Abstract
Tumor-associated macrophages and tumor-infiltrating lymphocytes are associated with survival in solid malignancies. Given the physiological link to peripheral immune cell counts, we evaluated if peripheral immune cell counts were predictors of outcomes in endometrial cancer. A retrospective study was completed for endometrial cancer cases between 2000 and 2010. Kaplan-Meier, bivariate, and multivariable Cox proportion hazard analyses were performed examining the relations between survival and peripheral immune cell counts. Three hundred ten patients were identified. In bivariate analyses, high monocyte counts (> 0.7 × 109 cells/L) trended with decreased progression free survival (PFS) (p = 0.10) and poorer overall survival (OS) (p = 0.16). By contrast, high lymphocyte level (> 1.5 × 109 cells/L) was associated with improved PFS (p = 0.008) and OS (p = 0.006). These findings were consistent for type I and type II endometrial cancers. In a multivariable Cox model, high monocyte level was associated with a greater risk of disease recurrence (hazard ratio (HR) = 1.63, p < 0.035). Other significant predictors of recurrence were age, non-endometrioid histology, and the presence of lymph vascular space invasion (LVSI). In a multivariable Cox model, high lymphocyte count trended with a lower risk of death (HR = 0.66, p = 0.07). Age, surgical stage, non-endometrioid histology, and LVSI were also associated with death in this model. In this sample of endometrial cancer patients, we found that high preoperative lymphocyte counts were associated with improved overall improved survival. High monocyte counts were associated with poorer disease-free survival outcomes. Further studies that focused on understanding tumor-antagonizing and pro-tumoral effects of lymphocytes and monocytes, respectively, in endometrial cancer are recommended.
Keywords: Endometrial cancer, Immune system, Peripheral lymphocytes, Peripheral monocytes
Introduction
Endometrial cancer is a major cause of morbidity and death in the USA with an estimated 61,880 new cases and 12,160 deaths in 2019. It is the fourth most frequent cancer in women and the most common gynecologic malignancy with a lifetime incidence of 2.7% https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html. Because abnormal uterine bleeding is often the sentinel signal for endometrial cancer, most patients are diagnosed at an early stage with invasion limited to the uterus. Widely accepted prognostic factors include (a) histological subtype, (b) depth of myometrial invasion (MI), (c) lymphovascular space invasion (LVSI), (d) cervical involvement, (e) lymph node metastasis, (f) peritoneal metastasis, and (g) patient age [1–7]. However, there is variability in survival outcomes among women with similar levels of prognostic factors, and as a result, a better understanding of underlying pathophysiology as well as identification of new, more specific biomarkers to guide treatment planning and surveillance is sought.
To this end, advances in cancer immunology have been made over the past decade demonstrating a critical role of the immune system in cancer progression and invasion for many solid tumors, including endometrial cancer. It is now recognized that cancer progression likely arises from complex biomolecular cell signaling cascades, initiated by the response of tumor cells to the surrounding tissue milieu, including tumor-infiltrating macrophages, neutrophils, and lymphocytes, as well as the local proliferation of stromal cells and fibroblasts [8]. Bidirectional molecular communication between immune cells and tumor cells stimulates anti- or pro-tumoral immune cell phenotypes [8–12]. The individual contribution to cancer progression or cancer regression from each immune cell type is currently an area of active research. Macrophages and lymphocytes, in particular, have been previously suggested as key immune cells in the tumor microenvironment.
With respect to lymphocytes, there are increasing lines of evidence that tumor-infiltrating lymphocytes (TILs) are associated with tumor regression and improved prognosis for several solid tumors [13–16]. The exact underlying mechanism, however, is complicated by the contributions of different T-cell lineages such as CD4+ and CD8+ cells, of which the respective actions have not been fully elucidated. In the case of endometrial cancer, intratumoral CD8+ T lymphocytes have been linked to improved OS and identified as an independent prognostic factor [17, 18]. Additionally, there are strong data supporting CD8+ T cells as key mediators of tumor regression in metastatic melanoma. This work has led to development of new technologies using autologous adoptive T-cell therapies, which have had promising durable clinical responses [19], and the development of new immunotherapeutics aimed at targeting checkpoint inhibition in lymphocytes.
Tumor-associated macrophages (TAMs) are also identified as critical in the biology of breast [20, 21], cervical [22], endometrial [23–25], and renal carcinomas [26, 27]. Macrophages are derived from differentiated monocytes in the peripheral circulation, with the capacity to be functionally activated by local cytokine concentrations and cell-receptor signaling to M1 (antitumor) or M2 (pro-tumoral) phenotypes. Prior studies have linked TAMs with increased tumor aggressiveness and poorer overall prognosis [28–30] consistent with an M2 functional phenotype. In the case of endometrial cancer, TAMs have been correlated with increased MI, LVSI, angiogenesis and lymph node metastasis [11, 31–33], and type II histology [34].
