Abstract
Abdala is a COVID-19 vaccine produced in Pichia pastoris and is based on the receptor-binding domain (RBD) of the SARS-CoV-2 spike. Abdala is currently approved for use in multiple countries with clinical trials confirming its safety and efficacy in preventing severe illness and death. Although P. pastoris is used as an expression system for protein-based vaccines, yeast glycosylation remains largely uncharacterised across immunogens. Here, we characterise N-glycan structures and their site of attachment on Abdala and show how yeast-specific glycosylation decreases binding to the ACE2 receptor and a receptor-binding motif (RBM) targeting antibody compared to the equivalent mammalian-derived RBD. Reduced receptor and antibody binding is attributed to changes in conformational dynamics resulting from N-glycosylation. These data highlight the critical importance of glycosylation in vaccine design and demonstrate how individual glycans can influence host interactions and immune recognition via protein structural dynamics.
Keywords: Abdala, Glycomics, mass photometry, SARS-CoV-2, vaccine
Introduction
As of the end of 2023, the severe acute respiratory syndrome (SARS) coronavirus (CoV)-2 outbreak was attributed to over 7 million deaths worldwide by the World Health Organisation (WHO). The rapid development, approval and deployment of multiple COVID-19 vaccines was critical for limiting viral spread and deaths, with over 13 billion vaccine doses administered to date. The major SARS-CoV-2 immunogen and neutralisation target for vaccine design is the surface “spike” glycoprotein, which recognises host cells via binding between the spike (S) receptor-binding domain (RBD) and the human angiotensin-converting enzyme-2 (ACE2) (Yan et al 2020). The use of full-length S trimers as immunogens required extensive protein engineering for stabilisation of pre-fusion states that resulted in altered glycosylation, protein dynamics and overall antigen structural integrity compared to spike trimers on infectious virions (Brun et al 2021; Burnap and Struwe 2022).
The SARS-CoV-2 spike is a trimeric class I fusion glycoprotein comprised of an S1 outer domain, containing the RBD and N-terminal domain (NTD), and an S2 domain responsible for host membrane fusion. Spikes are heavily glycosylated, with 22 N-glycosylation sites per monomer, equating to 66 N-glycans per S trimer with each RBD domain containing 2 N-glycans, N331 and N343.Glycans at positions N343 (RBD), N234 (NTD) and N165 (NTD) have been shown to modulate protein structural states through “up” and “down” RBD dynamics (Casalino et al 2020; Sztain et al 2021; Ives et al 2024). Varying O-glycosylation has been reported, with a known functional role in modulation of furin cleavage (Shajahan et al 2020; Sanda et al 2021; Zhang et al 2021; Gonzalez-Rodriguez et al 2023). Glycosylation is therefore particularly important for cellular processing and functional dynamics of COVID immunogens.
Effective worldwide vaccination relies on distributable and cost-effective production of stable vaccines. The Abdala COVID-19 vaccine, produced by the Center for Genetic Engineering and Biotechnology in Cuba (Limonta-Fernández et al 2022), is based on a recombinantly produced RBD subunits expressed in Pichia pastoris, a yeast system previously used for vaccines, including SARS-CoV, hepatitis B and other SARS-CoV-2 candidates (W. H. Chen et al 2014; Chen et al 2017; Chen et al 2020; Chen et al 2022; Cregg et al 1987). Clinical trials of Abdala showed it to be cost-effective, stable, safe and efficacious, fulfilling the WHO criteria for COVID-19 vaccines, and Abdala is currently authorised for emergency use in multiple countries (Hernández-Bernal et al 2022; Más-Bermejo et al 2022; Hernández et al 2023).
Glycoproteins produced in P. pastoris have different glycan structures to those found on circulating infectious viruses as well as recombinantly produced spikes or RBDs from mammalian systems, such as Chinese hamster ovary (CHO) or human embryonic kidney (HEK) cell lines (Bretthauer and Castellino 1999). In contrast to mammalian glycosylation, yeast glycans are generally high-mannose structures extending from a Man8GlcNAc2 core (Hamilton and Gerngross 2007). Therapeutic glycoproteins from P. pastoris remain largely uncharacterised at the glycan level but are recognised as important for vaccine immunogenicity and/or half-life, particularly through mannose-dependent clearance mechanisms, which has led to efforts for glycoengineering P. pastoris to produce human complex-type N-glycans (Lee et al 2002; Choi et al 2003; Jacobs et al 2009). P. pastoris have hyper-mannosylated glycans, however the extent of mannosylation, degree of N-glycan phosphorylation and precise structural information of the individual glycans and their protein site of attachment are currently undefined for Abdala. Equally, the effect of yeast glycosylation and comparisons with mammalian-derived RBD in antibody and receptor binding, which is critical for immunogenicity and overall vaccine efficacy, are unknown.
Here, we combine mass spectrometry (MS) methods in glycomics, glycoproteomics and hydrogen-deuterium exchange (HDX) to provide a comprehensive structural and dynamical understanding of the Abdala RBD vaccine. Using mass photometry (MP), a label-free single molecule imaging method (Soltermann et al 2020; Burnap and Struwe 2022), we quantify binding to ACE2 and an RBD-targeting antibody. Comparison with HEK cell derived RBD, we show how differences in site-specific glycosylation affect antibody and receptor binding that arise from conformational dynamics of the receptor binding motif (RBM) within the RBD glycoprotein. Taken together, these data represent the first full site-specific glycan characterisation of a structure-based vaccine from P. pastoris that uncovers how subtle changes in RBD glycosylation can shape immunogen functionality.
Results
Batch-to-batch characterisation of Abdala N-glycosylation
SDS-PAGE of Abdala and HEK-derived RBD (RBDHEK), showed Abdala to exhibit a broader mass distribution that migrated at a higher molecular weight, between 40 and 70 kDa, compared to RBDHEK which migrated at approximately 37 kDa (Fig. 1A). The theoretical masses are 25.9 kDa for RBDHEK and 26.1 kDa for Abdala, and following PNGaseF treatment both proteins had similar migration patterns, confirming differences in observed mass are from N-glycosylation. Additionally, the measured mass by MP confirmed a difference of approximately 11 kDa between RBDHEK (37 kDa) and three separate production batches of Abdala (48 ± 1 kDa) (Fig. 1B, Supplementary Fig. S1). MP also showed a minor population of dimeric species for both RBDHEK and Abdala, at approximately 19.6% and 5.6%, respectively. Dimer formation was consistently lower across three batches of Abdala compared to RBDHEK (Supplementary Fig. S1) but was not detected by size exclusion chromatography. Our results were consistent with a previous report that detected dimerization of HEK derived RBD using native MS (Roberts et al 2021). To quantify N-glycosylation across Abdala batches, we analysed fluorescently labelled N-glycans via high performance liquid chromatography (HPLC) (Fig. 1C) (Burnap and Struwe 2022). As a control, we included a glycoengineered form of RBD that was recombinantly expressed in the presence of kifunensine to contain predominantly Man9GlcNAc2N-glycans, which assists preliminary assignments of Abdala. Expectedly, Abdala profiles were distinct from kifunensine-derived RBD as N-glycans eluted later than the major Man9GlcNAc2 peak, indicative of hyper-mannosylated structures. RBDHEK N-glycan profiles contained 11 major N-glycan peaks all eluting earlier than Man9GlcNAc2 (<26 min), suggestive of complex-type N-glycans (Supplementary Fig. S2A). Batch-to-batch variation was minimal across the 9 major N-glycan HPLC peaks, with a mean coefficient of variation equal to 22%. The relative abundances of peaks 4 and 6 varied most across the three batches (Fig. 1C, Supplementary Fig. S2B, Supplementary Table S1).
