Abstract
The implementation of innovative techniques to achieve low-salt strategies in cured products is a critical issue faced by the food industry. This study aimed to investigate the impact of ultrasound treatment on the quality of the low-salt air-dried fish. The results showed that compared to traditional liquid curing, ultrasound-assisted curing significantly increased the NaCl transfer rate, improved tenderness, and improved water retention and in vitro digestibility (p < 0.05). Microscopic observations revealed that ultrasound treatment substantially disrupted the muscle fiber structure, enlarging the spaces between fibers. Furthermore, ultrasound-assisted curing led to the unfolding of the spatial structure of myofibrillar proteins, enhanced intermolecular hydrophobic interactions, and promoted protein oxidation (p < 0.05), which are fundamental reasons for the improvement in fish quality. Thus, ultrasound treatment had a positive effect on fish curing, with the optimal parameters identified as 590 W for 78 min. Overall, the findings of this study provide evidence for the application of low-salt processing technology in fish products.
Keywords: Ultrasound, Air-dried fish, Salt reduction, Myofibrillar proteins
1. Introduction
Fish is regarded as a high-value food due to its abundance of unsaturated fatty acids and proteins [1]. Given its unique growing environment and high susceptibility to spoilage, preservation methods, such as curing and air-drying, are commonly employed to extend fish's shelf life. The curing process typically involves substantial amounts of salt to prevent microbial spoilage. In addition to its preservative function, salt enhances the flavor of air-dried fish and contributes to the formation of a compact gel structure in the fish meat [2]. However, excessive salt consumption is closely associated with chronic diseases, including cardiovascular, renal and cancer-related conditions [3]. Numerous health authorities, such as the World Health Organization (WHO), the China Health and Wellness Commission, and the Canadian Health Check™ Program, advocate for low-salt products to mitigate the health risks associated with high salt intake. Moreover, consumer preferences are shifting, with a recent hedonic test on low-salt ham showing a growing rejection of high-salt foods [2]. Consequently, there is an urgent need to transition from traditional high-salt products to low-salt alternatives and enhance the curing techniques used for dried fish.
The implementation of salt reduction strategies in fish product processing often faces considerable challenges, primarily because the perception of salty flavor and the extractability of myofibrillar proteins (MPs) are closely tied to salt content [4], [5]. One proposed approach to reduce overall salt content involves introducing calcium chloride (CaCl2), magnesium chloride (MgCl2) and potassium chloride (KCl) as partial or complete substitutes for sodium chloride (NaCl). However, this substitution is not without limitations and may adversely affect the organoleptic properties (e.g., flavor, texture) and shelf life of the product [6]. Specifically, using KCl as a substitute for NaCl can affect the flavor of meat products [7], and substituting more than 50 % of NaCl with metal salt mixtures may introduce a bitter taste, significantly reducing consumer acceptance [8]. Given these challenges, recent research has shifted toward exploring more advanced and comprehensive salt reduction strategies. Non-thermal physical technologies (e.g., ultrasound, high-pressure processing, electrostatic fields) and electro-processing techniques (e.g., pulsed light, pulsed electric fields) have emerged as promising methods for reducing salt in meat products. Among these, ultrasonic technology stands out due to its excellent safety record, cost-effectiveness, and high processing efficiency, making it a promising tool for food processing [6], [9].
Ultrasonic technology induces strong mechanical forces (e.g., microfluidics and shear) and chemical reactions (e.g., free radical generation) through cavitation, which effectively alters the molecular structure of MPs and enhances the penetration and distribution of salts in muscle tissues [4]. This provides technical support for developing high-quality, low-salt fish and meat products. Additionally, ultrasonic treatment significantly improves protein solubility, viscoelasticity, gelation ability, flavor retention and surface properties, which helps offset the quality losses associated with reduced salt content [3]. This opens new avenues for producing salt-reduced fish and meat products. In summary, the combined use of new processing technologies, particularly ultrasonic technology, offers a scientific and practical solution to the salt reduction challenge in fish products [10], promoting the development of healthier food options and meeting the growing consumer demand for low-salt, high-quality meat products.
Grass carp, a herbivorous freshwater species, is an economically significant fish characterized by its rapid growth rate and the relatively low cost of aquaculture, making it highly valuable for commercial purposes [11]. Furthermore, its smooth texture and nutrient-rich composition make it an essential raw material for the production of dried fish. While ultrasound-assisted marination technology has been employed in meat processing, research exploring its potential to reduce salt content during the marination of grass carp remains limited. Based on the optimal process of ultrasound-assisted air-drying of fish in a previous study, the present study aimed to further evaluate the effects of ultrasound-assisted curing on the quality of freshwater fish (grass carp), specifically in terms of water retention, tenderness and digestive characteristics. Additionally, the mechanism by which ultrasound-enabled low-salt curing enhances fish quality was assessed by examining the structure and microscopic properties of MPs. This study may provide both the technical parameters and theoretical foundation for ultrasound-assisted low-salt curing technology in freshwater fish.
2. Materials and methods
2.1. Material preparation
Grass carp (Ctenopharyngodon idellus, body weights 3.0–3.5 kg) were purchased from the Hongjin Agricultural Wholesale Market (Huangshi, China). The processing of the live fish was performed by the trained staff at the market, following standard commercial procedures and rendered unconscious by a hard blow to the back of the head. Scales, gills and viscera were removed, and the fish were thoroughly rinsed with running water to remove blood. After processing, the surface of the fish was wiped dry [10].
2.2. Curing and ultrasonication
The marination process of fish was carried out by liquid static marinade, i.e., the fish was placed in a polypropylene container with the marinade liquid. The marination treatments were divided into three groups: (i) distilled water control group (CK), (ii) 5.5 % (w/v) NaCl marinade group with static marinade (ST), and (iii) 5.5 % NaCl marinade group with ultrasonic treatment (UT) [9]. Each group was independently configured according to the fish to marinade ratio of 100:250 (w/v). The marination process parameters were determined based on the single factor and response surface optimization methodology, please refer to the supplementary experiments for specific optimization details. The marination process conditions were set as follows: the concentration of NaCl marinade was 5.5 % (w/v), and for the ultrasonic treatment group, the duration of the treatment was 78 min, and the ultrasonic power was set at 590 W and a 2-second ultrasonic interval was used. During the ultrasonication process, an ultrasonic equipment (Hielscher GmbH, Teltow, Germany) (model UP400S, frequency of 24 kHz, amplitude of 120 μm), equipped with a probe of 40 mm diameter, was used and the ultrasonic probe was immersed in the solution. After the pickling process, the samples were suspended to remove excess surface moisture, followed by air drying at 4 °C until the moisture content dropped below 60 %. This process was used to optimize the pickling effect while maintaining the desired texture of the fish flesh.
