Highlights
-
•
Azithromycin is a preferred antibiotic for typhoid due to its broad-spectrum activity.
-
•
Ceftriaxone should be approached with caution due to the occurrence of resistance.
-
•
Fluoroquinolones are the least preferred choice in treating Salmonella infections.
-
•
Amoxicillin is the best alternative agent to fluoroquinolones for treating children.
-
•
Supportive therapies are highly warranted to minimize the episodes of typhoid fever.
Keywords: Salmonella, Typhoid, Efficacy, Safety, Diarrhea, Antibiotics
Abstract
A systematic review was conducted to critically analyze the outbreaks, efficacy, and safety of drugs used to treat various Salmonella infections. Four drugs—azithromycin, ceftriaxone, ciprofloxacin, and amoxicillin—are commonly used to treat Salmonella infections, and all four drugs were included in this review. This review found that, of these, azithromycin and ceftriaxone were more effective in treating Salmonella infections based on the patient's length of stay in the hospital and the rate at which the fever was resolved. Fluoroquinolones are also effective in treating Salmonella infection but are not approved for use in children. Azithromycin was found to be the physicians’ preferred choice of medication for Salmonella infection due to its less resistance development. Almost all these drugs produce varying degrees of adverse events, but they are mild to moderate. However, azithromycin was shown to be comparatively safer than the other three drugs in terms of side effects, adverse events, and relapse associated with Salmonella treatment. Developing effective and safe therapies for all strains of Salmonella remains a priority, especially given the increasing prevalence of antibiotic-resistant variants.
Introduction
Salmonellosis
Salmonella species are gram-negative, motile, acid-labile, facultative intracellular microorganisms commonly associated with salmonellosis, an infection prevalent in low- and high-resource countries [[1], [2], [3]]. Salmonellosis in humans typically presents as self-limiting food poisoning (gastroenteritis) but can also lead to serious systemic infections, such as enteric fever, which require antibiotic treatment [2].
Types and causative agents of salmonellosis
According to the Centers for Disease Control and Prevention (CDC), there are two species within the genus Salmonella: Salmonella enterica and Salmonella bongori. S. enterica is further categorized into six subspecies: enterica (I), salamae (II), arizona (IIIa), diarizonae (IIIb), houtenae (IV), and indica (VI). On the other hand, S. bongori has no subspecies [3].
Salmonellosis is caused by a subspecies of S. enterica and affects a wide range of hosts, including humans, mammals, birds, and fish. Salmonella serovars within subspecies S. enterica are divided into two groups based on their clinical syndromes, such as typhoidal Salmonella and nontyphoidal Salmonella (NTS). Typhoidal Salmonella is caused by S. Typhi and S. Paratyphi A, B, and C (Figure 1). Humans serve as hosts to S. Typhi and S. Paratyphi A, causing enteric fever, whereas S. Paratyphi B and C cause a typhoid fever-like illness in other animals, primarily, higher primates. In addition, nontyphoidal Salmonella typically leads to gastroenteritis, with host immunity determining the frequency of invasive illness. Individuals with conditions such as HIV infection, falciparum malaria, malnutrition, and other immunocompromised states are more susceptible to invasive NTS [4,5].
Figure 1.
Classification of Salmonella infections.
Recent outbreaks of Salmonella
According to the Salmonella Surveillance Overview by the CDC, Salmonella is known to cause more than 1.35 million illnesses per year, with 26,500 hospitalizations and 420 deaths in the United States [6]. Several outbreaks of Salmonella have been reported worldwide, with the most recent one reported in November 2023. The Salmonella outbreak was linked to cantaloupes, and, as of December 15, 2023, 302 cases of illness have been reported with 129 cases involving hospitalization, and six reported deaths according to the CDC. Data show that Minnesota reported the highest number of outbreaks (26 cases) of Salmonella linked to cantaloupes of 42 states in the United States [7]. In relation to Salmonella infection due to cantaloupes, 97 cases were reported in New South Wales in 2015.
In 2015, there were 44 cases of an outbreak connected to frozen raw breaded chicken products in four provinces of Canada: Ontario, Quebec, Newfoundland, Labrador, and Nova Scotia. In South Australia in 2016, raw mung bean sprouts were identified as the source of infection, resulting in 230 reported cases of Salmonella. In addition, 97 cases of an outbreak linked to cantaloupes were reported in New South Wales in the same year [8].
