Abstract
Despite progress in the diagnosis, prevention and therapy for hospital-acquired infections, ventilator-associated pneumonia (VAP) continues to complicate the course of a significant proportion of patients receiving mechanical ventilation. Mortality rates among patients with VAP have been reported to be as high as 72%, and the morbidity associated with VAP is also considerable, adding days to the hospital stay and increasing health care costs. Appropriate initial antimicrobial therapy for patients with VAP has been shown to reduce mortality rates and improve outcomes; therefore, rapid identification of infected patients and timely, accurate selection of effective antimicrobial agents are important clinical goals. The primary organisms responsible for VAP include Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. However, aetiologies differ considerably between intensive care units, and the increase in antibiotic resistance and nosocomial outbreaks worldwide have presented clinicians with a serious dilemma with respect to selecting appropriate empirical therapy. To date, no optimal antimicrobial regimen for the treatment of VAP has been identified, largely because none of the currently marketed antibiotics has a sufficiently extended spectrum of activity to cover all of the potential key pathogens. More active, less toxic antibacterial agents are still needed, in particular to combat problematic pathogens such as multiresistant Gram-negative bacilli and resistant Gram-positive organisms (e.g. methicillin-resistant S aureus).
Keywords: antibiotic resistance, nosocomial infection, ventilator-associated pneumonia
Introduction
Pneumonia is the single most common nosocomial infection among patients in intensive care units (ICUs) [1,2]. Rates of pneumonia are considerably higher among patients hospitalized in ICUs than in hospital wards, and the risk for developing pneumonia is 3-fold to 10-fold higher for intubated patients receiving mechanical ventilation [1,2]. Of hospital-acquired infections, nosocomial pneumonia is reported to be the leading cause of death, being responsible for half of the hospital-acquired infections that result in death [3,4]. However, whether patients with ventilator-associated pneumonia (VAP) have associated mortality is controversial. Indeed, in a large matched cohort study of patients with early onset VAP [5], an association between VAP and poor clinical and economic outcomes was demonstrated, but hospital mortality was not attributable to VAP in this analysis. On the other hand, there does appear to be a correlation between severity of illness at admission and survival [6]. Reported mortality in VAP patients ranges from 33% to 72% [4], with the upper range reflecting the increased risk for mortality among the elderly, patients with impaired cardiopulmonary function, immunocompromised patients, patients who require prolonged intubation, and those at risk for infection with Pseudomonas aeruginosa or methicillin-resistant Staphylococcus aureus (MRSA) [7].
Fiel [7] recently showed that a twofold reduction in mortality could be achieved in patients with nosocomial pneumonia with prompt use of appropriate antibiotics, but what are the appropriate therapeutic options for VAP, which is often a polymicrobial infection [8]? The published literature indicates that Gram-negative bacteria account for between 55% and 85% of cases of nosocomial pneumonia [8] but that the Gram-positive pathogen S aureus is the second most prevalent organism, accounting for 10–20% of all nosocomial pneumonias. Moreover, the growing incidence of methicillin-resistant strains of S aureus has important implications for the design of treatment regimens. Clearly, an agent, or agents, with broad-spectrum activity against both Gram-positive and Gram-negative pathogens is needed for optimal management of these infections. In this review we examine the incidence, aetiology and diagnosis of VAP, and address current therapeutic options.
Pathophysiology
There are several factors that potentially contribute to the high rates of VAP in hospitalized patients. First, hospitals contain clusters of highly vulnerable patients, many of whom will have predisposing pulmonary conditions that compromise defence mechanisms in their airways. Although the respiratory tract is designed to prevent the entry of pathogenic organisms into the lungs and to eradicate such pathogens should they bypass the upper airway host defences, these defence mechanisms can be overwhelmed by, for example, a large aspirated inoculum or an inherently virulent organism.
Second, the most common means of acquiring pneumonia is via aspiration [9]. Aspiration is promoted by supine position and by upper airway and gastrointestinal intubation; aspiration in mechanically ventilated patients occurs around the outside of the endotracheal tube rather than through the lumen. Leakage around the endotracheal cuff can be demonstrated in most patients. Given that as many as 45% of healthy individuals aspirate during sleep, it is not surprising that aspiration is even more common among patients with abnormal swallowing, impaired gag reflexes, compromised consciousness due to medication or anaesthesia, delayed gastric emptying, or decreased gastric motility.