Mechanistically, leukocytes extravasate from peripheral blood into target tissues, including solid tumors where key cell-receptor- and cell-cell-mediated signaling occurs. Because of the relatively short half-life of immune cells and ongoing dynamic interaction of cellular exchanges with tumor cells, it is expected that constant recruitment of immune cells from the periphery to the tumor microenvironment occurs. Consequently, it is plausible that direct qualitative and quantitative interactions at the tumor level are reflected by respective peripheral immune cell counts.
The aim of this study was to explore the relation between preoperative circulating immune cell counts on progression-free survival (PFS) and overall survival (OS) in women with endometrial cancer. Our focus is on lymphocytes and monocytes, given their clinical significance in other solid tumor malignancies.
Materials and Methods
Study Eligibility
Following Internal Review Board (IRB) approval (Protocol CCCWFU#99A13), a search of the Wake Forest University Comprehensive Cancer Center Tumor Bank registry was completed for all patients diagnosed with endometrial cancer from January 2000 to December 2010. A total of 331 patients were identified as possible candidates. Patient inclusion criteria included surgical staging at Wake Forest Baptist Hospital and a complete blood count within 6 weeks prior to surgery. Exclusion criteria included any of the following: (a) known history of immune compromised status (e.g., HIV, chronic corticosteroid use), (b) chronic inflammatory diseases, (c) presence of second primary cancer at the time of diagnosis, (d) final pathology revealing noninvasive endometrial lesions, (e) uncommon or rare histological subtype, (f) prior neoadjuvant therapy, and (g) loss to follow-up. Of the eligible cases, the following information was extracted from the medical records: (a) patient demographics, (b) final pathology report, (c) preoperative complete blood count, (d) review of past medical history and medications prior to surgical staging, and (e) lengths of PFS and OS.
Clinical Data
Archived medical records and the Wake Forest Tumor Registry data were reviewed, and appropriate clinical information was extracted. Demographic data included age at time of surgery, past medical history and current medications, body mass index (BMI), and ethnicity. Final pathology records were reviewed to determine FIGO staging, depth of MI, presence of LVSI, histological subtype, grade, and presence or absence of lymph node metastasis. Preoperative laboratory data including hemoglobin, total white blood count (WBC), neutrophils, monocytes, lymphocytes, and platelets were collected. If multiple CBC results were available, the laboratory report closest to the time of surgery was used. Cell counts were dichotomized for hemoglobin, neutrophils, platelets, monocytes, and lymphocytes into low versus high levels. For hemoglobin, we used the cutpoint of 12 g/dL. For neutrophils, we used the cutpoint of <= vs. > 7 × 109 cells/L, and for platelets, we used a cutpoint of <= vs. > 400 × 109 cells/L. For monocytes, we used a cutpoint of <= vs. > 0.7 × 109 cells/L as suggested from a previous study [35]. The cutpoint selected for lymphocytes was <= vs. > 1.5 × 109 cells/L, which roughly correlated to a median split in our sample. Depth of MI was grouped as < 2/3 or ≥ 2/3 invasion into uterine myometrium. Histological subtypes included in the study were (a) endometrioid (referred to as type I endometrial cancer) or (b) serous, clear cell, or poorly differentiated adenocarcinoma (collectively referred to as type II endometrial cancer). All preoperative cell counts and hemoglobins were measured within 6 weeks of diagnosis.
Statistical Analysis
The continuous variables of age at diagnosis, BMI, and WBC count were summarized using means and standard deviations, while the remaining categorical variables were summarized using frequencies and percents. Spearman’s correlation coefficients were used to assess associations among preoperative immune cell counts and other prognostic covariates. PFS and OS were both assessed from date of diagnosis.
To determine the relations between monocyte and lymphocyte levels and survival (both PFS and OS), we used Kaplan-Meier life table analyses and the corresponding logrank statistics to evaluate the bivariate associations between low and high levels of monocytes and survival and between low and high levels of lymphocytes and survival. We also assessed whether there was evidence of statistical interaction between histological subtype and monocyte level, or histological subtype and lymphocyte level, with respect to the outcomes of PFS and OS.
Next, we modeled the joint effects of monocyte and lymphocyte levels on recurrence and death in the multivariable setting, using multivariable Cox proportional hazard models. In addition to monocyte and lymphocyte levels, we included covariates of age at diagnosis, race/ethnicity (white versus non-white), FIGO stage (stage I versus stages II–IV), grade (grade 1 versus grades 2–3), histological subtype (type I versus type II cancer), depth of MI (< 2/3 versus ≥ 2/3 invasion), LVSI (present versus absent), hemoglobin (low versus high), neutrophils (low versus high), and platelets (low versus high).
