate, the purified glucanosyltransferases were incubated with a polysaccharide or oligosaccharide and then subjected to a western blot analysis with antibody directed against the polysaccharide. The lichenin and polysaccharides from the triple mutant growth medium used for the analysis were treated with 0.5 M NaBH4 in 1 M NaOH at 65 °C for 1 h and the reaction was terminated by adding glacial acetic acid until a pH of 5.0 was reached. To assess the formation of an enzyme:lichenin intermediate, a reaction assay was set up with 13 μl of 10 mg/ml lichenin or polysaccharides from the ∆gel-1, ∆gel-2, ∆gel-5 triple mutant, 2 μl of 1 M sodium acetate pH 5.0, and 5 μl of purified GEL-1 or GEL-2 and incubated at room temperature for 24 h. The reaction was stopped by the addition of 10 μl of 4× NuPAGE SDS sample buffer (Thermo Fisher, Carlsbad, CA, USA) and heated to 90o C for 15 min. The sample was loaded on 12 % SDS-PAGE gel and subjected to electrophoresis at 120 V. The glycoproteins were then transferred to a nitrocellulose filter. The filter was blocked overnight in 5 % non-fat milk in PBS and subjected to a western blot analysis. The blot was developed using primary monoclonal mouse antibodies directed against lichenin (BioSupplies Australia Pty Ltd., Victoria, Australia) and secondary HRP-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA). The presence of lichenin attached to the denatured enzyme on the nitrocellulose filter was taken as evidence of an enzyme lichenin intermediate. The assay for presence of an enzyme:laminarin intermediate was similar to that for lichenin except that 10 mg laminarin was used instead of lichenin as a polysaccharide donor and an anti-laminarin antibody (BioSupplies Australia Pty Ltd., Victoria, Australia) was used as a monoclonal primary antibody in the western blot.
3. Results
3.1. GH72 enzymes are needed for vegetative growth
Three of the five GH72 enzymes encoded in N. crassa are expressed in the vegetative hyphae and a deletion mutant lacking all three of these enzymes grows slowly with a tight colonial phenotype (Ao and Free, 2017). Fig. 1 panel A shows Petri dishes with a wildtype isolate and a ∆gel-1, ∆gel-2, ∆gel-5 triple deletion mutant at two days post inoculum. The wildtype isolate grew across the Petri dish within the first 24 h and produced abundant conidia by two days, while the triple deletion mutant barely grew past the inoculation site. Fig. 1 panel B shows the hyphal morphology at the growing edge of the colony and demonstrates that the ∆gel-1, ∆gel-2, ∆gel-5 triple deletion mutant is unable to extend hyphae over the substratum.
Fig. 1.
Phenotype of ∆gel-1, ∆gel-2, ∆gel-5 mutant. Panel A. Wildtype and mutant conidia were placed in the middle of Petri dishes containing Vogel's sucrose agar growth medium and allowed to grow for 2 days. Panel B. The growing edges of wildtype and mutant colonies were photographed after 12 h of growth on Vogel's sucrose agar. The hyphae from the mutant are unable to extend across the medium. Scale bar represents 1 mm.
3.2. Characterization of GEL-1 as a β-1,3-glucananse and a β-glucanosyltransferase
To characterize the enzymatic activity of GEL-1 (NCU08909), a codon-optimized version of the gene was cloned and expressed in E. coli as a maltose-binding protein MBP:GEL-1 chimeric protein. The MBP:GEL-1 produced in E. coli was found in inclusion bodies and efforts to solubilize the chimeric protein were unsuccessful. Thus, the gel-1 gene with a HIS6 tag replacing the GPI-anchor was expressed in N. crassa. The HIS6-tagged version of GEL-1 was isolated from cell extracts as indicated in the materials and methods section. Fig. 2 shows a Coomassie-stained polyacrylamide gel and western blot of the purified GEL-1 at the expected molecular weight of approximately 65 kDa. The gel shows the presence of some higher molecular weight proteins, and a proteomic analysis of the sample suggested that it contained three proteins involved in the modification and folding of ER glycoproteins, NCU03982 (gpr-78/hsp70), NCU09265 (cnx-1/calreticulin) and NCU02349 (gt24–1/UDP-glucose glycoprotein glycosyltransferase), which might be co-purifying with GEL-1 because of their roles in assisting with GEL-1 modification and folding.