Third, the dominant organisms in nosocomial pneumonia are aerobic Gram-negative bacilli [10-12]. These bacteria presumably reach the lower airway via aspiration of gastric contents or of upper airway secretions. Oropharyngeal colonization with Gram-negative bacilli is unusual in otherwise healthy, nonhospitalized individuals. In moderately ill patients, however, the carriage rate is around 16%, rising to almost 75% in severely ill patients [13]. Thus, the propensity for colonization of the upper airways directly correlates with severity of illness. In addition to severity of illness, several other factors have been identified as being associated with Gram-negative oropharyngeal colonization, as shown in Table 1. Other means by which pneumonia can be acquired include aspiration from the stomach or nose and paranasal sinuses. Aspiration of gastric contents can be minimized by maintaining the patient in a semi-recumbent position, but this is not an effective measure for minimizing oropharyngeal aspiration.
Table 1.
Risk factors for oropharyngeal colonization by Gram-negative bacilli
| Life-threatening illness | Pulmonary disease |
| Prolonged hospitalization/intensive care unit stay | Smoking |
| Advanced age | Uraemia |
| Antibiotic exposure | Alcoholism |
| Intubation | Coma |
| Major surgery | Multiple organ failure |
| Malnutrition | Neutropenia |
Incidence
The reported frequencies of VAP vary from 8% to 28%. In the National Nosocomial Infections Surveillance system [14], rates of VAP range from five cases per 1000 ventilator days in paediatric patients to 16 cases per 1000 ventilator days in patients with thermal injury or trauma. Kollef [15] reported an incidence of 22% among cardiothoracic patients, as compared with 14% among other surgical patients and 9.3% in medical patients, demonstrating that rates of VAP are generally higher among surgical than among medical patients.
Although nosocomial pneumonia accounts for only about 15% of hospital-acquired infections, it is the most frequent lethal nosocomial infection [2,16]. Mortality rates for nosocomial pneumonia are reported to range from 20% to 71%, whereas mortality rates for nosocomial pneumonia acquired in the ICU range from 20% to 40% [2,16]. The main risk factors for mortality among patients with nosocomial pneumonia include severity of underlying illness, inappropriate antibiotic therapy, advanced age, and infection with a high-risk pathogen such as P aeruginosa.
Each episode of nosocomial pneumonia will prolong a hospital stay by 7–9 days, resulting in increased hospital costs. In a study conducted by Rello and coworkers [5] in which patients with VAP were matched to 2243 control individuals without VAP, the patients with VAP had a significantly longer duration of mechanical ventilation (14.3 ± 15.5 days versus 4.7 ± 7.0 days; P < 0.001), ICU stay (11.7 ± 11.0 days versus 5.6 ± 6.1 days; P < 0.001), and hospital stay (25.5 ± 22.8 days versus 14.0 ± 14.6 days; P < 0.001). In addition, VAP in these patients was associated with increased hospital costs in excess of US$40,000 per patient (104,983 ± 91,080 versus 63,689 ± 75,030 [in US$]; P < 0.001).
Ibrahim and coworkers [11] found that hospital mortality was significantly greater in patients with early-onset nosocomial pneumonia (which they defined as occurring within 96 hours of ICU admission) and late-onset nosocomial pneumonia (defined as occurring after 96 hours of ICU admission) than in ICU patients who did not develop pneumonia. This indicates that both early-onset and late-onset pneumonia are associated with increased hospital mortality rates and length of stay. Ibrahim and coworkers also found that prior hospitalization and antibiotic use probably contributes to development of early-onset pneumonia due to MRSA and other resistant organisms. However, typically, antibiotics can help to prevent early-onset VAP and have a stronger association with late-onset disease [17].
Aetiology
The dominant organisms in nosocomial pneumonia are aerobic Gram-negative bacilli. Several studies have reported that more than 60% of VAP is caused by aerobic Gram-negative bacilli [18,19]. The predominant Gram-negative bacilli that cause VAP are P aeruginosa, Acinetobacter spp., Proteus spp., Escherichia coli, Klebsiella spp. and Haemophilus spp. More recently, however, studies have highlighted an increased prevalence of Gram-positive organisms in this setting, with S aureus being the predominant Gram-positive isolate (Table 2). For example, S aureus was responsible for most episodes of nosocomial pneumonia in the EPIC (European Prevalence of Infection in Intensive Care) study [19], accounting 31% of the 836 cases in which pathogens were identified. Anaerobic bacteria may be found in 20–30% of cases, but they are not generally isolated when using standard diagnostic specimen sources. When they are found it is usually as part of a polymicrobial infection including Gram-negative bacilli or S aureus, and their role is unclear [16]. Legionella accounts for approximately 4% of all nosocomial infections, based on a multihospital autopsy study of patients with lethal nosocomial pneumonia. Large outbreaks of Legionnaires' disease in hospitals are generally associated with contaminated water supplies that are distributed via air conditioning systems or showerheads [20].