In bivariate and multivariable analyses of survival, we examined whether there were significant interactions between histological subtype, tumor grade, and immune cell counts with respect to prediction of recurrence and death. We also examined model results after stratifying on these covariates (histologic subtype and tumor grade) dichotomously. In both approaches, we found no evidence of significantly differing associations between immune cell counts and survival by levels of these covariates. Thus, all results are presented for the total sample combined, and not stratified by histological subtype or tumor grade.
All statistical analyses were done in SAS version 9.4 (Cary, NC). We used a two-tailed alpha level of 0.05 throughout to indicate significance.
Results
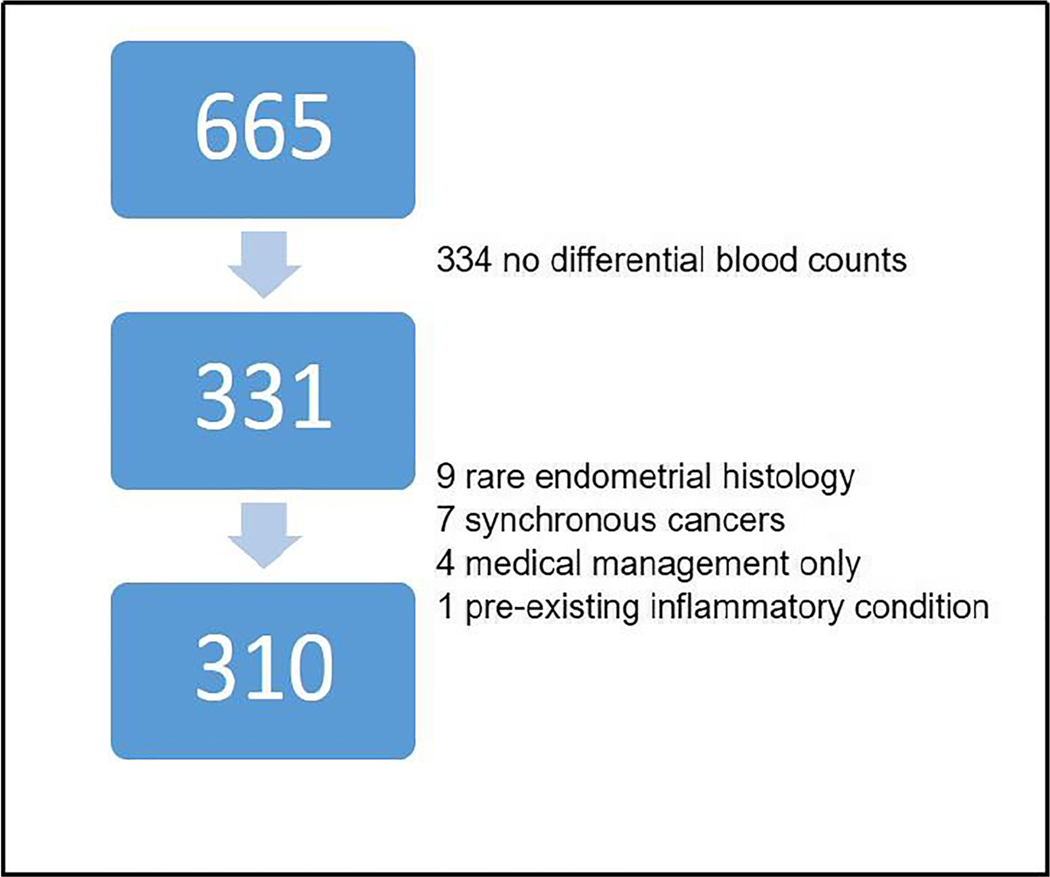
During the 11-year study period from January 2000 through December 2010, we successfully identified 665 patients diagnosed with endometrial cancer who were surgically staged. Three hundred and thirty one of these patients had corresponding preoperative complete blood counts with differential counts collected within 6 weeks prior to primary surgery. Of those, 7 were found to have synchronous cancers (e.g., ovarian and endometrial cancer), 9 were found to have mixed or rare endometrial histology, 4 patients were managed nonsurgically, and 1 patient was found to have a preexisting inflammatory condition. These 21 cases were excluded from our sample. The remaining 310 patients constituted our sample for analysis below (see Fig. 1).
Fig. 1.
