Abstract
Objective: To compare the efficacy and safety of 12-24 hours versus 72 hours of intravenous terlipressin therapy in patients with acute esophageal variceal bleeding (AVB). Data sources: A systematic search was conducted using PubMed, Scopus, Cochrane Library, Google Scholar, Web of Science, VHL, and ClinicalTrials.gov for studies published up to February 24, 2024. The search terms included “terlipressin,” “variceal bleeding,” “short-course,” and “72-hour treatment.” Study selection and data extraction: Randomized controlled trials (RCTs) comparing 12 to 24 hours with 72 hours of terlipressin therapy in patients with AVB were included. Studies not meeting these criteria or focusing on unrelated outcomes were excluded. Two authors conducted data extraction and bias assessment independently, with discrepancies resolved by a third reviewer. Baseline characteristics and outcomes (rebleeding and mortality within 5 days) were recorded. Results: Four RCTs with 469 patients were included in the analysis. There were no significant differences observed in 5-day rebleeding rates (OR = 0.943; 95% CI [0.384, 2.317]; P = 0.898) or mortality rates (OR = 0.386; 95% CI [0.066, 2.260]; P = 0.291) between terlipressin treatment durations of 12 to 24 hours and 72 hours within the first 5 days posttreatment. In addition, no heterogeneity was found in both variables (P > 0.1). Conclusion: This meta-analysis indicates that there is no significant difference in rebleeding rates or mortality between 12 to 24 hours and 72 hours of terlipressin therapy for AVB within 5 days posttreatment. Shorter treatment durations may offer advantages in terms of resource utilization and adverse event risk but require further validation through studies involving larger patient populations.
Keywords: esophageal variceal, terlipressin, meta-analysis, bleeding, vasopressors
Introduction
Esophageal variceal bleeding (EVB) is a common and serious complication of cirrhosis. 1 EVB affects around 30% of individuals with compensated cirrhosis and 60% with decompensated cirrhosis. 2 Annually, esophageal variceal bleeding (EVB) affects 10% to 20% of patients with cirrhosis, with each incident potentially leading to in-hospital mortality, 3 and it remains a major cause of death among these patients, although there has been a significant decrease in mortality rates over the past 2 decades 4 and women experienced a reduced likelihood of death compared with men.5,6 The main therapies for acute variceal bleeding (AVB) involve endoscopic variceal band ligation (EVL) and intravenously administering vasoactive medications like terlipressin, somatostatin, and octreotide. 7
Multiple studies compared the efficacy and safety of different vasoactive medications. Terlipressin has more prolonged hemodynamic effects on portal venous flow and portal pressure in patients with bleeding varices compared with octreotide, 8 but it is associated with the risk of causing ischemic injury, such as myocardial infarction, skin necrosis, and bowel ischemia.9,10 In addition, it is known to be linked with hyponatremia. 11 Sridharan and Sivaramakrishnan 12 conducted a meta-analysis in 2019 and the results advocated the use of terlipressin as the best agent. On the contrary, another meta-analysis published in 2019 by Zou et al 13 supported the use of octreotide. A more recent retrospective real-world data study concluded that terlipressin and octreotide have similar outcomes in terms of control of bleeding, hospital stay, mortality, and adverse effects when used as adjuvant therapy for the management of variceal bleeding. 14
Current guidelines advise using a vasoactive agent before performing endoscopic procedures. Research by Lo et al found that vasoactive agents effectively controlled variceal bleeding within 48 hours in 90% of cases. However, using vasoactive agents alone resulted in a 15% rebleeding rate within 5 days, while combining vasoactive agents with endoscopic methods during initial hemostasis prevented rebleeding. 15 The ideal length of pharmacotherapy following successful endoscopic hemostasis remains uncertain, although early use of vasoactive agents is widely accepted as standard practice. After endoscopic hemostasis has been achieved, the Baveno VII consensus statements and the American Association for the Study of Liver Diseases (AASLD) advise that vasoactive medications be administered for a period of 2 to 5 days.7,16
Several studies have indicated that there is no substantial difference in rebleeding rates or mortality between patients treated with terlipressin for 12 to 24 hours compared with those treated for 72 hours within 5 days.17 -20 In addition, 1 study followed patients for 30 days, 18 while another followed them for 42 days, 19 both yielding similar results regarding the efficacy of 12-24 hours versus 72 hours of terlipressin treatment. This indicates that using vasoactive drugs for a shorter period can be equally effective in controlling rebleeding and mortality rates as a longer treatment course.18 -21 Our study aimed to evaluate potential variations in rebleeding rates and mortality among patients who undergo shorter-duration (12-24 hours) versus longer-duration (72 hours) intravenous terlipressin therapy for AVB.