In 2016, a Salmonella outbreak affected multiple states in the United States, with 27 patients infected with Salmonella Virchow. During the same year, a significant number of individuals, 907 patients, were infected with the Salmonella Poona serotype outbreak in the United States, which was traced back to cucumbers imported from Mexico. In 2018, a large number of typhoid fever cases were documented in Syria at the Al-Hol Camp among Iraqi and Syrian refugees. In India in 2018, Woraiyur reported 40 cases of typhoid fever, attributed to contaminated water [8].
Amid these outbreaks, there were instances where the Salmonella outbreak was linked to antimicrobial-resistant strains. According to the Morbidity and Mortality Weekly Report, in 2018, an outbreak of S. enterica serotype Newport with decreased susceptibility to azithromycin was reported in Mexico. This infection affected 255 individuals in the United States, resulting in the hospitalization of 60 individuals and the death of two [9]. Since 2016, Pakistan has been experiencing an outbreak of extensively drug-resistant typhoid cases, with the situation worsening over the years. From 2016 to 2018, of 8188 cases reported in the Sindh province of Pakistan, 5274 cases were attributed to extensively drug-resistant S. enterica serovar typhi, Salmonella paratyphi A, and Salmonella paratyphi B [10]. The development of resistance against fluoroquinolones and third-generation cephalosporin by the Salmonella strains is concerning because these are the main first-line agents used against typhoid fever [11]. Limited information is available regarding the mortality rate of this typhoid fever outbreak, but one study suggests a mortality rate of 1.8%, which may be underestimated because it only includes hospitalized patients and not the general population of Pakistan [12].
Although there are drugs available for the treatment of Salmonella infection, to date, these drugs have not been able to control the outbreaks of Salmonella infections. This is due to their varying safety and efficacy profiles, which makes it harder for health care providers to choose suitable medication. Therefore, a systematic review was conducted to assess the effectiveness and safety of drugs commonly used in the treatment of various Salmonella infections.
Methods
A systematic review provides health care practitioners with the most current and comprehensive information for analyzing and evaluating the reliability and clinical significance of a topic or intervention. It presents crucial information concisely, incorporating thorough data and a methodical literature search while avoiding selection bias that may occur in other types of reviews. This type of review also involves synthesizing previous study results, often accompanied by a meta-analysis to combine and analyze data for an overall outcome. Ultimately, the synthesized information is used to draw conclusions and make recommendations.
Outcome assessment
The focus of this review is to assess the effectiveness and safety of drugs commonly used in the treatment of various Salmonella infections. To evaluate the effectiveness of these drugs, a combination of clinical and microbiological responses, fever clearance time, and culture and sensitivity tests (before and after completing antibiotic treatment) were taken into account. Drug safety is determined by a decrease in adverse drug effects or adverse events compared with drugs previously approved by the US Food and Drug Administration (FDA).
Data extraction
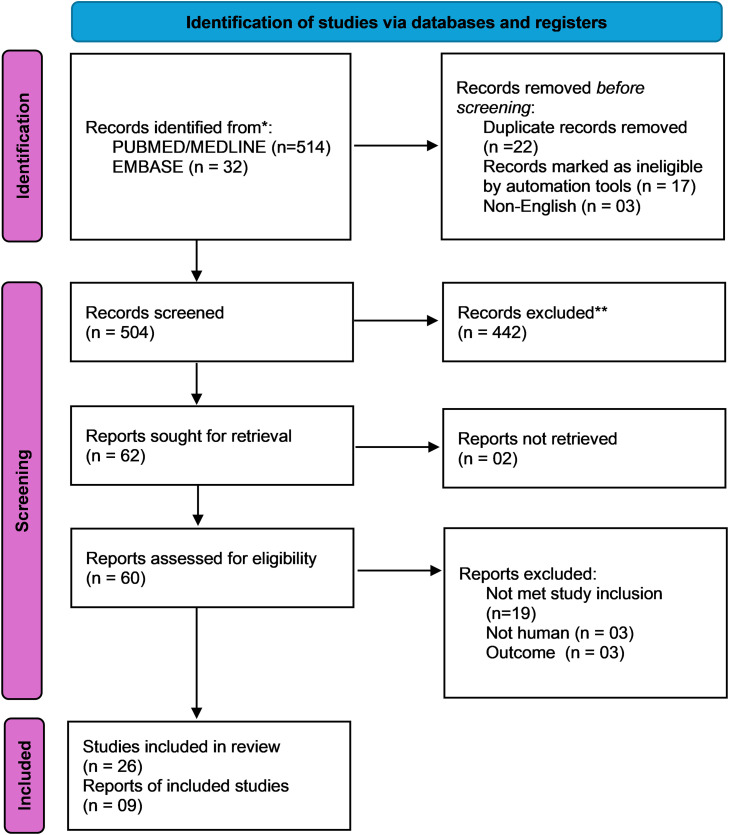
The information gathered for this review primarily focuses on the most recent therapies for Salmonella infections. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline was used to identify publications and reviews that evaluated randomized controlled trials. The main data sources used were PubMed, Nvivo, Mendeley, Evernote, CiteUlike, Biohunter, DelveHealth, Scicurve, MEDLINE, the Cochrane Library, Scopus, Web of Science, and Google Scholar. Articles related to animals were excluded. According to Center Watch, the medications used in our study were approved by the US FDA. Four medications were licensed to treat Salmonella infections, resulting in the publication of 546 articles. Finally, 35 articles were selected for review (Figure 2). Each author retrieved relevant material from the publications separately. Any conflicts were resolved through consensus. All identified studies were evaluated for inclusion by two independent investigators (PS and CSI), with any disagreements resolved by a third reviewer (PM). The retrieved data included the study phase, area, subject conditions (mean age, gender, duration of infection, culture and sensitivity test, body temperature, and comorbidities), and outcome measures. This information was gathered and compiled in paragraphs that provided a thorough introduction to each medication. The review protocol is currently registered in PROSPERO under reference number CRD42024580893 and publicly available.