Study schema. Six hundred and sixty five patients with endometrial cancer who underwent surgical management between 2000 and 2010 were identified. Three hundred and thirty four of these patients did not have a preoperative complete blood count (CBC) or had a CBC without differential blood counts of immune cells. Of the remaining 331 patients, 21 were excluded for reasons listed above. The remaining 310 patients constituted our study population
Demographic and other notable patient and tumor characteristics are depicted in Table 1. At the time of diagnosis, the mean patient age and BMI of the cohort were 63.8 years old (± 12.4 years) and 34.5 kg/m2 (± 10.2 kg/m2), respectively. Sixty four percent of the patients were obese (BMI = > 30 kg/m2), and 87% were Caucasian (including Hispanic and non-Hispanic whites). Endometrioid or type I cancer (85%) was the most common histology, followed by serous (10%), undifferentiated (3%), and clear cell (2.0%) tumors (the latter three groups are collectively referred to as type II). Most patients in this study were diagnosed with early stage disease (71% FIGO stage I vs. 29% stages II–IV). Forty-one percent of the patients had low-grade tumors, while 59% of patients had high grade (exactly half of the high-grade tumors were grade 2, and the remaining half were grade 3). LVSI and deep myometrial invasion (MI, defined as ≥ 2/3 invasion) were present in 26% and 23% of patients, respectively. Twenty-two percent of patients had positive regional lymph nodes. Approximately 69% of the patients had high preoperative lymphocyte levels, and 28% had high monocyte levels. Just over a quarter of patients (25.5%) had hemoglobin levels = > 12 g/dL. Approximately 19% of the patients had high preoperative neutrophil levels, and 14% had high platelet levels.
Table 1.
Patient demographics
| Category | N (%) | Mean (SD) |
|---|---|---|
| Age | 63.8 (± 12.4) | |
| ≥ 60 | 109 (35.2) | |
| < 60 | 201 (64.8) | |
| BMI | 34.5 (± 10.2) | |
| ≥ 30 | 289 (64) | |
| < 30 | 104 (36) | |
| Ethnicity | ||
| Caucasianβ | 263 (86.5) | |
| African American | 34 (11.2) | |
| Other | 7 (2.3) | |
| Histology | 263 (84.8) | |
| Endometrioid | 263 (84.8) | |
| Serous | 32 (10.3) | |
| Clear cell | 6 (2.0) | |
| Poorly differentiated | 9 (2.9) | |
| FIGO stage | ||
| Stage I | 219 (70.7) | |
| Stage II | 23 (7.4) | |
| Stage III | 45 (14.5) | |
| Stage IV | 23 (7.4) | |
| Grade | ||
| Grade 1 | 128 (41.2) | |
| Grade 2 | 91 (29.4) | |
| Grade 3 | 91 (29.4) | |
| Myometrial invasion | ||
| < 1/3 | 160 (53.9) | |
| ≥ 1/3, < 2/3 | 68 (22.9) | |
| ≥ 2/3 | 69 (23.2) | |
| Lymphovascular space invasion | ||
| Absent | 217 (73.8) | |
| Present | 77 (26.2) | |
| Lymph node metastasis | ||
| Not present | 242 (78.1) | |
| Present | 68 (21.9) | |
| WBC (cells/L) | 7.88 × 109 (3.34) | |
| Hemoglobin level | ||
| High | 231 (74.5%) | |
| Low | 79 (25.5%) | |
| Lymphocyte level | ||
| High | 213 (68.7%) | |
| Low | 97 (31.3%) | |
| Monocyte level | ||
| High | 88 (28.4%) | |
| Low | 222 (71.6%) | |
| Neutrophil level | ||
| High | 58 (18.7%) | |
| Low | 252 (81.3%) | |
| Platelet level | ||
| High | 43 (14.0%) | |
| Low | 265 (86.0%) |
= Including Hispanic and non-Hispanic
The correlations among preoperative leukocyte counts and other prognostic covariates were evaluated (Table 2). Lymphocyte levels were positively correlated with BMI (r = 0.12), histology type (r = 0.12), and WBC (r = 0.26) levels. Lymphocyte levels were negatively correlated with myometrial invasion (r = −0.12) and lymph node metastasis (r = −0.11). Monocyte levels were significantly positively correlated with BMI (r = 0.20), platelet level (r = 0.18), neutrophil level (r = 0.32), and WBC (r = 0.48) counts. Monocytes were significantly negatively correlated with hemoglobin level (r = −0.17). Lymphocyte and monocyte levels were not significantly correlated with each other (r = 0.10, p = 0.08.).
Table 2.