Methods
Search Strategy and Study Selection
Our study design compared the outcomes of 2 groups: 1 receiving terlipressin for 12 to 24 hours and the other for 72 hours, specifically assessing rebleeding and mortality rates within 5 days posttreatment. We ensured that the search criteria were independent of factors such as gender, ethnicity, language, or socioeconomic status.
Our inclusion criteria were strict, focusing only on studies that directly compared the efficacy of terlipressin between the 12 to 24 hours and 72 hours treatment durations for AVB. We excluded studies that did not meet this comparison, as well as those focusing on different medical conditions, comparisons with other medications, discussions solely on AVB without treatment comparisons, overlapping research, and review papers. Additional details regarding our search phrases can be found in the supplemental file.
On February 24, 2024, we conducted a systematic review following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) criteria, 22 focusing on randomized controlled trials (RCTs) involving patients with AVB who received terlipressin. To gather relevant data, we searched multiple databases, including PubMed, Scopus, VHL, Cochrane Controlled Register of Trials, Web of Science, Clinicaltrials.gov, and Google Scholar using specific search phrases related to terlipressin and esophageal varices. The research protocol was registered with PROSPERO under the registration number CRD42024517636.
Remove Duplicate and Screening Studies
Database results were imported into EndNote version 9, and duplicates were removed as part of the initial screening process. Two independent reviewers then applied preestablished inclusion and exclusion criteria to filter titles and abstracts. Subsequently, selected publications were thoroughly reviewed by each author based on predefined standards. Exclusions were categorized based on research design, duplicated material, and the inability to compare terlipressin treatment durations of 12-24 hours versus 72 hours. Any discrepancies or disagreements during the screening process were resolved by a third author, who made the final decisions regarding the inclusion or exclusion of studies.
Risk of Bias and Quality Assessment
Two authors utilized the Cochrane Risk of Bias 2.0 (RoB 2.0) method to assess biases present in the included papers. This method encompasses various forms of biases, such as issues with randomization, treatment variations, missing data, imprecise outcome assessment, and biased reporting, providing a comprehensive evaluation. 23 Bias risk was categorized into 3 levels: high, some concerns, or low. 23 Any disagreements in the evaluation process were resolved through discussion. The graphical representation displays the assessment of each study’s bias level.
Data Extraction
As part of the data extraction process, 2 investigators independently entered data into a Microsoft Excel spreadsheet. The recorded baseline characteristics encompassed various parameters such as trial name, nation, year, research design, type of RCT, participant count, gender distribution, age, hemoglobin level, Child-Pugh score, child class, varices grade, MELD score, and cirrhosis etiology. In addition, binary results data (such as death within 5 days and rebleeding within 5 days) were collected and categorized using numerical values. Any discrepancies during the data extraction process were resolved through discussion. We converted the data that we found in median and IQR to mean and SD by the method mentioned by Wan et al. 24
Primary Outcome and Statistical Analysis
The primary objective of our study was to investigate the impact of terlipressin administration for 12-24 hours versus 72 hours on rebleeding rates within 5 days in patients with AVB. The secondary objective was to compare the 5-day mortality between the 2 treatment durations. We utilized odds ratios (OR) and 95% confidence intervals (CI) to compare outcomes for categorical factors between these 2 treatment groups.
To account for potential differences in participant characteristics and research designs, we employed a random-effects model during our meta-analysis. This approach ensures that our findings can be extrapolated across various national contexts. Heterogeneity among the studies was assessed using the Q statistic, the I2 test, and the associated P-value. Heterogeneity was deemed significant if the P-value was below 0.1. Our original plan included evaluating publication bias if the number of included trials exceeded 10; however, due to the limited number of trials (only 4 were included in the review), we were unable to perform this assessment. 25 The analysis of our data was performed using Version 3 of the Comprehensive Meta-Analysis Tool.