Fig. 2.
Purification profile of GEL-1 using Ni-NTA column. Panel A shows a Coomassie-stained gel of the purification and Panel B shows a western blot using antibody directed against the HIS6 tag. Lane 1: Cell lysate from control cells not expressing a HIS6-tagged protein. Lane 2: MW markers. Lane 3: cell extract containing GEL-1:HIS6. Lane 4: unbound protein after passage through the affinity column. Lanes 5–10: Elution fractions 2, 6, 9, 10, 14 and 18, respectively. GEL-1:HIS6 was eluted at the expected size of ∼65 kDa (arrow) and elution fraction 6 was used for enzymatic studies.
The ability of the purified GEL-1 to function as a glycosylhydrolase was tested by incubating the purified GEL-1 with oligosaccharide and polysaccharide substrates (laminaritriose, laminaritetraose, laminaripentaose, laminarihexaose, laminarin (β-1,3-glucan), and lichenin). After allowing the enzyme to digest these substrates, the samples were evaluated for the presence of digestion products using a TLC assay (Fig. 3). The purified enzyme was able to release smaller oligosaccharides from laminaritetraose (lane 4 blue arrows), laminaripentaose (lane 6 blue arrows) and laminarihexaose (lane 8 blue arrows). As expected, we found that the purified enzyme was able to digest laminarin and release small oligosaccharides (lane 11 blue arrows). We did not find evidence for purified GEL-1 being able to digest lichenin (lane 13).
Fig. 3.
Thin layer chromatography (TLC) analysis demonstrating the hydrolase and transferase activities of purified GEL-1. Lanes 1 and 2 contain laminaritriose without enzyme (lane 1) and with GEL-1 (lane 2). Lanes 3 and 4 contain laminaritetraose without (lane 3) and with GEL-1 (lane 4). Lanes 5 and 6 contain lamarinipentaose without (lane 5) and with GEL-1 (lane 6). Lanes 7 and 8 contain laminarihexaose without (lane 7) and with GEL-1 (lane 8). Lane 9 contains ladder laminarinoses (biose to hexaose). Lanes 10 and 11 contain laminarin without enzyme (lane 10) and with GEL-1 (lane 11). Lanes 12 and 13 contain lichenin without enzyme (lane 12) and with GEL-1 (lane 13). Lane 14 contains GEL-1 enzyme without polysaccharides. Blue arrows indicate hydrolytic products, and the black arrows indicate transferase products after enzymatic action by the purified enzyme. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
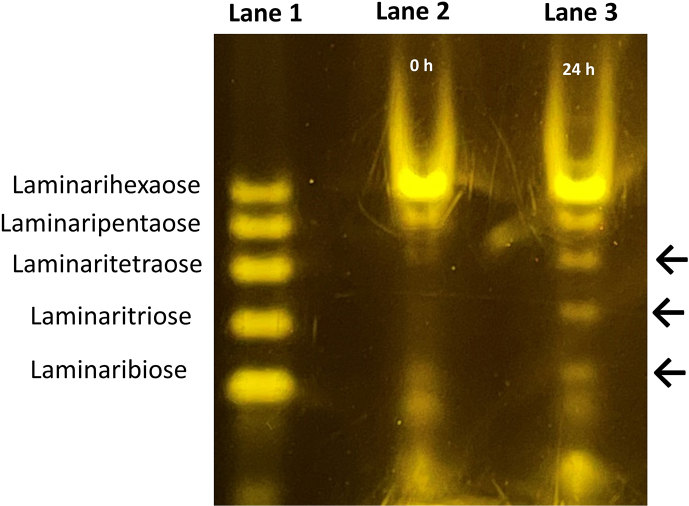
To further characterize the hydrolytic activity of GEL-1, we analyzed the results of GEL-1 hydrolytic assay with the PACE assay. As shown in Fig. 4, incubation of GEL-1 with laminarihexaose generated laminaribiose, laminaritriose, and laminaritetraose digestion products (arrows in Fig. 4). We also used a TLC assay to further characterize the GEL-1 digestion products from the polysaccharides found in the medium from the ∆gel-1, ∆gel-2, ∆gel-5 mutant (arrows in Fig. 5). We found that GEL-1 was able to release laminaribiose and laminaritriose from the N. crassa polysaccharides, consistent with the results seen in Fig. 3. Our results demonstrate that the enzyme recognizes and hydrolyzes β-1,3-glucans with four or more sugars and releases disaccharide and trisaccharide products. The PACE assay with laminarihexaose showing the release of laminaribiose, laminaritriose, and laminaritetraose (Fig. 4) indicates that GEL-1 can cleave laminarihexaose at a position of 2 or 3 sugars from the end of the oligosaccharide.