Fig. 1.

HPLC-based analysis of Abdala N-glycans. (A) SDS-PAGE of RBDHEK and three separate production batches of Abdala +/− PNGaseF digestion. (B) Mass photometry of RBDHEK and Abdala. (C) HPLC of N-glycans from three production batches of Abdala and a kifunensine-derived RBD control.
N-glycan structural analysis by ion mobility-MS/MS
Ion mobility (IM)-MS/MS of Abdala N-glycans revealed detailed information of their hyper-mannosylated structures as well as the extent of phosphorylation (Fig. 2A). N-glycan ions were predominantly doubly charged and were detected as deprotonated [M-2H]2− species. The mass difference between each peak was ~81 m/z, corresponding to a hexose (presumably mannose) residue (162 Da). The overall monosaccharide compositions correspond to Man9-16GlcNAc2Phos2 and their proposed structures are schematically represented in the spectrum. The MS spectra of the three Abdala batches were consistent and it underscored batch-to-batch production consistency with near identical glycan peak distribution and abundances (Supplementary Fig. S3A). One notable difference was that Abdala batch 1 had a greater Man10/Man11 ratio compared to batches 2 and 3, which indicates HPLC peaks 4 and 6 are likely Man10GlcNAc2Phos2 and Man11GlcNAc2Phos2, respectively.
Fig. 2.
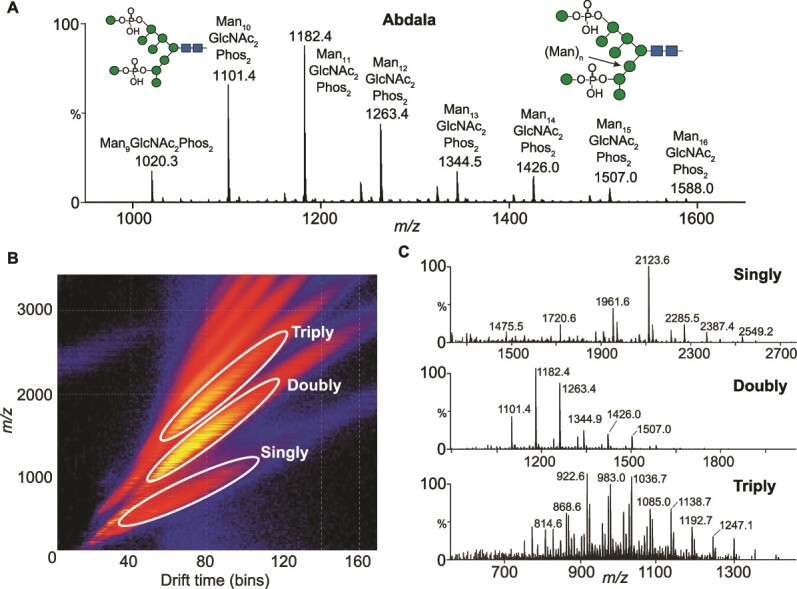
IM-MS of Abdala N-glycans. (A) MS spectrum of Abdala N-glycans (batch 1). (B) Representative drift plot of Abdala N-glycans showing the distribution of singly, doubly and triply charged ions. (C) Corresponding extracted MS spectra of singly, doubly and triply charged N-glycans. Glycan compositions and ion assignments are annotated in supplementary tables 2–5.
IM separation (Fig. 2B) and extraction of singly, doubly and triply charged ions revealed additional structural information, specifically the presence of glycans with 1–3 phosphate groups and up to 22 mannose residues. Singly charged ions had a major series of Man5-14GlcNAc2Phos1 detected as [M–H]− ions and a minor series of Man9-13GlcNAc2Phos2 as [M–H + Na]− (Fig. 2C (top spectrum), Supplementary Table S2). The major N-glycan ion was 2123.6, which is Man10GlcNAc2Phos1, followed by Man9GlcNAc2Phos1 (1961.6 m/z) and Man11GlcNAc2Phos1 (2285.5 m/z). IM extraction of doubly charged ions uncovered additional mannose residues beyond those shown in Fig. 2a, specifically N-glycans with 2 phosphates and up to 21 mannose residues (Man9-21GlcNAc2Phos2). Ions were detected as [M–2H]2− and/or [M–H + Na + H2PO4]2− species (Fig. 2C (middle spectrum), Supplementary Table S3). The spectrum of doubly charged ions is similar to the MS spectra of all N-glycans shown in Fig. 2A, as Man9-16GlcNAc2Phos2 are the major N-glycan structures present on Abdala. Triply charged ions were predominantly Man10-22GlcNAc2Phos3 N-glycans that were detected as [M – 3H]3−, [M – 2H + H2PO4]3− and [M – 2H + Na + H2PO4]3− ions (Fig. 2C bottom spectrum, Supplementary Table S4). A minor amount of N-glycans with two phosphates were also detected as Man11-19GlcNAc2Phos2. Overall, Abdala glycans were Man5-22GlcNAc2, with the dominant species being di-phosphorylated species (Supplementary Table S5).
MS-based fragmentation revealed structural information of the underlying glycan (representative MS/MS spectra from Abdala batch 1 are shown). Collision induced dissociation of all N-glycans resulted primarily in the neutral loss of mannose residues with minimal diagnostic A-type cross-ring fragments (Fig. 3). One cross-ring fragment was found, specifically 0,2AR of the reducing end GlcNAc. Fragmentation across glycan species revealed similar spectra, with all ions increasing by the mass of mannose residues, suggestive of the core structure of all compounds to be the same (Man10-14GlcNAc2Phos2). Depicted structures were based on a core of Man8GlcNAc2 with further mannose residues being added to the 3-antenna (Byrd et al 1982). According to the literature, a small percentage structures appear to have additional α1 → 2-linked mannose residues attached to each antenna but this was not confirmed in our study (Gemmill and Trimble 1999). The positions of the mannosyl-phosphate groups in yeasts appear to be as shown in Fig. 3 and fragment ions at m/z 259 and 241 (Supplementary Fig. S3B) in our spectra are consistent with the presence of mannose-phosphate groups (Jigami and Odani 1999).
Fig. 3.

Fragmentation spectra of Abdala N-glycans. MS/MS fragmentation spectra of Man10-14GlcNAc2Phos2 N-glycans showing characteristic loss of mannose and N-acetylglucosamine (GlcNAc) residues. Proposed N-glycan structures are shown. Precursor ions are [M – 2H]2− species.
IM-MS analysis was also conducted on RBDHEK N-glycans. The total acquired MS spectra (Supplementary Fig. S4A), as well as mobility extracted doubly (Supplementary Fig. S4B) and triply (Supplementary Fig. S4C) charged ions were primary doubly charged complex type N-glycans, specifically fucosylated, bi and tri-antennary structures with terminal sialyation, with Hex5GlcNAc4Fuc1NeuAc2 as the most abundant glycan (Supplementary Fig. S4B). IM extraction of triply charged ions revealed a minor presence of tetra-antennary, complex glycans with varying levels of terminal sialyation (Supplementary Fig. S4C). While multiple studies have performed glycan analyses on recombinant full-length spikes or isolated S1 domains, glycoproteomics has been the method of choice to characterise RBD N-glycosylation (Gstöttner et al 2021; Hsu et al 2022). Our IM-MS analysis of RBDHEK aligns with previous bottom-up glycoproteomic studies that show RBD N-glycans as primarily bi- or tri-antennary with varying degrees of sialyation. Glycan analysis via IM-MS revealed a greater degree of sialyation than previously shown, potentially due to a reduced loss of labile sialic acids during ionisation of glycopeptides.