Results
Study Characteristics
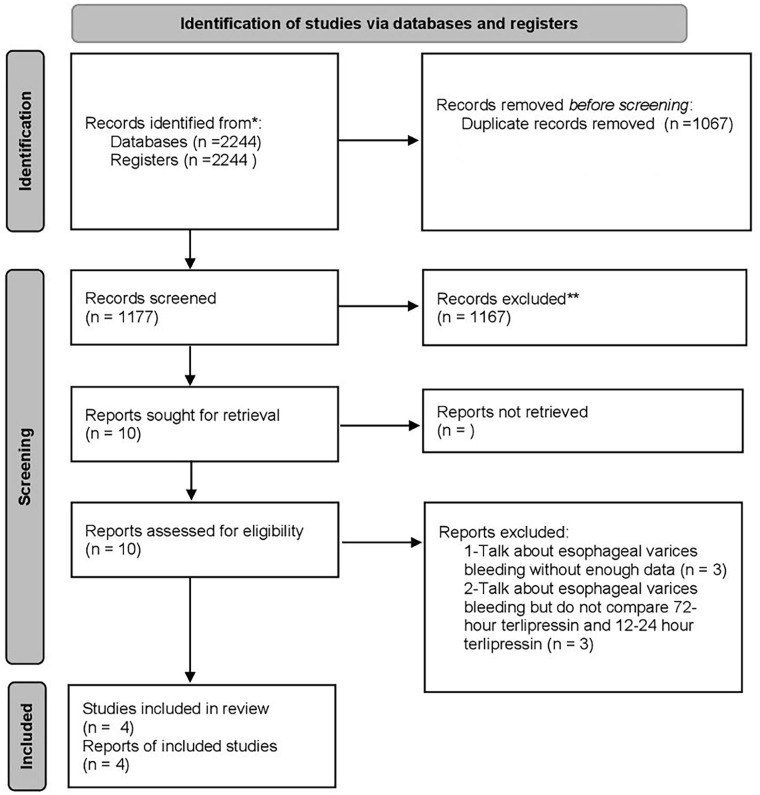
During our initial search across 7 databases, we identified 2244 articles. After eliminating duplicates, 1067 articles were excluded. Further screening based on titles and abstracts narrowed down the selection to 10 articles for full-text review. Upon thorough examination, 4 studies were found to meet our inclusion criteria;17 -20 3 were conducted in Pakistan and 1 in India. We excluded 3 studies due to insufficient data on esophageal variceal bleeding and 3 others because they did not compare terlipressin administration within 12-24 hours versus 72 hours.
The PRISMA flow diagram in Figure 1 illustrates the process of study screening and selection. 26 In addition, a comprehensive summary of the studies included in the meta-analysis can be found in Table 1
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram illustrates the process of study screening and selection.
Table 1.
General Characteristics of the Included Studies.
| Study name | Vaishnav et al 19 | Azam et al 18 | Salim et al 17 | Zaman et al 20 |
|---|---|---|---|---|
| Country | India | Pakistan | Pakistan | Pakistan |
| Year | 2023 | 2012 | 2017 | 2019 |
| Study Design | RCT, Pilot | RCT, Double blind | RCT | RCT |
| Number of Participants | ||||
| 12- to 24-hour terlipressin | 74 | 65 | 65 | 50 |
| 72-hour terlipressin | 75 | 65 | 25 | 50 |
| Gender | ||||
| Male | ||||
| 12- to 24-hour terlipressin | 63 (85.1%) | 48 (73.85%) | 51 (57%) | 58 (58%) |
| 72-hour terlipressin | 67 (89.3%) | 49 (75.3%) | ||
| Female | ||||
| 12- to 24-hour terlipressin | 11 (14.9%) | 17 (26.15%) | 39 (43%) | 42 (42%) |
| 72-hour terlipressin | 8 (10.7%) | 16 (27.7%) | ||
| Age, mean ± SD | ||||
| 12- to 24-hour terlipressin | 43.83 (11.72) | 49.8 (11.2) | 51.35 (11.46) | 55.16 (5.56) |
| 72-hour terlipressin | 41.67 (8.31) | 49.7 (12.1) | 53.56 (11.11) | |
| Hemoglobin (g/dL), mean ± SD | ||||
| 12- to 24-hour terlipressin | 8.0 (2.8) | 9.4 (2.4) | ||
| 72-hour terlipressin | 7.4 (2.3) | 9.4 (2.3) | ||
| Child-Pugh score, mean ± SD | ||||
| 12- to 24-hour terlipressin | .66 (2.27) | 8.9 (1.7) | 8.9 (1.4) | |
| 72-hour terlipressin | 8 (1.51) | 8.4 (1.6) | 9.32 (1.65) | |
| Child Class | ||||
| Child A | ||||
| 12- to 24-hour terlipressin | 21 (28.4%) | 7 (10.8%) | 0 (0%) | |