2.3. Quality indicators
2.3.1. Salt content determination
The NaCl content in fish meat was determined using the argentometric method, in accordance with the national food standard for the determination of chloride content (GB5009.44–2016) in China [10].
2.3.2. Cooking loss (CL)
The CL value of fish meat was determined using the method described by Liu et al [12]. The dorsal muscle of the fish was split, and samples (3 × 1.5 × 1.5 cm) were placed in a polyethylene bag and boiled in a water bath at 85 °C. Heating was ceased when the center of the samples reached 75 °C, as measured with a thermocouple thermometer. The samples were then cooled to room temperature, and residual water was removed from the surface using absorbent paper. The weights of the samples before and after cooking were recorded, and CL (%) was calculated as the percentage of weight loss, expressed as: (weight before cooking − weight after cooking)/(weight before cooking) × 100.
2.3.3. Shear force
The fish meat shear force was evaluated using a texture meter (FTC Corporation, Washington, USA) with reference to the method of Cai et al [13]. Briefly, the steamed-treated fish meat was cut into 3 cm × 1 cm × 1 cm sized pieces along the direction of the muscle fibers, and the maximum shear force value of the meat in the vertical muscle fiber direction was determined. A force of 250 N was applied by a mass spectrometer (equipped with a 500 N pressure transducer), the starting force was set at 0.2 N, the test and post-test speeds were 2 mm/s, and the return distance was set at 30 mm.
2.4. MP structural analysis
2.4.1. Preparation of MPs
Fish MPs were extracted following the method of Guo et al with slight modifications [14]. The fish back muscle was minced using a meat grinder, and 5 g of meat paste was added to four volumes (w/v) of 20 mM PBS (containing 0.1 M NaCl, 2 mM MgCl2 and 1 mM EDTA, pH 7.0) for homogenization (Denson Biotechnology Co., Ltd., Shanghai, China). The mixture was homogenized twice for 12 s each, followed by centrifugation at 4500 × g, 4 °C for 15 min. After each homogenization, the supernatant was discarded to obtain the precipitate. The precipitate was then resuspended in four volumes of PBS, and the homogenization and centrifugation steps were repeated twice. The final homogenate was filtered through four layers of 80-mesh gauze to remove connective tissue, and the precipitate obtained after centrifugation constituted the MPs extract.
2.4.2. Myofibrillar fragmentation index (MFI)
The extract MPs were adjusted to 0.5 mg/mL with PBS and the absorbance values were measured at 540 nm. MFI was expressed as 200 times the absorbance value [15].
2.4.3. Carbonyl and sulfhydryl content
MPs carbonyl and sulfhydryl levels were measured according to the kit manufacturer's instructions (Jiancheng Biological Research Institute, Nanjing, China) [16].
2.4.4. Determination of surface hydrophobicity
The MPs were diluted to 5 mg/mL using PBS. Then 1 mL of the MPs solution (with PBS as control) was added to 200 μL of bromophenol blue solution (BPB, 1 mg/mL) and mixed thoroughly. The solution was shaken for 10 min in the darkand then centrifuged (4000 rpm, 15 min, 4℃). After centrifugation, the supernatant was diluted 10-fold with PBS, and the absorbance value was measured at 595 nm [17]. The surface hydrophobicity of the MPs was calculated by the following formula:
2.4.5. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The method of Zhang et al was followed with minor modifications [18]. In brief, the MP concentration was adjusted to 5 mg/mL using PBS buffer, and the protein samples were mixed with loading buffer in a 1:1 (v/v) ratio. The mixture was then heated at 100 °C for 5 min. A molecular weight marker ranging from 10 kDa to 150 kDa was employed as a standard. The protein samples underwent SDS-PAGE using a 5 % concentration gel and a 12 % separation gel. The stacking gel was run at a constant voltage of 80 V, while the resolving gel was operated at 150 V. Following electrophoresis, the gels were stained with R-250 on a shaker for 2 h and subsequently destained until clear protein bands were visible. Gel images were captured using a gel imaging system (Bio-Rad, CA, USA), and the bands were quantified by scanning with Image J software to obtain grayscale values for data analysis.
2.4.6. Fourier transform infrared spectroscopy (FTIR)
MPs were freeze-dried and ground into powder, and 1 mg of MP powder was accurately weighed and ground with potassium bromide in the ratio of 1:100 (w:w) and pressed into tablets, and the samples were analyzed using a FTIR spectrometer (Thermo Fisher, MA, USA) across the spectral range of 400–4000 cm−1, with a resolution of 4 cm−1 and 32 scans. Baseline correction, deconvolution and Gaussian function fitting were applied to the amide I region (1600–1700 cm−1) to calculate the proportions of α-helix, β-sheet, β-turn and random coil structures in the proteins [19].
2.4.7. Fluorescence spectra
MP fluorescence spectra were analysed by using Zhang et al with slight modification [19]. The MP solution was diluted to 0.5 mg/mL with PBS buffer, and fluorescence spectra were obtained from 300-400 nm using a fluorescence spectrophotometer (Hitachi, Tokyo Metropolitan, Japan) with an excitation wave of 283 nm and excitation and emission slit widths of 10 nm.
2.5. Hematoxylin-eosin (HE) staining
Fish samples (1 cm × 1 cm × 1 cm) were fixed in 10 times volume of fixative (4 % paraformaldehyde) for 24 h. Sections were embedded by paraffin, sectioned, stained with HE, and sealed with neutral gum after dehydration in ethanol and xylene. The microstructure of the fish tissue was obtained by observation under a microscope (Nikon, Tokyo, Japan) at 200 × magnification [20].
2.6. Scanning electron microscopy (SEM)
Microstructure of the fish samples was observed using a scanning electron microscope (TM3030, Hitachi, Japan). Pre-processing steps, including sample fixation, ethanol dehydration, cleaning, freeze-drying, sectioning and gold plating, were performed according to the procedure outlined by Cai et al [13]. The final samples were scanned at 500 × magnification to obtain images.
