Abstract
To examine alterations in brain activation associated with pharmacologically induced memory impairment, we used functional MRI (fMRI) to study the effects of lorazepam and scopolamine on a face–name associative encoding paradigm. Ten healthy young subjects were scanned on four occasions, 2 weeks apart; they were administered i.v. saline during two placebo-scanning sessions and then alternately administered i.v. lorazepam (1 mg) or scopolamine (0.4 mg) in a double-blind, randomized, cross-over design. Both the extent and magnitude of activation within anatomic regions of interest (ROIs) were examined to determine the reproducibility of activation in the placebo sessions and the regional specificity of the pharmacologic effects. Activation within all ROIs was consistent across the two placebo scans during the encoding of novel face–name pairs (compared with visual fixation). With the administration of either lorazepam or scopolamine, significant decreases were observed in both the extent and magnitude of activation within the hippocampal, fusiform, and inferior prefrontal ROIs, but no significant alterations in activation in the striate cortex were found. Both medications impaired performance on postscan memory measures, and significant correlations between memory performance and extent of activation were found in hippocampal and fusiform ROIs. These findings suggest that pharmacologic effects can be detected with fMRI by using a reproducible experimental paradigm and that medications that impair memory also diminish activation in specific brain regions thought to subserve complex memory processes.
An essential aspect of episodic memory is the ability to form new associations between previously unrelated items of information. Converging evidence from animal electrophysiologic studies (1–3) and human lesion studies (4–6) suggests that the hippocampus plays a critical role in forming these new associations in memory. Recently, a number of functional imaging studies also have demonstrated that memory tasks requiring associative or relational processing activate the hippocampus and related structures in the medial temporal lobe (7, 8).
We previously have examined associative learning with functional MRI (fMRI) by using a face–name paired associate task. Faces and names are inherently unrelated and require the formation of an association across the verbal and visual domains. We found activation in a specific functional network, which included the hippocampus, fusiform gyrus, and prefrontal cortex, during the encoding of novel face–name associations (9). Using this paradigm, we sought to test the hypothesis that pharmacologically induced memory impairment is associated with alterations of fMRI activation in these brain regions. To detect and quantify such alterations in vivo, we collected fMRI data in a blinded, placebo-controlled trial of two drugs known to produce impairments in episodic memory, lorazepam and scopolamine.
Lorazepam and other benzodiazepines bind to a specific site on γ-aminobutyric acid (GABA) receptors and are thought to impair memory by enhancing the inhibitory influence of GABAergic neurons (10). Scopolamine is a potent antagonist of the muscarinic acetylcholine receptor and is thought to impair memory by blocking cholinergic transmission (10). Considerable work has been done to examine the effects of these medications on cellular physiology and animal behavior (11) and on human memory performance (12–15). Both benzodiazepines and scopolamine have been shown to selectively impair the ability to encode new information, with relative sparing of semantic and procedural memory (13, 16, 17). Less is known regarding the regional specificity of these medication effects on functional brain activity, although recently, several studies have used functional imaging to examine effects of lorazepam or scopolamine on memory-related activation (18–21). The majority of these studies have used positron-emission tomography (PET) imaging techniques (18–20), with the exception of one recent fMRI study, which examined the effects of these medications on priming (21). These previous studies did not, however, employ cognitive tasks that produced significant activation within the hippocampus and, thus, could not examine alterations in hippocampal activity with drug administration.
We hypothesized that the administration of lorazepam or scopolamine, during the encoding of face–name associations, would decrease activation in the same functional network thought to subserve this task, namely, the hippocampus and the fusiform and prefrontal cortices, and would impair performance on postscan face–name retrieval tasks. To permit accurate interpretation of the pharmacologic effects, it was also important to evaluate the reproducibility of the fMRI activity with this task under placebo conditions. Very little work examining the test–retest reliability of fMRI activation with cognitive paradigms has been published to date (22, 23), and, to our knowledge, no previous fMRI studies have examined pharmacologic effects on episodic memory in the context of reliability data in the same subjects. We therefore used a within-subject, repeated-measures design, using an anatomically based region of interest (ROI) analysis method, to examine both the test–retest reliability of fMRI activation and the sensitivity of fMRI in detecting pharmacologically induced alterations in memory-related activation.
Methods
Subjects.
Ten right-handed, native English-speaking, healthy, male subjects (ages 23–35) participated in this study. Subjects were recruited by advertisements at local universities. All subjects provided informed consent in accordance with the Human Subjects Committee guidelines of the Massachusetts General Hospital, Boston.