Spearman’s correlation coefficients of preoperative lymphocyte and monocyte cell counts with prognostic covariates
| Variable | Lymphocyte correlation coefficient (p value) | Monocyte correlation coefficient (p value) |
|---|---|---|
| Age | −0.06 (0.29) | −0.06 (0.29) |
| BMI | 0.12 (0.04) | 0.20 (0.0008) |
| Ethnicity (white vs. non-white) | −0.02 (0.73) | 0.03 (0.59) |
| Stage (1 vs. higher) | 0.08 (0.14) | −0.03 (0.55) |
| Grade (1 vs. higher) | 0.06 (0.31) | −0.02 (0.73) |
| MI (< 2/3 mm vs. >= 2/3 mm) | −0.12 (0.03) | 0.04 (0.53) |
| Histology subtype (endometrioid vs. other) | 0.12 (0.03) | 0.007 (0.90) |
| LVSI | −0.05 (0.44) | 0.05 (0.38) |
| Lymph node metastasis | −0.11 (0.05) | 0.08 (0.15) |
| Neutrophil level | −0.02 (0.79) | 0.32 (< 0.0001) |
| Lymphocyte level | 1.00 | 0.10 (0.08) |
| Monocyte level | 0.10 (0.08) | 1.00 |
| Platelet level | 0.05 (0.42) | 0.18 (0.001) |
| WBC | 0.26 (< 0.0001) | 0.48 (< 0.0001) |
| Hemoglobin level | 0.07 (0.23) | −0.17 (0.003) |
Next, we examined the unadjusted bivariate associations between monocyte and lymphocyte levels and survival. This was done using Kaplan-Meier analysis. The maximum length of follow-up time in our cohort was 14.7 years. Using the cutpoint of <= vs. > 0.7 × 109 cells/L, we found a trend between high monocyte levels and worse PFS and OS (p = 0.10, Fig. 2). Among the 82 women with high monocyte levels for whom we had recurrence data, 58% survived to at least 5 years without progression (n = 34 progression events by 5 years). A total of 38 progression events occurred among the 82 women in this group; the remaining women were censored. Among the 206 women with low monocyte level and with the required recurrence data, 71% survived without progression to 5 years (n = 58 events by 5 years). A total of 81 progression events occurred among the 206 women in this group during follow-up. For OS, 70.0% of the 88 women with high monocytes survived to 5 years (n = 26 deaths by 5 years; 34 deaths total), while 79% of 222 women survived to 5 years in the low monocyte group (n = 46 deaths by 5 years; 75 deaths total).
Fig. 2.
Kaplan-Meier life table analyses evaluating the bivariate associations between monocyte cell count level and PFS (1A) and OS (1B).a Monocyte level and PFS. High monocyte level (n = 82 women) was nonsignificantly associated with worse PFS compared to low monocyte level (n = 206 women) (p = 0.10). b Monocyte level and OS. High monocyte level (n = 88 women) was nonsignificantly associated with worse OS compared to low monocyte level (n = 222 women) (p = 0.16). Crossover in the data at the extreme limits (> 14 yrs) is attributed to sparse data
A cutpoint of <= vs. > 1.5 × 109 cells/L was used for lymphocytes. Figure 3 shows that high preoperative lymphocyte level was significantly associated with improved PFS (p = 0.008) and improved OS (p = 0.006). Among the 195 women with high lymphocyte levels and recurrence data, 72% survived without progression to 5 years (n = 53 events by 5 years; 71 events during total follow-up), while 56% of the 93 women with low lymphocyte levels and recurrence data were progression-free at 5 years (40 progression events by 5 years; 48 total events observed). For OS, the corresponding percentages for 5-year survival were 81% among the 213 women in the high lymphocyte group (39 deaths by 5 years; 65 deaths total) and 65.0% among the 97 women in the low lymphocyte group (n = 33 deaths by 5 years; 44 total observed).
Fig. 3.
Kaplan-Meier life table analyses evaluating the bivariate associations between lymphocyte cell count level and PFS (2A) and OS (2B). a Lymphocyte level and PFS. High lymphocyte level (n = 195 women) was significantly associated with improved PFS (p = 0.008) compared to low lymphocyte level (n = 93 women). b Lymphocyte level and OS. High lymphocyte level (n = 213 women) was significantly associated with improved OS compared to low lymphocyte level (n = 97 women) (p = 0.006). Crossover in the data at the extreme limits (> 14 yrs) is attributed to sparse data
We then modeled the relation between monocyte and lymphocyte level on recurrence and death in the multivariate setting using Cox proportional hazard models. Results from these models are shown in Tables 3 (recurrence) and 4 (death). High monocyte count was associated with a greater risk of recurrence after adjustment of other covariates (adjusted HR = 1.63, 95%CI, 1.15–2.55, p = 0.035). Additional significant predictors of recurrence were increasing age, non-endometrioid histology, presence of LVSI, and advanced surgical stage.
Table 3.
