Abstract
Background
Recent trials suggest that angiotensin-converting enzyme inhibitors (ACEI) are effective in prevention of ischemic stroke, as measured by reduced stroke incidence. We aimed to compare stroke severity between stroke patients who were taking ACEI before their stroke onset and those who were not, to examine the effects of pretreatment with ACEI on ischemic stroke severity.
Methods
We retrospectively studied 126 consecutive patients presenting within 24 hours of ischemic stroke onset, as confirmed by diffusion-weighted magnetic resonance imaging (DWI). We calculated the NIHSS score at presentation, as the primary measure of clinical stroke severity, and categorized stroke severity as mild (NIHSS [less than or equal to] 7), moderate (NIHSS 8–13) or severe (NIHSS [greater than or equal to] 14). We analyzed demographic data, risk-factor profile, blood pressure (BP) and medications on admissions, and determined stroke mechanism according to TOAST criteria. We also measured the volumes of admission diffusion- and perfusion-weighted (DWI /PWI) magnetic resonance imaging lesions, as a secondary measure of ischemic tissue volume. We compared these variables among patients on ACEI and those who were not.
Results
Thirty- three patients (26%) were on ACE-inhibitors. The overall median baseline NIHSS score was 5.5 (range 2–21) among ACEI-treated patients vs. 9 (range 1–36) in non-ACEI patients (p = 0.036). Patients on ACEI prior to their stroke had more mild and less severe strokes, and smaller DWI and PWI lesion volumes compared to non-ACEI treated patients. However, none of these differences were significant. Predictably, a higher percentage of patients on ACEI had a history of heart failure (p = 0.03). Age, time-to-imaging or neurological evaluation, risk-factor profile, concomitant therapy with lipid lowering, other antihypertensives or antithrombotic agents, or admission BP were comparable between the two groups.
Conclusion
Our results suggest that ACE-inhibitors may reduce the clinical severity of stroke, as measured by NIHSS score. Further, larger-scale, prospective studies areneeded to validate our findings, and to elucidate the mechanism(s) of ACEImediated benefits in patients with ischemic stroke.
Background
Data from the heart outcomes prevention evaluation study (HOPE) suggest that angiotensin-converting enzyme inhibitors (ACEI) are effective in prevention of ischemic stroke, as measured by reduced stroke incidence in subjects randomized to treatment with ACEI [1]. In this trial, the use of the ACEI, ramipril, resulted in a 32% reduction in ischemic stroke risk despite minimal reduction in blood pressure (BP) [1], leading some to suggest that ACEI may also exert direct neuroprotective effects.
To further elucidate if ACEI have potential neuroprotective effects, we tested whether their use prior to ischemic stroke onset might also reduce the severity of stroke. We examined clinical and admission magnetic resonance imaging (MRI) data from patients with ischemic stroke to determine the effects of prestroke use of ACEI on stroke severity.
Methods
Study design and patient selection
We retrospectively reviewed our prospectively collected stroke database over a 30-month period from 1998 to 2000, and identified consecutive patients who presented with acute ischemic stroke within 24 hours of onset and had DWI/PWI upon presentation. Onset time was defined, as the last time the patient was known to be in his/her usual state of health. The diagnosis of ischemic stroke was confirmed by diffusion-weighted imaging (DWI) showing evidence of acute cerebral infarction, combined with serial neurological examinations performed by stroke-trained neurologists. We included patients who had received thrombolytic, endovascular or experimental neuroprotective treatment. We only excluded patients who had transient ischemic attacks (TIAs), in whom DWI/PWI was negative.
Data collection and assessments
We retrieved the following data for each patient: (1) demographics; (2) risk factors for stroke, i.e. hypertension (HTN), diabetes mellitus (DM), hyperlipidemia, coronary artery disease (CAD), atrial fibrillation (AF), heart failure (CHF), history of TIA and smoking, as reported by the patient andhis/her family; (3) vital signs at presentation (BP and temperature); (4) blood glucose level at admission; (5) medications upon admission, with particular attention to antiplatelets, anticoagulants, lipid-lowering agents, and antihypertensives including ACEI. We did not collect information about the duration of medication(s) use, daily use or compliance. Patients and families were only questioned about patient's use of medication(s), including ACEI, in the week before stroke; (6) the baseline National Institute of Health Stroke Scale (NIHSS) score [2], which was recorded by stroke-trained neurolgistscertified in the application of NIHSS at admission; and (7) time from strokedetection to imaging.