| 72-hour terlipressin | 17 (22.7%) | 7 (10.8%) | 0 (0%) | |
| Child B | ||||
| 12- to 24-hour terlipressin | 41 (55.4%) | 30 (46.2%) | 36 (55.4%) | |
| 72-hour terlipressin | 48 (64%) | 41 (63%) | 13 (52%) | |
| Child C | ||||
| 12- to 24-hour terlipressin | 12 (16.2%) | 28 (43%) | 29 (44.6%) | |
| 72-hour terlipressin | 10 (13.3%) | 17 (26.2%) | 12 (48%) | |
| Grade of varices | ||||
| Grade I | ||||
| 12- to 24-hour terlipressin | 0 (0%) | 1 (1.5%) | ||
| 72-hour terlipressin | 0 (0%) | 0 (0%) | ||
| Grade II | ||||
| 12- to 24-hour terlipressin | 9 (13.8%) | 1 (1.5%) | ||
| 72-hour terlipressin | 6 (9.2%) | 1 (4%) | ||
| Grade III | ||||
| 12- to 24-hour terlipressin | 25 (38.5%) | 28 (43%) | ||
| 72-hour terlipressin | 25 (38.5%) | 13 (52%) | ||
| Grade IV | ||||
| 12- to 24-hour terlipressin | 31 (47.7%) | 35 (53.8%) | ||
| 72-hour terlipressin | 34 (52.3%) | 11 (44%) | ||
| MELD score, mean ± SD | ||||
| 12- to 24-hour terlipressin | 13.7 (5.07) | 20 (2) | ||
| 72-hour terlipressin | 14.23 (3.86) | 19 (3) | ||
| Etiology of cirrhosis | ||||
| Alcohol | ||||
| 12- to 24-hour terlipressin | 36 (48.6%) | 4 (6.2%) | ||
| 72-hour terlipressin | 32 (42.7%) | 11 (16.9%) | ||
| Hepatitis B virus | ||||
| 12- to 24-hour terlipressin | 4 (5.4%) | 6 (9.2%) | ||
| 72-hour terlipressin | 16 (21.3%) | 10 (15.4%) | ||
| Hepatitis C virus | ||||
| 12- to 24-hour terlipressin | 7 (9.5%) | 42 (64.6%) | ||
| 72-hour terlipressin | 7 (9.3%) | 33 (50.8%) | ||
| NAFLD | ||||
| 12- to 24-hour terlipressin | 7 (9.5%) | |||
| 72-hour terlipressin | 5 (6.7%) | |||
| Others | ||||
| 12- to 24-hour terlipressin | 20 (27.0%) | 13 (20%) | ||
| 72-hour terlipressin | 15 (20.0%) | 11 (17%) |
Patient Characteristics and Quality Assessment
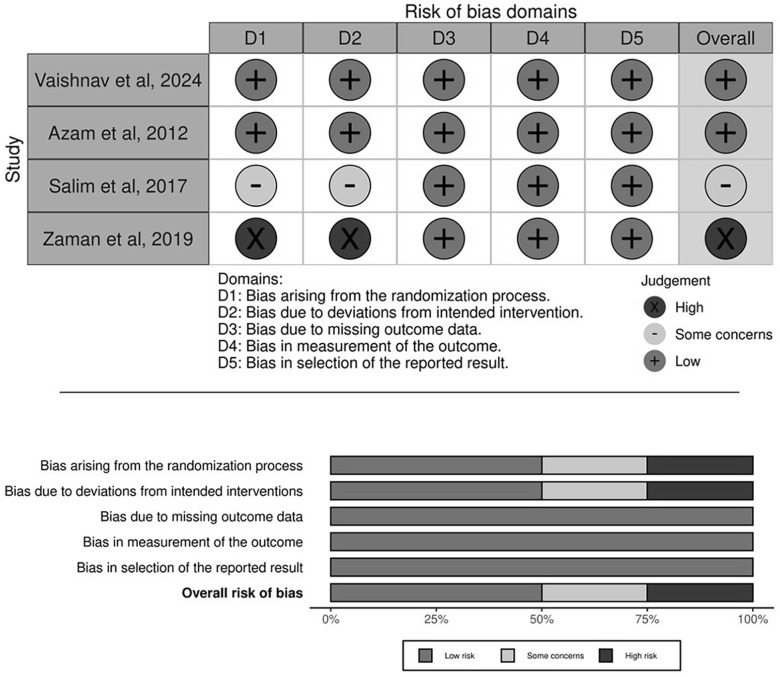
Our systematic review included 469 patients, of whom 278 (71.6%) were male and 91 (28.4%) were female. All patients had achieved endoscopic hemostasis before the inclusion. Among these patients, 215 (45.8%) received intravenous terlipressin treatment for 72 hours, while 254 (54.2%) received treatment for 12-24 hours. In terms of the quality of studies included, 2 studies in our analysis showed a low risk of bias, 1 study raised some concerns, and 1 study showed high risk of bias, as assessed by ROB2. 27 For a detailed overview of the findings, refer to Figure 2.