Recurrence risk: multivariable Cox proportional hazard analysis
| Variable | HR (95% CI) | p value |
|---|---|---|
| Age (per 1 yr increase) | 1.04 (1.02–1.07) | < 0.0001 |
| Ethnicity | ||
| Caucasian vs. other* | 0.76 (0.44–1.33) | 0.34 |
| Stage | ||
| 1 vs. 2–4 | 0.56 (0.32–0.99) | 0.045 |
| Grade | ||
| 1 vs. 2–3 | 0.78 (0.48–1.27) | 0.31 |
| Histology | ||
| ED vs. non-EDβ Myometrial invasion |
0.49 (0.28–0.85) | 0.01 |
| > 2/3 vs. < 2/3 | 0.90 (0.53–1.51) | 0.68 |
| LVSI | ||
| Present vs. absent | 2.28 (1.36–3.85) | 0.002 |
| Hemoglobin | 0.70 (0.43–1.14) | 0.015 |
| Neutrophil levelΩ | 1.60 (0.93–2.73) | 0.09 |
| Monocyte levelΩ | 1.63 (1.15–2.55) | 0.035 |
| Lymphocyte levelΩ | 0.70 (0.46–1.25) | 0.10 |
| Platelet levelΩ | 0.67 (0.35–1.28) | 0.22 |
Caucasian includes Hispanic White and non-Hispanic
ED = Endometrioid, non-ED = Serous, clear cell and poorly differentiated
Comparing high level to low level as defined by relevant cutpoint
High lymphocyte count trended with a lower risk of death in the multivariable analysis (adjusted HR = 0.66, 95%CI, 0.42–1.03, p = 0.07, Table 4). Increasing age, higher surgical stage, higher grade, non-endometrioid histology, and presence of LVSI were also predictors of death.
Table 4.
Death risk: multivariable Cox proportional hazard analysis
| Variable | HR (95% CI) | p value |
|---|---|---|
| Age (per 1 yr increase) | 1.05 (1.02–1.07) | < 0.0001 |
| Ethnicity | ||
| Caucasian vs. other* | 1.35 (0.74–2.47) | 0.33 |
| Stage | ||
| 1 vs. 2–4 | 0.54 (0.30–0.97) | 0.04 |
| Grade | ||
| 1 vs. 2–3 | 0.56 (0.32–0.97) | 0.04 |
| Histology | ||
| ED vs. non-EDβ | 0.41 (0.23–0.72) | 0.002 |
| Myometrial invasion | ||
| > 2/3 vs. < 2/3 | 0.71 (0.41–1.23) | 0.23 |
| LVSI | ||
| Present vs. absent | 2.00 (1.18–3.38) | 0.01 |
| Hemoglobin | 0.65 (0.38–1.11) | 0.11 |
| Neutrophil levelΩ | 1.28 (0.70–2.34) | 0.43 |
| Monocyte levelΩ | 1.28 (0.78–2.11) | 0.33 |
| Lymphocyte levelΩ | 0.66 (0.42–1.03) | 0.07 |
| Platelet levelΩ | 1.09 (0.56–2.13) | 0.80 |
Caucasian includes Hispanic White and non-Hispanic
ED = Endometrioid, non-ED = serous, clear cell and poorly differentiated
Comparing high to low level as defined by relevant cutpoint
Discussion
The complex interaction between immune cells and cancer cells has been previously identified as a critical modifier of solid tumor biology and has been linked to long-term prognosis. Understanding the impact of circulating immune cells on endometrial cancer outcomes and the potential response of these cancers to immunotherapy are promising treatment vistas. We completed a retrospective study aimed to discover the relation between preoperative circulating immune cell count survival in women with endometrial cancer. Our results suggest that monocytes and lymphocytes have opposing effects on survival outcomes for type I and type II endometrial cancer.
The multivariable Cox proportional models showed that high preoperative circulating monocyte levels were significantly predictive of disease recurrence, and high circulating lymphocyte levels positively trended toward improved overall survival. It is likely that collinearity in our multivariable model led to the emergence of monocyte level as significant for recurrence (when it was not in the bivariate setting). While regression diagnostic statistics did not indicate a high degree of collinearity among the predictors in our model, even a modest degree of collinearity can alter findings regarding statistical significance. We note that the direction of association for both monocytes and lymphocytes with the survival outcomes remained consistent across bivariate and multivariable analyses.
It is well recognized with the advent of advanced immune cell markers that virtually all solid neoplastic lesions contain immune cells of varying densities and types including macrophages, neutrophils, T and B lymphocytes, and NK cells [36]. Immunoevasion by cancer cells is an emerging hallmark of cancer [37]. However, direct studies of the tumor microenvironment require invasive sampling along with advanced cell sorting techniques limiting the clinical utility of these measurements. Peripheral immune cell counts, on the other hand, can be measured easily and noninvasively and represent a supply of immune cells responsive to recruitment. Sensing chemokine and chemotactic gradients, peripheral immune cells migrate from the circulation and extravasate into target tissues. Due to the chronic nature of solid neoplastic tumors, combined with the short half-life of immune cells, a relative recruitment from the peripheral circulation to the tumor microenvironment is anticipated to be proportionally reflective of the immune cell populations. To this end, peripheral immune counts are clinically significant and can shed light on immune cell involvement within neoplastic lesions.