Potential subjects underwent a screening visit that included medical history, physical and neurological examination, blood and urine laboratory analyses, and electrocardiogram. Subjects were excluded if they had any significant medical, neurological, or psychiatric illness or if they were taking any prescription or over-the-counter medications with known central nervous system effects.
Study Design.
Each subject was scanned on four consecutive occasions, with a 2-week interval between each scanning session. Each scanning session took place at the same time of day and was conducted in an identical fashion so that the subjects were blinded as to which substance was administered. Subjects were given i.v. saline as a placebo during the first and second sessions in a single-blinded design. During the third and fourth sessions, subjects were alternately given i.v. lorazepam (1 mg) or scopolamine (0.4 mg) in a double-blind, randomized, cross-over design. We chose this design to examine test–retest reliability effects without the potential confounding effects of intervening drug administration.
At each session, subjects first had an i.v. line placed in the left antecubital vein to allow blood drawing for pharmacokinetic sampling and drug infusion. Thirty minutes after infusion, subjects were placed in the scanner for structural image acquisition. The functional imaging protocol began 60 min after drug infusion. Every 30 min during each session, subjects were asked to rate sedation and dry mouth. Heart rate, expired CO2, and O2 saturation were recorded throughout each session.
After the completion of each scanning session, subjects were taken to a testing room for the administration of the postscan memory tests and additional physiological monitoring. Subjects were observed on each occasion for 3 h after drug infusion and released after a final examination by a physician.
Cognitive Paradigm.
Functional images were acquired during the encoding of face–name associations. The fMRI activation task consisted of three conditions presented in blocks: (i) Novel face–name pairs, unfamiliar faces paired with fictional first names each shown only once (5-sec duration) during the scanning session; (ii) Repeated face–name pairs, two repeated face–name pairs alternating throughout each block; (iii) Fixation, subjects examined a white fixation cross (+) presented in the center of the visual field on a black background presented throughout the block to provide focused visual attention. It should be noted that the Repeated blocks were included as an additional “control” condition, which had visual complexity similar to the Novel stimuli and differed from the Novel condition only in the familiarity of the face–name pairs.
Four equivalent face–name stimulus sets, each consisting of 84 novel face–name pairs and 2 repeated face–name pairs, were developed and validated for this study, based on similar stimuli used in previous experiments (9). The order of the stimulus sets was randomized per subject. The visual stimuli were presented by using a Macintosh computer (Apple +) connected to a Sharp 2000 color liquid crystal display projector. Images were projected through a collimating lens (Buhl Optical, Pittsburgh) onto a screen attached to the head coil during functional data acquisition.
To ensure that subjects remained attentive to the stimuli, they were instructed to press a button with their right hand to indicate the gender of the face–name pair and were asked to try to remember which name was associated with each face for later testing. Reaction time and gender-determination accuracy were recorded for each session.
Postscan Testing.
Immediately after each imaging session, subjects were tested for their memory of a subset of the face–name pairs presented during that imaging session. Two memory tests were administered. (i) Face recognition and name recall. This task evaluated face recognition and free recall for the name by using 20 faces: 14 faces from the set of novel face–name pairs that were presented during that scanning session, 7 distracter faces (not presented during the session), and the 2 repeated face–name pairs presented throughout the session. Subjects were asked to indicate if they had seen the face before with a yes/no answer and, if yes, to recall the name associated with the face. (ii) Forced choice face–name recognition. This test evaluated recognition for the face–name pairs. A different subset of 14 novel faces shown during that scanning session was presented, each with one correct and one incorrect name written underneath, and subjects were asked to press a button corresponding to the correct name.
Image Acquisition.
Structural data were acquired on a 1.5-tesla sonata imager (Siemens Medical Systems, Iselin, NJ) by using an MP-RAGE sequence with repetition time TR = 7.25 msec, echo time TE = 3.0 msec, and flip angle = 7°; 128 sagittal slices, thickness = 1.33 mm, each slice = 256 × 256 with a pixel size of 1.00 × 1.00 mm. Four structural scans were acquired for each subject, one on each scanning session. The four sets of images for each subject were aligned and averaged together (24).