Figure 2.
Risk of bias assessment of included studies using ROB2 tool.
Rebleeding
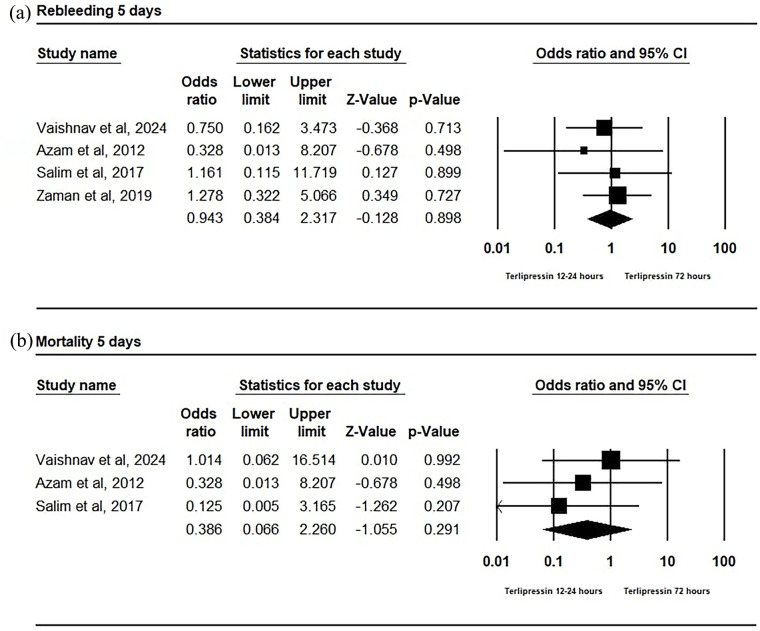
In our analysis of the early rebleeding period (5 days) within the included RCTs, no statistically significant difference was found between the 2 groups with an OR of 0.943 (95% CI [0.384, 2.317]; P = 0.898). There was also no significant heterogeneity observed (P = 0.87; I2 = 0.00; τ2 = 0.00), with a mean effect size of 0.94 and a 95% CI ranging from 0.38 to 2.31 (Figure 3a).
Figure 3.
(a) Forest plot of 5-day rebleeding odds ratio. (b) Forest plot of 5-day mortality odds ratio.
Mortality
The mortality rate within 5 days showed no statistically significant difference between the 2 groups with an OR of 0.386 (95% CI [0.066, 2.260]; P = 0.291). There was no significant heterogeneity observed (P = 0.62; I2 = 0.00; τ2 = 0.00), with a mean effect size of 0.39 and a 95% CI ranging from 0.07 to 2.26 (Figure 3b).
Discussion
Variceal bleeding is a significant complication of portal hypertension that is often associated with cirrhosis and advanced liver disease.11,28 Primary treatments for AVB are EVL and intravenous vasoactive medications like terlipressin, vasopressin, somatostatin, and octreotide. 7 The ideal duration of pharmacotherapy after successful endoscopic hemostasis is uncertain, but starting vasoactive agents early is standard practice, typically lasting 2 to 5 days according to Baveno VII consensus, the Chinese Society of Gastroenterology, and AASLD guidelines.7,16,29 Evidence in the literature indicates that using vasoactive drugs for a shorter period can be just as effective as a longer treatment when it comes to reducing rebleeding and mortality rates.17 -19 This practice could reduce the treatment burden and lower the chances of terlipressin-related adverse events. Our study aimed to explore the difference in rebleeding rates and mortality within a 5-day period among patients receiving shorter-duration (12-24 hours) versus longer-duration (72 hours) intravenous terlipressin therapy for AVB.