Figure 2.
PRISMA flow diagram for the selection of articles for the review.
Search strategy
The following search strategy was used to collect articles for this review (“salmonella infections”[MeSH Terms] OR (“salmonella”[All Fields] AND “infections”[All Fields]) OR “salmonella infections”[All Fields] OR (“salmonella”[All Fields] AND “infection”[All Fields]) OR “salmonella infection”[All Fields]) AND ((y_5[Filter]) AND (ffrft[Filter]) AND (randomizedcontrolledtrial[Filter]) AND (fft[Filter])).
Inclusion and exclusion criteria
This review included all studies that examined the efficacy and safety profiles of the four drugs approved by the US FDA within the search period: azithromycin, ceftriaxone, ciprofloxacin, and amoxicillin. These four drugs are commonly used for the treatment of Salmonella infection either alone or in combination. Most people recover from Salmonella infection within 4-7 days without antibiotics, with the support of drinking extra fluids as long as the diarrhea lasts. Antibiotic treatment is recommended for people with severe illness, and combination therapy is preferred when the chance of resistance to single-drug therapies is high. Excluded from this review were studies on non-randomized controlled trials, incomplete data, studies with unavailable full texts, frequency of Salmonella recurrences, studies of unsatisfactory quality, studies with non-randomized sample selection, research on the frequency of risk factors, and studies not available in English.
Data analysis
The focus of the analysis was on the treatment of Salmonella infections, with the culture and sensitivity test being the primary measure of effectiveness for binary outcomes. We examined the rate of initial infections and recurrence, as well as the drug's safety profile in terms of side effects and adverse events, by evaluating the frequency of recorded incidents. Heterogeneity was assessed using the SD of studies. In a sensitivity analysis, we examined the results by subgrouping trials that studied similar treatments, assuming a sufficient number of trials were available.
Management of Salmonella infections
This section focuses comprehensively on pharmacotherapy management of Salmonella infection. It summarizes the treatment options available for the management of Salmonella infections. There are four drugs available for the treatment of these infections: azithromycin, ceftriaxone, fluoroquinolone ciprofloxacin, and amoxicillin. The efficacy and safety of all these drugs are discussed in this section.
Pharmacological management
This section will focus on the efficacy and safety of the drugs commonly used for the treatment of various Salmonella infections and the information is comprehensively presented.
Azithromycin
Azithromycin, an azalide macrolide, has emerged as a promising treatment for typhoid fever. For four decades, ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole-resistant S. typhi have been the preferred treatments for typhoid fever. However, the rise of multidrug-resistant strains of the virus has made the search for other treatments necessary [12]. Fluoroquinolones, although effective, have not been approved for use in children. Fluoroquinolone-resistant Salmonella has also been reported mostly due to topoisomerase genes gyrA, gyrB, parC, and parE; plasmid-mediated quinolone resistance (PMQR); and chromosomally encoded efflux mechanisms [13]. Therefore, azithromycin has proved to be effective against uncomplicated Salmonella infections, specifically, S. enteritidis and S. typhimurium, in animal studies given the trends of antimicrobial resistance. Azithromycin 500 mg orally (maximum) as a once-daily dose for 5-7 days is administered to the patient [14]. Azithromycin showed a remarkable capacity to accumulate at elevated intracellular concentrations, attaining intracellular values that were 50-100 times higher than the levels found in serum [15].