Functional data were acquired by using gradient echo T2* weighted [blood oxygen level-dependent method (BOLD) with TR = 2,500 msec, TE = 40 msec, and flip angle = 90; voxel dimensions = 3.125 × 3.125 × 6.0 mm]. Twenty-nine slices (5-mm thick; 1-mm gap between slices) were acquired in a coronal orientation, perpendicular to the anterior commissure–posterior commissure (AC-PC) line. This orientation was chosen to maximize the in-plane resolution within the hippocampus. Images were acquired beginning at the occipital pole. Scanning time for each functional run was 4 min and 15 sec, consisting of 102 time points (4 for T1 stabilization and 98 for functional data collection). A total of six functional runs per session were acquired.
Image Analysis.
Each of the six functional runs was motion-corrected to the first run by using afni (http://afni.nimh.nih.gov/afni/index.shtml) and then spatially smoothed by using a two-dimensional Hanning filter (full width at half maximum = 4.6 mm). The stimulus effects at each voxel were estimated by fitting the amplitudes of two boxcar functions (one for the Novel condition, one for the Repeated condition) convolved with a gamma function to the BOLD signal across all runs (25). The boxcar was delayed by 5 sec with respect to block onset to account for the hemodynamic delay. A baseline offset and linear trend were also fit for each run. The residual error was used to estimate the variance of the noise (25).
Manually drawn ROIs were outlined for each subject, using the averaged structural MRI scan described above. Anatomic ROIs were chosen on the basis of the activation pattern seen in a previous experiment using this paradigm and included striate cortex, fusiform gyrus, hippocampus, and dorsolateral and inferior prefrontal cortices (9). Regions were drawn, by a skilled operator, on multiple slices of the MRI displayed in three planes (26, 27).
The functional volumes from each of the four imaging sessions were aligned to the structural volume to determine which functional voxels fell within the anatomically defined ROIs (24). Each ROI was constrained further by removing any voxels that did not show a significant activation (P < 0.01) in an “omnibus” test of task-related activity (i.e., a significant response to the Novel and/or Repeated conditions compared with Fixation), using an F statistic, with the sign based on the sign of the response to the Novel condition. The voxels showing a significant response in the omnibus test then were averaged together to derive percent signal change measures for the Novel vs. Fixation and Novel vs. Repeated contrasts.
Statistical Analysis of Activation Within ROIs.
Both the extent and magnitude of activation were examined within each ROI to test the reproducibility of fMRI activation during the placebo conditions and the pharmacologic effects on this pattern of fMRI activation. Extent of activation was defined as the percentage of voxels activated over the significance threshold of 0.01 within the anatomic ROI (i.e., number of significantly activated voxels divided by the total number of voxels within that anatomic region). Magnitude of activation was defined as the percent signal change during the Novel or Repeated conditions within voxels that were activated in any of the four sessions (to guard against the possibility that activation might be present in a different location within the ROI across the four sessions).
Linear mixed-effects models were applied separately to each ROI to statistically evaluate effects of placebo and drug administration on fMRI activity in each region. These mixed-effects models estimated fixed effects for the placebo and drug sessions while controlling for random effects of individuals and intra-individual correlations (28–31). Each initial mixed-effects model for each ROI included main effects for session and brain hemisphere as well as session-by-hemisphere interactions. A likelihood–ratio test was used to assess the significance of the session-by-hemisphere interactions. Interaction terms were retained only if they significantly improved the fit when compared with that of the simpler model, which included main effects only. Session-order effects also were assessed by likelihood–ratio tests.
Four contrasts of estimated session effects were examined for each ROI: (i) the difference between the first and second placebo sessions (for reliability assessment); (ii) the effect of lorazepam compared with a weighted combination of the first and second placebo sessions; (iii) the effect of scopolamine compared with a weighted combination of the first and second placebo sessions; and (iv) a comparison of the effects of lorazepam vs. scopolamine. A conservative Bonferroni correction was applied to the resultant P values for these contrasts, inflating each by a factor of 4 (i.e., the number of nonsimultaneous contrasts tested).
Whole-Brain, Voxel-by-Voxel Analysis.
To ascertain whether there were any additional brain regions not included in the ROIs that showed differences between placebo sessions (for reliability assessment) or showed alterations with administration of drug, a secondary analysis examining the whole brain, voxel-by-voxel, was performed. This analysis used a paired t test statistic, comparing sessions by using a random-effects model. Regions were considered to show a statistical difference between sessions if they contained 10 or more contiguous voxels that exceeded a significance threshold of 0.01 and were greater than a 10-mm distance from the nearest peak activation.