Site-specific N- and O-glycan analysis
Having characterised Abdala and RBDHEKN-glycans, we next identified their sites of attachment via bottom-up glycoproteomics. It is important to note that both Abdala and RBDHEK contain amino acids 319–541 present on the native Wuhan-Hu-1 SARS-CoV-2 virus, but the Abdala immunogen contains additional sequences at both termini (Limonta-Fernández et al 2022) (Fig. 4A). There are two N-glycan sites in this region, N331 and N343 which are distal to the RBM region responsible for ACE2 binding. The additional Abdala N-terminal sequence is NWSFFSNIGGSSGGS, which is flexible and polar, and added to prevent protein aggregation (Limonta-Fernández et al 2022). This introduces an additional N-glycan sequon (N-X-S/T, X ≠ P) at the first residue, NWS. The Abdala C-terminal sequence is GGSGGSSSSSSSSSSIEHHHHHH and was added to improve His-tag protein purification (Limonta-Fernández et al 2022). Both Abdala and RBDHEK have the endogenous N331 and N343 N-glycan glycosylation sites. Furthermore, the spike RBD is known to have varying levels of O-glycosylation, with a report showing up to 8 glycoforms at T323 with a core 1, di-sialyated O-glycan as the most abundant structure (Roberts et al 2021). P. pastoris has a distinct type of protein O-glycosylation in the form of O-mannosylation, where up to 6 mannose residues can be added primarily to S/T residues, primarily in α1,2 linkages (Radoman et al 2021). Importantly, O-mannosylation is not present on human-derived glycoproteins, and has been shown to enhance antigen immunogenicity specifically through CD4+ T cell responses, highlighting the importance of characterising the extent of O-glycosylation in addition to N-glycosylation on Abdala (Luong et al 2007).
Fig. 4.
Glycoproteomic characterisation of Abdala. (A) Schematic representation of Abdala amino acid sequence, highlighting N- and C-terminal regions, RBM and the major N- and O-glycans identified from this study. (B) Pie charts reflecting site-specific glycosylation analysis by glycoproteomics. Glycans are characterised by the number of mannose residues and phosphate groups. (C) MS/MS spectra of a glycopeptide containing Man13GlcNAc2Phos2 at N343 (top). O-mannosylation at position S469 (middle) and O-mannosylation in the C-terminal extension (bottom). (D) AlphaFold model of Abdala showing the location of N-glycans, RBM and Abdala N- and C-termini.
Consistent with IM-MS data of released N-glycans, glycoproteomics of the three Abdala batches identified Man9-15GlcNAc2 with between 0–2 phosphate groups as the major species at N343 (Fig. 4B, green pie charts). The majority glycans contained two phosphates (56%) followed by one (33%) and zero (11%) (Fig. 4B, orange pie charts). Consistent with the HPLC data, glycoproteomics identified more similarity between batches 2 and 3 than batch 1. Opposed to glycan MS, the bottom-up analysis did not detect glycopeptides containing three phosphate groups, which is most likely due to their low abundance and poor detection efficiency. The presence of phosphorylated glycans was evidenced by 243 m/z (HexPhos) and 405 m/z (Hex2Phos) oxonium ion fragments in the MS/MS spectra (Fig. 4C, top spectrum). Chymotrypsin, alpha-lytic or ProAlanase proteases were predicted to generate theoretical peptides spanning N331 with Abdala, but we did not identify any glycopeptides spanning this site, nor did we detect the equivalent non-glycosylated peptide(s). The final N-glycosylation site at the N-terminal sequon (NWS) was only detected as unoccupied (Supplementary Fig. S5A). On the other hand, analysis of RBDHEK identified complex-type glycans at positions N331 and N343, consisting of bi-, tri- and tetra-antennary structures (Supplementary Fig. S6A). While bi- and tri-antennary glycans were also most abundant in the IM-MS analysis, a lesser degree of sialyation was observed by glycoproteomics with the most abundant glycoform harbouring a single sialic acid (Supplementary Fig. S6B). We did not detect any non-glycosylated peptides spanning both N331 and N343 sites and contrary to a previous report we only identified one O-glycan at position T430 on RBDHEK (Supplementary Fig. S6B) (Roberts et al 2021).
Multiple O-mannosylated peptides were observed on Abdala and their presence was evidenced by hexose oxonium ion fragments (162 m/z, with and without the loss of H2O) within the MS/MS spectra. Two mannose residues were identified at S469/T470 (Fig. 4C, middle spectrum), while one mannose residue was identified at position T393 (Supplementary Fig. S5B). A longer Man6 glycan was detected within the C-terminal region of Abdala (SSIEHHHHHH), highlighting a previously unknown site arising from the addition of a repeating serine sequence (Fig. 4C, bottom spectrum). Importantly, O-mannosylated peptides were also observed in a non-glycosylated state with varying degrees of glycan occupancy. To understand the extent of glycan occupancy, the abundances of non-glycosylated peptides were summed and made relative to their glycosylated counterparts. The O-glycan sites had different degrees of occupancy: T393 was <1% occupied, S469/T470 were approximately 2.1% occupied while the Man6 glycan at S544 within the C-terminal region SSIEHHHHHH had an occupancy of 12.7%. Overall, O-mannosylation is relatively low on Abdala.
Quantifying receptor and antibody binding by mass photometry
Glycomics and glycoproteomics show clear differences between Abdala and RBDHEK, including the presence of O-mannosylation. Although RBD N-glycans are distal to the ACE2 binding interface, molecular dynamics simulations have shed light on the role N343 plays in maintaining RBD structural integrity via shielding of the hydrophobic RBD core (Ives et al 2024). Secondly, it is thought that N343 can influence opening and closing of the RBD in the context of pre-fusion spike trimers (Sztain et al 2021). The extent of glycan-driven structural change in isolated RBD as a result of the presence of hyper-mannosylated N-glycans is unknown. Quantifying ACE2 binding would inform whether the structural integrity (i.e. protein folding) of Abdala is maintained and comparable to RBDHEK.
Elicitation of high-affinity, neutralising antibodies in response to Abdala is critical for vaccine efficacy. The primary mode of SARS-CoV-2 neutralisation is through direct interaction between antibodies and the RBM, through similar contact residues with ACE2 binding (Chen et al 2023; Shitaoka et al 2023). Class 3/4 neutralising antibodies however, defined through binding distal to the RBM, have distinct neutralisation mechanisms with mAb S309 as a notable example that makes direct contact with the N343 glycan (Chen et al 2023; Shitaoka et al 2023). Due to Abdala glycans being greatly different to those derived from mammalian culture, and glycans found on the spike of live virus, antibodies targeting Abdala glycan epitopes are unlikely to be effective in combatting SARS-CoV-2 infection. A quantitative understanding of binding between Abdala and RBDHEK is important for understanding possible differences in functionality between glycovariants, in particular testing if phosphorylated oligomannose N-glycans or O-mannosylation alters receptor or antibody binding.