A previous study reported that azithromycin was the most active macrolide against S. typhi, with a minimum inhibitory concentration (MIC) of 8 mg/l [16,17]. In a study involving human volunteers, it was found that the neutrophil concentration of azithromycin exceeds the MIC of S. typhi by up to 20 times, which is desirable [18].
Furthermore, azithromycin was effective in treating animal tissue infection caused by S. enteritidis. This finding raised questions about the possible future use of azithromycin. Azithromycin has the advantage of once-daily dosing due to its long half-life, which would directly improve patient adherence. In a randomized controlled trial in the United Kingdom in 2019 involving 81 participants, 500 mg of azithromycin was reported to be an effective treatment for fully sensitive strains of S. typhi. However, treatment with azithromycin resulted in extended fever clearance and extended bacteremia (median 90.8 hours [95% confidence interval: 65.9-93.8] compared with 20.1 hours [95% confidence interval: 7.8-24.3], P <0.001) compared with ciprofloxacin [18]. Research also reported that azithromycin has no adverse side effects and was found to be safe for children.
Azithromycin is administered either alone or in combination with other drugs. For instance, a combination of azithromycin and ciprofloxacin is used in some clinical settings to combat Salmonella infections. Combination therapy is specifically employed for treating particular forms of Salmonella infections, such as those that are life-threatening or resistant to single-drug treatments. It should be noted that the azithromycin and ciprofloxacin combination is not typically the preferred choice for treating Salmonella infections. Although both antibiotics are effective against Salmonella, they act through different mechanisms and may have varying side effects, which limits their use in combination therapy [[16], [17], [18]].
Ceftriaxone
According to recent guidelines, ceftriaxone is one of the preferred antibiotics for the treatment of typhoid fever and bacterial enteric infections in immunocompromised individuals. This recommendation applies to those who have not developed resistance to ceftriaxone and did not acquire the infection from Pakistan or Iraq, where there is a high prevalence of extensively drug-resistant S. typhi [10,19].
Ceftriaxone is a third-generation cephalosporin with a broad spectrum, capable of combating gram-negative and gram-positive bacteria, including the gram-negative Salmonella species. It has high plasma protein binding and a half-life of 6-9 hours. Comparable to other β-lactams, ceftriaxone works by inhibiting the cross-linking of peptidoglycan, which is crucial for the structure of the bacterial cell wall [20].
The use of ceftriaxone as a primary antibiotic treatment for typhoid fever dates back to 1986. At that time, chloramphenicol was commonly used for typhoid fever treatment, but its frequent administration and lengthy duration prompted the search for a more effective antibiotic. In a study comparing ceftriaxone to chloramphenicol in 59 patients with typhoid fever, it was found that ceftriaxone achieved satisfactory clinical and antibiotic effects. Although the efficacy of ceftriaxone was initially lower at 79% than chloramphenicol's 90%, blood cultures on day 3 showed that ceftriaxone-treated patients had negative results for S. typhi, whereas 65% of chloramphenicol-treated patients were still positive. This indicates that ceftriaxone is superior in terms of treatment duration efficacy and safety because it did not cause significant bone marrow suppression such as chloramphenicol [21].
A previous study investigated the effects of third- and fourth-generation cephalosporins on Salmonella typhimurium L forms, a strain known to cause diarrhea in infants and young children. The study revealed that this strain is susceptible to third-generation cephalosporins, such as ceftriaxone [22]. Another study comparing the effectiveness of ceftriaxone with chloramphenicol in treating typhoid fever confirmed that ceftriaxone can reduce fever in patients 1 day faster than chloramphenicol. In Indonesia, Dasopang et al. [23] found that ceftriaxone treatment for typhoid fever is more cost-effective than chloramphenicol, with patients saving Rp. 20,289.
However, recent cases in China have shown ceftriaxone-resistant Salmonella in children. The resistance rate was reported to be 5.7%, but another study found a resistance rate of 25.9%. The study identified the gene blaCTA-M-55 as responsible for ceftriaxone resistance, which may be transferred between bacteria in the human intestinal tract [24].
Ceftriaxone injection comes in the form of a powder to be mixed with liquid or as a premixed product to be injected intravenously over a period of 30 or 60 minutes. It can also be administered intramuscularly. Unlike other medications available for the treatment of Salmonella infection, ceftriaxone is only available in parenteral formulation, which limits the self-administration of the drug by the patient [20,21].