Results
All subjects completed the protocol without serious adverse events. Subjects did, however, report a greater subjective sense of sedation in both drug conditions than in the placebo sessions (P < 0.05). An increased sensation of “dry mouth” was reported in the scopolamine condition compared with both the placebo and lorazepam conditions (P < 0.01).
The order of stimulus set presentation and of session also was examined by using the mixed-effects model. There were no significant effects of order of set presentation on reaction time, postscan memory performance, or fMRI activation in any ROI. Similarly, no significant session order effects were found.
The effect of right vs. left hemisphere also was examined. Only the hippocampal ROI showed a consistent asymmetry across the sessions, with greater activation in the left than right hemisphere (P < 0.03). No significant session-by-hemisphere interaction was found in any ROI (i.e., there were no placebo or pharmacologic effects that showed hemispheric asymmetry). The data below, therefore, present only the main effects of session, controlling for hemisphere.
Test–Retest Reliability of fMRI Activation.
The activation pattern for the Novel vs. Fixation contrast was highly consistent across the two placebo sessions (see Fig. 1). Significant activation was observed in the striate, fusiform, hippocampal, and prefrontal ROIs during both placebo sessions. No significant differences were found in any ROI for either the extent variable (percent voxels activated over threshold within the ROI) or in the magnitude variable (percent signal change for activated voxels within the ROI) between the first and second placebo sessions for the Novel vs. Fixation contrast (see Table 1). The whole-brain, voxel-by-voxel paired t test method also showed no significant differences between the first and second placebo sessions for the Novel vs. Fixation contrast.
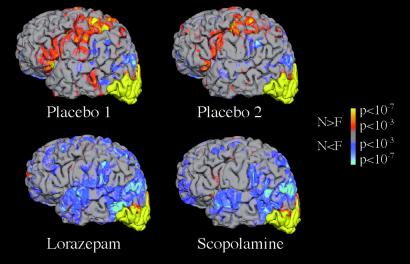
Figure 1.
Group fMRI data (n = 10) for the Novel face–name pairs vs. Fixation contrast is shown superimposed on a three-dimensional reconstruction of an individual subject's structural data in lateral view of left hemisphere. Activation shown in orange to yellow represents voxels with greater activity in the Novel compared with Fixation conditions. Activation shown in blue represents voxels with greater activity in Fixation condition compared with Novel. All four sessions show a similar pattern of activation in the occipital cortex.
Table 1.
ROI activation data for the Novel vs. Fixation (NvF) contrast across four scanning sessions
| Region | Extent of activation, % voxels activated,
NvF
|
Magnitude of activation, % signal change, NvF
|
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo 1 | Placebo 2 | Lorazepam | Scopolamine | Placebo 1 | Placebo 2 | Lorazepam | Scopolamine | |
| Striate | 66.0 (5.1) | 64.3 (4.4) | 60.3 (3.9) | 62.2 (6.7) | 0.29 (0.02) | 0.29 (0.02) | 0.31 (0.03) | 0.30 (0.04) |
| Fusiform | 33.3 (2.8) | 33.9 (3.0) | 20.9§ (2.9) | 21.1§ (3.4) | 0.20 (0.02) | 0.22 (0.03) | 0.13‡ (0.02) | 0.12§ (0.03) |
| Hippocampal | 15.3 (3.6) | 10.7 (2.3) | 6.1† (1.9) | 6.0‡ (1.5) | 0.08 (0.01) | 0.06 (0.01) | 0.03* (0.01) | 0.03† (0.01) |
| Dorsolateral prefrontal | 9.8 (2.7) | 10.2 (2.9) | 6.7 (1.8) | 6.7 (2.3) | 0.07 (0.02) | 0.07 (0.02) | 0.03* (0.02) | 0.03* (0.02) |
| Inferior frontal | 17.6 (5.3) | 18.8 (6.3) | 6.8‡ (2.9) | 10.2* (3.9) | 0.09 (0.02) | 0.08 (0.02) | 0.00§ (0.02) | 0.03† (0.02) |
Percent signal change is calculated for all voxels that activated in any of the four sessions. Numbers represent means with SEMs shown in parentheses. Significance values are based on a linear mixed-effects model, and are corrected for multiple comparisons:
, P < 0.05;
, P < 0.01;
, P < 0.001;
, P < 0.0001.