Table 2.
Common causative pathogens associated with ventilator-associated pneumonia
| Frequency [n (%)] | |||
| Pathogen | Trouillet [12] (n = 245) | Rello [10,51] (n = 301) | Ibrahim [11] (n = 420) |
| Pseudomonas aeruginosa | 39 (15.9) | 102 (33.9) | 130 (30.9) |
| Acinetobacter baumannii | 22 (9.0) | 38 (12.6) | 16 (3.8) |
| Haemophilus influenzae | 15 (6.1) | 26 (8.6) | 19 (4.5) |
| Streptococcus pneumoniae | 3 (1.2) | 25 (8.3) | 6 (1.4) |
| MRSA | 20 (8.2) | 10 (3.3) | 81 (19.3) |
| MSSA | 32 (13.1) | 38 (12.6) | 62 (14.7) |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
There is significant variability in aetiology between hospitals, as shown in Fig. 1, which shows the different aetiologic patterns for multiresistant pathogens in different institutions among patients who were mechanically ventilated for more than 7 days and had prior antibiotic exposure. Similarly, in a study conducted by Valles and coworkers [21] in patients with hospital-acquired pneumonia requiring ICU admission, significant variations in aetiology were observed between hospitals, particularly affecting the incidence of pneumonias caused by Aspergillus spp. and Legionella pneumophila. This study highlights the need for good local epidemiologic data as part of effective therapeutic decision making.
Figure 1.
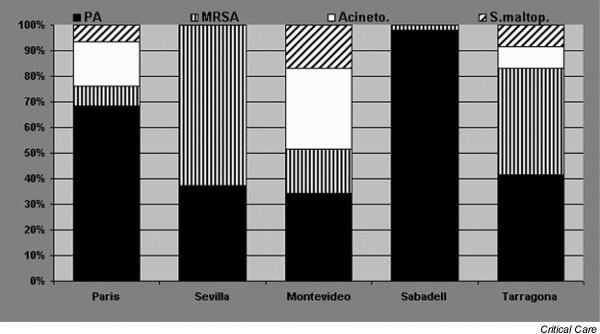
Different aetiologic patterns for multiresistant pathogens in different institutions among patients who were mechanically ventilated for more than 7 days and had prior antibiotic exposure. Acineto, Acinetobacter baumannii; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa; S.maltop, Stenotrophomonas maltophilia.
Underlying disease may predispose patients to infection with specific organisms. For example, patients with chronic obstructive pulmonary disease are at increased risk for H influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae infections; cystic fibrosis increases the risk of P aeruginosa and S aureus infections; and trauma and neurologic patients are at increased risk for S aureus infection [16,22].
The time of onset of VAP also appears to correlate with certain pathogens [12,16]. Early-onset VAP, defined as occurring during the first 4 days of mechanical ventilation, is often associated with high rates of S pneumoniae, H influenzae, methicillin-sensitive S aureus and susceptible Enterobacteriaceae. Many of these pneumonias probably reflect infection that was incubating in the community and presented early during hospitalization. Late-onset VAP, defined as developing 5 or more days after initiation of mechanical ventilation, is more frequently caused by enteric Gram-negative organisms, including P aeruginosa, Acinetobacter or Enterobacter spp., or by MRSA [12,16].
The rise in the number of multidrug-resistant pathogens in recent years has led to the development of infections that are difficult to treat and have limited physicians' treatment options. In particular, MRSA is now responsible for a significant proportion of nosocomial pneumonias, and MRSA strains with reduced susceptibility to vancomycin (glycopeptide-intermediate S aureus) are causing concern. Glycopeptide-intermediate S aureus has been identified in Japan, the USA and Europe [23]. In addition, glycopeptides are suboptimal (50% overall mortality rate) as therapy for MRSA pneumonia [24-27]. The incidence of multiresistant pathogens is linked to local factors and varies from institution to institution. Clinicians must therefore be aware of the common organisms associated with both early-onset and late-onset VAP in their own hospitals if they are to avoid administering inappropriate initial therapy. In addition, ICUs must collect epidemiologic data and be vigilant with respect to local susceptibility patterns.