Ciprofloxacin
Fluoroquinolones are broad-spectrum antibiotics with good oral bioavailability, indicated for the treatment of pneumonia, gastroenteritis, urinary tract infections, and gonococcal infections. Ciprofloxacin, a fluoroquinolone, exhibits excellent activity against aerobic gram-negative organisms, making it a mainstay for treating severe Salmonella infections and typhoid fever in adults [25]. In addition, ciprofloxacin is known to have potential anti-biofilm effects because biofilms are common factors in developing antimicrobial agent tolerance [26]. However, the efficacy of ciprofloxacin in treating Salmonella has declined due to increased levels of resistance to fluoroquinolones and treatment failure from reduced susceptibility to ciprofloxacin. The development of resistance to ciprofloxacin typically involves mutations in gyrA within the quinolone resistance determining region of subunit A of DNA gyrase in quinolone-resistant S. enterica [25,26]. Single-point mutations in the quinolone resistance determining region of the gyrA gene, particularly, between amino acids 67 and 106, are associated with decreased susceptibility to ciprofloxacin, whereas double mutations at residues 83 and 87 are observed in highly resistant clinical S. enterica serovar typhimurium isolates. Overactivation of multidrug efflux pumps and decreased outer membrane permeability also contribute to Salmonella resistance. PMQR occurs when the organism acquires plasmid-encoded proteins protecting fluoroquinolones. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Salmonella enteric serotype Derby (S. Derby) identified three S. Derby isolates of 826 non-typhoidal Salmonella isolates, with ciprofloxacin MIC of 4 ug/ml. These isolates shared the same genetic structure of quinolone resistance, including silent gyrA mutation and three PMQR genes [26]. As a result, fluoroquinolone is no longer the first-line treatment for Salmonella due to reduced effectiveness.
Furthermore, the safety of fluoroquinolones can be evaluated based on their adverse effects. Adverse effects of ciprofloxacin at therapeutic doses are usually mild, causing gastrointestinal disturbances, such as nausea and diarrhea. Serious side effects may include prolonged QT interval, photosensitivity, and hyper-/hypoglycemia. FDA warnings also include tendinitis and tendon rupture due to the two- to four-fold increased risk of tendinopathy associated with fluoroquinolones [25]. Tendinopathy onset is highest within the first month after drug exposure and commonly affects the Achilles tendon, causing severe pain. The incidence of tendinopathy can be up to 2% in patients aged 65 years and older. The use of steroids and advanced age can increase the risk of tendinitis due to collagen degradation caused by upregulation of matrix metalloproteinases. In addition, fluoroquinolone use is associated with aortic aneurysm and aortic dissection, with increased risk during prolonged therapy and in older patients. Ciprofloxacin should be immediately discontinued if aortic dissection is suspected [26].
Amoxicillin
According to recent guidelines for the treatment of Salmonella infection, amoxicillin is considered one of the possible alternative agents, although it is not the first line of defense. Amoxicillin is a commonly used β-lactam antimicrobial drug approved by the FDA for primary care settings in the treatment of various infectious diseases. It offers additional coverage against certain gram-negative pathogens while remaining effective against a wide range of gram-positive bacteria. Amoxicillin has demonstrated effectiveness against Salmonella species, Actinomyces species, Shigella species, and some strains of Escherichia coli. It is often the preferred or alternative antibiotic within its class due to its high rate of absorption through oral administration [27].
Amoxicillin is commonly used in combination with clavulanic acid, a β-lactamase inhibitor, to prevent degradation by β-lactamase-producing bacteria, which are resistant to a broad range of β-lactam antibiotics, including penicillin [27]. Combining amoxicillin with clavulanic acid increases its effectiveness by reducing susceptibility to β-lactamase resistance.
Research published in 1980 found that using ampicillin or amoxicillin to treat uncomplicated Salmonella gastroenteritis was ineffective and significantly increased the risk of bacteriologic and clinical relapse [28]. However, according to recent guidelines, amoxicillin can be used as an alternative agent to treat Salmonella infection in children, although its usefulness is limited due to high rates of multidrug resistance. Fluoroquinolones are effective in treating Salmonella infection but are not approved for use in children. For children with Salmonella infection, a dose of 100 mg/kg of amoxicillin in thrice daily doses orally can be given, with a maximum daily dose of 3 g. For adults, a dose of 1 g of amoxicillin can be given thrice daily orally, and the treatment duration for adults and children is 10-14 days [28].
Prenatal exposure to antibiotics, specifically, amoxicillin, may have adverse effects on children if mothers take it during pregnancy, potentially by altering the gut microbiome. These findings raise questions about the relationship between changes in the mother's microbiome during pregnancy and significant malformations. However, earlier studies concluded that exposure to amoxicillin and clavulanic acid, either as a group or individual medications, during the first trimester of pregnancy was not linked to major malformations in general or by organ systems [[27], [28], [29]]. The efficacy and safety of the drugs used for the treatment of Salmonella infection are summarized in Table 1.