Significant or near-significant differences were observed, however, across the two placebo sessions in several ROIs for the Novel vs. Repeated contrast. On inspection, it was apparent that the signal during the Novel blocks remained constant between the first and second placebo sessions, but there was a significant decrease in the signal during the Repeated blocks from the first to the second placebo scan. This resulted in a systematic increase in the extent and magnitude of Novel vs. Repeated activation from the first to the second placebo session. Because the Novel vs. Repeated contrast did not yield reliable activation across the two placebo conditions, only the Novel vs. Fixation contrast was used to assess pharmacologic effects.
Pharmacologic Effects on fMRI Activation.
Regionally specific effects of both medications were observed on the pattern of fMRI activation. Significant reductions in both extent and magnitude of activation were observed in fusiform (P < 0.0001), hippocampal (P < 0.0015), and inferior prefrontal cortices (P < 0.002), with the administration of both lorazepam and scopolamine compared with placebo by using the mixed-effects model corrected for multiple comparisons (see Table 2). The reduction of activation for the Novel vs. Fixation contrast was particularly striking in the medial fusiform gyrus and anterior hippocampus with the administration of either lorazepam or scopolamine (see Figs. 2 and 3, respectively). The dorsolateral prefrontal ROI showed a significant reduction in the magnitude of the percent signal change (P < 0.02) but a nonsignificant decrease in extent of activation (P = 0.22) for both medications. There were no significant differences between lorazepam and scopolamine in the extent or magnitude of activation in any ROI.
Table 2.
Behavioral measures obtained during and post scan across four scanning sessions
| Behavioral measure | Placebo 1 | Placebo 2 | Lorazepam | Scopolamine |
|---|---|---|---|---|
| Reaction time for gender classification, sec | 0.946 (0.065) | 0.996 (0.065) | 1.247§ (0.115) | 1.081 (0.064) |
| Face recognition, % correct | 84.2 (2.6) | 79.6 (3.4) | 72.4 (4.5) | 71.0* (4.3) |
| Free recall of name, % correct | 26.5 (3.3) | 25.6 (3.6) | 14.9† (3.5) | 16.2* (2.4) |
| Forced choice name recognition, % correct | 88.1 (2.6) | 89.4 (3.6) | 71.6§ (5.5) | 80.8 (4.4) |
Behavioral data during scanning (reaction time) and post-scan memory measures are shown as means with SEMs in parentheses. Significance values are based on a linear mixed-effects model, and are corrected for multiple comparisons:
, P < 0.05;
, P < 0.01;
, P < 0.0001.
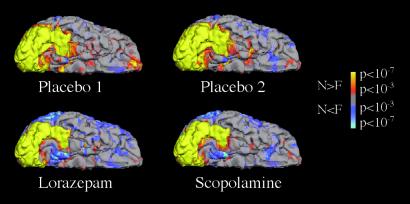
Figure 2.
Group fMRI data for the Novel vs. Fixation contrast is shown in the ventral view of the right hemisphere. All four sessions show a similar pattern of activation in striate and lateral extrastriate cortices, but there is reduced activation in the medial inferior temporal cortex, confined within the fusiform gyrus, that was observed with the administration of both lorazepam and scopolamine.
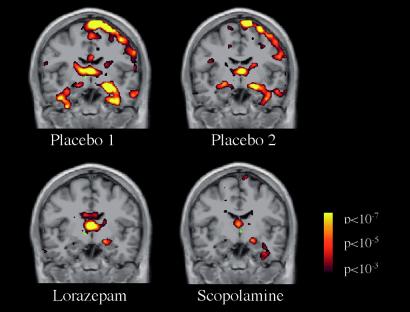
Figure 3.
Group fMRI data for the Novel vs. Fixation contrast shown on a coronal image at the level of the anterior hippocampus, showing reduced activation in the lorazepam and scopolamine sessions.
In contrast, striate cortex showed no significant differences in extent or magnitude of activation for the Novel vs. Fixation contrast when placebo was compared with either lorazepam or scopolamine. The magnetic resonance signal time courses for the striate cortex showed a consistent paradigm-linked pattern across all four sessions, in contrast to the hippocampus, which showed a marked difference in the signal time course between the placebo sessions and the administration of both medications (see Fig. 4).
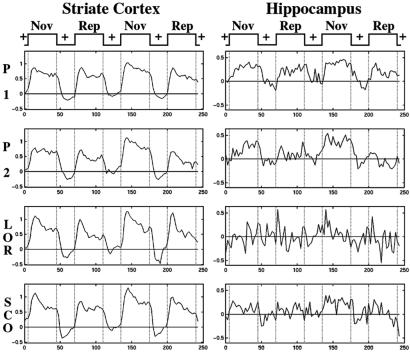
Figure 4.