Diagnosis
Diagnosis of bacterial pneumonia in severely ill, mechanically ventilated patients remains difficult for the clinician. The criteria used most often for clinical diagnosis of nosocomial pneumonia are fever, with a temperature > 100.4°F (38°C); leucocytosis or leucopenia; a new or increasing pulmonary infiltrate on chest film; purulent tracheobronchial secretions; and a sputum Gram stain with many polymorphonuclear leucocytes, fewer than 10 epithelial cells, and a predominant pathogen. VAP is pneumonia in persons in whom a device was used to assist or control respiration continuously through a tracheostomy or by endotracheal intubation within the 48 hours period before the onset of infection. Incidence should be reported as days/1000 ventilation days [16,28]. However, this clinical picture can often be confused with a variety of other infections and noninfectious pulmonary processes in the ventilated ICU patient [28].
Radiographical evidence of pneumonia in ventilated patients is also notoriously inaccurate. In a study of autopsy proven VAP, Wunderink and coworkers [29] found that only air bronchograms correlated with pneumonia in the total study population and that no specific roentgenographic sign correlated with pneumonia in patients with adult respiratory distress syndrome. The diagnoses most frequently confused with nosocomial pneumonia, based on radiographical appearance, include adult respiratory distress syndrome, congestive heart failure, atelectasis, pulmonary embolism and neoplastic infiltration [28].
The use of lung tissue for diagnosis of pneumonia is not recommended in most ventilated patients, although it can be useful in some immunocompromised patients. Several autopsy studies that compared clinical diagnosis with histopathologic examinations identified errors in diagnosis and treatment of pneumonia in 29–38% of patients [30,31]. In a study conducted by Fagon and coworkers [32] that compared clinical diagnosis with histopathologic and bronchoscopic bacteriologic criteria in ventilated patients, the clinical diagnosis was correct in 62% of cases and therapeutic treatment plan was appropriate in only 33% of cases.
Given the difficulty in diagnosing pneumonia in the ventilated patient clinically, alternative methods of diagnosis have been sought. Although simple qualitative culture of endotracheal aspirates is a technique with a high percentage of false-positive results due to bacterial colonization of the proximal airways observed in most patients in the ICU, some studies using quantitative culture techniques suggest that endo-tracheal aspirate cultures may have an acceptable overall diagnostic accuracy, similar to that with several other, more invasive techniques [28]. Although quantitative endotracheal aspirate cultures can correctly identify patients with pneumonia, it should be borne in mind that the microbiologic results cannot be used to infer which micro-organisms present in the trachea are really present in the lungs.
Blind and bronchoscopic sampling of lower airways has also been studied extensively. Fibreoptic bronchoscopy permits direct access to the lower airways for sampling bronchial and parenchymal tissues at the site of lung inflammation [28]. To reach the bronchial tree, however, the bronchoscope must traverse the endotracheal tube and proximal airways, where contamination is likely to occur. Therefore, distal secretions directly aspirated through the bronchoscope suction channel are frequently contaminated, limiting their clinical specificity [28]. Nevertheless, the use of invasive techniques such as fibreoptic bronchoscopy, together with quantitative cultures of bronchoscopic samples obtained with bronchoalveolar lavage or protected specimen brush, can help to guide the choice of antibiotic therapy and confirm the diagnosis of VAP. What is clear is that avoiding delay in sampling and initiating therapy quickly are more important than the type of quantitative technique used.
Treatment strategy and impact of appropriate antibiotics
In an effort to help physicians to manage VAP, the Tarragona strategy – the basic principles of which are outlined in Table 3 – has been proposed. Clearly, clinicians must be aware of the common pathogens that are associated with nosocomial pneumonia in their hospitals so that they can avoid administering inadequate antibiotic therapy. Several researchers have shown that inadequate antimicrobial therapy is an important factor in the emergence of infections due to resistant organisms [33,34]. Factors that contribute to inadequate therapy for hospitalized patients include prior antibiotic exposure, prolonged length of stay, prolonged mechanical ventilation and presence of invasive devices. Clinicians can improve antibiotic therapy, and therefore outcome, in hospitalized patients by using empiric combination antibiotic therapy based on individual patient characteristics, the predominant bacterial flora and their local antibiotic susceptibility profiles. Therapy can then be narrowed once culture results are obtained. The effectiveness of antibiotics to treat nosocomial infections can be preserved by antibiotic cycling – that is, withdrawing an antibiotic or the entire class from use and then reintroducing it at a later time point [35,36].
Table 3.