Here, we us MP to quantify binding affinities (Kd values) via single molecule counting, as we have done previously (Soltermann et al 2020; Burnap and Struwe 2022; Kofinova et al 2024). MP has the advantage over conventional biophysical methods in its ability to quantify each Kd in a multivalent interaction, which are characteristic of IgGs. To ensure accurate quantification, all proteins were first measured individually to confirm the concentration (particle counts), expected mass distribution (monomer vs. oligomers) and purity (Supplementary Figs S1 and S7A). Prior to each measurement, proteins were mixed at a 1:1 molar ratio and measured after 5 minutes incubation at room temperature. We first calculated the dissociation constant between Abdala and a monomeric form of ACE2 (mACE2). Abdala bound mACE2 with a Kd = 55.5 ± 6.1 nM, which was 60% weaker than RBDHEK (Kd = 22 ± 1.8 nM (Fig. 5A, Supplementary Fig. S7B). The interaction between RBD and mACE2 can also be expressed in terms of occupancy whereby the sum of all mACE2 counts, including species with 0 and 1 RBD molecule bound, is compared to the total counts of RBD-bound species. RBDHEK exhibited a binding occupancy of 33% compared to Abdala at 24% (Fig. 5A, Supplementary Fig. S7C).
Fig. 5.

Quantifying Abdala interactions with mass photometry. (A) MP RBDHEK and Abdala with monomeric ACE2 (mACE2) at a 1:1 molar ratio. (B) MP of RBDHEK and Abdala with a receptor-binding motif targeting antibody at a 1:1 molar ratio.
To determine whether Kd differences were consistent in the context of antibody binding, we tested an RBM targeting monoclonal antibody, which is currently under investigation (Supplementary Fig. S7D). Each IgG bound two RBDs when mixed and measured at an equimolar ratio following equilibration at room temperature. In line with the ACE2 results, the antibody bound Abdala with weaker affinities compared to RBDHEK, with an average percent occupancy of bound mAb compared to free mAb equal to 82% (Abdala) compared to 89% (Supplementary Figs S7E and F). For RBDHEK, the first Kd1 was 0.34 ± 0.07 nM (mAb1:RBD1) with the second Kd2 = 0.72 ± 0.11 nM (mAb1:RBD2). Abdala had a Kd1 = 0.76 ± 0.12 nM (mAb1:RBD1), which is 55% weaker compared to mAb1:RBD1HEK. The second interaction (mAb1:RBD2) was an order of magnitude weaker (Kd2 = 2.01 ± 0.44 nM) but still in the low nM range (Fig. 5B).
Probing RBD dynamics by HDX-MS
We compared dynamics of Abdala and RBDHEK by following the HDX of approximately 80 peptides spanning 83% of the protein sequences with a redundancy of 5.8, including the O-mannosylated peptides (S469/T470 and S543/544) (Supplementary Fig. S8). We tracked HDX at 4 °C, 23 °C and 28 °C and over 5 time points (10s, 100 s, 1000s, 10,000 s and 9 h) (Supplementary Table S6 and S7). We observed an increase in HDX with Abdala that arises from an increase in flexibility, dynamics and solvent accessibility, specifically regions spanning residues 349–361, 407–415 and 454–471 (Fig. 6A). Conversely, a decrease in HDX was observed between residues 472–487 indicating conformational restriction and reduced accessibility compared to RBDHEK (Supplementary Figs S9 and S10). Mapping the effects on the structure of the RBD-ACE2 complex (pdb:6M0J), reveals how Abdala N-glycans induce a destabilization of the adjacent protein segment (+20% of relative fractional uptake (RFU)), and the long-range allosteric propagation of destabilizing effects results in the rigidification of the RBM segment that directly engages ACE2 (−25% RFU) Notably, decreased HDX in the RBM was observed across multiple time intervals (Fig. 6B). Overall, the reduced HDX in the Abdala RBM supports the observed reduction in binding to ACE2 and an RBM-targeting mAb compared to RBDHEK by MP.
Fig. 6.

Differences in conformational dynamics probed by HDX-MS. (A) Protein regions with differences in HDX superimposed onto the crystal structure of RBD with ACE2 (pdb:6M0J). Red and pink indicate areas with increased dynamics (HDX) and blue indicates reduced dynamics (HDX) of Abdala compared to RBDHEK. N-glycan positions are indicated by asterisks. RBD-ACE2 interface with RBD contact residues (inset). (B) Deuterium uptake plots of peptides spanning regions with differences in HDX (colour coded as in panel A).
Discussion
Here, we characterise the glycosylation of the COVID-19 vaccine Abdala which uncovers distinct structures, namely phosphorylated hyper-mannosylated N-glycans and the presence of O-mannosylation. Despite the distance of these glycan sites to the RBM, we quantify how differences in glycosylation have a tractable effect in ACE2 binding as well as a neutralising IgG antibody as compared to the mammalian-derived equivalent RBD. Variations in binding affinities can be attributed to changes in protein conformational dynamics, which we believe to be caused by P. pastoris N- and O-glycosylation. While P. pastoris derived glycans are vastly different to the glycan structures observed on mammalian-derived proteins, and therefore glycans found on virions, Abdala has been shown to be efficacious in reducing severe illness and death caused by SARS-CoV-2.
Although Abdala has hyper-mannosylated, primarily phosphorylated Man9-16GlcNAc2 structures, it remains a viable immunogen. To this point, separate parallel efforts by the Argentinian AntiCovid Consortium are ongoing that also uses the RBD subunit expressed in P. pastoris as a vaccine candidate. It was recently demonstrated that an enzymatically deglycosylated RBD from P. pastoris elicited a 10-fold greater anti-RBD IgG titre in a preclinical mouse model, which plausibly results from altered protein half-life (Idrovo et al 2023). The effect of yeast glycans on protein half-life are well known, primarily driven by the action of carbohydrate receptors, specifically mannose-binding, promoting glycoprotein clearance (Stahl et al 1976; Lee et al 2002; Cummings 2022). In our hands, both PNGaseF and EndoH treatment of the Abdala vaccine led to evident protein precipitation (data not shown), highlighting the expected role that glycans play in protein solubility (Solá and Griebenow 2009). Abdala is stable up to 25–37 °C as demonstrated in an accelerated thermal stress study conducted at five temperatures for 15 days (Izquierdo et al 2022). Protein stability was evaluated by circular dichroism spectroscopy analysis, mass spectrometry (including disulfide bond assignment), functionality by immunogenicity assays and inhibiting RBD-ACE2 binding (Solá and Griebenow 2009). It is reasonable that glycosylation contributes to these results.
The observation that Abdala binds ACE2 and an IgG with lower affinities was not expected based on the view that N- and O-glycans are not close to the RBM, a region that shares 100% sequence homology between Abdala and RBDHEK. Although the measure Kd values with MP are all in the nM range, Abdala binding to mACE2 and IgG was approximately twice as weak compared to RBDHEK. We cannot rule out these differences arise from the additional C- and N-termini, in addition to changes in N-/O-glycosylation. We postulated changes in binding at the RBM is driven by altered protein structural dynamics within the core RBD. This hypothesis was influenced by molecular dynamics simulations showing the RBD N343 glycan, which is amphipathic in nature when mammalian-derived, is integral to preserving structural integrity of the RBD hydrophobic core. Loss of this glycan was shown to consistently trigger conformational change (Ives et al 2024). It could therefore be envisaged that replacement of a complex, amphipathic glycan on RBDHEK, with a larger phosphorylated hyper-mannosylated structure would disrupt the structural integrity of the RBD core in a similar manner.