Table 1.
The efficacy and safety of the drugs used for the treatment of Salmonella infection.
| Name of the drugs | Study & author | Study design | Efficacy | Safety |
|---|---|---|---|---|
| Azithromycin | Giri et al. [29] Girgis et al. [12] |
This is a double-blind randomized, placebo-controlled trial of azithromycin or trimethoprim. A total of 326 patients older than 2 years old and younger than 65 years old were the participants involved. The patients with temperatures more than or equal to 38.0°C for more than 4 days without any localized signs were presented to two Kathmandu hospitals in Nepal. The primary end point was fever clearance time, whereas secondary end points included treatment failure and adverse reactions. This is a randomized control trial in Egypt on azithromycin vs ciprofloxacin. Female and male patients older than 18 years old, have a fever of ≥38.5°C plus a history of fever for at least 4 days, in addition to two or more of the following: abdominal tenderness, hepatomegaly (>2 cm below the right costal margin), splenomegaly (>2 cm below the left costal margin), and rose spots were admitted to Abbassia Fever Hospital Egypt. This study consisted of 64 subjects with positive blood or stool cultures for S. typhi or S. paratyphi, 36 individuals received azithromycin and 28 individuals received ciprofloxacin. |
The median fever clearance time for all patients was 2.7 days (95% CI, 2.6-3.3 days) in the trimethoprim arm and 2.1 days (95% CI, 1.6-3.2 days) in the azithromycin arm. On analysis, it was found that azithromycin resulted in speedier fever clearance time in individuals with sterile blood cultures and fewer relapses of culture-confirmed enteric fever. All individuals treated with azithromycin or ciprofloxacin were cured and no relapses were found. After the initiation of therapy, the following times (mean ± SD [range]) saw defervescence (highest daily temperatures of ≤38°C) azithromycin for 3.8 ± 1.1 (2-7) days and ciprofloxacin for 3.3 ± 1.0 (1-5) days. The culture of blood and stools of all patients were negative, except the 1 individual who had a positive blood culture on day 4 of treatment with azithromycin. |
Between azithromycin and trimethoprim, the hazard ratio of treatment failures by 28 days was 0.62 (95% CI, 0.37-1.05; P = 0.073). There were significantly lower relapses and fewer AEs associated with azithromycin. A total of 11 patients required hospital admission due to grade 3 or 4 AEs, high-grade or persistent fever, or IV rescue therapy administration on one occasion. Two were in the azithromycin arm and nine were in the SXT arm. In all treatment groups, the same number of mild-to-moderate AEs were recorded; these events were all transient and self-limited. |
| Ceftriaxone | Islam et al. [21] Dasopang et al. [23] |
This is a randomized clinical trial that included patients who fit the inclusion criteria: age 6 months to 60 years; fever for >4 days; diarrhea, defined as more than three liquid stools in 24 hours; and a somatic O agglutinin titer of >80 for S. typhi, as determined by the Widal test. These patients were requested to stay in the hospital for 14 days and they were randomly assigned using sealed envelopes that contained a numeric treatment code from a table of random numbers, chloramphenicol or ceftriaxone. The samples taken for analysis were venous blood, stool, and urine samples. This is a cross-sectional study that included 30 patients who were diagnosed with typhoid fever in Indonesia, ranging from age 0 to 25 years, many of whom were 12-16 years old. A total of 13 of these patients were treated with chloramphenicol, whereas the other 17 were treated with ceftriaxone. |
Blood cultures obtained on the third day of treatment showed that 20 patients receiving chloramphenicol were still positive, whereas all the patients receiving ceftriaxone turned up negative. This study found that ceftriaxone was more effective based on the patient's length of stay in the hospital and the rate at which the fever was resolved. In both cases, ceftriaxone was quicker than chloramphenicol in achieving the target. Chloramphenicol, on average, took 57% longer than ceftriaxone to achieve discharge, which is about 2.36 days longer. In terms of the resolution of the fever, it was found that ceftriaxone on average, only took 2.3 days to resolve the fever, compared with chloramphenicol, which used an average of 3.5 days. |
Patients who received chloramphenicol as their treatment were found to have a significantly lower median hematocrit (30.5%) on the last day than ceftriaxone (34.5%) and the leukocyte count for chloramphenicol was also lower than those treated with ceftriaxone (P = 0.02). The ceftriaxone was well-tolerated by the study participants and no side effects or harmful effects were reported. |