2.7. In vitro static digestion
The digestive solution and static simulated digestion were prepared with reference to the methods of Brodkorb et al [21]. Simulated saliva (SSF), simulated gastric fluid (SGF) and simulated gastrointestinal fluid (SGF) and the specific parameters are shown in Table S1. The simulated digestive solution was stored at 4℃ after preparation, and preheated to 37℃ before digestion. For oral digestion, the MPs solution was adjusted to 30 mg/mL. Then 5 mL of MPs solution was added to 4 mL of SSF preheated to 37℃, and the pH of the mixture was adjusted to 7.0, then 25 μL of 0.3 M CaCl2 and 975 μL of distilled water were added sequentially, and then the mixture was digested and reacted for 2 min on a shaking table (37℃, 120 rpm). For gastric digestion, 8 mL of SGF was added to the sample after simulated oral digestion, and after adjusting the pH of the mixture to 3.0, 5 μL of 0.3 M CaCl2, 1895 μL of distilled water and 100 μL of pepsin solution (final concentration of 20 U/mL) were added in turn to reach a final volume of 10 mL. Then, the mixture was placed on a shaking table (37℃, 120 rpm) for a digestion reaction for 2 h. Immediately after digestion, the sample was removed, and the pH of the mixture was adjusted to 3.0. For intestinal digestion, the gastrointestinal two-step digestion sample after enzyme inactivation treatment was taken, 16 mL of SIF was added, and the pH of the mixture was adjusted to 7.0 by mixing, and then 40 μL of 0.3 M CaCl2, 3960 μL of distilled water, 0.1813 g of porcine bile salts, and 100 μL of trypsin solution (final concentration of 100 U/mL) were added in turn to reach a final volume of 20 mL. The mixture was then placed in a shaker (37℃, 120 rpm) for 1 h. Immediately after digestion, the samples were removed and heated at 95 ℃ for 5 min.
2.8. Determination of digestibility and particle size
The digestibility was expressed as the ratio of protein concentration before and after digestion, and the protein concentration was determined by BCA kit (Jiancheng Biological Research Institute, Nanjing, China). The concentration of MPs was adjusted to 1 mg/mL using PBS, and the particle size of MPs of each sample was determined using a laser particle size analyzer (Malvern, Malvern City, UK). The refractive index of the particles to be measured was set at 1.46, and the refractive index of the dispersed phase was set at 1.33. Particle size was expressed as the volume-averaged diameter (D43). The determination was repeated six times for each sample.
2.9. Statistical analysis
Each experiment was repeated three times independently and results were expressed as mean ± standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA), followed by Duncan's multiple range test with the significance level set at p < 0.05 (SPSS Inc., Chicago, IL, USA). All images were plotted with Origin 2022 Pro software (OriginLab Corp., Northampton, MA, USA).
3. Results and discussion
3.1. Quality characteristics
Ultrasound-assisted curing process promotes salt diffusion to shorten curing time. As shown in Fig. 1a, ultrasonic treatment significantly increased the salt level of fish tissue and significantly shortened the curing time by 40 min for the same salt concentration ultrasonic treatment. This may be due to the cavitation effect by ultrasound, which induced micro-vibration and formed microchannels at the fish interface, and facilitated salt penetration into fish tissues [22]. Ultrasonication can improve the production efficiency of cured air-dried fish. Water-holding capacity (WHC) and tenderness are usually considered as the most important indicators of meat quality and are also directly related to other physical attributes [9]. As shown in Fig. 1b, the cooking loss of fish using ultrasound-assisted marination (UT) was significantly lower than that of the other groups (p < 0.01), and the cooking loss of ultrasound-treated fish was reduced by 2.76 % compared with that of the static marination (ST). Shear force, an important indicator of tenderness, was significantly lower in the UT group than in the CK and ST groups (p < 0.01), with a reduction of 59.58 % and 41.78 %, respectively. The results suggest that the curing process with ultrasonic treatment usually increases fish WHC and improves muscle tenderness more than the curing process alone. However, the improvement effect of ultrasound was significantly correlated with ultrasound power Pan et al found that low levels of ultrasonication (less than 300 W) did not significantly (p > 0.05) affect muscle WHC and shear force [23]. The ultrasound power used in this study (590 W) improved the quality characteristics of cured air-dried fish, consistent with the findings of Pan et al [23]. Similar results have been observed in chicken, beef and fish [9], [24], [25].