Our HDX-MS results indeed uncovered regions of Abdala with greater deuterium uptake in regions proximal to the beta-sheet core, suggestive of a destabilizing effect, that propagates to the RBM, explaining differences in ACE2 and IgG binding affinities. The impact of changes in N-glycosylation on protein structure and dynamics, as tracked by HDX-MS, has also been exemplified in other proteins, highlighting N-glycosylation to regulate long-range protein conformational dynamics, impacting ligand binding and enzymatic activity (Lee et al 2020; Whittington et al 2024). Alterations in protein dynamics in regions distal to N-glycans within the RBD N-terminus suggests allosteric effects from P. pastoris N-glycosylation that are transmitted to regions directly engaging with ACE2 and the RBM-targeting mAb investigated in our study.
In conclusion, Abdala glycosylation modulates protein structural dynamics and binding to RBM targeting proteins. While N-glycan differences modulate Abdala protein function in the context of receptor and antibody binding, neutralising epitopes are preserved as evidenced by clinical trials validating Abdala to be efficacious in eliciting cross-neutralising antibody responses and reducing severe illness and death caused by SARS-CoV-2.
Materials and methods
Protein expression
Vector pCAGGS containing the SARS-CoV-2, Wuhan-Hu-1 Spike Glycoprotein receptor binding domain (RBD) was a kind gift from the Krammer Laboratory, Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York. pHL-sec vector encoding C-terminally His-tagged monomeric (a.a 19–611) ACE2 was a kind gift from the Zitzmann Laboratory, Department of Biochemistry, University of Oxford. The production of Abdala RBD in P. pastoris has been described extensively elsewhere (Limonta-Fernández et al 2022). Three production lots of Abdala RBD were assessed in this manuscript to compare consistency in produced protein product, one lot from a 75 L production scale and two lots from a 3,000 L production scale. RBD and monomeric ACE2 were transiently expressed in HEK293F (FreeStyle™, Thermo Fisher Scientific). Cells were cultured in Freestyle 293 expression media (ThermoFisher Scientific) and incubated at 37 °C, 8% CO2 and 120 rpm. Transfection was achieved using FreeStyle™ MAX reagent (Invitrogen) and OptiMEM™ (Gibco) following a published protocol (Schaub et al 2021). For the kifunensine control RBD protein, kifunensine was added at time of transfection at a final concentration 10 μM. Five days post transfection, cell culture supernatant was harvested by centrifugation at 3,000 × g for 10 min and then filtered using 0.45 μM pore size filters (Merck). The supernatant was supplemented with 10 mM imidazole prior to purification using a HisTrap HP, 5 mL column (Cytiva) connected to an ÄKTA pure protein purification system (Cytiva). Both RBD and monomeric ACE2 were further purified by size exclusion chromatography (SEC) using a Superose 6 increase 10/300 GL column (GE Healthcare) equilibrated in Dulbecco’s phosphate-buffered saline (DPBS, pH 7.4, ThermoFisher Scientific). SEC fractions were pooled and concentrated using Amicon molecular weight cut-off centrifugal filters (GE Healthcare). Protein concentrations were determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific) at absorbance 280 nM and corrected for protein molecular weight and extinction coefficient.
Glycan HPLC
Approximately 10 μg of RBDHEK and Abdala was loaded onto SDS-PAGE gels, run at 200 V for 30 min and Coomassie stained. Gel bands were excised and de-stained in 50:50 Acetonitrile (MeCN): water. PNGase F (generated in-house (Loo et al 2002)) was added to each gel-band and incubated for 16 h at 37 °C. Released N-glycans were labelled with 2-aminoanthranilic acid (2-AA) as previously described (Alonzi et al 2008). Briefly, glycans were resuspended in 30 μL of HPLC-grade H2O followed by addition of 80 μL of labelling mixture (30 mg/mL 2-AA and 45 mg/mL sodium cyanoborohydride in a solution of sodium acetate trihydrate [4% w/v] and boric acid [2% w/v] in methanol). N-glycans were incubated at 80 °C for 1 h. Excess label was removed using Spe-ed Amide-2 cartridges (Applied Separation) as described (Alonzi et al 2008).
Fluorescently labelled N-glycans were profiled by hydrophilic interaction liquid chromatography- high-performance liquid chromatography (HILIC-HPLC) using a 2.1 mm × 10 mm Acquity BEH Amide Column (1.7 μm particle size) (Waters, Elstree, UK) attached to an Agilent 1260 Infinity II (Agilent, Manchester, UK). Mobile phase was solvent A: 50 mM ammonium formate, pH 4.4 and solvent B: MeCN. The gradient was: (t = 0): 22.0% A, 78.0% B (flow rate of 0.5 mL/min); t = 38.5: 44.1% A, 55.9% B (0.5 mL/min); t = 39.5: 100% A, 0% B (0.25 mL/min); t = 44.5: 100% A, 0% B (0.25 mL/min); t = 46.5: 22.0% A, 78.0% B (0.5 ml/min), t = 48: 22.0% A, 78.0% B (0.5 mL/min). Fluorescence was measured using an excitation wavelength of 360 nm and a detection wavelength of 425 nm.
Ion mobility – MS/MS
IM-MS/MS measurements were performed on a Synapt G2 instrument (Waters, Manchester, UK). For each analysis, 2 μL of N-glycan sample material was ionized by nano-electrospray ionization (nano-ESI) from gold-coated borosilicate glass capillaries prepared in-house (Hernández and Robinson 2007). The instrument was set in sensitivity mode with the following: capillary voltage 0.8–1.2 kV, sample cone 65 V, extraction cone 3.3 V, source temperature 80 °C. Collision-induced dissociation was performed both before and after mobility separation in the trap and transfer cells respectively with argon as the collision gas. The instrument was externally mass-calibrated with sodium iodide and the mobility cell was calibrated with dextran ((Glc2–13) (from Leuconostoc mesenteroides). Data acquisition and processing were carried out using Waters Driftscope (version 2.8) software and MassLynx™ (version 4.1). The scheme devised by Domon and Costello (Domon and Costello 1988) was used to name the fragment ions with the following exception: the subscript R (for reducing terminal) is used when general reference is made for loss or fragmentation of a GlcNAc residue from the reducing terminus of the glycan in order to avoid confusion caused by the subscript number changing as the result of altered chain lengths. Interpretation of the spectra followed rules developed earlier in this laboratory (Harvey 2005b, 2005a, 2005c; Harvey et al 2008).
Glycoproteomics
Approximately 5 μg protein was loaded and run on an SDS-PAGE. Gel bands were excised and washed sequentially with HPLC grade water followed by 1:1 (v/v) MeCN/H2O. Gel bands were dried (via vacuum centrifuge), treated with 10 mM dithiothreitol (DTT) in 100 mM NH4HCO3 and incubated for 45 min at 56 °C with shaking. DTT was removed and 55 mM iodoacetamide (in 100 mM NH4HCO3) was added and incubated for 30 minutes in the dark. All liquid was removed and gels were washed with 100 mM NH4HCO3/MeCN as above. Gels were dried and 12.5 ng/μL trypsin, chymotrypsin or alpha lytic protease was added separately and incubated overnight at 37 °C. Samples were then washed and (glyco) peptides were extracted and pooled with sequential washes with 5% (v/v) formic acid (FA) in H2O and MeCN. Dried samples were reconstituted in 2% MeCN, 0.05% trifluoroacetic acid and run by LC–MS.