The Tarragona strategy
| Point | Details |
| 1 | Antibiotic therapy should be started immediately |
| 2 | Antibiotic choice can be targeted, in some cases, based on direct staining |
| 3 | The prescription should be modified in accordance with microbiologic findings |
| 4 | Prolonging antibiotic treatment does not prevent recurrence |
| 5 | Patients with chronic obstructive pulmonary disease or 1 week of intubation should receive combination therapy because of the risk for ventilator-associated pneumonia caused by Pseudomonas aeruginosa |
| 6 | Methicillin-resistant Staphylococcus aureus is not anticipated in the absence of antibiotic exposure, whereas methicillin-sensitive S aureus should be strongly suspected in comatose patients |
| 7 | Therapy against yeast is not required, even in case of colonization with Candida spp. |
| 8 | Vancomycin administration for Gram-positive pneumonias is associated with very poor outcome |
| 9 | The specific choice of agent should avoid the regimen to which each patient has previously been exposed |
| 10 | Guidelines should be updated regularly and customized in accordance with local patterns |
Modified from Sandiumenge and coworkers [41].
The impact antibiotic therapy has on the outcome of VAP has been assessed by several researchers [6,37-39], whose work has become the basis for the concept that inadequate antibacterial therapy is associated with increased mortality rates. In a study conducted by Dupont and coworkers [40], 20 patients were given initial antibiotic therapy immediately after bronchial sampling. If all of the significant organisms were susceptible to at least one of the antibiotics used, then initial therapy was considered to be appropriate. Antibiotic therapy was adapted if necessary when the results of the susceptibility testing were available (48–72 hours later). Dupont and colleagues found that initial antibiotic therapy was appropriate in only half of the patients, but when initial antibiotic therapy was appropriate the patients experienced a shorter stay (12 ± 11 days versus 20 ± 24 days) in the ICU. Mechanical ventilation was also shorter for appropriately treated patients. The pathogens most associated with inappropriate initial treatment were oxacillin-resistant S aureus and P aeruginosa.
Successful treatment of patients with VAP remains difficult and complex. Two factors appear to contribute to the difficulty in selecting antibiotics for critically ill patients. First, VAP is most likely to result from highly resistant organisms, especially in those patients who were previously treated with antibiotics [12]. Second, multiple organisms are frequently cultured from the pulmonary secretions of ventilated patients considered to have acquired pneumonia [32]. Thus, because of the emergence of multiresistant, extended spectrum, lactamase-producing, Gram-negative bacilli in many institutions and the increasing role played by Gram-positive bacteria such as MRSA, even a protocol combining ceftazidime or imipenem and amikacin would not ensure adequate coverage of all cases of VAP in these ICUs. Clearly, there is a need for an agent with effective coverage of both the Gram-positive and Gram-negative pathogens associated with VAP.
The 1996 American Thoracic Society guidelines [16] make recommendations for antimicrobial therapy for VAP based on assessment of disease severity, the presence or absence of risk factors for specific organisms and time of onset of the pneumonia. However, one limitation of these guidelines is that they do not take into account local susceptibilities; given the range of bacteria that cause VAP and that their susceptibilities vary widely among hospitals, selection of initial antimicrobial therapy should be tailored to local patterns of antimicrobial resistance [10,41].
Indeed, heavy use of third-generation cephalosporins and aztreonam has been linked to the emergence of extended-spectrum β-lactamases, with resulting drug resistance issues [42]. Similarly, overuse of the fluoroquinolones, particularly the older class members that have less activity against, for example, S pneumoniae, has led to the emergence of fluoroquinolone-resistant pneumococci [43]. Moreover, a study conducted by Trouillet and coworkers [33] comparing patients who developed VAP caused by piperacillin-resistant P aeruginosa with those who developed piperacillin-sensitive P aeruginosa showed that previous fluoroquinolone use was an independent risk factor for piperacillin-resistant P aeruginosa VAP. Since 2000, two new classes of antibiotics have been approved for the treatment of Gram-positive bacteria: the oxazolidinones (e.g. linezolid) and the cyclic lipopeptides (daptomycin). In US hospitals 50% of S aureus isolates are methicillin resistant and 30% of enterococci are vancomycin resistant, and so new investigational drugs are extremely important. Even more recently introduced agents such as linezolid, which is indicated for the treatment of nosocomial pneumonia caused by MRSA [25,26], is beginning to lose its effectiveness against staphylococci [44].