Fig. 1.
(a) Influence of ultrasonic treatment on the NaCl content of cured air-dried fish. (b) Effect of ultrasonic treatment on cooking loss and shear force of cured air-dried fish. Different letters indicate significant differences (p < 0.05).
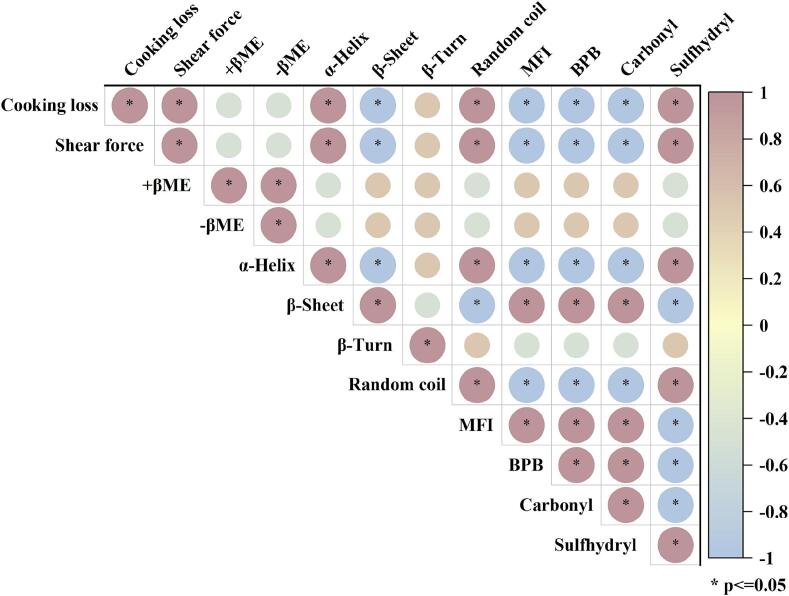
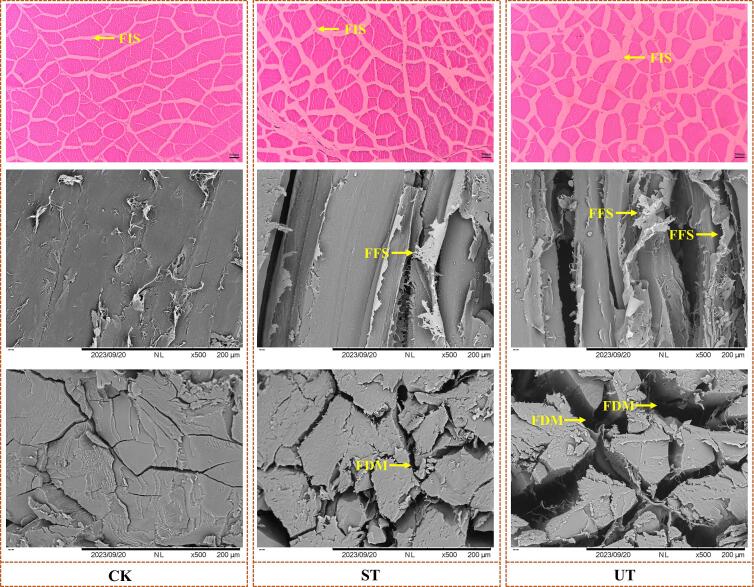
The mechanism by which the ultrasound affects WHC seems to be well established and is usually attributed to the ultrasonic treatment, which leads to deterioration and cavitation of the muscle fiber structure, increasing the interstitial space of the myofibrils, which provides accommodating space for the migration of water. Fig. 5 demonstrates that ultrasonication increased the gaps between muscle fibers, supporting this observation. Furthermore, the correlation analysis in Fig. 6 indicates a significant positive relationship between water-holding capacity and muscle fiber stability. In addition, the ultrasound-assisted curing process promotes uniform and rapid salt dispersion between muscle fibers, and osmotic pressure drives water retention in the interstitial spaces of myofibers [24]. This is consistent with the results of this study (Fig. 1a), where ultrasonication significantly enhanced salt diffusion in the muscle. It has been proposed that there is an inverse relationship between protein solubility and cooking losses due to the denaturation of MPs, whereby less water is retained in their structure (as a result of weakened capillary properties), and excess interstitial water is removed by cooking, thereby increasing cooking losses [25]. It is worth noting that this observation seems to be complemented by the results of shear force. Shear force was found to be inversely proportional to the integrity of myofibrils (Fig. 1b and Fig. 6), lower shear force indicates an increase in the degree of myofibril fracture, confirmed by the results of the MFI (Fig. 3a). The structural alterations in muscle fibers are closely linked to sensory attributes because they directly affect the mechanical properties of the muscle tissue. Ultrasonication-induced modifications, such as disruption of muscle fiber integrity, loosening of myofibrillar networks, and partial degradation of myofibrillar proteins, can reduce the overall toughness of the muscle, leading to improved tenderness [10], [13]. These changes facilitate the breakdown of the muscle fibers during mastication (Fig. 2 and Fig. 3a), which enhances chewiness and reduces the perception of toughness. However, the fracture of myofiber structure inevitably increases protein solubility, which is an important factor affecting fish quality. Overall, ultrasound-assisted curing had a positive impact on air-dried fish quality, including reduced curing time, improved tenderness and WHC.
Fig. 5.
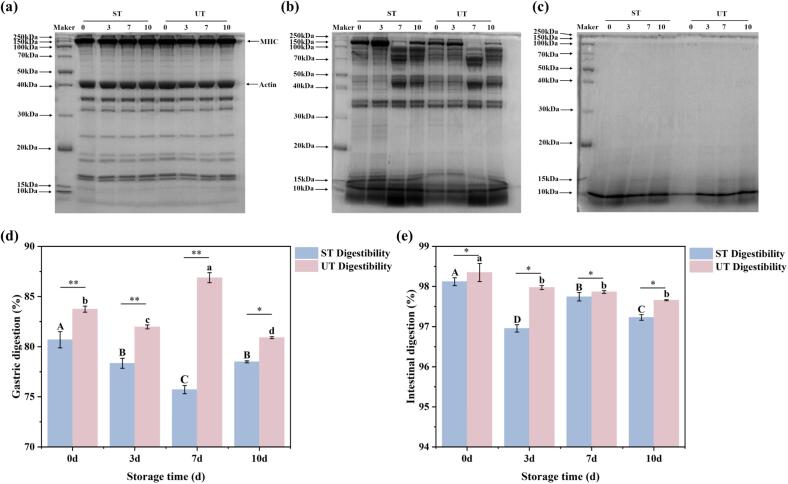
Effect of ultrasonic treatment on the digestive properties of air-dried fish. (a-c) SDS-PAGE profiles of air-dried fish MPs before digestion, gastric digestion and intestinal digestion. (d) Stomach digestibility. (e) Intestinal digestibility. Different letters indicate significant differences (p < 0.05).
Fig. 6.
Correlation analysis of ultrasound treatment on quality and protein structure of cured air-dried fish. Where red colour indicates positive correlation blue colour indicates negative correlation. “*” indicates significant correlation (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
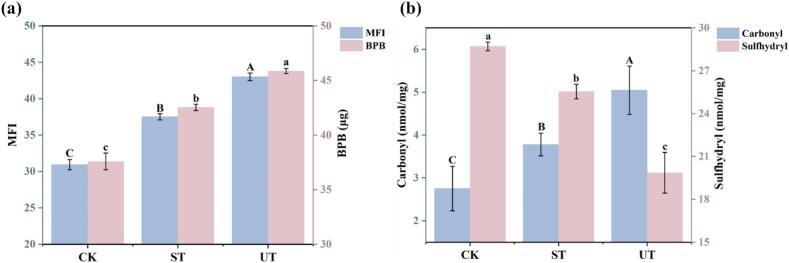
(a) Effect of sonication on myofibrillar fragmentation index (MFI) and hydrophobicity. (b) Effect of sonication on MPs carbonyl and sulfhydryl levels. Different letters indicate significant differences (p < 0.05).
Fig. 2.
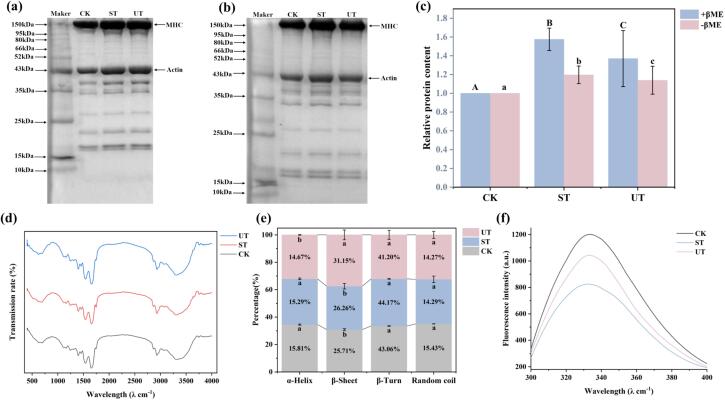
Effect of ultrasound treatment on the structure of myofibrillar proteins of air-dried fish. (a-b) SDS-PAGE plots of MPs in reducing condition (+βME) and non-reducing condition (−βME). (c) Calculation of relative actin content based on Images J. (d) FTIR scanning spectrogram. (e) Characterization of protein secondary structure. (f) Fluorescence intensity of tryptophan residues. Different letters indicate significant differences (p < 0.05).