Fig. 4.
Hydrolase activity of GEL-1 on laminarihexaose (1 mg/ml) using polyacrylamide carbohydrate gel electrophoresis (PACE). Lane 1 contains laminarinose ladder. Lane 2 contains the undigested laminarihexoase prior to the addition of GEL-1 (0 h). Lane 3 shows the presence of digestion products from GEL-1 enzyme activity (24 h). Black arrows indicate the hydrolytic products due to enzymatic action of GEL-1 purified protein.
Fig. 5.

Thin layer chromatography (TLC) analysis of products generated by digestion of ΔGH72 polysaccharides by purified GEL-1. Lane 1 shows maltodextrin marker (MD). Lane 2 contains undigested polysaccharides prior to the addition of GEL-1 (Control/0 h). Lane 3 shows the small oligosaccharide digestion products (Assay/24 h). The arrows point to the digestion products.
The assays in which the purified enzyme was incubated with laminaritetraose, laminaripentaose, and laminarihexaose also provided evidence that GEL-1 can function as a glucanosyl transferase. In the TLC assays for these substrates, we found the presence of larger oligosaccharides which could only be formed as a result of transferase activity. The black arrows in Fig. 3 point to these larger oligosaccharides. The formation of these larger oligosaccharides demonstrates that the enzyme can use the substrate β-1,3-glucan as a donor polysaccharide and as an acceptor polysaccharide to create a larger β-1,3-glucan. A time course of the digestion of laminarihexaose by GEL-1 showed that the release of smaller oligosaccharides from hydrolase activity (Fig. 6, blue arrows) and the formation of a larger oligosaccharide from transferase activity (Fig. 6, black arrow) occurs slowly over the 24-h assay period (Fig. 6).
Fig. 6.
Time-course assay for purified GEL-1 enzymatic activity on laminarihexaose from 0 h to 24 h indicating that the hydrolase activity of GEL-1 can be seen as soon as 2 h and transferase activity can be detected after 8 h of incubation. LD: ladder laminarinoses (biose, triose, tetraose, pentaose and hexaose). 0 h (prior to addition of enzyme) through 24 h: Assay timepoints. Blue arrows indicate hydrolase activity, and the black arrow indicates transferase activity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Characterization of GEL-2 as a laminarinase/licheninase and a laminarin/lichenin transferase
In order to characterize GEL-2, a codon-optimized version of the gene was used to generate a chimeric MBP:GEL-2 gene and was expressed in E. coli. The initial cloning and purification of the expressed MBP:GEL-2 has been previously reported (Patel and Free, 2022). To assess hydrolase activity from GEL-2, we used our TLC assay. We incubated the purified MBP:GEL-2 with oligosaccharide and polysaccharide substrates (laminaritriose, laminaritetraose, laminaripentaose, laminarihexaose, laminarin (β-1,3-glucan), and lichenin) and looked for the release of small oligosaccharides (Fig. 7). We found that GEL-2 had hydrolase activity on laminaripentaose (lane 6 blue arrow) and laminarihexaose (lane 8 blue arrow), We also found that GEL-2 released smaller oligosaccharides from the laminarin (lane 11 blue arrows). We were unable to detect any differences in the laminarin digestion patterns produced by GEL-1 and GEL-2 for laminarin substrates. The TLC assay also showed that GEL-2 was capable of digesting lichenin and releasing smaller oligosaccharides from this substrate (lane 13 blue arrows), a hydrolytic activity not seen in GEL-1 (Fig. 3, lane 13). We conclude that GEL-2 can hydrolyze lichenin as well as laminarin polysaccharides. Because shorter lichenin oligosaccharides are not commercially available, we were unable to further characterize which linkage(s) in the lichenin polysaccharide (a repeating polymer of β-1,4-glucose-β-1,4-glucose-β-1,3-glucose units) GEL-2 might be cleaving.
Fig. 7.