Our meta-analysis found that there is no statistically significant difference in 5-day rebleeding rates or mortality between patients receiving 12- to 24-hour versus 72-hour terlipressin treatment for AVB. Although the differences in our study weren’t statistically significant, we noticed trends showing lower rates of bleeding again and lower chances of death with shorter terlipressin treatment. Even small improvements in outcomes matter a lot in health care. This trend might be because shorter terlipressin treatment helps stabilize bleeding and reduces the risk of terlipressin-related adverse effects. Terlipressin has been associated with many adverse effects, such as ischemic injuries and hyponatremia.9 -11
In 2021, a meta-analysis study by Huaringa-Marcelo et al, compared vasoactive agents with endoscopic therapy for treating AVB in cirrhotic patients. They categorized agents into 2 groups: terlipressin and vasopressin (T-V), and octreotide and somatostatin (O-S). Their results indicated that while mortality rates were similar between T-V and O-S treatments, the T-V group had a higher risk of adverse events. Specifically, terlipressin did not show a significant difference in early or late rebleeding rates, blood transfusion requirements, or hospital stay duration compared with other agents, but it was associated with a higher incidence of adverse events such as chest pain, abdominal pain, diarrhea, and hyponatremia. However, due to study limitations, the overall confidence in the evidence, particularly regarding bleeding control and rebleeding outcomes, was rated as low. 30 Another meta-analysis in 2021, by Yeh et al, compared 3 days or less of vasoconstrictor therapy with a longer course (3-5 days) following EVL. Their analysis revealed no significant differences in rebleeding or mortality rates at 5 days and 6 weeks between the 2 treatment approaches. 31
Our meta-analysis differs in that it specifically compared the effects of different durations of terlipressin administration, rather than comparing terlipressin to other vasoactive agents or assessing the effect of treatment duration using different vasoconstrictor agents. Our focus was on identifying whether shorter treatment durations of terlipressin could achieve similar outcomes with potentially fewer adverse effects and costs. In addition, 2 randomized clinical trials, Poudel et al 32 comparing 2-day with 5-day and Bruha et al 33 comparing 5- and 10-day demonstrated that lengthening the duration of treatment with terlipressin did not prove a significant decrease in mortality or bleeding recurrence, rather it may increase the risk of adverse events. Of note, these 2 trials were not included in our meta-analysis due to the difference in the treatment duration studied.
Azam et al compared the efficacy of 24- and 72-hour terlipressin treatments in 130 patients divided equally into the 2 groups. Their study showed that the rates of rebleeding at 5 and 30 days, as well as the mortality rate at 30 days, were similar between both groups. 18 Salim et al 17 compared 12-hour with 72-hour duration of terlipressin treatment in 90 patients and showed similar 5-day rebleeding rates among the 2 groups. Similarly, Zaman et al 20 randomized 100 patients into a 24-hour treatment group or 72-hour treatment group of terlipressin and concluded similar 5-day rebleeding rates among the 2 groups. More recently, Vaishnav et al 19 concluded that there were no significant differences in rebleeding rates or mortality at both 5 and 42 days among 150 patients who were randomly assigned to either 24-hour or 72-hour terlipressin treatment.
Based on the current findings, health care providers may think about the advantages of using shorter durations (12-24 hours) of terlipressin therapy for specific patient groups while waiting for more conclusive evidence. Shorter treatment durations may reduce the treatment burden, minimize the risk of adverse effects, and lower health care costs without compromising clinical outcomes such as rebleeding and mortality. Adjusting the duration of treatment based on individual patient needs and responses could become a practical strategy.
To our knowledge, this is the first meta-analysis to compare the short and usual course of terlipressin specifically. All the studies included in our analysis were randomized, we also considered variations in measuring variables and incorporated random effects to ensure the findings could be applied across diverse populations.
The present meta-analysis has a few limitations. First, the small number of included studies, consisting of only 4 RCTs, and the fact that the participants were predominantly of South Asian descent, may restrict the generalizability of the findings to broader populations, emphasizing the need for further research. Second, we observed heterogeneity among the included studies in terms of baseline patient characteristics, bleeding severity, and outcome definitions, which could potentially influence the pooled effect estimates. Third, due to the short-term follow-up duration in the studies, our understanding of the impact of terlipressin duration on long-term outcomes, such as rebleeding rates and mortality beyond the initial 5-day period, remains limited. Fourth, incomplete reporting of adverse events across the studies introduced variability and hindered a more comprehensive comparison of safety profiles, such as length of hospital stay.