Magnetic resonance signal averaged time courses taken from voxels that activated during both placebo scans (P 1 and P 2), within the striate cortex (Left) and hippocampus (Right), across the four imaging sessions. The signal in striate remains paradigm-linked across all four sessions, whereas the signal in the hippocampus shows an altered pattern in the lorazepam (LOR) and scopolamine (SCO) sessions.
Whole-brain, voxel-by-voxel analyses revealed only one additional region, not included within the ROIs, that was activated during both placebo sessions and showed a significant reduction with the administration of lorazepam and scopolamine. This region was located in the left medial orbital cortex (BA 10/11; peak coordinates: −7, 53, −10). In addition, significant “deactivations” (i.e., a relative decrease in signal during the Novel condition compared with Fixation) were observed in lateral temporal, lateral parietal, and precuneus regions with the administration of both medications (areas shown in blue in Fig. 1). These deactivations will be discussed in detail in another paper.
Reaction time for gender determination was slowed significantly by lorazepam administration compared with both placebo sessions and the scopolamine session (P < 0.01). To determine whether the correlation between drug effect and reaction time confounded the evaluation of drug effects on fMRI activation, reaction time was included as a covariate in the linear mixed-effects model. Significant pharmacologic effects still were observed in the fusiform, hippocampal, and inferior prefrontal ROIs with reaction time included in the model.
Postscan Memory Testing.
Performance on all postscan memory measures was consistent across the two placebo sessions (see Table 2). However, both lorazepam and scopolamine significantly impaired performance, compared with placebo, on postscan memory testing, particularly on free recall for the name (see Table 2). There were no significant differences between lorazepam and scopolamine on any postscan memory measure.
Correlational analyses were performed to examine the relationship between extent of fMRI activation and memory performance. The strongest correlations across the four sessions were found between the percentage of correct answers on the face-recognition task and extent of activation in the fusiform gyrus (r = 0.62; P < 0.00001; Pearson's correlation) and the hippocampus (r = 0.36; P < 0.001).
Discussion
These findings indicate that pharmacologically induced memory impairment on a paired associate learning task produced a regionally specific alteration in the pattern of fMRI activation. A decrease in both the extent and magnitude of activation was observed in the hippocampus, the fusiform gyrus, and the inferior prefrontal cortex. These results are particularly striking given the reliable activation observed in all of these regions across the two placebo sessions. Moreover, we did not observe any pharmacologic effect on activation in the striate cortex, suggesting that global effects on cerebral blood flow or oxygen utilization were not responsible for the reduced activation in other regions.
These findings are consistent with previous neuroimaging studies that have shown regional alterations in activation associated with pharmacologic manipulation. These studies, primarily using PET techniques, have examined the effects of scopolamine or benzodiazepines on baseline metabolism (18, 20, 32) and/or on task-related activation (19–21, 33). Grasby et al. (20) studied the effects of scopolamine by using PET during a supraspan verbal memory task and found a decrease in task-related PET activation in the right anterior cingulate and bilateral prefrontal cortex. Rosier et al. studied the effects of diazepam (33) and, later, scopolamine (19) on PET activation during the encoding of abstract visual shapes and reported decreased task-related activation in the fusiform gyri with both medications. Using fMRI, Thiel et al. (21) compared the effects of scopolamine and lorazepam to placebo during a verbal priming task and reported that both medications altered priming performance and had a similar effect on fMRI activation. Furey et al. (34) examined the effects of enhancing cholinergic transmission with physostigmine and reported increased fMRI activation in ventral extrastriate cortex during the encoding component of a working memory task. Our fMRI study demonstrates reliable fMRI activation in the hippocampus during an encoding task across two placebo sessions and reductions in hippocampal activation (and in memory performance) with administration of scopolamine and lorazepam.