Thin layer chromatography (TLC) analysis demonstrating the hydrolase and transferase activities of purified GEL-2. Lanes 1 and 2 contain laminaritriose without enzyme (lane 1) and with GEL-2 (lane 2). Lanes 3 and 4 contain laminaritetraose without (lane 3) and with GEL-2 (lane 4). Lanes 5 and 6 contain lamarinipentaose without (lane 5) and with GEL-2 (lane 6). Lanes 7 and 8 contain laminarihexaose without (lane 7) and with GEL-2 (lane 8). Lane 9 contains ladder laminarinoses (biose to hexaose). Lanes 10 and 11 contain laminarin without enzyme (lane 10) and with GEL-2 (lane 11). Lanes 12 and 13 contain lichenin without enzyme (lane 12) and with GEL-2 (lane 13). Lane 14 contains GEL-2 enzyme without polysaccharides. Blue arrows indicate hydrolytic products, and the black arrows indicate transferase products after enzymatic action by the purified enzyme. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
GEL-2 TLC assays (Fig. 7) where the purified enzyme was incubated with laminaripentaose and laminarihexaose provides evidence that GEL-2 is a glucanosyltransferase. The TLC assays for these substrates show that larger oligosaccharides were generated when GEL-2 was incubated with these substrates. The larger oligosaccharides could only be formed as a result of glucanosyltransferase activity. The black arrows in Fig. 7 point to these larger oligosaccharides. The formation of these larger oligosaccharides demonstrates that the enzyme can use the substrate β-1,3-glucan as a donor polysaccharide and as an acceptor polysaccharide to create a larger β-1,3-glucan.
To further demonstrate that GEL-2 functions as a transferase, we looked at the ability of purified GEL-2 to form enzyme:lichenin and enzyme:laminarin intermediates. We incubated purified MBP:GEL-2 with lichenin and with polysaccharides from the triple mutant growth medium and used anti-lichenin antibody to assess the presence of GEL-2:lichenin intermediates in western blot experiments. As shown in Fig. 8 (panel A), a GEL-2:lichenin intermediate was detected when lichenin was used as a substrate (lane 3) and when growth medium polysaccharides were used as substrate (lane 2). These results showing the presence of a enzyme:lichenin intermediate provide strong evidence for GEL-2 functioning as a lichenin transferase and that the lichenin released into the medium is recognized as a substrate by GEL-2. We carried out similar experiments using antibody directed against laminarin to look for the ability of GEL-2 to form substrate intermediates with laminarin and growth medium polysaccharides (Fig. 8 Panel B). We found that GEL-2 formed an enzyme:substrate intermediate with both of these substrates (lanes 2 and 3). This provides evidence that β-1,3-glucan released by the triple mutant is a substrate for GEL-2 (lane 2). Previous analyses of the N. crassa cell wall polysaccharides have shown that β-1,3-glucan (laminarin) and lichenin are major cell wall components (Ao and Free, 2017; Maddi and Free, 2010; Maddi et al., 2012; Fu et al., 2014). Based on our experiments, we conclude that GEL-2 can use both laminarin (β-1,3-glucan) and lichenin (mixed β-1,3−/β-1,4-glucan) as substrates for hydrolysis and transferase activities. Our results suggest that GEL-2 functions to crosslink β-1,3-glucan and lichenin into the fungal cell wall polysaccharide matrix. To the best of our knowledge, this is the first report showing that a sub-family 2 glucanosyltransferase may function to incorporate lichenin into the fungal cell wall.
Fig. 8.
Western blot analysis of purified MBP:GEL-2 when incubated with lichenin, laminarin and polysaccharides from ΔGH72 medium indicating the presence of enzyme:lichenin and enzyme:laminarin intermediates. Panel A: Western blot analysis of GEL-2:lichenin intermediate. Lane 1: control lane having MBP:GEL-2 without added polysaccharide. Lane 2: MBP:GEL-2 with ΔGH72 polysaccharides. Lane 3: MBP:GEL2 with lichenin (1 mg/ml). Panel B: Western blot analysis of GEL-2:laminarin intermediate. Lane 1: control lane having MBP:GEL-2 without polysaccharides. Lane 2: MBP:GEL-2 with ΔGH72 polysaccharides. Lane 3: MBP:GEL-2 with laminarin (1 mg/ml). The western blots were done with anti-lichenin (panel A) and anti-laminarin antibodies (panel B).