Samples were analysed using an Ultimate 3,000 UHPLC coupled to an Orbitrap Q Exactive mass spectrometer (Thermo Fisher Scientific). (Glyco)peptides were loaded onto a 75 μm × 2 cm pre-column and separated on a 75 μm × 15 cm Pepmap C18 analytical column (Thermo Fisher Scientific). Buffer A was 0.1% FA in H2O and buffer B was 0.1% FA in 80% MeCN with 20% H2O. A 40-minute linear gradient (0% to 40% buffer B) was used. Data was collected in data-dependent acquisition mode with a mass range 375 to 1500 m/z and at a resolution of 70,000. For MS/MS scans, stepped HCD normalized energy was set to 27, 30 and 33% with orbitrap detection at a resolution of 35,000.
Glycopeptide data was analysed with Byonic (Protein Metrics). Digestion was set to RK, TASV and FYWML for trypsin, alpha-lytic and chymotrypsin digests, respectively and fully specific with a maximum of two miss cleavages allowed. Carbamidomethylation (57.02 Da) was set as a fixed modification, while methionine oxidation (15.99 Da), deamidation (0.98 Da) and Gln - > pyro-glutamate (−17.03 Da) were set as variable modifications. The Byonic in-built common human N-linked (182 glycans), and O-linked glycan (9 glycans) databases were used to identify glycopeptides for RBDHEK. The Byonic in-built yeast glycan library was used to identify glycopeptides for Abdala, containing high mannose structures with and without phosphate groups and O-mannosylation. Byonic output files were imported into Byologic for quantification (Protein Metrics). A minimum Byonic threshold score of 300 was used for glycopeptide identification. All glycopeptide assignments were manually validated. For quantification, the extracted ion chromatogram intensities for each glycopeptide were summed and plotted relative to the total intensity for each glycosite.
Mass photometry
Mass photometry measurements were conducted using a Refeyn TwoMP system (Refeyn Ltd) as previously described (Soltermann et al 2020). High Precision No. 1.5H glass coverslips were cleaned via sonication in Milli-Q H2O, followed by isopropanol and Milli-Q H20 then dried under nitrogen flow. Sample chambers were assembled using silicone gaskets (CultureWell™ reusable gasket, 3 mm diameter × 1 mm depth, Grace Bio-Labs). Coverslips were placed on the MP sample stage and a single gasket was filled with 5–20 μL DPBS (without calcium, without magnesium, pH 7.4 ThermoFisher Scientific) to find focus and ensure low background signal-to-noise. For interaction experiments, proteins were mixed at a 1:1 molar ratio and equilibrated for 5 min prior to data acquisition.
Acquisition settings within AcquireMP (v2.5.0, Refeyn Ltd) were as follows: small field of view, frame binning = 14, frame rate = 685.0 Hz, pixel binning = 6, exposure time 1.41 ms and movies were taken over 60 seconds. Mass calibration was conducted using an in-house protein standard. Data was analysed using DiscoverMP (v2.5.0, Refeyn Ltd). Molecule counts were used to determine levels of ACE2-RBD occupancy. The interaction between RBD and ACE2 is represented as % total occupancy, which was calculated using the sum of all ACE2 counts including species with 0 and 1 RBD molecules bound and expressed as a percentage of RBD bound counts compared to total ACE2 counts. The interaction between RBD and RBM targeting mAb is represented as % total occupancy, which was calculated using the sum of all mAb counts including species with 0, 1 and 2 RBD molecules bound and expressed as a percentage of 1 and 2 RBD bound counts compared to total mAb counts. Calculation of approximate Kd values was done as previously described (Soltermann et al 2020; Kofinova et al 2024). Representative histograms with overlaid kernel density estimates were generated in R (v4.2.1) using event exports from DiscoverMP.
Hydrogen-deuterium exchange (HDX)-MS
Prior to conducting HDX-MS experiments, peptides were identified by digesting RBDHEK and Abdala using the same protocol and identical liquid chromatographic (LC) gradient as detailed below and performing MSE analysis with a Synapt G2-Si mass spectrometer (Waters), applying collision energy ramping from 20 to 30 kV. Sodium iodide was used for calibration and leucine enkephalin was applied for mass accuracy correction. MSE runs were analysed with ProteinLynx Global Server (PLGS) 3.0 (Waters) and peptides identified in 3 out of 4 runs, with at least 0.2 fragments per amino acid (at least 2 fragments in total) and at least 1 consecutive product, with mass error below 10 ppm were selected in DynamX 3.0 (Waters).
HDX was conducted with differential temperature labelling, in a similar manner as previously described (Baggen et al 2023; Calvaresi et al 2023). RBDHEK and Abdala (20 uM) were diluted 1:20 in deuterated PBS buffer (95% final D2O fraction, pH read 7.3) and the exchange reaction was conducted for 10 s on ice, for 10 s, 100 s (1 min 40 s), 1000 s (16 min 40 s) and 10,000 s (2 h 46 min 40 s) at 23 °C, and for 9 h at 28 °C. The exchange reactions were quenched by 1:1 dilution into ice-cold 100 mM phosphate buffer containing 3 M Urea and 70 mM tris (2-carboxyethyl) phosphine (TCEP, final pH read 2.3). Samples were held on ice for 30 s and snap-frozen in liquid nitrogen and kept frozen at −80 °C until LC–MS analysis. Triplicates were performed for time points 10 s on ice and 9 h at 28 °C; duplicates were performed for all the other time points. Maximally labelled samples were produced by labelling RBDs with 3 M fully deuterated urea in D2O and 4 mM TCEP, resulting in a final deuterium content as for the other labelled samples. The maximally labelled samples were quenched after 6 h by 1:1 dilution with ice-cold 100 mM phosphate buffer (final pH read 2.3), held for 30 s on ice and snap-frozen in liquid nitrogen and kept frozen at −80 °C until LC–MS analysis.
Frozen protein samples were quickly thawed and injected into an Acquity UPLC M-Class System with HDX Technology (Waters). Proteins were on-line digested at 20 °C into a home-made Pepsin column and trapped/desalted with solvent A (0.23% formic acid in water, pH 2.5) for 3 min at 200 μL/min and at 0 °C through an Acquity BEH C18 VanGuard pre-column (1.7 μm, 2.1 mm × 5 mm, Waters). Peptides were eluted into an Acquity UPLC BEH C18 analytical column (1.7 μm, 2.1 mm × 100 mm, Waters) with a 7 min-linear gradient raising from 8 to 35% of solvent B (0.23% formic acid in acetonitrile) at a flow rate of 40 μL/min and at 0 °C. Then, peptides went through electrospray ionization in positive mode and underwent MS analysis with ion mobility separation.
Peptide level deuterium uptake was calculated with DynamX 3.0 and data visually inspected and curated. The threshold for the statistically significant difference in HDX was established at the significance level of 99%, based on an approach described earlier (Houde et al 2011).