We did not detect any significant differences in the pattern of fMRI activation in response to lorazepam and scopolamine, despite the fact that these medications are known to impair memory through different neurotransmitter systems. Both drugs, however, impaired memory to a similar degree, as assessed by postscan testing. This finding is consistent with another recent study, which directly compared the effects of lorazepam and scopolamine on episodic memory and event-related potentials (13). Similarly, Thiel et al. (21) reported similar effects of lorazepam and scopolamine on behavioral measures of priming and on the pattern of fMRI activation. Thus, one possibility is that fMRI activation may reflect the neural correlates of behavior rather than the underlying mechanism of memory impairment. Future studies modifying the degree of memory impairment by varying the dose of these medications may elucidate the relationship between pharmacologic mechanism, degree of memory impairment, and pattern of activation. It also should be noted that, although these drugs exhibit activity in different neurotransmitter systems, there are populations of GABAergic and cholinergic neurons located in close proximity to each other within the hippocampus and the neocortical areas examined in our ROIs (11). The spatial resolution of fMRI may not be able to detect such differences and may reflect only the net effect of neural activity and blood-flow changes in these regions.
Although overall sedation and decreased attention during encoding may have played a role in the decreased activation observed (13), after controlling for the drug effects on reaction time (a sensitive measure of sedation), we still observed significant decreases in activation in the fusiform, hippocampal, and inferior prefrontal ROIs. Moreover, we did not observe any difference with either drug in activation within visual cortex. Global impairments in attention and arousal may have played a role in the “deactivations” (i.e., the relative decrease in signal during the Novel condition compared with Fixation) that were observed in several large regions of heteromodal association cortex during the drug conditions. This will be discussed in detail in a separate paper.
To date, very few studies have examined the test–retest reliability of fMRI activity by using memory paradigms or other complex cognitive designs (22, 23). One of the strengths of this study is that we were able to examine both the reliability of fMRI activation with placebo administration and pharmacologic effects on these activations within the same individuals. As described above, we observed good reliability in both the extent and magnitude of activation in all ROIs for the Novel vs. Fixation comparison. Moreover, the pattern of activation we observed in both placebo sessions is quite consistent with our previous report using a very similar paradigm in an entirely different set of subjects, with a different scanner (3-tesla scanner; General Electric) (9). These findings suggest that the pattern of fMRI activation with this associative encoding paradigm is robust over time within individuals, as well as across subjects and image-acquisition systems.
Although the test–retest reliability in both extent and magnitude of activation was excellent for the Novel vs. Fixation contrast, the reliability for the Novel vs. Repeated contrast was quite variable. This result appeared to be due to a systematic decrease from the first to the second session in the magnetic resonance signal throughout the Repeated blocks. We hypothesize that this systematic decrease in signal was a result of habituation to the Repeated block design. Thus, we used only the Novel vs. Fixation data to assess pharmacologic effects. In future experiments that use a Novel vs. Repeated block design for the longitudinal evaluation of subjects, it may be prudent to expose subjects to this design before the first scanning session.
We chose to use a ROI-based method for the primary analysis to capitalize on the intrasubject repeated-measures study design and to allow an anatomically constrained analysis of specific brain regions both within and across individuals. Using a whole-brain, voxel-based analysis of group data requires that the data sets be transformed into a common stereotatic space and, thus, alters the anatomic resolution, which is particularly important in small and anatomically variable regions such as the hippocampus. The disadvantage of the ROI method, however, is that it requires a priori hypotheses about specific regions. We, therefore, sought to determine whether there were other large regions that showed significant changes in activation with drug administration by using a whole-brain, voxel-by-voxel analysis. Only one additional region, in orbital cortex, showed significant activation during placebo sessions and a significant decrease in activation with administration of both medications. As mentioned above, additional regions in heteromodal-association cortices in the parietal and temporal cortices, which did not activate during placebo sessions, showed evidence of “deactivation” with drug administration. It should be possible to develop ROI methods to examine these regions in more detail.
In summary, our findings suggest that pharmacologic effects can be detected by fMRI in a reproducible experimental paradigm by using an associative encoding task, and that activation is decreased in specific brain regions thought to subserve this cognitive function. Further work exploring optimal analysis methods is required, but the reliability and validity of these data, using medications known to impair memory, suggest that fMRI eventually may prove a useful tool for screening compounds that are being developed to enhance memory and treat cognitive impairment.
Acknowledgments
We thank Mary Foley for assistance with image acquisition, Chris Rosenbaum for assistance with clinical monitoring, and John Vetrano for assistance with pharmacy procedures. This study was supported by Glaxo Smith Kline and National Institutes of Health Grants K23-NS02189 (to R.A.S.) and P01-AG04953 (to M.S.A.).