3.2. MPs structure
MPs are the main component of proteins in the muscle, especially in fish muscle accounting for up to 50–70 % [24]. Therefore, the physicochemical characteristics of MPs are the core factors affecting the quality of fish meat. Myosin (50–60 %) and actin (20 %) were the absolute components of MPs, accounting for 70–80 % [23]. The electropherograms of the proteins (Fig. 2 a and b) showed the distribution of the proteins in the fish muscle samples, and the protein bands of different molecular weights were evenly distributed in the reduced (a) and non-reduced (b) states, and the protein bands did not show any significant differences among the groups. Among them, myosin heavy chain (MHC, 200 kDa) and actin (45 KDa) showed distinct target bands, consistent with the results of studies. Since the SDS-PAGE results could not directly show the protein cross-linking or degradation differences, the status was assessed by comparing the spectral band densities (Fig. 2c). The difference in band densities between the ST and UT groups was not significant (p > 0.05), but the band densities tended to be higher. The increased band density indicated enhanced protein extraction, this may be becausethe salt from the curing process disrupts the original structure and composition of the myofibers, leading to myofibril separation and lysis.
Analyzing the secondary structure of the protein based on the amide band of FTIR (1600–1700 cm−1) showed that the ultrasound-mediated curing process resulted in a shift from α-helix to β-sheet of fish MPs (Fig. 2e). Hydrogen bonding within the peptide chains of proteins plays a key role in the stability of the α-helix, while hydrogen bonding between peptide chains is essential for the maintenance of the β-sheet structure. The decrease in the proportion of α-helix suggests that ultrasonication promotes the breakage of hydrogen bonds within the peptide chain, leading to the exposure of reactive groups, disrupting the ordered structure of the MPs and decreasing the stability of the proteins [26]. The conversion of protein secondary structure from α-helix to β-sheet was also observed during ultrasound-assisted thawing of duck meat, and this conversion was favorable for increasing the WHC of the meat [27], consistent with the results of this study. Therefore, hydrogen bond breaking and rearrangement-induced protein secondary structure conversion is a key factor affecting the WHC of meat. Endogenous fluorescence originates from aromatic amino acid residues (e.g., tryptophan) within the structure of MPs, and can reflect protein molecular environmental variables and characterize the tertiary structure of proteins [28]. The tryptophan of MPs has a maximum absorption peak near 332 nm, and although no shift in the absorption peak caused by the sonication and curing processes was observed, the sonication and curing processes significantly reduced the fluorescence intensity of the tryptophan (Fig. 2f). This was attributed to the structural unfolding of the protein, resulting in the exposure of hydrophobic amino acids on its side chains. Meanwhile, the enhanced electrostatic interactions between the protein molecules induced their aggregation, and the exposed tryptophan residues were encapsulated in the aggregates, thus reducing the fluorescence [28]. Enhanced hydrophobic interactions were observed in the present study, which may lead to the encapsulation of tryptophan by hydrophobic groups inside the protein structure, reducing the fluorescence intensity of tryptophan. However, this wrapping behavior causes the formation of protein aggregates, and the changes in the protein primary and secondary structure do not support this view. Another view is that salt penetration triggers a change in solvent polarity and reduces the stability of tryptophan chromophores in protein molecules. In addition, protein oxidation induces a modifying effect on tryptophan, reducing its fluorescence intensity [29]. The changed carbonyl and sulfhydryl levels (Fig. 3b) confirmed the enhancement of protein oxidation. Therefore, the decrease in fluorescence intensity triggered by the curing process may be attributed to the oxidative modification of tryptophan, and the higher fluorescence intensity of UT tryptophan than ST may be due to the reduction of the aggregation and masking between protein molecules through intermolecular forces by ultrasound.
Fig. 2 Moving to 3.2 MPs structure
3.3. Intermolecular interactions and protein oxidation of MPs
Myofibrillar Fragmentation Index (MFI) refers to the proportion of 1 to 4 myofibril segment fragments within the myofibrillar structure. It is utilized to characterize the integrity and extent of damage to the internal structure of myofibrils [29]. As illustrated in Fig. 3a, the MFI value for the UT was 43.0 ± 0.53, significantly higher than those of CK and ST groups, exceeding by 28.1 % and 12.8 % (p < 0.05), respectively. Similarly, Cao et al. (2021) reported that ultrasound can damage muscle fibers. Ultrasonic treatment enhanced the solubility and fragmentation of MPs, as verified by the structural characteristics of the MPs (Fig. 4). Furthermore, the increase in MFI values was positively correlated with improved tenderness in fish meat, and the reduction in shear force levels observed in this study supports this result (Fig. 6).
Fig. 4.
Effect of ultrasonication on the microstructure of cured air-dried fish muscle tissue (HE staining, 500 μm and scanning electron microscopy, 200 μm). FIS, fiber interstitial space, FFS, fiber fragments, FDM, fiber depolymerization.
It is imperative to emphasize that ultrasound can also exert its influence on the aggregation and dispersion of MPs through mechanisms involving protein oxidation and intermolecular interactions [22], [23]. Surface hydrophobicity is a pivotal intermolecular force underpinning the stability of protein tertiary structure, with its variations serving as indicators of conformational shifts within the protein architecture [23]. As shown in Fig. 3a, ultrasonic treatment markedly enhanced the surface hydrophobicity of MPs compared with CK and ST (p < 0.05). Recent investigations have demonstrated that ultrasound disrupts intermolecular hydrogen bonds, electrostatic interactions and hydration forces, thus instigating a reorganization of protein aggregates and prompting the translocation of hydrophobic moieties to the surface. This phenomenon is further accompanied by the outward migration of hydrophobic cores, culminating in an elevation of hydrophobicity. Under typical conditions, hydrophobic residues are sequestered by water molecules, clustering within the protein matrix and driving the peptide chains into intricate folds [22]. Nevertheless, ultrasonic treatment augments the exposure of hydrophobic groups, thereby facilitating the disintegration and solubilization of proteins in aqueous environments.