Supplementary Material
Acknowledgments
We would like to thank Arthur Huang (Chang Gung Memorial Hospital, Taiwan), Tiong Tan, Lisa Schimanski and Alain Townsend (MRC Weatherall Institute of Molecular Medicine, University of Oxford) for providing a SARS-CoV-2 spike receptor-binding motif (RBM) targeting antibody.
Contributor Information
Sean A Burnap, Department of Biochemistry, Dorothy Crowfoot Hodgkin Building, University of Oxford, South Parks Road, Oxford, OX1 3QU, United Kingdom; The Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, South Parks Road, Oxford, OX1 3QU, United Kingdom.
Valeria Calvaresi, Department of Biochemistry, Dorothy Crowfoot Hodgkin Building, University of Oxford, South Parks Road, Oxford, OX1 3QU, United Kingdom; The Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, South Parks Road, Oxford, OX1 3QU, United Kingdom.
Gleysin Cabrera, Center for Genetic Engineering and Biotechnology, Avenida 31 e/ 158 y 190. Cubanacán. Playa. Havana, 11600, Cuba.
Satomy Pousa, Center for Genetic Engineering and Biotechnology, Avenida 31 e/ 158 y 190. Cubanacán. Playa. Havana, 11600, Cuba.
Miladys Limonta, Center for Genetic Engineering and Biotechnology, Avenida 31 e/ 158 y 190. Cubanacán. Playa. Havana, 11600, Cuba.
Yassel Ramos, Center for Genetic Engineering and Biotechnology, Avenida 31 e/ 158 y 190. Cubanacán. Playa. Havana, 11600, Cuba.
Luis Javier González, Center for Genetic Engineering and Biotechnology, Avenida 31 e/ 158 y 190. Cubanacán. Playa. Havana, 11600, Cuba.
David J Harvey, Department of Biochemistry, Dorothy Crowfoot Hodgkin Building, University of Oxford, South Parks Road, Oxford, OX1 3QU, United Kingdom; The Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, South Parks Road, Oxford, OX1 3QU, United Kingdom.
Weston B Struwe, Department of Biochemistry, Dorothy Crowfoot Hodgkin Building, University of Oxford, South Parks Road, Oxford, OX1 3QU, United Kingdom; The Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, South Parks Road, Oxford, OX1 3QU, United Kingdom.
Author contributions
S.A.B performed protein production, glycomics, glycoproteomics and mass photometry data acquisition and analysis. V.C conducted HDX data acquisition and analysis. G.C, S.P, M.L. and Y.R performed sample production and processing. L.J.G, D.J.H and W.B.S conceived the paper and supervised the project. S.A.B and W.B.S wrote the manuscript.
CRediT statement
Sean Burnap (Data curation [equal], Formal analysis [equal], Investigation [equal], Writing–original draft [equal], Writing–review & editing [equal]), Valeria Calvaresi (Formal analysis [equal], Investigation [equal], Writing–review & editing [equal]), Gleysin Cabrera (Writing–review & editing [equal]), Satomy Pousa (Investigation [supporting], Resources [supporting], Writing–review & editing [supporting]), Miladys Limonta (Writing–review & editing [equal]), Yassel Ramos (Writing–review & editing [equal]), Luis González (Writing–review & editing [equal]), David J. Harvey (Data curation [equal], Investigation [equal], Writing–review & editing [equal]), Weston B. Struwe (Conceptualization [lead], Funding acquisition [lead], Supervision [lead], Writing–original draft [equal], Writing–review & editing [equal])
Funding
This work was supported by funding from the UK Research and Innovation Future Leaders Fellowship MR/V02213X/1 and the University of Oxford’s COVID-19 Research Response Fund to W.S. and S.A.B.
Conflict of interest
W.B.S. is a shareholder of Refeyn Ltd.
Data availability
Mass spectrometry raw data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier <PXD058644> (Vizcaíno et al 2014) All other data included in the manuscript and supplemental materials is available upon request.
References
- Alonzi DS, Neville DCA, Lachmann RH, Dwek RA, Butters TD. 2008. Glucosylated free oligosaccharides are biomarkers of endoplasmic- reticulum alpha-glucosidase inhibition. Biochem J. 409:571–580. 10.1042/BJ20070748. [DOI] [PubMed] [Google Scholar]
- Baggen J, et al. 2023. TMEM106B is a receptor mediating ACE2-independent SARS-CoV-2 cell entry. Cell. 186:3427–42.e22. 10.1016/j.cell.2023.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretthauer RK, Castellino FJ. 1999. Glycosylation of Pichia pastoris -derived proteins. Biotechnol Appl Biochem. 30:193–200. 10.1111/j.1470-8744.1999.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Brun J, et al. 2021. Assessing antigen structural integrity through glycosylation analysis of the SARS-CoV-2 viral spike. ACS Cent Sci. 7:586–593. 10.1021/acscentsci.1c00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnap SA, Struwe WB. 2022. Mass photometry reveals SARS-CoV-2 spike stabilisation to impede ACE2 binding through altered conformational dynamics. Chem Commun. 58:12939–12942. 10.1039/D2CC04711J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Tarentino AL, Maley F, Atkinson PH, Trimble RB. 1982. Glycoprotein synthesis in yeast. Identification of Man8GlcNAc2 as an essential intermediate in oligosaccharide processing. J Biol Chem. 257:14657–14666. 10.1016/S0021-9258(18)33332-5. [DOI] [PubMed] [Google Scholar]
- Calvaresi V, et al. 2023. Structural dynamics in the evolution of SARS-CoV-2 spike glycoprotein. Nat Commun. 14:1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalino L, et al. 2020. Beyond shielding: the roles of Glycans in the SARS-CoV-2 spike protein. ACS Cent Sci. 6:1722–1734. 10.1021/acscentsci.0c01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, et al. 2014. Yeast-expressed recombinant protein of the receptor-binding domain in SARS-CoV spike protein with deglycosylated forms as a SARS vaccine candidate. Hum Vaccin Immunother. 10:648–658. 10.4161/hv.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, et al. 2017. Optimization of the production process and characterization of the yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1), a SARS vaccine candidate. J Pharm Sci. 106:1961–1970. 10.1016/j.xphs.2017.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, et al. 2020. Yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1) formulated with aluminum hydroxide induces protective immunity and reduces immune enhancement. Vaccine. 38:7533–7541. 10.1016/j.vaccine.2020.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, et al. 2022. Yeast-expressed recombinant SARS-CoV-2 receptor binding domain RBD203-N1 as a COVID-19 protein vaccine candidate. Protein Expr Purif. 190:106003. 10.1016/j.pep.2021.106003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. 2023. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat Rev Immunol. 23:189–199. 10.1038/s41577-022-00784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BK, et al. 2003. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci USA. 100:5022–5027. 10.1073/pnas.0931263100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, et al. 1987. High–level expression and efficient assembly of hepatitis B surface antigen in the methylotrophic yeast, Pichia Pastoris. Bio/Technology. 5:479–485. [Google Scholar]
- Cummings RD. 2022. The mannose receptor ligands and the macrophage glycome. Curr Opin Struct Biol. 75:102394. 10.1016/j.sbi.2022.102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon B, Costello CE. 1988. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 5:397–409. 10.1007/BF01049915. [DOI] [Google Scholar]