4. Discussion
Multiple GH72 family hydrolases/transferases are encoded in the genomes of ascomycetes fungi. The GH72 hydrolases/transferases are found exclusively in the fungal kingdom. The crystal structure of Gas1p from S. cerevisiae has been shown to have an extended binding pocket capable of accommodating up to ten β-1,3-glucose residues (Hurtado-Guerrero et al., 2009). These enzymes are expressed redundantly, and in varying combinations, at different points of fungal life cycles (Ao and Free, 2017; Mazan et al., 2011). GH72 enzymes have been previously demonstrated to function as β-1, 3 glucan hydrolases/ transferases involved in crosslinking polysaccharides together to create a cell wall matrix (Aimanianda et al., 2017; Mazan et al., 2011; Mouyna et al., 2000; Gastebois et al., 2010a; Hartland et al., 1996; Goldman et al., 1995; Gow and Lenardon, 2023; Popolo et al., 2017). GH72 enzymes are important for cell wall biogenesis in S. cerevisiae, C. albicans, and A. fumigatus. There are five GH72 enzymes in the S. cerevisiae and C. albicans genomes (Gastebois et al., 2010a; Gastebois et al., 2010b; Calderon et al., 2010; Ragni et al., 2007b). Two GH72 enzymes, Gas1p and Gas5p, are expressed in S. cerevisiae vegetative growth and deletion of Gas1p results in a growth defect and a reduction in cell wall glucan (Rolli et al., 2011; Ram et al., 1998). During sporulation in S. cerevisiae, Gas2p and Gas4p are expressed and the double mutant lacking these two enzymes is affected in sporulation (Ragni et al., 2007a; Rolli et al., 2011). The GH72 enzymes Phr1p and Phr2p are required for normal morphogenesis and virulence in C. albicans (Fonzi, 1999). In A. fumigatus, GEL4 has been found to be essential for vegetative growth and GEL2 is required for virulence (Gastebois et al., 2010a; Mouyna et al., 2005). These studies demonstrate the importance of GH72 enzymes in cell wall biogenesis and show that they are often expressed in cell type-specific combinations as redundant or partially redundant cell wall crosslinking enzymes.
We previously presented a model for GH72 enzyme function in which the enzymes functioned to crosslink cell wall polysaccharides to N-linked galactomannans that had been processed by GH76 family mannanases (Patel and Free, 2019; Kar et al., 2019). The model was derived from studies in which secreted proteins from various N. crassa mutants were used to look for the attachment of lichenin to cell wall glycoproteins (Kar et al., 2019). We tried to repeat some of these studies with our purified GEL-1 and GEL-2 enzymes and did not find any evidence for GEL-1 or GEL-2 being able to attach laminarin or lichenin to cell wall glycoproteins. The current data supports the role of GEL-1 and GEL-2 in crosslinking polysaccharides together but suggests that the enzymes do not use glycoproteins as acceptors for the transferase activity.
When the three N. crassa GH72 genes expressed during vegetative growth are mutated, the mutant has a tight colonial growth phenotype as seen in Fig. 1 (Ao and Free, 2017). The N. crassa GH72 enzymes have an N-terminal signal peptide to direct them into the secretory pathway and a C- terminal GPI- anchor to target them to the plasma membrane and cell wall. The exocytosis of cell wall crosslinking enzymes, including GH72 enzymes, has been shown to occur at the tips of the growing hyphae (Martinez-Nunez and Riquelme, 2015; Riquelme et al., 2011; Verdin et al., 2019). Once released into the cell wall space, the enzymes are thought to actively participate in crosslinking the cell wall polysaccharides together to create a polysaccharide matrix. As part of our research, we demonstrated that loss of the three major GH72 family enzymes expressed in the vegetative hyphae results in the release of β-1,3-glucan and lichenin into the growth medium, providing verification that these enzymes are needed for the incorporation of these polysaccharides into the cell wall matrix. In the present study, we observed hydrolase and transferase activity for two N. crassa GH72 enzymes, GEL-1 and GEL-2.