The unfolding of the three-dimensional structure of MPs results in the exposure of amino acid side chains previously concealed within the protein motifs. These liberated side chains exhibit increased susceptibility to oxidation, culminating in an augmented carbonyl content alongside a concomitant reduction in sulfhydryl levels [28]. Consequently, carbonyl and sulfhydryl groups are routinely employed as biomarkers to assess the degree of protein oxidation. As illustrated in Fig. 3b, ultrasonic treatment markedly increased the carbonyl content of MPs while significantly diminishing sulfhydryl levels in comparison to CK and ST (p < 0.05). The formation of carbonyls arises from the oxidative transformation of amino acid residues within the protein, whereas the depletion of sulfhydryls is primarily due to oxidative modifications affecting the cysteine residues embedded in the protein matrix [30]. It is unequivocal that protein oxidation instigates modifications to the chemical properties and spatial configurations of amino acid side chains, thereby influencing the unfolding and aggregation of muscle proteins [30]. The structural changes observed in our study is consistent with the report (Fig. 2). It is generally believed that protein oxidation negatively impacts meat quality, including loss of freshness, rancidity, the development of off-flavors, reduced water-holding capacity, diminished gel-forming ability, and decreased nutritional value [31] (Günal-Köroğlu, et al., 2025). An alternative view, however, posits that the effects of oxidation are degree-dependent; low levels of oxidation may actually improve muscle quality. For example, oxidation-induced protein degradation can enhance meat tenderness [32], and the release of small molecules during oxidation can enrich the aroma of the meat [33]. In the present study, we observed that ultrasonication intensified the oxidation process of muscle proteins (MP) during fish marination. However, protein oxidation did not negatively affect the water retention and tenderness of the fish (Fig. 1). These results suggest that the modifications in the fish muscle proteins are a combined outcome of oxidation and ultrasound treatment. Nonetheless, the structural changes induced by oxidation appear to be masked by the mechanical modifications brought about by the ultrasound. The influence of ultrasonic treatment on sulfhydryl levels warrants a multifaceted analysis that transcends a singular focus on protein oxidation. Sulfhydryl groups are indispensable for the maintenance of the spatial architecture of proteins, serving as critical indicators of protein unfolding, disulfide bond formation and conformational alterations [23]. This importance is underscored by the capacity of sulfhydryls to facilitate the formation of disulfide bonds through the oxidation of cysteine residues situated on adjacent polypeptide chains, thereby acting as pivotal determinants in the stabilization of both tertiary and quaternary structures [22]. Nevertheless, Pan et al have found that ultrasonic treatment imparted a robust mechanical effect, increasing the sulfhydryl content by promoting the exposure of these groups − an observation that stands in stark contrast to the findings of the current study [23]. Thus, the effects of ultrasound on sulfhydryl levels present a dual nature. Ultrasonic treatment may catalyze protein unfolding, leading to subunit dissociation, cleavage of disulfide bonds, and the exposure of internal thiol groups, ultimately culminating in the unmasking of sulfhydryls. This exposure of inherently unstable moieties may inevitably precipitate oxidative losses. Consequently, the observed sulfhydryl levels emerge as a complex interplay between structural integrity and oxidative dynamics within proteins.
Fig. 3 Moving to 3.3
3.4. Tissue microstructure of air-dried fish
Tissue sections and microstructure revealed that muscle fibers in the CK group were uniformly distributed and aligned, and the fiber shape was complete (Fig. 4a). The marinated groups (ST and UT) exhibited different degrees of myofiber damage, the structure and smoothness of myofibers were destroyed, fragments appeared, the fiber surface gradually became rough, and the fiber gap increased (Fig. 4b and c). Especially in the UT group, depolymerization of myofibers and dispersion of fiber filaments were observed, which could not maintain normal muscle bundles. The results indicated that ultrasound was able to disrupt the structural integrity of myofibrils, leading to myofibrillar rupture. The exposure of the internal motifs of the protein structure, which in turn affects the intermolecular forces and the primary, secondary and tertiary structure of the protein, confirms the results as shown in Fig. 2.
Fig. 4 Moving to 3.4
3.5. In vitro simulated digestion of air-dried fish
Protein digestibility constitutes a pivotal metric for evaluating both the rate and extent of protein digestion within food matrices, thereby representing one of the most critical attributes for delineating the nutritional characteristics of dietary sources [34]. In this context, the present study seeks to elucidate the effects of ultrasonic treatment on the nutritional profile of marinated and air-dried fish through an in vitro digestion model. It is widely acknowledged that proteins with elevated digestibility may have enhanced bioavailability, signifying a greater propensity for digestion and subsequent absorption by the human. As depicted in Fig. 5a-c, the MPs exhibited pronounced disparities in molecular weight subsequent to in vitro gastrointestinal simulated digestion. Prior to digestion, the protein bands for ST and UT were similarily distributed. However, following gastric digestion, the density of protein bands for UT was markedly lower than that of ST, particularly for those exceeding 40 kDa, where the difference was even more pronounced. In the aftermath of intestinal digestion, the protein bands in both ST and UT (greater than 20 kDa) were nearly undetectable, while the intensity of UT protein bands below 20 kDa was significantly reduced as compared to those of ST. In parallel, the gastrointestinal digest of MPs subjected to ultrasound-assisted curing showed a significantly enhanced profile compared to ST (Fig. 5 d and e). Particle size constitutes a critical parameter for delineating the spatial dimensions of proteins, encapsulating not only the molecular weight distribution but also the digestibility profile of the protein. Analyzing the particle size of the MPs throughout the simulated digestion process revealed a marked decrease in size with prolonged digestion time. Particularly, most of the UT particle sizes were significantly smaller than those of ST, especially in the later stages of intestinal digestion, where the particle size of UT exhibited a reduction by a factor of 1.73 (Table 1).
Table 1.
Effect of ultrasound assisted pickling on the particle size of MPs in dry fish.
| Digestive model | Particle size (μm) |
||||
|---|---|---|---|---|---|
| 0 d | 3 d | 7 d | 10 d | ||
| Pre-digestion |
ST | 200.37 ± 21.77aA | 158.72 ± 6.15bA | 210.08 ± 2.83aA | 139.30 ± 2.49bA |
| UT | 140.72 ± 3.20bB | 140.47 ± 0.56bA | 159.19 ± 1.86aB | 119.68 ± 2.43cB | |
| Gastric digestion | ST | 54.71 ± 0.90bA | 60.02 ± 4.20aA | 35.42 ± 0.51dA | 41.39 ± 0.25cA |
| UT | 46.26 ± 0.92aB | 40.20 ± 0.55bB | 32.83 ± 1.32cB | 39.95 ± 0.31bB | |
| Intestinal digestion | ST | 12.47 ± 1.37cA | 12.98 ± 0.38cA | 48.86 ± 1.73aA | 43.86 ± 1.71bA |
| UT | 8.30 ± 0.10cA | 8.74 ± 0.11cB | 33.15 ± 2.47aB | 25.29 ± 2.00bB | |
Note: Different lowercase letters on the same line indicate significant differences between storage times in the treatment groups (p < 0.05); Different capital letters indicate significant differences (p < 0.05) between the ST and UT groups under the same storage time, same as below.
Fig. 5 Moving to 3.5
The digestibility of proteins and their particle size are profoundly influenced by the microstructural characteristics of the food matrix, as a stable network architecture can significantly hinder digestive efficiency [34]. Yang et al reported that ultrasound treatment increased the β-sheet content within proteins, thereby rendering the secondary structure of the digestive products more dynamic, which in turn exerted a beneficial impact on protein digestion within the gastrointestinal tract [35]. The cavitation effects induced by ultrasound appear to facilitate substantial structural modifications in proteins, leading to the exposure of reactive residues on their surfaces. This exposure not only diminishes the rigidity of the protein structure but also enhances its flexibility, thereby promoting interactions between proteins and digestive enzymes. Consequently, this mechanism results in improved protein digestibility [35]. In conclusion, an assessment of ultrasound treatment's influence on fish protein utilization, evaluated from both digestibility and nutritional perspectives, may yield positive implications.