The investigational new drug tigecycline, the first of a new synthetic class of antibiotics called the glycylcyclines, has an extended broad spectrum of activity that suggests it may be an effective agent for the treatment of VAP [45]. Its MIC90 (concentration at which 90% of isolates are inhibited) values have been shown to be significantly lower than those for vancomycin, linezolid and quinupristin/dalfopristin against clinically important Gram-positive and Gram-negative aerobic bacteria, including S pneumoniae, H influenzae, M catarrhalis, Neisseria gonorrhoeae, most Enterobacteriaceae [including extended spectrum β-lactamase-producing strains], and Enterococcus spp. and S aureus, including methicillin-resistant strains [46]. Against P aeruginosa, tigecycline exhibits modest activity (MIC90 ≥ 8 mg/l) [47]. Of particular importance, tigecycline does not exhibit cross-resistance with other classes of antimicrobial agents [46]. Clinical trials of tigecycline in patients with VAP are awaited.
In addition to developing new antimicrobial agents, alternative approaches to the management of VAP are also being explored. For example, we know that different strains of P aeruginosa have different expressions of virulence. Hauser and coworkers [48] showed that secretion of type III proteins is associated with worse outcomes for patients with VAP caused by P aeruginosa and hypothesized that antibodies targeted against these proteins could be effective in prevention or therapy for VAP in such patients. Similarly, Schulert and coworkers [49] have shown ExoU to be marker of highly virulent strains of P aeruginosa, which again offers the intriguing possibility of immunotherapy for P aeruginosa induced VAP.
Finally, prevention of VAP should be our ultimate goal. Measures to prevent VAP can target invasive devices, such as ensuring adequate pressure in the endotracheal cuff, removal of nasogastric and/or endotracheal tubes, subglottic drainage, oral intubation, drainage of the condensate from the ventilator circuits, and humidification with heat–moisture exchangers. VAP prevention measures should also target the potential pathogens and include hand washing, formal infection control programmes, avoidance of unnecessary antibiotics, and use of routine parenteral antibiotics in comatose patients. Finally, to protect the patient from VAP, health care providers should wear gowns and gloves, provide adequate nutritional support, and limit the magnitude of aspiration by placing patients in a semi-upright position [50].
Conclusion
VAP continues to present a major therapeutic challenge to clinicians, particularly when patient management is complicated by the presence of underlying conditions, which are frequently present. The significant morbidity and mortality associated with VAP require early, appropriate and adequate antimicrobial therapy, ideally with an agent that has good activity against both Gram-positive and Gram-negative pathogens. Ready availability of local patterns of antimicrobial resistance can help physicians in their decision making for empirical therapy, which in turn should improve quality of care and outcomes.
Abbreviations
ICU = intensive care unit; MIC90 = concentration at which 90% of isolates are inhibited; MRSA = methicillin-resistant Staphylococcus aureus; VAP = ventilator-associated pneumonia.
Competing interests
This article was sponsored by an educational grant from Wyeth. JR serves on the advisory board for Wyeth, Merck, Astra-Zenca, Pfizer and Basilea.
Acknowledgments
Acknowledgement
The author wishes to acknowledge the contributions of Susan J Watson and Annie Jones to the preparation of this manuscript.
References
- Craven D, Steger K, Barber T. Preventing nosocomial pneumonia: state of the art and perspectives for the 1990s. Am J Med. 1991;91:44S–53S. doi: 10.1016/0002-9343(91)90343-V. [DOI] [PubMed] [Google Scholar]
- Fein A, Grossman R, Ost D, Farber B, Cassiere H. In Diagnosis and Management of Pneumonia and Other Respiratory Infections. 2. Caddo, OK, USA: Professional Communications, Inc; 2000. Nosocomial or hospital-acquired pneumonia; pp. 125–138. [Google Scholar]
- Baughman R, Tapson V, McIvor A. The diagnosis and treatment challenges in nosocomial pneumonia. Diagn Microbiol Infect Dis. 1999;33:131–139. doi: 10.1016/S0732-8893(98)00161-8. [DOI] [PubMed] [Google Scholar]