Structural analysis of GH72 enzymes divides the enzymes into two sub-families, Sub-family 1 contains a CBM43 carbohydrate domain in addition to the catalytic domain (Hurtado-Guerrero et al., 2009). The CBM43 domain has been shown to function as a β-1,3-glucan binding domain (Aimanianda et al., 2017). In N. crassa, GEL-1 is the only GH72 family enzyme from sub-family 1. Sub-family 2 enzymes contain the catalytic domain but lack a CBM43 domain. The N. crassa GEL-2 is an example of a GH72 sub-family 2 enzyme. Our results demonstrate that both sub-family 1 and sub-family 2 enzymes recognize β-1,3-glucan (laminarin) as a substrate for hydrolysis and for transferase activities. Our results demonstrate that in N. crassa a sub-family 2 enzyme, GEL-2, has a more relaxed substrate specificity and can function as a hydrolase and transferase for lichenin (mixed β-1,3−/β-1,4-glucan) as well as for β-1,3-glucan. β-1,3-glucan and lichenin have been found to be the major components of the N. crassa cell wall. Having GH72 sub-family 2 enzymes capable of crosslinking lichenin into the cell wall matrix would be important for N. crassa cell wall biogenesis and for cell wall formation in related fungi with lichenin as a component of their cell walls. The role of GH72 enzymes in crosslinking β-1,3-glucans into the cell wall has been well established. To the best of our knowledge, our report is the first to touch on the topic of how lichenin becomes incorporated into the cell wall. While all fungal cell walls contain β-1,3-glucan as a major component, many fungal cell walls contain multiple types of additional polysaccharides, including mixed β-1,3−/β-1,4-glucans, α-1,3-glucans, and β-1,6-glucans. Our results strongly suggest that sub-family 2 glucanosyltransferases function to crosslink lichenin into the cell wall. We hypothesize that these enzymes, having a relaxed specificity, might function in the incorporation of other types of polysaccharides into cell wall matrices.
The following are the supplementary data related to this article.
Supplementary Fig. S1.

Amino acid sequences for native GEL-1 and HIS6-tagged GEL-1.
Supplementary Fig. S2.

GEL-1 nucleotide sequences for HIS6-tagged GEL-1 (NCU08909).
Supplementary material 1
Funding sources
Funding for this research was provided by NSF grant # MCB 2125018 and by the UB Foundation.
CRediT authorship contribution statement
Apurva Chatrath: Writing – review & editing, Methodology, Investigation, Formal analysis, Conceptualization. Pavan Patel: Writing – review & editing, Methodology, Investigation, Conceptualization. Protyusha Dey: Writing – review & editing, Investigation. Stephen J. Free: Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aimanianda V., Simenel C., Garnaud C., Clavaud C., Tada R., Barbin L., Mouyna I., Heddergott C., Popolo L., Ohya Y., Delepierre M., Latge J.P. The dual activity responsible for the elongation and branching of beta-(1,3)-glucan in the fungal Cell Wall. mBio. 2017;8 doi: 10.1128/mBio.00619-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao J., Free S.J. Genetic and biochemical characterization of the GH72 family of cell wall transglycosylases in Neurospora crassa. Fungal Genet. Biol. 2017;101:46–54. doi: 10.1016/j.fgb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatzer M., Beauvais A., Henrissat B., Latge J.P. Revisiting old questions and new approaches to investigate the fungal cell wall construction. Curr. Top. Microbiol. Immunol. 2020;425:331–369. doi: 10.1007/82_2020_209. [DOI] [PubMed] [Google Scholar]
- Bowman B.J., Draskovic M., Freitag M., Bowman E.J. Structure and distribution of organelles and cellular location of calcium transporters in Neurospora crassa. Eukaryot. Cell. 2009;8:1845–1855. doi: 10.1128/EC.00174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon J., Zavrel M., Ragni E., Fonzi W.A., Rupp S., Popolo L. PHR1, a pH-regulated gene of Candida albicans encoding a glucan-remodelling enzyme, is required for adhesion and invasion. Microbiology. 2010;156:2484–2494. doi: 10.1099/mic.0.038000-0. [DOI] [PubMed] [Google Scholar]