4. Conclusions
The findings of this study showed that the ultrasound-assisted curing process for air-dried fish not only accomplished the goal of salt reduction but also significantly enhanced the tenderness of the fish, augmented its water retention capacity, and elevated its digestibility. More specifically, ultrasound treatment promoted the conversion of α-helices into β-sheets, intensified hydrophobic interactions, exposed tryptophan residues, and facilitated protein oxidation. These transformations in the spatial configuration and intermolecular interactions of the proteins lead to notable alterations in the characteristics of MPs. In conclusion, the implementation of ultrasound technology in the meat curing process holds tremendous potential for broader applications, warranting further exploration and utilization.
CRediT authorship contribution statement
Jun Liu: Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Changxin Xie: Visualization, Software, Resources, Methodology, Investigation, Data curation, Conceptualization. Wenhan Ma: Investigation. Xue Xiao: Investigation. Weiwei Dong: Software, Resources. Youwei Chen: Resources, Methodology. Yuanliang Hu: Supervision, Resources, Project administration. Yanli Feng: Software, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Xiang Yu: Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
This study was financially supported by the Hubei Normal University 2023 Talent Introduction Project (HS2023RC084), Hubei Provincial Natural Science Foundation Project (2024AFB162), Hubei Key Laboratory of Edible Wild Plants Conservation and Utilization Open Fund (EWPL202304), Hubei Provincial Department of Education's Science Research Program for Young Talents (Q20232506), and Centralized Local Science and Technology Development Funds (Laboratory Major Scientific and Technological Achievement Transformation) Project (2024BSB020).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2024.107214.
Contributor Information
Jun Liu, Email: JLiu@hbnu.edu.cn.
Yuanliang Hu, Email: ylhu@hbnu.edu.cn.
Yanli Feng, Email: fengyanli@hbnu.edu.cn.
Xiang Yu, Email: yuxiang25cn@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Faisal M., Rima N.N., Riya K.K., Sarker P.K., Siddique M.A.B., Albeshr M.F., Arai T., Yu J., Hossain M.B. Assessing health hazards of dried fish consumption from coastal markets in a developing nation. J Agr Food Res. 2024;18 doi: 10.1016/j.jafr.2024.101385. [DOI] [Google Scholar]
- 2.Zhao X., Zhou C., Xu X., Zeng X., Xing T. Ultrasound combined with carrageenan and curdlan addition improved the gelation properties of low-salt chicken meat paste. LWT - Food Sci Technol. 2022;172 doi: 10.1016/j.lwt.2022.114230. [DOI] [Google Scholar]
- 3.Jiang S., Li Q., Wang T., Huang Y., Guo Y., Meng X. Utilizing ultrasound combined with quinoa protein to improve the texture and rheological properties of Chinese style reduced-salt pork meatballs (lion’s head) Ultrason Sonochem. 2024;109 doi: 10.1016/j.ultsonch.2024.106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X., Yang S., You J., Yin T., Xiong S., Liu R. Changes in gelation properties of silver carp myosin treated by combination of high intensity ultrasound and NaCl. Foods. 2022;11:3830. doi: 10.3390/foods11233830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan D., Xu W., Yu Q., You J., Gao R., Bao Y. Pre-rigor salting improves gel strength and water-holding of surimi gel made from snakehead fish (Channa argus): The role of protein oxidation. Food Chem. 2024;450 doi: 10.1016/j.foodchem.2024.139269. [DOI] [PubMed] [Google Scholar]
- 6.Fan S., Guo J., Wang X., Liu X., Chen Z., Zhou P. Effects of lipoxygenase/linoleic acid on the structural characteristics and aggregation behavior of pork myofibrillar protein under low salt concentration. LWT - Food Sci Technol. 2022;161 doi: 10.1016/j.lwt.2022.113359. [DOI] [Google Scholar]
- 7.Zheng J., Han Y., Ge G., Zhao M., Sun W. Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocolloid. 2019;96:36–42. doi: 10.1016/j.foodhyd.2019.05.003. [DOI] [Google Scholar]
- 8.Petit G., Jury V., de Lamballerie M., Duranton F., Pottier L., Martin J. Salt intake from processed meat products: benefits, risks and evolving practices. Compr Rev Food Sci F. 2019;18:1453–1473. doi: 10.1111/1541-4337.12478. [DOI] [PubMed] [Google Scholar]
- 9.Çimen N., Unal K., Alp H. Effects of ultrasound-assisted marination on spent hen meats: Microstructure, textural and technological properties. Food Biosci. 2024;61 doi: 10.1016/j.fbio.2024.104563. [DOI] [Google Scholar]
- 10.Bai H., Li L., Wu Y., Chen S., Zhao Y., Cai Q., Wang Y. Ultrasound improves the low-sodium salt curing of sea bass: Insights into the effects of ultrasound on texture, microstructure, and flavor characteristics. Ultrason Sonochem. 2023;100 doi: 10.1016/j.ultsonch.2023.106597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X., Feng L., Jiang W., Wu P., Liu Y., Ren H., Jin X., Zhang R., Zhou X. From antioxidant to muscle enhancer: Resveratrol's role in grass carp (Ctenopharyngodon idella) nutrition. Aquacult Rep. 2024;39 doi: 10.1016/j.aqrep.2024.102499. [DOI] [Google Scholar]
- 12.Liu J., Hu Z., Liu D., Zheng A., Ma Q. Glutathione metabolism-mediated ferroptosis reduces water-holding capacity in beef during cold storage. Food Chem. 2023;398 doi: 10.1016/j.foodchem.2022.133903. [DOI] [PubMed] [Google Scholar]
- 13.Cai L., Cao M., Cao A., Regenstein J., Li J., Guan R. Ultrasound or microwave vacuum thawing of red seabream (Pagrus major) fillets. Ultrason Sonochem. 2018;47:122–132. doi: 10.1016/j.ultsonch.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y., Kong B., Xia X., Yu T., Liu Q. Changes in physicochemical and protein structural properties of common carp (Cyprinus carpio) muscle subjected to different freeze-thaw cycles. J Aquat Food Prod t. 2014;23:579–590. doi: 10.1080/10498850.2012.741663. [DOI] [Google Scholar]
- 15.Cao C., Xiao Z., Tong H., Tao X., Gu D., Wu Y., Xu Z., Ge C. Effect of ultrasound-assisted enzyme treatment on the quality of chicken breast meat. Food Bioprod Process. 2021;125:193–203. doi: 10.1016/j.fbp.2020.11.005. [DOI] [Google Scholar]