Abbreviations
- fMRI
functional MRI
- ROI
region of interest
- GABA
γ-aminobutyric acid
- PET
positron-emission tomography
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bunsey M, Eichenbaum H. Behav Neurosci. 1993;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- 2.Wood E R, Dudchenko P A, Eichenbaum H. Nature (London) 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki W A, Eichenbaum H. Ann NY Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- 4.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Schacter D L, Church B. J Int Neuropsychol Soc. 1995;1:434–442. doi: 10.1017/s1355617700000539. [DOI] [PubMed] [Google Scholar]
- 6.Chalfonte B L, Verfaellie M, Johnson M K, Reiss L. Memory. 1996;4:591–614. doi: 10.1080/741940998. [DOI] [PubMed] [Google Scholar]
- 7.Henke K, Buck A, Weber B, Wieser H G. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Schacter D L, Wagner A D. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Sperling R A, Bates J F, Cocchiarella A J, Schacter D L, Rosen B R, Albert M S. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardman J G, Limbird L E, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw–Hill; 1996. pp. 149–150. [Google Scholar]
- 11.Hasselmo M E, Wyble B P, Wallenstein G V. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Robbins T W, Semple J, Kumar R, Truman M I, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Psychopharmacology. 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- 13.Curran H V, Pooviboonsuk P, Dalton J A, Lader M H. Psychopharmacology (Berlin) 1998;135:27–36. doi: 10.1007/s002130050482. [DOI] [PubMed] [Google Scholar]
- 14.Bishop K I, Curran H V. Psychopharmacology (Berlin) 1998;140:345–353. doi: 10.1007/s002130050775. [DOI] [PubMed] [Google Scholar]
- 15.Wagemans J, Notebaert W, Boucart M. Psychopharmacology (Berlin) 1998;138:326–333. doi: 10.1007/s002130050678. [DOI] [PubMed] [Google Scholar]
- 16.Ghoneim M M, Mewaldt S P. Psychopharmacologia. 1975;44:257–262. doi: 10.1007/BF00428903. [DOI] [PubMed] [Google Scholar]
- 17.Ghoneim M M, Mewaldt S P. Psychopharmacology (Berlin) 1977;52:1–6. doi: 10.1007/BF00426592. [DOI] [PubMed] [Google Scholar]
- 18.Molchan S E, Matochik J A, Zametkin A J, Szymanski H V, Cantillon M, Cohen R M, Sunderland T. Neuropsychopharmacology. 1994;10:191–198. doi: 10.1038/npp.1994.21. [DOI] [PubMed] [Google Scholar]
- 19.Rosier A M, Cornette L, Dupont P, Bormans G, Mortelmans L, Orban G A. Eur J Neurosci. 1999;11:3701–3714. doi: 10.1046/j.1460-9568.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 20.Grasby P M, Frith C D, Paulesu E, Friston K J, Frackowiak R S, Dolan R J. Exp Brain Res. 1995;104:337–348. doi: 10.1007/BF00242019. [DOI] [PubMed] [Google Scholar]
- 21.Thiel C M, Henson R N, Morris J S, Friston K J, Dolan R J. J Neurosci. 2001;21:6846–6852. doi: 10.1523/JNEUROSCI.21-17-06846.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machielsen W C, Rombouts S A, Barkhof F, Scheltens P, Witter M P. Hum Brain Mapp. 2000;9:156–164. doi: 10.1002/(SICI)1097-0193(200003)9:3<156::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGonigle D J, Howseman A M, Athwal B S, Friston K J, Frackowiak R S, Holmes A P. NeuroImage. 2000;11:708–734. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- 24.Collins D L, Neelin P, Peters T M, Evans A C. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 25.Burock M A, Dale A M. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killiany R J, Moss M B, Albert M S, Sandor T, Tieman J, Jolesz F. Arch Neurol. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- 27.Killiany R J, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman B T, Albert M S. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 28.Lange N, Laird N M. J Am Stat Assoc. 1989;84:241–247. [Google Scholar]
- 29.Laird N M, Ware J H. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 30.Lange N, Ryan L. Ann Stat. 1989;17:624–642. [Google Scholar]
- 31.Pinheiro J C, Bates D M. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 32.Wang G J, Volkow N D, Levy A V, Felder C A, Fowler J S, Pappas N R, Hitzemann R J, Wong C T. J Nucl Med. 1999;40:715–720. [PubMed] [Google Scholar]
- 33.Rosier A, Cornette L, Dupont P, Bormans G, Michiels J, Mortelmans L, Orban G A. Proc Natl Acad Sci USA. 1997;94:7627–7632. doi: 10.1073/pnas.94.14.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furey M L, Pietrini P, Haxby J V. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]