In conclusion, our analysis of 4 clinical trials showed that there was no significant difference in mortality or bleeding control between using terlipressin for 12-24 hours compared with 72 hours in cirrhotic patients with AVB. This suggests that shorter treatment could be better to reduce hospital stays, lower strain on health care resources, and decrease the risk of hospital-acquired infections. However, more research with larger groups of patients is needed to better understand the implications of shorter terlipressin duration on clinical practice.
Supplemental Material
Supplemental material, sj-docx-1-pmt-10.1177_87551225241311444 for Comparison of 12- to 24-Hour Versus 72-Hour Intravenous Terlipressin in Patients With Acute Esophageal Variceal Bleeding: A Systematic Review and Meta-analysis by Mohammad Al Hayek, Bisher Sawaf, Shahem Abbarh, Sudheer Dhoop, Abdallah Khashan, Ahmed Hassan, Alhasan Saleh Alzubi, Abdelrahman F. Abdelwahed, Abdussalam I. A. Alzein, Mohamedhen Vall Nounou, Yaseen Alastal and Muhammed Elhadi in Journal of Pharmacy Technology
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammed Elhadi  https://orcid.org/0000-0001-6406-4212
https://orcid.org/0000-0001-6406-4212
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Shaheen AA, Nguyen HH, Congly SE, Kaplan GG, Swain MG. Nationwide estimates and risk factors of hospital readmission in patients with cirrhosis in the United States. Liver Int. 2019;39(5):878-884. doi: 10.1111/liv.14054 [DOI] [PubMed] [Google Scholar]
- 2. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92(7):1081-1091. [PubMed] [Google Scholar]
- 3. D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38(3):599-612. doi: 10.1053/jhep.2003.50385 [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98(3):653-659. doi: 10.1111/j.1572-0241.2003.07294.x [DOI] [PubMed] [Google Scholar]
- 5. Sohal A, Chaudhry H, Dhaliwal A, et al. Gender differences in esophageal variceal bleeding in the United States. Ann Med. 2022;54(1):2115-2122. doi: 10.1080/07853890.2022.2104920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haukeland JW, Småstuen MC, Pålsdatter PP, et al. Effect of gender on mortality and causes of death in cirrhotic patients with gastroesophageal varices. PLoS ONE. 2020;15(3):e0230263. doi: 10.1371/journal.pone.0230263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310-335. doi: 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 8. Baik SK, Jeong PH, Ji SW, et al. Acute hemodynamic effects of octreotide and terlipressin in patients with cirrhosis: a randomized comparison. Am J Gastroenterol. 2005;100(3):631-635. doi: 10.1111/j.1572-0241.2005.41381.x [DOI] [PubMed] [Google Scholar]
- 9. Kim HR, Lee YS, Yim HJ, et al. Severe ischemic bowel necrosis caused by terlipressin during treatment of hepatorenal syndrome. Clin Mol Hepatol. 2013;19(4):417-420. doi: 10.3350/cmh.2013.19.4.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed AI, Kaleem MZ, Abbarh S, et al. Terlipressin-induced skin necrosis in cirrhotic patients—a case report and comprehensive literature review. Clin Case Rep. 2024;12(10):e9141. doi: 10.1002/ccr3.9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743-752. doi: 10.1016/j.jhep.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 12. Sridharan K, Sivaramakrishnan G. Vasoactive agents for the management of variceal bleeding: a mixed treatment comparison network meta-analysis and trial sequential analysis of randomized clinical trials. Drug Res (Stuttg). 2019;69(9):487-495. doi: 10.1055/a-0846-3071 [DOI] [PubMed] [Google Scholar]
- 13. Zou Z, Yan X, Lu H, et al. Comparison of drugs facilitating endoscopy for patients with acute variceal bleeding: a systematic review and network meta-analysis. Ann Transl Med. 2019;7(23):717. doi: 10.21037/atm.2019.12.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rehman H, Rehman ST, Zulfiqar S, Awan S, Abid S. Real-world comparison of terlipressin vs. octreotide as an adjuvant treatment in the management of variceal bleeding. Sci Rep. 2024;14(1):6692. doi: 10.1038/s41598-024-56873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lo GH, Chen WC, Wang HM, et al. Low-dose terlipressin plus banding ligation versus low-dose terlipressin alone in the prevention of very early rebleeding of oesophageal varices. Gut. 2009;58(9):1275-1280. doi: 10.1136/gut.2008.165910 [DOI] [PubMed] [Google Scholar]