- Gemmill TR, Trimble RB. 1999. Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim Biophys Acta. 1426:227–237. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez E, et al. 2023. O-linked Sialoglycans modulate the proteolysis of SARS-CoV-2 spike and likely contribute to the mutational trajectory in variants of concern. ACS Cent Sci. 9:393–404. 10.1021/acscentsci.2c01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstöttner C, et al. 2021. Structural and functional characterization of SARS-CoV-2 RBD domains produced in mammalian cells. Anal Chem. 93:6839–6847. 10.1021/acs.analchem.1c00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SR, Gerngross TU. 2007. Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr Opin Biotechnol. 18:387–392. 10.1016/j.copbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. 2005a. Fragmentation of negative ions from carbohydrates: part 2. Fragmentation of high-mannose N-linked glycans. J Am Soc Mass Spectrom. 16:631–646. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. 2005b. Fragmentation of negative ions from carbohydrates: part 1. Use of nitrate and other anionic adducts for the production of negative ion electrospray spectra from N-linked carbohydrates. J Am Soc Mass Spectrom. 16:622–630. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. 2005c. Fragmentation of negative ions from carbohydrates: part 3. Fragmentation of hybrid and complex N-linked glycans. J Am Soc Mass Spectrom. 16:647–659. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Royle L, Radcliffe CM, Rudd PM, Dwek RA. 2008. Structural and quantitative analysis of N-linked glycans by matrix-assisted laser desorption ionization and negative ion nanospray mass spectrometry. Anal Biochem. 376:44–60. 10.1016/j.ab.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Hernández H, Robinson CV. 2007. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protoc. 2:715–726. 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- Hernández-Bernal F, et al. 2022. Safety, tolerability, and immunogenicity of a SARS-CoV-2 recombinant spike RBD protein vaccine: a randomised, double-blind, placebo-controlled, phase 1-2 clinical trial (ABDALA study). EClinicalMedicine. 46:101383. 10.1016/j.eclinm.2022.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Bernal F, et al. 2023. A phase 3, randomised, double-blind, placebo-controlled clinical trial evaluation of the efficacy and safety of a SARS-CoV-2 recombinant spike RBD protein vaccine in adults (ABDALA-3 study). Lancet Reg Health–Am. 21:100497. 10.1016/j.lana.2023.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde D, Berkowitz SA, Engen JR. 2011. The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies. J Pharm Sci. 100:2071–2086. 10.1002/jps.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-P, et al. 2022. Structural remodeling of SARS-CoV-2 spike protein glycans reveals the regulatory roles in receptor-binding affinity. Glycobiology. 33:126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrovo-Hidalgo T, et al. 2023. Deglycosylated RBD produced in Pichia pastoris as a low-cost sera COVID-19 diagnosis tool and a vaccine candidate. Glycobiology. 34:cwad089. [DOI] [PubMed] [Google Scholar]
- Ives CM, et al. 2024. Role of N343 glycosylation on the SARS-CoV-2 S RBD structure and co-receptor binding across variants of concern. elife. 13:RP95708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M, et al. 2022. Demonstrating “Abdala” subunit vaccine Thermostability study. Bioprocess J. 21. [Google Scholar]
- Jacobs PP, Geysens S, Vervecken W, Contreras R, Callewaert N. 2009. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat Protoc. 4:58–70. 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- Jigami Y, Odani T. 1999. Mannosylphosphate transfer to yeast mannan. Biochim Biophys Acta. 1426:335–345. [DOI] [PubMed] [Google Scholar]
- Kofinova Z, Karunanithy G, Ferreira AS, Struwe WB. 2024. Measuring protein-protein interactions and quantifying their dissociation constants with mass photometry. Curr Prot. 4:e962. [DOI] [PubMed] [Google Scholar]
- Lee SJ, et al. 2002. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 295:1898–1901. 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- Lee S-M, et al. 2020. Calcitonin receptor N-glycosylation enhances peptide hormone affinity by controlling receptor dynamics. J Mol Biol. 432:1996–2014. 10.1016/j.jmb.2020.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limonta-Fernández M, et al. 2022. An engineered SARS-CoV-2 receptor-binding domain produced in Pichia pastoris as a candidate vaccine antigen. New Biotechnol. 72:11–21. 10.1016/j.nbt.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T, Patchett ML, Norris GE, Lott JS. 2002. Using secretion to solve a solubility problem: high-yield expression in Escherichia coli and purification of the bacterial glycoamidase PNGase F. Protein Expr Purif. 24:90–98. 10.1006/prep.2001.1555. [DOI] [PubMed] [Google Scholar]
- Luong M, Lam JS, Chen J, Levitz SM. 2007. Effects of fungal N- and O-linked mannosylation on the immunogenicity of model vaccines. Vaccine. 25:4340–4344. 10.1016/j.vaccine.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más-Bermejo PI, et al. 2022. Cuban Abdala vaccine: effectiveness in preventing severe disease and death from COVID-19 in Havana, Cuba; a cohort study. Lancet Reg Health–Am. 16:100366. 10.1016/j.lana.2022.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoman B, et al. 2021. The degree and length of O-glycosylation of recombinant proteins produced in Pichia pastoris depends on the nature of the protein and the process type. Biotechnol J. 16:e2000266. 10.1002/biot.202000266. [DOI] [PubMed] [Google Scholar]
- Roberts DS, et al. 2021. Structural O-Glycoform heterogeneity of the SARS-CoV-2 spike protein receptor-binding domain revealed by top-down mass spectrometry. J Am Chem Soc. 143:12014–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanda M, Morrison L, Goldman R. 2021. N- and O-glycosylation of the SARS-CoV-2 spike protein. Anal Chem. 93:2003–2009. 10.1021/acs.analchem.0c03173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub JM, et al. 2021. Expression and characterization of SARS-CoV-2 spike proteins. Nat Protoc. 16:5339–5356. 10.1038/s41596-021-00623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan A, Supekar NT, Gleinich AS, Azadi P. 2020. Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology. 30:981–988. 10.1093/glycob/cwaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitaoka K, et al. 2023. Structural basis of spike RBM-specific human antibodies counteracting broad SARS-CoV-2 variants. Commun Biol. 6:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solá RJ, Griebenow K. 2009. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci. 98:1223–1245. 10.1002/jps.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltermann F, et al. 2020. Quantifying protein-protein interactions by molecular counting with mass photometry. Angew Chem Int Ed Engl. 59:10774–10779. 10.1002/anie.202001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P, et al. 1976. Evidence for specific recognition sites mediating clearance of lysosomal enzymes in vivo. Proc Natl Acad Sci USA. 73:4045–4049. 10.1073/pnas.73.11.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztain T, et al. 2021. A glycan gate controls opening of the SARS-CoV-2 spike protein. Nat Chem. 13:963–968. 10.1038/s41557-021-00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno JA, et al. 2014. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol. 32:223–226. 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington C, et al. 2024. Impact of N-glycosylation on protein structure and dynamics linked to enzymatic C–H activation in the M. Oryzae lipoxygenase. Biochemistry. 63:1335–1346. 10.1021/acs.biochem.4c00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, et al. 2020. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 367:1444–1448. 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. 2021. Furin cleavage of the SARS-CoV-2 spike is modulated by O-glycosylation. Proc Natl Acad Sci USA. 118:e2109905118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mass spectrometry raw data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier <PXD058644> (Vizcaíno et al 2014) All other data included in the manuscript and supplemental materials is available upon request.