- Colot H.V., Park G., Turner G.E., Ringelberg C., Crew C.M., Litvinkova L., Weiss R.L., Borkovich K.A., Dunlap J.C. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.H., Deserres F.J. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 1970;27:79–143. [Google Scholar]
- Fonzi W.A. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of beta-1,3- and beta-1,6-glucans. J. Bacteriol. 1999;181:7070–7079. doi: 10.1128/jb.181.22.7070-7079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C., Sokolow E., Rupert C.B., Free S.J. The Neurospora crassa CPS-1 polysaccharide synthase functions in cell wall biosynthesis. Fungal Genet. Biol. 2014;69:23–30. doi: 10.1016/j.fgb.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastebois A., Fontaine T., Latge J.P., Mouyna I. beta(1-3)Glucanosyltransferase Gel4p is essential for aspergillus fumigatus. Eukaryot. Cell. 2010;9:1294–1298. doi: 10.1128/EC.00107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastebois A., Mouyna I., Simenel C., Clavaud C., Coddeville B., Delepierre M., Latge J.P., Fontaine T. Characterization of a new beta(1-3)-glucan branching activity of Aspergillus fumigatus. J. Biol. Chem. 2010;285:2386–2396. doi: 10.1074/jbc.M109.077545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R.C., Sullivan P.A., Zakula D., Capobianco J.O. Kinetics of beta-1,3 glucan interaction at the donor and acceptor sites of the fungal glucosyltransferase encoded by the BGL2 gene. Eur. J. Biochem. 1995;227:372–378. doi: 10.1111/j.1432-1033.1995.tb20399.x. [DOI] [PubMed] [Google Scholar]
- Gow N.A.R., Lenardon M.D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2023;21:248–259. doi: 10.1038/s41579-022-00796-9. [DOI] [PubMed] [Google Scholar]
- Gow N.A.R., Latge J.P., Munro C.A. The fungal Cell Wall: structure, biosynthesis, and function. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.funk-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartland R.P., Fontaine T., Debeaupuis J.P., Simenel C., Delepierre M., Latge J.P. A novel beta-(1-3)-glucanosyltransferase from the cell wall of Aspergillus fumigatus. J. Biol. Chem. 1996;271:26843–26849. doi: 10.1074/jbc.271.43.26843. [DOI] [PubMed] [Google Scholar]
- Hurtado-Guerrero R., Schuttelkopf A.W., Mouyna I., Ibrahim A.F., Shepherd S., Fontaine T., Latge J.P., van Aalten D.M. Molecular mechanisms of yeast cell wall glucan remodeling. J. Biol. Chem. 2009;284:8461–8469. doi: 10.1074/jbc.M807990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M., Yamashita K., Takahashi M., Fukumori F., Ichiishi A., Fujimura M. Deletion and expression analysis of beta-(1,3)-glucanosyltransferase genes in Neurospora crassa. Fungal Genet. Biol. 2013;52:65–72. doi: 10.1016/j.fgb.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Kar B., Patel P., Ao J., Free S.J. Neurospora crassa family GH72 glucanosyltransferases function to crosslink cell wall glycoprotein N-linked galactomannan to cell wall lichenin. Fungal Genet. Biol. 2019;123:60–69. doi: 10.1016/j.fgb.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Klis F.M., Boorsma A., de Groot P.W. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- Latge J.P. The cell wall: a carbohydrate Armour for the fungal cell. Mol. Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Lesage G., Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006;70:317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi A., Free S.J. Alpha-1,6-Mannosylation of N-linked oligosaccharide present on cell wall proteins is required for their incorporation into the cell wall in the filamentous fungus Neurospora crassa. Eukaryot. Cell. 2010;9:1766–1775. doi: 10.1128/EC.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi A., Fu C., Free S.J. The Neurospora crassa dfg5 and dcw1 genes encode alpha-1,6-mannanases that function in the incorporation of glycoproteins into the cell wall. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin B.S., Frietag M., Selker E.U. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa. Fungal Genet. Newsl. 1997;44:34–36. [Google Scholar]
- Martinez-Nunez L., Riquelme M. Role of BGT-1 and BGT-2, two predicted GPI-anchored glycoside hydrolases/glycosyltransferases, in cell wall remodeling in Neurospora crassa. Fungal Genet. Biol. 2015;85:58–70. doi: 10.1016/j.fgb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Mazan M., Ragni E., Popolo L., Farkas V. Catalytic properties of the gas family beta-(1,3)-glucanosyltransferases active in fungal cell-wall biogenesis as determined by a novel fluorescent assay. Biochem. J. 2011;438:275–282. doi: 10.1042/BJ20110405. [DOI] [PubMed] [Google Scholar]