- Lode H, Raffenberg M, Erbes R, Geerdes-Fenge H, Mauch H. Nosocomial pneumonia: epidemiology, pathogenesis, diagnosis, treatment and prevention. Curr Opin Infect Dis. 2000;13:377–384. doi: 10.1097/00001432-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH, VAP Outcomes Scientific Advisory Group Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- Rello J, Rue M, Jubert P, Muses G, Sonora R, Valles J, Niederman MS. Survival in patients with nosocomial pneumonia: impact of severity of illness and the etiologic agent. Crit Care Med. 1997;25:1862–1867. doi: 10.1097/00003246-199711000-00026. [DOI] [PubMed] [Google Scholar]
- Fiel S. Guidelines and critical pathways for severe hospital-acquired pneumonia. Chest. 2001;(Suppl):412S–418S. doi: 10.1378/chest.119.2_suppl.412S. [DOI] [PubMed] [Google Scholar]
- Lynch J. Hospital-acquired pneumonia: risk factors, microbiology, and treatment. Chest. 2001;(Suppl):373S–384S. doi: 10.1378/chest.119.2_suppl.373S. [DOI] [PubMed] [Google Scholar]
- Torres A, Serra-Batlles J, Ros E, Piera C, Puig de la Bellacasa J, Cobos A, Lomena F, Rodriguez-Roisin R. Pulmonary aspiration of gastric contents in patients receiving mechanical ventia-tion: the effect of body position. Ann Intern Med. 1992;116:540–543. doi: 10.7326/0003-4819-116-7-540. [DOI] [PubMed] [Google Scholar]
- Rello J, Sa-Borges M, Correa H, Leal SR, Baraibar J. Variations in etiology of ventilator-assocatied pneumonia across four treatment sites: implications for antimicrobial prescribing paractices. Am J Respir Crit Care Med. 1999;160:608–613. doi: 10.1164/ajrccm.160.2.9812034. [DOI] [PubMed] [Google Scholar]
- Ibrahim E, Ward S, Sherman G. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000;177:1434–1442. doi: 10.1378/chest.117.5.1434. [DOI] [PubMed] [Google Scholar]
- Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, Gibert C. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- Johanson W, Pierce A, Sanford J. Changing pharyngeal bacterial flora of hospitalized patients: emergence of Gram-negative bacilli. N Engl J Med. 1969;281:1137–1140. doi: 10.1056/NEJM196911202812101. [DOI] [PubMed] [Google Scholar]
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- Kollef M. Ventilator-associated pneumonia: a multivariate analysis. JAMA. 1993;270:1965–1970. doi: 10.1001/jama.270.16.1965. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventative strategies. Am Rev Resp Crit Care Med. 1995;153:1711–1725. doi: 10.1164/ajrccm.153.5.8630626. [DOI] [PubMed] [Google Scholar]
- Rello J, Diaz E, Roque M, Valles J. Risk factors for developing pneumonia within 48 hours of intubation. Am J Respir Crit Care Med. 1999;159:1742–1746. doi: 10.1164/ajrccm.159.6.9808030. [DOI] [PubMed] [Google Scholar]
- Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, Celis R, Rodriguez-Roisin R. Incidence, risk, and prognostic factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- Spencer R. Predominant pathogens found in the European Prevalence of Infection in Intensive Care Study. Eur J Clin Microbiol Infect Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- Goetz A, Yu V. Screening for nosocomial legionellosis by culture of the water supply and targeting of high-risk patients for specialized laboratory testing. Am J Infect Control. 1991;63:458–463. doi: 10.1016/0196-6553(91)90040-j. [DOI] [PubMed] [Google Scholar]
- Valles J, Mesalles E, Mariscal D, del Mar Fernandez M, Pena R, Jimenez JL, Rello J. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Int Care Med. 2003;29:1981–1988. doi: 10.1007/s00134-003-2008-4. [DOI] [PubMed] [Google Scholar]
- Antonelli M, Moro ML, Capelli O, De Blasi RA, D'Errico RR, Conti G, Bufi M, Gasparetto A. Risk factors for early-onset pneumonia in trauma patients. Chest. 1994;105:224–228. doi: 10.1378/chest.105.1.224. [DOI] [PubMed] [Google Scholar]
- Kono K, Arakawa K. Methicillin-resistant Staphylococcus aureus (MRSA) isolated in clinics and hospitals in the Fukuoka city area. J Hosp Infect. 1995;29:265–273. doi: 10.1016/0195-6701(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid versus vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. doi: 10.1378/chest.124.5.1789. [DOI] [PubMed] [Google Scholar]
- Rello J, Torres A, Ricart M, Valles J, Gonzalez J, Artigas A, Rodriguez-Roisin R. Ventilator-associated pneumonia by Staphylococcus aureus : comparison of methicillin-resistant with methicillin-sensitive episodes. Am J Respir Crit Care Med. 1994;150:1545–1549. doi: 10.1164/ajrccm.150.6.7952612. [DOI] [PubMed] [Google Scholar]
- Kollef MH, Rello J, Cammarata SK, Croos-Dabrera RV, Wunderink RG. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Int Care Med. 2004;30:388–394. doi: 10.1007/s00134-003-2088-1. [DOI] [PubMed] [Google Scholar]