Outcome measures
We used the NIHSS score at presentation as the primary measure of clinical stroke severity, and categorized stroke severity as mild (NIHSS score = 7), moderate (NIHSS score 8–13) or severe (NIHSS score = 14). We measured the total DWI and PWI lesion volumes, as secondary radiological measures of stroke severity, in 110/126 patients. All MRI studies were performed on a Siemens Medical Systems Vision 1.5-T MR whole body scanner with echoplanar imaging capabilities. An experienced researcher blinded to clinical data and patient's identity, performed MRI measurements. The volume of the perfusion abnormality was measured on relative Mean Transit Time (rMTT) maps. The specific MRI sequence parameters, imaging processing and volumetric analysis are described in details in previous publications [3,4]. We classified stroke mechanisms, after completing the diagnostic work-up, according to the Trial of Org 10172 in Acute Treatment (TOAST) criteria [5].
Statistical analysis
We divided patients into 2 groups, those who were taking ACEI before their stroke onset and those who were not. We compared inter-group differences between individual categorical variables by using student's t-test or Wilcoxon rank sum test for continuous variables, and Fisher's exact test forcategorical comparisons, as appropriate. We compared the median baseline NIHSS scores with the Mann-Whitney U test to evaluate the severity of the stroke in both groups. The Cochran-Mantel-Haenszel row mean score rank test, adjusted for various confounding variables (age, sex, risk factors, use of concomitant medications, time-from-stroke-to-evaluation, and stroke mechanism/subtype) was used to control for the differences in NIHSS scores between ACEI and non-ACEI users. A p-value of < 0.05 was considered statistically significant for all analyses.
Results
Patient characteristics (demographic and clinical Features)
A total of 126 patients met all of our inclusion and none of the exclusion criteria, and were included in subsequent analyses. Approximately, 26% (33 patients) were on ACEI before stroke onset. Fourteen were taking lisinopril (20 to 40 mg per day), 13 enalapril (10 to 40 mg per day), 5 captopril (75 to 150 mg per day) and 1 accupril (40 mg per day). None of our patients was taking perindopril or ramipril, or a combination of different ACEI. Table 1 summarizes the demographic and clinical features of patients in both ACEI- and non ACEI-treated groups. There were no significant differences in the mean age or sex distribution between the 2 groups. Slightly higher percentages of ACEI-treated patients had history of HTN, DM, hyperlipidemia, and smoking in comparison to the non-ACEI group. A slightly higher percentage of non-ACEI group reported history of prior TIA. However, none of these differences were statistically significant. There was a trend towards a higher frequency of cardiac disease among ACEI-treated patients. This was mostly driven by a significantly higher percentage of heart failure among ACEI-treated patients.
Table 1.
Comparison of demographic and clinical features between ACEI- and non ACEI-treated groups.
| Number of patients | ACEI group 33 (26%) | Non-ACEI group 93 (74%) | p-value |
| Sex (women/men) | 13/20 | 43/50 | |
| • % women | 39% | 46% | 0.55 |
| • % men | 61% | 54% | 0.55 |
| Mean age (year) ± SD | 73 ± 11 | 70 ± 13 | 0.23 |
| Risk factors: | |||
| History of hypertension | 78% | 65% | 0.19 |
| History of diabetes | 32% | 24% | 0.36 |
| History of hyperlipidemia | 28% | 22% | 0.48 |
| History of cardiac disease | 39% | 21% | 0.06 |
| • CHF | 23% | 9% | 0.03* |
| • AF | 10% | 7% | 0.69 |
| • CAD | 6% | 5% | 0.98 |
| History of smoking | 16% | 11% | 0.53 |
| History of prior TIA | 10% | 16% | 0.39 |
| Concomitant medications: | |||
| Antiplatelets | 37% | 41% | 0.68 |
| Anticoagulants | 12% | 10% | 0.74 |
| Statins | 20% | 21% | 0.99 |
| Other BP lowering agents | 56% | 52% | 0.84 |
| • Diuretics | 25% | 18% | 0.45 |
| • B-blockers | 18% | 27% | 0.35 |
| • Ca++ blockers | 13% | 13% | 0.99 |
| • ARB | 0% | 4% | 0.57 |
| Time from stroke-to evaluation | |||
| • 0–6 h | 61% | 63% | 0.83 |
| • 6–25 h | 39% | 37% | 0.83 |
| Clinical features: | |||
| NIHSS score, median | 5.5 | 9 | 0.036* |
| SBP (mean ± SD), mmHg | 162 ± 27 | 158 ± 31 | 0.35 |
| DBP (mean ± SD), mmHg | 84 ± 16 | 81 ± 20 | 0.38 |
| Temperature (mean ± SD), F° | 97 ± 7 | 98 ± 6 | 0.34 |
A roughly equal percentage of patients in both groups presented to our emergency room and were imaged within 6 hours from stroke onset (61% in ACEI-treated patients vs. 63% in non-ACEI group; p = 0.83). The mean time from stroke-symptom onset to evaluation was 10.9+5.2 h in ACEI-treated patients vs. 11.3+6.4 h in non-ACEI group (p = 0.62). There were no significant differences in admission temperature (97+7 F° vs. 98+6 F°; p = 0.34) or glucose levels (137+23 mg/dL vs. 144+29 mg/dL; p = 0.26) between the 2 groups. The mean SBP upon admission was 162+27 mmHg in ACEI-treated patients vs. 158+31 mmHg in non-ACEI group, and the mean DBP was 84+16 vs. 81+20 mmHg. None of these differences were statistically significant.