- 16. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C. Baveno VII–renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959-974. doi: 10.1016/j.jhep.2021.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salim A, Malik K, Haq IU, Butt AK, Alam A. Comparison of 12-hour with 72-hour terlipressin therapy for bleeding esophageal varices. J Coll Physicians Surg Pak. 2017;27(6):334-337. [PubMed] [Google Scholar]
- 18. Azam Z, Hamid S, Jafri W, et al. Short course adjuvant terlipressin in acute variceal bleeding: a randomized double blind dummy controlled trial. J Hepatol. 2012;56(4):819-824. doi: 10.1016/j.jhep.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 19. Vaishnav M, Biswas S, Shenoy A, et al. Comparison of 1-day versus 3-day intravenous terlipressin in cirrhosis patients with variceal bleeding: a pilot randomised controlled trial. Aliment Pharmacol Ther. 2024;59(5):645-655. doi: 10.1111/apt.17868 [DOI] [PubMed] [Google Scholar]
- 20. Zaman M, Zaidi AR, Hyder A, Kumar M, Amin J, Malik K. Frequency of rebleeding between short course terlipressin different courses (24 hours) and usual course (72 hours) terlipressin in adult cirrhotic patients presenting with acute variceal rebleeding. Med Forum. 2019;30(2):130-133. [Google Scholar]
- 21. Lo GH, Perng DS, Chang CY, Tai CM, Wang HM, Lin HC. Controlled trial of ligation plus vasoconstrictor versus proton pump inhibitor in the control of acute esophageal variceal bleeding. J Gastroenterol Hepatol. 2013;28(4):684-689. doi: 10.1111/jgh.12107 [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 24. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Page MJ, Higgins JPT, Sterne JAC. Assessing risk of bias due to missing results in a synthesis. In: Higgins JT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019:349-374. [Google Scholar]
- 26. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55-61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362(9):823-832. doi: 10.1056/NEJMra0901512 [DOI] [PubMed] [Google Scholar]
- 29. Qi X, Bai Z, Zhu Q, et al. Practice guidance for the use of terlipressin for liver cirrhosis-related complications. Therap Adv Gastroenterol. 2022;15:17562848221098253. doi: 10.1177/17562848221098253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huaringa-Marcelo J, Huaman MR, Brañez-Condorena A, et al. Vasoactive agents for the management of acute variceal bleeding: a systematic review and meta-analysis. J Gastrointestin Liver Dis. 2021;30(1):110-121. doi: 10.15403/jgld-3191 [DOI] [PubMed] [Google Scholar]
- 31. Yeh JH, Lo GH, Huang RY, Lin CW, Wang WL, Perng DS. Short-course vasoconstrictors are adequate for esophageal variceal bleeding after endoscopic variceal ligation: a systematic review and meta-analysis. Sci Prog. 2021;104(3):368504211031711. doi: 10.1177/00368504211031711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poudel RC, Dhibar DP, Sharma N, Sharma V, Taneja S, Prakash A. Rational for continuing terlipressin after endoscopic variceal ligation in acute variceal haemorrhage needs further evidence: a pilot study. Arq Gastroenterol. 2022;59(1):89-96. doi: 10.1590/s0004-2803.202200001-16 [DOI] [PubMed] [Google Scholar]
- 33. Bruha R, Marecek Z, Prochazka V, et al. Double-blind randomized multicenter study comparing the efficacy and safety of 10-day to 5-day terlipressin treatment of bleeding esophageal varices. Hepatogastroenterology. 2009;56(90):390-394. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pmt-10.1177_87551225241311444 for Comparison of 12- to 24-Hour Versus 72-Hour Intravenous Terlipressin in Patients With Acute Esophageal Variceal Bleeding: A Systematic Review and Meta-analysis by Mohammad Al Hayek, Bisher Sawaf, Shahem Abbarh, Sudheer Dhoop, Abdallah Khashan, Ahmed Hassan, Alhasan Saleh Alzubi, Abdelrahman F. Abdelwahed, Abdussalam I. A. Alzein, Mohamedhen Vall Nounou, Yaseen Alastal and Muhammed Elhadi in Journal of Pharmacy Technology