| Ciprofloxacin | Khadka et al. [30] | This is a cross-sectional study where blood specimens were taken from clinically suspected enteric fever patients at the outpatient department of Kathmandu Model Hospital in 2018. The signs and symptoms were examined by the physician. 5 mL of blood samples were taken from patients >5 years of age and 3 mL for <5 years of age for culture. A total of 706 blood samples were collected and among them, 46 samples were culture positive for S. enterica. Patients who were already receiving antibiotics, pregnant women, and patients who had a fever >14 days were excluded from this study. | Among 46 samples, 95.7% of the isolates were found to be nalidixic acid resistant, and only one of the isolates was found to be susceptible to all three fluoroquinolones (ciprofloxacin, ofloxacin, and levofloxacin). It was found that 54.3% (n = 25) of isolates had intermediate susceptibility to ciprofloxacin, and the remaining 43.5% (n = 20) were resistant to ciprofloxacin. | The ciprofloxacin was well-tolerated by the study participants, with no major side effects or harmful effects reported. |
| Amoxicillin | Nelson et al. [28] | This is a randomized and double-blind study that involved 44 infants and children who are diagnosed with uncomplicated Salmonella gastroenteritis. The study population was treated with ampicillin (15 patients), amoxicillin (15 patients) or placebo (14 patients). | The findings showed that antibiotic therapy did not show a significant reduction in the length of diarrhea (means 8.8, 7.3, and 7.2 days, respectively). Relapse in bacteria was not seen in individuals who received a placebo, but 8 patients who received ampicillin (53%) and 8 patients who received amoxicillin (53%) experienced bacteriologic relapse (P = 0.003). However, the study shows that ampicillin and amoxicillin were still effective in vitro against the salmonella isolated in relapse. | Around 53% of patients with amoxicillin had a substantial increase in the risk of bacteriologic and symptomatic relapse (P = 0.003). |
AEs, adverse events; CI, confidence interval.
In addition to the pharmacotherapy for treating Salmonella infection, water, sanitation, and hygiene (WASH) infrastructure, encompassing water, sanitation, and hygiene facilities, is a cornerstone of public health and sustainable development. Access to clean water and proper sanitation reduces the spread of waterborne diseases, improves hygiene practices, and enhances overall well-being. Adequate WASH infrastructure empowers communities, particularly, women and children, by reducing the time spent on water collection and improving sanitation conditions. It also contributes to economic growth by promoting healthier populations and reducing health care costs. Investing in WASH infrastructure is not only a matter of human rights but also a strategic investment in the future of our communities.
Conclusion
Salmonella has proved to be a pathogen of concern for human health when not treated properly or when antimicrobial stewardship is not practiced. It can cause symptoms ranging from mild gastroenteritis to severe systemic diseases, such as enteric fever. Recent outbreaks may be caused by contaminated food, especially in regions with poor sanitation. In addition, there has been an emergence of antibiotic resistance within certain communities, which complicates treatment. To address this, an understanding of the mechanisms of Salmonella infection and the development of its antimicrobial properties are necessary to develop effective prevention and treatment strategies. Vigilant surveillance of antimicrobial stewardship is crucial in combating antibiotic resistance.
The treatment of Salmonella infections typically involves non-pharmacologic methods, such as rehydration therapy, antidiarrheal therapy, and zinc supplementation. Pharmacologic treatment, including antibiotics, is preferred in cases of typhoid fever. Azithromycin, among other antibiotics, has emerged as a preferred option for typhoid fever due to its ability to accumulate intracellularly and its broad-spectrum activity. However, the use of ceftriaxone should be approached with caution due to the occurrence of resistance. Similarly, fluoroquinolones have decreased efficacy in treating Salmonella infections due to widespread resistance, whereas amoxicillin can be used as an alternative agent, particularly, in children.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
Not applicable.
Author contributions
P.S. was the lead author responsible for conceptualization of the work and incorporation of all intellectual content including feedback from the other authors. L.S.Y. and C.S.Y. led the acquisition of the data, reviewed data quality, verified with different data sources, performed the statistical analyses, and developed the data visualizations. I.K. and F.H.S.E wrote the manuscript. P.S., L.S.Y., C.S.Y., I.K., F.H.S.E., and P.M. contributed to the conceptualization and provided intellectual input into shaping the manuscript. All authors provided valuable input to the interpretation of the data and critically reviewed the paper for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Palanisamy Sivanandy, upon reasonable request.