A roughly equal percentage of patients in each group were using antiplatelets, anticoagulants, statins and other BP lowering agents. Similarly, the frequency of using other classes of antihypertensive agents was not significantly different in either group. Four patients were taking angiotensin receptor blockers (ARBs) at the time of their stroke. None of these 4 patients was on ACEI. They were all included in non ACEI-treated group for purposes of statistical analysis.
Patient outcomes
Approximately, 48% of ACEI-treated patients had baseline NIHSS score = 7 compared with 40% of non-ACEI group (p = 0.42); 28% had NIHSS score between 8 – 13 vs. 20% in non-ACEI group (p = 0.46); and 24% had NIHSS score = 14 vs. 40% in non-ACEI users (p = 0.18).
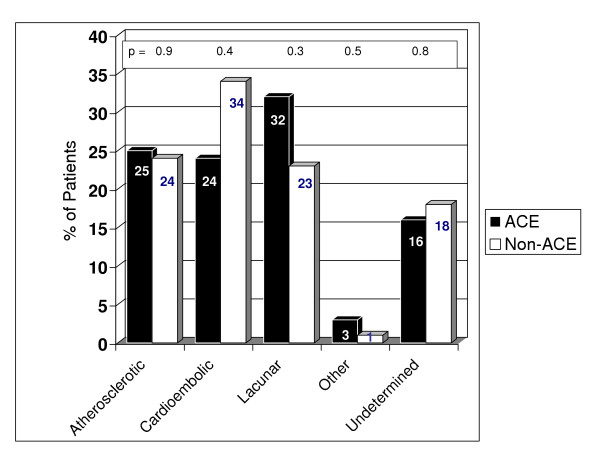
Figure 1 shows the distribution of stroke mechanisms, according to TOAST criteria, among ACEI- and non ACEI-treated patients. The stroke mechanisms were roughly equivalent in both groups. Although, cardioembolic cause was more frequent among non ACEI-treated patients (34% vs. 24%) and lacunar etiology was more commonly seen among patients who were taking ACEI prior to stroke onset (32% vs. 23%), these differences were not statistically significant.
Figure 1.
Comparison of stroke mechanisms between ACEI- and non ACEI-treated patients.
The overall median NIHSS score at admission was significantly lower in ACEI-treated patients (5.5; range 2 – 21) than in non-ACEI patients (9; range 1 – 36; p = 0.036). This difference remained statistically significant after controlling for confounding variables, such as history of hypertension, TIA, DM hyperlipidemia and cardiac disease, including CHF, stroke mechanism, onsetto- evaluation time, and concomitant medications, using the Cochran-Mantel-Haenszel row mean score test using ranks adjusted for these covariates (p = 0.042). Similarly, the median NIHSS score at admission was lower in the ACEI group when the analysis was limited to patients with non-lacunar strokes (8.5 vs. 12; p = 0.03) or to those who presented within 6 hours of stroke symptom onset (6 vs. 8; p = 0.046).