- Bodi M, Ardanuy C, Rello J. Impact of Gram-positive resistance on outcome of nosocomial pneumonia. Crit Care Med. 2001;(Suppl):N82–N86. doi: 10.1097/00003246-200104001-00005. [DOI] [PubMed] [Google Scholar]
- Chastre J, Fagon J. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- Wunderink R, Woldenberg L, Zeiss J. The radiologic diagnosis of autopy-proven ventilator-associated pneumonia. Chest. 1992;101:458–463. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- Bell RC, Coalson JJ, Smith JD, Johanson WG., Jr Multiple organ system failure and infection in adult respiratory distress syndrome. Ann Intern Med. 1983;99:293–298. doi: 10.7326/0003-4819-99-3-293. [DOI] [PubMed] [Google Scholar]
- Andrews CP, Coalson JJ, Smith JD, Johanson WG., Jr Diagnosis of nosocomial bacterial pneumonia in acute, diffuse lung injury. Chest. 1981;80:254–258. doi: 10.1378/chest.80.3.254. [DOI] [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, Gibert C. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quanitative culture techniques. Am Rev Respir Dis. 1989;139:877–884. doi: 10.1164/ajrccm/139.4.877. [DOI] [PubMed] [Google Scholar]
- Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C. Pseudomonas aeruginosa ventilator-associated pneumonias: comparson of episodes due to piperacillin-resistant versus piperacillin-sensitive organisms. Clin Infect Dis. 2002;34:1047–1054. doi: 10.1086/339488. [DOI] [PubMed] [Google Scholar]
- Kollef M. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalised patients. Clin Infect Dis. 2000;(Suppl 4):S131–S138. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- Gruson D, Hilbert G, Vargas F, Valentino R, Bebear C, Allery A, Bebear C, Gbikpi-Benissan G, Cardinaud JP. Rotation and restricted use of antibiotics in a medical intensive care unit. Am J Respir Crit Care Med. 2000;162:837–843. doi: 10.1164/ajrccm.162.3.9905050. [DOI] [PubMed] [Google Scholar]
- Bantar C, Vesco E, Heft C, Salamone F, Krayeski M, Gomez H, Coassolo MA, Fiorillo A, Franco D, Arango C, et al. Replacement of broad-spectrum cephalosporins by piperacillin-tazobactam: impact on sustained high rates of bacterial resistance. Antimicrob Agents Chemother. 2004;48:392–395. doi: 10.1128/AAC.48.2.392-395.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lerma F. Modification of empiric antibiotic therapy in patients with pneumonia acquired in the intensive care unit. Intensive Care Med. 1996;22:387–394. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly EC. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997;111:676–685. doi: 10.1378/chest.111.3.676. [DOI] [PubMed] [Google Scholar]
- Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001;27:355–362. doi: 10.1007/s001340000640. [DOI] [PubMed] [Google Scholar]
- Sandiumenge A, Diaz E, Bodi M, Rello J. Treatment of ventilator-associated pneumonia: a patient-based approach based on the rules of the 'Tarragona strategy'. Intensive Care Med. 2003;29:876–883. doi: 10.1007/s00134-003-1715-1. [DOI] [PubMed] [Google Scholar]
- Gold H, Moellering R. Antimicrobial drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- Chen DK, McGeer A, de Azavedo JC, Low DE. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet. 2001;358:207–208. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- Chopra I. Glycylcyclines: third-generation tetracycline antibiotics. Curr Opin Pharmacol. 2001;1:464–469. doi: 10.1016/S1471-4892(01)00081-9. [DOI] [PubMed] [Google Scholar]
- Biedenbach DJ, Beach ML, Jones RN. In vitro antimicrobial activity of GAR-936 tested against antibiotic-resistant gram-positive blood stream infection isolates and strains producing extended-spectrum beta-lactamases. Diagn Microbiol Infect Dis. 2001;40:173–177. doi: 10.1016/S0732-8893(01)00269-3. [DOI] [PubMed] [Google Scholar]
- Gales AC, Jones RN. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn Microbiol Infect Dis. 2000;36:19–36. doi: 10.1016/S0732-8893(99)00092-9. [DOI] [PubMed] [Google Scholar]
- Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein sectretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Schulert GS, Feltman H, Rabin SD, Martin CG, Battle SE, Rello J, Hauser AR. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188:1695–1706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- Rello J, Diaz E. Pneumonia in the intensive care unit. Crit Care Med. 2003;31:2544–2551. doi: 10.1097/01.CCM.0000089928.84326.D2. [DOI] [PubMed] [Google Scholar]
- Rello J, Torres A. Microbial causes of ventilator-associated pneumonia. Semin Respir Infect. 1996;11:24–31. [PubMed] [Google Scholar]