As aforementioned, tumor-associated immune cells can operate in conflicting roles, either as tumor antagonizing or tumor promoting. This dual role for immune cells may be tied to their subclass role as innate or adaptive cells [37]. In the case of lymphocytes, adaptive immune cells, multiple lines of evidence demonstrate that T cells are critical for host antitumor responses [14–16]. Mechanistically, lymphocytes can respond to “neoantigens” containing epitopes cleaved from mutated proteins, prompting a direct cell-mediated cytotoxicity by granule exocytosis or by the Fas pathway [38–40]. This has led to the development of T-cell immunotherapies including adoptive T-cell therapy and immune checkpoint blockade approaches. Our work suggests that there is also a survival advantage for effective lymphocytes in type I and type II endometrial cancer and opens the question for the role in lymphocyte-directed therapies in endometrial cancer. No other immune cells in our study were linked to a survival advantage.
Historically immune responses in tumors were thought to reflect the host’s attempt to eradicate tumor cells. However, over the past decade, study of effector functions of various immune cell types along with pharmacologic inhibitors indicates that some immune cell types are capable of fostering a tumor-promoting role. The roster of possible immune cells includes macrophages and neutrophils, parts of the innate system [37]. Those cells are major sources of angiogenic, epithelial, and stromal growth factors as well as matrix remodeling enzymes needed for wound healing. Once recruited to the tumor microenvironment, innate cells can be subverted to support neoplastic progression. Recalling that monocytes represent undifferentiated macrophages in tissues, the multivariable Cox model predicts that high monocyte counts are significantly associated with tumor aggressiveness and a decreased PFS in type I and type II endometrial cancer, and our results are in line with a tumor-promoting role associated with macrophages in endometrial [34] and other solid tumor malignancies. Additionally, our model suggests that high neutrophil level may be associated with poorer survival.
Limitations and weaknesses of the current study pertain to its retrospective nature, specifically the inability to control for differences in surgical and postoperative management, including adjuvant therapies such chemotherapy and/or radiation therapy in the study subjects. While we did not include covariates related to surgical and postoperative therapies, because our main interest lay in examining preoperative (early) levels of immune cells, the absence of these other variables is not a severe limitation. Tumor cells and the immune response reflect a complex and dynamic interaction. Each preoperative cell count represents a single snapshot in a dynamic peripheral process and may fail to identify transient but critically important nuances at the tumor circulation interface. Another limitation of our study was the small sample sizes for some groups (e.g., non-endometrioid histology), precluding high statistical power for detecting small but potentially real differences in immune cell survival relationships across levels/strata of different covariates. Future studies incorporating more sophisticated phenotyping of leukocyte subsets, including stratification of monocyte polarization, and neutrophil and T-cell subpopulations may allow better discrimination of survival prognosis in women with endometrial cancer.
Strengths of the study include a moderately large overall sample size, clinically relevant outcomes for type I and type II endometrial cancer, and assessment of parameters with clinical utility. Our study results highlight the potential ability of preoperative peripheral lymphocyte and monocyte counts to serve as independent prognostic indicators in the management of endometrial cancer.
Our results indicate that higher lymphocytes and lower monocytes correspond to better survival in both type I and type II endometrial cancers. These results are in line with other studies suggesting a tumor-antagonizing role for lymphocytes and tumor-promoting role for monocytes/macrophages and to a lesser extent for neutrophils [14–16, 28–30, 41]. There might also be a role for lymphocyte immunotherapies in cases of endometrial cancer. Increased monocyte counts may have implications for surgical and medical management based on the patient’s preoperative blood work. Future investigations are recommended to focus on these immune cell types to further elucidate their dynamic interaction with endometrial tumor cells. Currently there has been no formal assessment of clinically relevant densities of specific immune cell types in the stromal compartment of endometrial cancer. A better understanding of the distribution of immune cells around the tumor border and within the stroma coupled to patient outcomes and peripheral immune counts may help to advance immunotherapy technologies and enable practical, noninvasive monitoring to direct therapy decisions.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict(s) of interest.