The DWI/PWI lesions volumetric measurements were performed in 87% of the patients (110/126). Sixteen patients were not included in MRI data analyses because their images were of poor quality to allow adequate quantitative measurements. As table 2 indicates, there were no significant differences between both groups with regard to the mean diffusion, perfusion or perfusion-diffusion (mismatch) lesion volumes.
Table 2.
Comparison of MRI between ACEI- and non ACEI-treated group
| ACEI group | Non-ACEI group | p-value | |
| DWI lesion volume (mean ± SD), cm3 | 25.2 ± 23.4 | 28.7 ± 25.0 | 0.55 |
| PWI lesion volume (mean ± SD), cm3 | 72.6 ± 56.6 | 75.1 ± 68.5 | 0.86 |
| Mismatch (PWI – DWI) volume | 47.6 ± 39.5 | 46.6 ± 28.2 | 0.93 |
Discussion
We found that the baseline NIHSS score was lower in patients who were taking ACEI prior to their stroke compared to those who were not taking ACEI at the time of stroke onset. The NIHSS is accepted widely for measuring acute stroke deficits to assess the degree of severity of neurological deficits from stroke and its reliability has been tested in several clinical trials [6-8].
We found no difference in admission BP between ACEI and non-ACEI users, suggesting that the beneficial effects of ACEI use may not be directly related to their BP-lowering effect. This is concordant with the results from the HOPE trial [1].
Several factors could potentially account for the observed difference in baseline NIHSS between ACEI and non-ACEI groups, such as differences in risk factors, stroke mechanism/subtype, or baseline hemodynamic parameters. The risk factors that influence ACEI use, such as history of HTN, CHF and DM, were dissimilarly distributed between the two groups, and their impact on stroke type and severity cannot be entirely excluded. However, ourfindings are unlikely to be related to differences in baseline risk factor profile between the ACEI- and non-ACEI treated patients since patients who were on ACEI had a higher prevalence of HTN, DM and heart failure, which may have biased our data toward higher stroke severity in ACEI-treated patients, and thus limited our ability to detect larger differences in favor of ACEI use. It is possible that ACEI use reflected a greater degree of medical attention and more aggressive risk factor reduction in these patients, which subsequently lessened stroke severity.
Recent studies have shown that TIAs before stroke can induce tolerance (ischemic preconditioning) to subsequent strokes by raising the threshold of brain tissue vulnerability, which results in smaller infarct volumes, and better recovery [9-11]. We found no significant differences in the frequency of prior history of TIAs, as reported by the patient or his/her family, between the ACEI and non-ACEI treated patients. In fact, a slightly higher percentage of patients in the non-ACEI group reported history of TIAs prior to their presenting stroke.
Some epidemiological studies show that greater stroke severity at onset is associated with a shorter interval between symptom onset and time to emergency department arrival [12], suggesting that the observed difference in baseline NIHSS could be attributed to dissimilar distribution of patients' arrival time to the hospital. However, a roughly identical proportion of patients in both groups presented to our hospital within 6 hours of stroke-symptom onset.
Since the observed beneficial effect of ACEI on stroke severity could potentially be secondary to ACEI effects on stroke mechanism, we examined the impact of ACEI use on stroke mechanism using TOAST criteria. We found a greater preponderance of lacunar strokes among ACEI-treated patients and cardioembolic strokes among non-ACEI patients. However, these differences were not significant and the difference in baseline NIHSSS remained significant even after excluding patients with non-lacunar strokes from analysis. This suggests that ACEI use did not seem to influence stroke mechanism in our cohort of patients, and that our findings were unrelated to the higher frequency of lacunar strokes among ACEI-treated patients. Similarly, the beneficial effect of ACEI in our patients is unlikely to be related to other concomitant treatments. Although, several patients in both groups were on statins, antithrombotics and other antihypertensive agents, we found no significant difference between ACEI- and non ACEI-treated patients receiving any of these classes of drugs. Finally, it is noteworthy that the difference in baseline stroke severity between ACEI and non-ACEI groups remained statistically significant after adjusting for the above confounding variables.