- 16.Rashid M.T., Liu K., Ning M., Ullah K., Wali A., Jatoi M.A., Muzaffar N. Enhanced antioxidant activity of selenium-enriched brown rice protein against oxidative stress in mammalian erythrocytes under various cooking conditions. J Agr Food Res. 2024;18 doi: 10.1016/j.jafr.2024.101520. [DOI] [Google Scholar]
- 17.Zhang D., Li H., Emara A.M., Hu Y., Wang Z., Wang M., He Z. Effect of in vitro oxidation on the water retention mechanism of myofibrillar proteins gel from pork muscles. Food Chem. 2020;315 doi: 10.1016/j.foodchem.2020.126226. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z., Yang Y., Tang X., Chen Y., You Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015;188:111–118. doi: 10.1016/j.foodchem.2015.04.129. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H., Tang D., Yang H., Liu X., Cheng J., Wang X., Zou J., Lin Y. Effects of high hydrostatic pressure assisted enzymatic tenderization on goose meat texture and myofibril protein. LWT - Food Sci Technol. 2023;184 doi: 10.1016/j.lwt.2023.114845. [DOI] [Google Scholar]
- 20.Liu J., Hu Z., Ma Q., Yang C., Zheng A., Liu D. Reduced water-holding capacity of beef during refrigeration is associated within creased heme oxygenase 1 expression, oxidative stress and ferroptosis. Meat Sci. 2023;202 doi: 10.1016/j.meatsci.2023.109202. [DOI] [PubMed] [Google Scholar]
- 21.Brodkorb A., Egger L., Alminger M., Alvito P., Assuncao R., Ballance S., Bohn T., Bourlieu-Lacanal C., Boutrou R., Carrière F., Clemente A., Corredig M., Dupont D., Dufour C., Edwards C., Golding M., Karakaya S., Kirkhus B., Le Feunteun S., Lesmes U., Macierzanka A., Mackie A.R., Martins C., Marze S., McClements D.J., Ménard O., Minekus M., Portmann R., Santos C.N., Souchon I., Singh R.P., Vegarud G.E., Wickham M., Weitschies W., Recio I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc. 2019;14:991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 22.Gu S., Zhu Q., Zhou Y., Wan J., Liu L., Zhou Y., Chen D., Huang Y., Chen L., Zhong X. Effect of ultrasound combined with glycerol-mediated low-sodium curing on the quality and protein structure of pork tenderloin. Foods. 2022;11:3798. doi: 10.3390/foods11233798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan Q., Zhou Y., Wang Y., Xu B., Li P., Chen C. Effects of ultrasound-assisted dry-curing on water holding capacity and tenderness of reduced‑sodium pork by modifying salt-soluble proteins. Food Chem. 2024;453 doi: 10.1016/j.foodchem.2024.139704. [DOI] [PubMed] [Google Scholar]
- 24.Liu D., Du L., Huang Q., Zhou M., Xiong G., Li C., Qiao Y., Wu W. Effects of ultrasound treatment on muscle structure, volatile compounds, and small molecule metabolites of salted Culter alburnus fish. Ultrason Sonochem. 2023;97 doi: 10.1016/j.ultsonch.2023.106440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehrabani A., Jebelli Javan A., Hesarinejad M.A., Mahdavi A., Parsaeimehr M. The combined effect of ultrasound treatment and leek (Allium ampeloprasum) extract on the quality properties of beef. Food Biosci. 2022;47 doi: 10.1016/j.fbio.2022.101622. [DOI] [Google Scholar]
- 26.Yin F., Bai X., Wang K., Ru A., Xu L., Tian W., Hao J., Zhu C., Zhao G. Mechanism of tumbling-curing to improve beef quality: Insights from the structural and functional properties of myofibrillar protein. LWT - Food Sci Technol. 2024;207 doi: 10.1016/j.lwt.2024.116692. [DOI] [Google Scholar]
- 27.Sun H., Zhao Y., Zhao J., Sun J. Ultrasound thawing for improving the eating quality and off-flavor of frozen duck meat and its possible mechanisms. LWT - Food Sci Technol. 2023;187 doi: 10.1016/j.lwt.2023.115314. [DOI] [Google Scholar]
- 28.Cheng Y., Zheng Y., Cai X., Wang L., Zhou C., Cao J., Tong C., Wang J., Sun Y., Wang Z., Barba F.J., Pan D., Wu Z., Xia Q. Effect of pre-acidification induction on the physicochemical features, myofibrillar protein microstructure, and headspace volatiles of ready-to-cook goose meat. Food Res Int. 2024;197 doi: 10.1016/j.foodres.2024.115166. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Zhu L., Song L., Song L., Shi S., Liu H., Wu J., Si K., Gong T., Liu H. Combined treatment of lactic acid-ultrasound-papain on yak meat and its tenderization mechanism. Meat Sci. 2023;196 doi: 10.1016/j.meatsci.2022.109043. [DOI] [PubMed] [Google Scholar]
- 30.Yu Q., Hong H., Liu Y., Monto A.R., Gao R., Bao Y. Oxidation affects pH buffering capacity of myofibrillar proteins via modification of histidine residue and structure of myofibrillar proteins. Int J Biol Macromol. 2024;260 doi: 10.1016/j.ijbiomac.2024.129532. [DOI] [PubMed] [Google Scholar]
- 31.Günal-Köroğlu D., Yılmaz H., Gultekin Subasi B., Capanoglu E. Protein oxidation: The effect of different preservation methods or phenolic additives during chilled and frozen storage of meat/meat products. Food Res Int. 2025;200 doi: 10.1016/j.foodres.2024.115378. [DOI] [PubMed] [Google Scholar]
- 32.Lund M.N., Heinonen M., Baron C.P., Estévez M. Protein oxidation in muscle foods: A review. Mol Nutr Food Res. 2011;55:83–95. doi: 10.1002/mnfr.201000453. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Xiang X., Wei S., Li S. Multi-omics revealed the formation mechanism of flavor in salted egg yolk induced by the stages of lipid oxidation during salting. Food Chem. 2023;398 doi: 10.1016/j.foodchem.2022.133794. [DOI] [PubMed] [Google Scholar]
- 34.Yu Z., Zhang H., Guo H., Zhang L., Zhang X., Chen Y. High intensity ultrasound-assisted quality enhancing of the marinated egg: Gel properties and in vitro digestion analysis. Ultrason Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Shao J., Duan Y., Geng F., Jin W., Zhang H., Peng D., Deng Q. Insights into digestibility, biological activity, and peptide profiling of flaxseed protein isolates treated by ultrasound coupled with alkali cycling. Food Res Int. 2024;190 doi: 10.1016/j.foodres.2024.114629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.