References
- 1.Boronow RC, Morrow CP, Creasman WT, Disaia PJ, Silverberg SG, Miller A, et al. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63(6):825–32. [PubMed] [Google Scholar]
- 2.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–41. [DOI] [PubMed] [Google Scholar]
- 3.DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in stage I endometrial cancer. Am J Obstet Gynecol. 1985;151(8):1009–15. [DOI] [PubMed] [Google Scholar]
- 4.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40(1):55–65. [DOI] [PubMed] [Google Scholar]
- 5.Orr JW Jr, Orr PF, Taylor PT. Surgical staging endometrial cancer. Clin Obstet Gynecol. 1996;39(3):656–68. [DOI] [PubMed] [Google Scholar]
- 6.Rungruang B, Olawaiye AB. Comprehensive surgical staging for endometrial cancer. Rev Obstet Gynecol. 2012;5(1):28–34. [PMC free article] [PubMed] [Google Scholar]
- 7.Poupon C, Bendifallah S, Ouldamer L, Canlorbe G, Raimond E, Hudry N, et al. Management and survival of elderly and very elderly patients with endometrial cancer: an age-stratified study of 1228 women from the FRANCOGYN group. Ann Surg Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 8.Zsiros E, Odunsi K. Tumor-associated macrophages: coconspirators and orchestrators of immune suppression in endometrial adenocarcinoma. Gynecol Oncol. 2014;135(2):173–5. [DOI] [PubMed] [Google Scholar]
- 9.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71. [DOI] [PubMed] [Google Scholar]
- 10.Vanderstraeten A, Tuyaerts S, Amant F. The immune system in the normal endometrium and implications for endometrial cancer development. J Reprod Immunol. 2015;109:7–16. [DOI] [PubMed] [Google Scholar]
- 11.Soeda S, Nakamura N, Ozeki T, Nishiyama H, Hojo H, Yamada H, et al. Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol. 2008;109(1):122–8. [DOI] [PubMed] [Google Scholar]
- 12.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7(5): 651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51): 18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral Tcells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. [DOI] [PubMed] [Google Scholar]
- 16.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1): 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung IK, Kim SS, Suh DS, Kim KH, Lee CH, Yoon MS. Tumor-infiltration of T-lymphocytes is inversely correlated with clinicopathologic factors in endometrial adenocarcinoma. Obstet Gynecol Sci. 2014;57(4):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114(1):105–10. [DOI] [PubMed] [Google Scholar]
- 19.Radvanyi LG. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. 2015;21(6):450–64. [DOI] [PubMed] [Google Scholar]
- 20.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79(5–6):991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14(2):425–31. [PubMed] [Google Scholar]
- 22.Fujimoto J, Sakaguchi H, Aoki I, Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60(10):2632–5. [PubMed] [Google Scholar]
- 23.Hashimoto I, Kodama J, Seki N, Hongo A, Miyagi Y, Yoshinouchi M, et al. Macrophage infiltration and angiogenesis in endometrial cancer. Anticancer Res. 2000;20(6C):4853–6. [PubMed] [Google Scholar]
- 24.Yang X, Dong Y, Zhao J, Sun H, Deng Y, Fan J, et al. Increased expression of human macrophage metalloelastase (MMP-12) is associated with the invasion of endometrial adenocarcinoma. Pathol Res Pract. 2007;203(7):499–505. [DOI] [PubMed] [Google Scholar]
- 25.Tong H, Ke JQ, Jiang FZ, Wang XJ, Wang FY, Li YR, et al. Tumor-associated macrophage-derived CXCL8 could induce ERalpha suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016. [DOI] [PubMed] [Google Scholar]
- 26.Hamada I, Kato M, Yamasaki T, Iwabuchi K, Watanabe T, Yamada T, et al. Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res. 2002;22(6C): 4281–4. [PubMed] [Google Scholar]
- 27.Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, et al. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102(7):1424–31. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, Schioppa T, Biswas SK, Marchesi F, Allavena P, Sica A. Tumor-associated macrophages and dendritic cells as prototypic type II polarized myeloid populations. Tumori. 2003;89(5):459–68. [DOI] [PubMed] [Google Scholar]
- 29.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. [DOI] [PubMed] [Google Scholar]
- 30.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. [DOI] [PubMed] [Google Scholar]
- 31.Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 2004;24(5C):3335–42. [PubMed] [Google Scholar]
- 32.Espinosa I, Jose Carnicer M, Catasus L, Canet B, D’Angelo E, Zannoni GF, et al. Myometrial invasion and lymph node metastasis in endometrioid carcinomas: tumor-associated macrophages, microvessel density, and HIF1A have a crucial role. Am J Surg Pathol. 2010;34(11):1708–14. [DOI] [PubMed] [Google Scholar]
- 33.Kubler K, Ayub TH, Weber SK, Zivanovic O, Abramian A, Keyver-Paik MD, et al. Prognostic significance of tumor-associated macrophages in endometrial adenocarcinoma. Gynecol Oncol. 2014;135(2):176–83. [DOI] [PubMed] [Google Scholar]
- 34.Kelly MG, Francisco AM, Cimic A, Wofford A, Fitzgerald NC, Yu J, et al. Type 2 endometrial cancer is associated with a high density of tumor-associated macrophages in the stromal compartment. Reprod Sci. 2015;22(8):948–53. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo K, Hom MS, Moeini A, Machida H, Takeshima N, Roman LD, et al. Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol. 2015;138(2):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–102. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 38.Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–32. [DOI] [PubMed] [Google Scholar]
- 39.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–70. [DOI] [PubMed] [Google Scholar]
- 40.Bobisse S, Foukas PG, Coukos G, Harari A. Neoantigen-based cancer immunotherapy. Ann Transl Med. 2016;4(14):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]