A recent prospective observational study of 507 patients with first-ever ischemic stroke showed that treatment with ACEI at the time of stroke onset is associated with reduced plasma concentration of C-reactive protein and better long-term outcomes [13], suggesting that ACEI may have anti-inflammatory properties and reduce the acute-phase inflammatory response after stroke onset. There are several other potential mechanisms by which ACEI may provide benefit to stroke patients. Experimental data suggest that the rennin angiotensin system modulates the atherosclerotic process, and that angiotensin II exerts pro-inflammatory actions in the vascular wall, which induce the production of reactive oxygen species and hydroxyl radicals, cytokines and adhesion molecules [14-19]. Angiotensin converting enzyme inhibitors could provide neuroprotection via blockade of angiotensin II-mediated endothelial dysfunction, lipid peroxidation and subsequent oxidative stress, and vascular smooth muscle intracellular calcium accumulation and hypertrophy [14-20]. Furthermore, ACEI may help maintain homeostatic balance of fibrinolytic and procoagulant factors [21] and increase cerebral blood flow [22]. Recent studies using transcranial Doppler ultrasonography have shown that perindopril can improve the cerebral vasomotor reactivity in patients with lacunar infarcts beyond any effect on BP [22], and that treatment with quinapril can ameliorate cerebrovascular reactivity caused by methionine-induced hyperhomocysteinemia in healthy volunteers [23].
We found that ACE I use had no effect on MRI measures of ischemic lesion volume. This discrepancy between the beneficial effects of ACEI on clinical, but not radiological, measures of stroke severity is reconcilable since the correlation between infarct volume and NIHSS is only moderate, particularly in non-dominant hemispheric strokes [24-26]. We explored the possibility that the lower NIHSS scores in ACEI-treated patients might be secondary to a higher percentage of non-dominant hemispheric strokes in this group [26]. However, we found no significant difference in the preponderance of non-dominant hemispheric strokes between the 2 groups (data not reported). Infarct location, not only size, is also an important determinant of the severity of clinical deficits and our small sample size may have limited our ability to detect a difference in favor of ACEI.
We acknowledge that our study has inherent limitations imposed by its retrospective nature, non-randomization of treatment allocation and small sample size. The small number of ACEI-treated patients does not allow us to test for possible differences among the various ACEIs or dose regimens. Similarly, we cannot be certain of the duration of treatment or compliance with daily use of ACEI in our patients. We used an arbitrary cut-off for NIHSS scores to categorize stroke severity. It is possible that different cut-off values could lead to different results. Most importantly, our study lacks follow-up data regarding the effect of ACEI use on long-term outcomes since a large percentage of our patients were either enrolled in experimental neuroprotective trials or treated with thrombolysis upon presentation.
Conclusion
Our results show that pre-stroke use of ACEI is associated with milder stroke severity, as assessed by NIHSS score. Our findings need to be prospectively validated in larger-scale randomised studies, and the mechanism(s) of ACEI-mediated benefits in patients with ischemic stroke need to be elucidated.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MS designed the study, collected and analyzed data, wrote the paper, and carried out critical revision of the manuscript. SS collected and analyzed data, and reviewed the manuscript. IL collected data and reviewed the manuscript. LC assisted with data interpretation and critically reviewed the manuscript. GS collected data, and carried out critical revision of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This study was supported, in part, by grants from the Harvard Center for Neurodegeneration (Dr Selim) and the Doris Duke Charitable foundation, the NINDS (1R01NS045049-01A1) and the Dana foundation (Dr Schlaug).
Contributor Information
Magdy Selim, Email: mselim@bidmc.harvard.edu.
Sean Savitz, Email: ssavitz@bidmc.harvard.edu.
Italo Linfante, Email: ilinfante@med.miami.edu.
Louis Caplan, Email: lcaplan@bidmc.harvard.edu.
Gottfried Schlaug, Email: gschlaug@bidmc.harvard.edu.