- Mouyna I., Hartland R.P., Fontaine T., Diaquin M., Simenel C., Delepierre M., Henrissat B., Latge J.P. A 1,3-beta-glucanosyltransferase isolated from the cell wall of aspergillus fumigatus is a homologue of the yeast Bgl2p. Microbiology. 1998;144(Pt 11):3171–3180. [Google Scholar]
- Mouyna I., Fontaine T., Vai M., Monod M., Fonzi W.A., Diaquin M., Popolo L., Hartland R.P., Latge J.P. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 2000;275:14882–14889. doi: 10.1074/jbc.275.20.14882. [DOI] [PubMed] [Google Scholar]
- Mouyna I., Morelle W., Vai M., Monod M., Lechenne B., Fontaine T., Beauvais A., Sarfati J., Prevost M.C., Henry C., Latge J.P. Deletion of GEL2 encoding for a beta(1-3)glucanosyltransferase affects morphogenesis and virulence in aspergillus fumigatus. Mol. Microbiol. 2005;56:1675–1688. doi: 10.1111/j.1365-2958.2005.04654.x. [DOI] [PubMed] [Google Scholar]
- Patel P.K., Free S.J. The genetics and biochemistry of Cell Wall structure and synthesis in Neurospora crassa, a model filamentous fungus. Front. Microbiol. 2019;10:2294. doi: 10.3389/fmicb.2019.02294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Free S.J. Characterization of Neurospora crassa GH16, GH17, and GH72 gene families of cell wall crosslinking enzymes. Cell Surf. 2022;8 doi: 10.1016/j.tcsw.2022.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidatala V.R., Mahboubi A., Mortimer J.C. Structural characterization of mannan cell wall polysaccharides in plants using PACE. J. Vis. Exp. 2017;128 doi: 10.3791/56424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo L., Degani G., Camilloni C., Fonzi W.A. The PHR family: the role of extracellular Transglycosylases in shaping Candida albicans cells. J. Fungi (Basel) 2017;3 doi: 10.3390/jof3040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni E., Coluccio A., Rolli E., Rodriguez-Pena J.M., Colasante G., Arroyo J., Neiman A.M., Popolo L. GAS2 and GAS4, a pair of developmentally regulated genes required for spore wall assembly in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:302–316. doi: 10.1128/EC.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni E., Fontaine T., Gissi C., Latge J.P., Popolo L. The gas family of proteins of Saccharomyces cerevisiae: characterization and evolutionary analysis. Yeast. 2007;24:297–308. doi: 10.1002/yea.1473. [DOI] [PubMed] [Google Scholar]
- Ram A.F., Kapteyn J.C., Montijn R.C., Caro L.H., Douwes J.E., Baginsky W., Mazur P., van den Ende H., Klis F.M. Loss of the plasma membrane-bound protein Gas1p in Saccharomyces cerevisiae results in the release of beta1,3-glucan into the medium and induces a compensation mechanism to ensure cell wall integrity. J. Bacteriol. 1998;180:1418–1424. doi: 10.1128/jb.180.6.1418-1424.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M., Yarden O., Bartnicki-Garcia S., Bowman B., Castro-Longoria E., Free S.J., Fleissner A., Freitag M., Lew R.R., Mourino-Perez R., Plamann M., Rasmussen C., Richthammer C., Roberson R.W., Sanchez-Leon E., Seiler S., Watters M.K. Architecture and development of the Neurospora crassa hypha -- a model cell for polarized growth. Fungal Biol. 2011;115:446–474. doi: 10.1016/j.funbio.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Rolli E., Ragni E., de Medina-Redondo M., Arroyo J., de Aldana C.R., Popolo L. Expression, stability, and replacement of glucan-remodeling enzymes during developmental transitions in Saccharomyces cerevisiae. Mol. Biol. Cell. 2011;22:1585–1598. doi: 10.1091/mbc.E10-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Ortiz-Castellanos L. Cell wall glucans of fungi. A review. Cell Surf. 2019;5 doi: 10.1016/j.tcsw.2019.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin J., Sanchez-Leon E., Rico-Ramirez A.M., Martinez-Nunez L., Fajardo-Somera R.A., Riquelme M. Off the wall: the rhyme and reason of Neurospora crassa hyphal morphogenesis. Cell Surf. 2019;5 doi: 10.1016/j.tcsw.2019.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1