References
- The Heart Outcome Prevention Evaluation Study Investigators Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Benfield A, Baird AE, Siewert B, Lovblad KO, Parker RA, Edelman RR, Warach S. The ischemic penumbra: Operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Yi C, Linfante I, Schlaug G. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: Prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002;33:2047–2052. doi: 10.1161/01.STR.0000023577.65990.4E. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke: Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–879. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Spilker J, Kongable G, Barch C, Braimah J, Brattina P, Daley S, Donnarumma R, Rapp K, Sailor S. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J Neurosci Nurs. 1997;29:384–92. doi: 10.1097/01376517-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Wilterdink JL, Bendixen B, Adams HP, Jr, Wolson RF, Clarke WR, Hansen MD. Effect of prior aspirin use on stroke severity in the trial of Org 10172 in acute ischemic stroke (TOAST) Stroke. 2001;32:2836–2840. doi: 10.1161/hs1201.099384. [DOI] [PubMed] [Google Scholar]
- Schaller B. Ischemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: its clinical relevance. Neurosci Lett. 2005;377:206–211. doi: 10.1016/j.neulet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Arboix A, Cabeza N, Garcia-Eroles L, Massons J, Oliveres M, Targa C, Balcells M. Relevance of transient ischemic attack to early neurological recovery after nonlacunar ischemic stroke. Cerebrovasc Dis. 2004;18:304–311. doi: 10.1159/000080356. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. MRI in Acute Stroke Study Group of the German Competence Network Stroke. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Rossnagel K, Jungehulsing GJ, Nolte CH, Muller-Nordhorn J, Roll S, Wegscheider K, Villringer A, Willich SN. Out-of-hospital delays in patients with acute stroke. Ann Emerg Med. 2004;44:476–483. doi: 10.1016/j.annemergmed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F. Angiotensin-converting enzyme inhibitor use isassociated with reduced plasma concentration of C-reactive protein in patients with first-ever ischemic stroke. Stroke. 2003;34:2922–2929. doi: 10.1161/01.STR.0000099124.84425.BB. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Manning WM, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Fukai N, Sato R, Sugiyama T, Ozawa N, Shichiri M, Hirata Y. Antioxidant effect of adrenomedullin on angiotensin II-induced reactive oxygen species generation in vascular smooth muscle cells. Endocrinology. 2004;145:3331–3337. doi: 10.1210/en.2003-1583. [DOI] [PubMed] [Google Scholar]
- Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- Skurk T, Van Harmelen V, Hauner H. Angiotensin II Stimulates the Release of Interleukin-6 and Interleukin-8 From Cultured Human Adipocytes by Activation of NF-{kappa}B. Arterioscler Thromb Vasc Biol. 2004;24:1199–1203. doi: 10.1161/01.ATV.0000131266.38312.2e. [DOI] [PubMed] [Google Scholar]
- Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- Michel JB. Renin-angiotensin system and vascular remodelling. Med Sci (Paris) 2004;20:409–413. doi: 10.1051/medsci/2004204409. [DOI] [PubMed] [Google Scholar]
- Francis GS. ACE inhibition in cardiovascular disease. N Engl J Med. 2001;342:201–202. doi: 10.1056/NEJM200001203420309. [DOI] [PubMed] [Google Scholar]
- Makris TK, Stavroulakis GA, Krespi PG, Hatzizacharias AN, Triposkiadis FK, Tsoukala CG, Votteas VV, Kyriakidis MK. Fibrinolytic/hemostatic variables in arterial hypertension: response to treatment with irbesartan or atenolol. Am J Hypertens. 2000;13:783–788. doi: 10.1016/S0895-7061(00)00262-4. [DOI] [PubMed] [Google Scholar]
- Walters M, Muir S, Shah I, Lees K. Effect of Perindopril on Cerebral Vasomotor Reactivity in Patients With Lacunar Infarction. Stroke. 2004;35:1899–1902. doi: 10.1161/01.STR.0000131748.12553.ed. [DOI] [PubMed] [Google Scholar]
- Chao CL, Lee YT. Impairment of cerebrovascular reactivity by methionine-induced hyperhomocysteinemia and amelioration by quinapril treatment. Stroke. 2000;31:2907–2911. doi: 10.1161/01.str.31.12.2907. [DOI] [PubMed] [Google Scholar]
- Mitsias PD, Jacobs MA, Hammoud R, Pasnoor M, Santhakumar S, Papamitsakis NI, Soltanian-Zadeh H, Lu M, Chopp M, Patel SC. Multiparametric MRI ISODATA ischemic lesion analysis: correlation with the clinical neurological deficit and single-parameter MRI techniques. Stroke. 2002;33:2839–2844. doi: 10.1161/01.STR.0000043072.76353.7C. [DOI] [PubMed] [Google Scholar]
- Tong DC, Yenari MA, Albers GW, O'Brien M, Marks MP, Moseley ME. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology. 1998;50:864–870. doi: 10.1212/wnl.50.4.864. [DOI] [PubMed] [Google Scholar]
- Fink JN, Selim MH, Kumar S, Silver B, Linfante I, Caplan LR, Schlaug G. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.STR.0000013069.24300.1D. [DOI] [PubMed] [Google Scholar]