References
- 1.Ajmera A, Shabbir N. StatPearls Publishing; Treasure Island: 2023. Salmonella. [PubMed] [Google Scholar]
- 2.Giannella RA. 4th ed. University of Texas Medical Branch at Galveston; Galveston: 1996. Salmonella. [PubMed] [Google Scholar]
- 3.Mkangara M. Prevention and control of human salmonella enterica Infections: an implication in food safety. Int J Food Sci. 2023;2023 doi: 10.1155/2023/8899596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohmann EL. Nontyphoidal salmonella: microbiology and epidemiology. UpToDate, https://www-uptodate-com.ezp2.imu.edu.my/contents/nontyphoidal-salmonella-microbiology-and-epidemiology?search=salmonella%20infection%20types%20&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H3; 2023 [accessed 10 December 2023].
- 5.Centers for Disease Control and Prevention . CDC; 2023. National enteric disease surveillance: salmonella surveillance overview. [Google Scholar]; https://www.cdc.gov/nationalsurveillance/pdfs/NationalSalmSurveillOverview_508.pdf; [accessed 12 December 2023].
- 6.Centers for Disease Control and Prevention . CDC; 2024. Where sick people lived. [Google Scholar]; https://www.cdc.gov/salmonella/turtles-08-24/map.html; [accessed 20 September 2024].
- 7.Popa GL, Papa MI. Salmonella spp. infection - a continuous threat worldwide. GERMS. 2021;11:88–96. doi: 10.18683/germs.2021.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plumb ID, Schwensohn CA, Gieraltowski L, Tecle S, Schneider ZD, Freiman J, et al. Outbreak of salmonella Newport infections with decreased susceptibility to azithromycin linked to beef obtained in the United States and soft cheese obtained in Mexico - United States, 2018–2019. MMWR Morb Mortal Wkly Rep. 2019;68:713–717. doi: 10.15585/mmwr.mm6833a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Typhoid fever-Pakistan, https://www.who.int/emergencies/disease-outbreak-news/item/27-december-2018-typhoid-pakistan-en; 2018 [accessed 14 December 2023].
- 10.Fasih F, Fatima A, Baig S, Naseem S, Tauheed MM, Gohar H. Antimicrobial susceptibility of bacteraemic isolates of Salmonella enterica serovar typhi and paratyphi infection in Pakistan from 2017–2020. J Pak Med Assoc. 2023;73:505–510. doi: 10.47391/JPMA.6083. [DOI] [PubMed] [Google Scholar]
- 11.Fatima M, Kumar S, Hussain M, Memon NM, Vighio A, Syed MA, et al. Morbidity and mortality associated with typhoid fever among hospitalized patients in Hyderabad district, Pakistan, 2017–2018: retrospective record review. JMIR Public Health Surveill. 2021;7:e27268. doi: 10.2196/27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girgis NI, Butler T, Frenck RW, Sultan Y, Brown FM, Tribble D, et al. Azithromycin versus ciprofloxacin for treatment of uncomplicated typhoid fever in a randomized trial in Egypt that included patients with multidrug resistance. Antimicrob Agents Chemother. 1999;43:1441–1444. doi: 10.1128/AAC.43.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjölund-Karlsson M, Howie RL, Crump JA, Whichard JM. Fluoroquinolone susceptibility testing of Salmonella enterica: detection of acquired resistance and selection of zone diameter breakpoints for levofloxacin and ofloxacin. J Clin Microbiol. 2014;52:877–884. doi: 10.1128/JCM.02679-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry CM, Thieu NT, Dolecek C, Karkey A, Gupta R, Turner P, et al. Clinically and microbiologically derived azithromycin susceptibility breakpoints for Salmonella enterica serovars Typhi and Paratyphi A. Antimicrob Agents Chemother. 2015;59:2756–2764. doi: 10.1128/AAC.04729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Li Y, Xu X, Liang B, Wu F, Yang X, et al. Antimicrobial resistance of Salmonella enterica serovar typhimurium in Shanghai. China. Front Microbiol. 2017;8:510. doi: 10.3389/fmicb.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidary M, Ebrahimi Samangani A, Kargari A, Kiani Nejad A, Yashmi I, Motahar M, et al. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J Clin Lab Anal. 2022;36:e24427. doi: 10.1002/jcla.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tack B, Phoba MF, Thong P, Lompo P, Hupko C, Desmet S, et al. Epidemiological cut-off value and antibiotic susceptibility test methods for azithromycin in a collection of multi-country invasive non-typhoidal Salmonella. Clin Microbiol Infect. 2022;28:1615–1623. doi: 10.1016/j.cmi.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Jin C, Gibani MM, Pennington SH, Liu X, Ardrey A, Aljayyoussi G, et al. Treatment responses to azithromycin and ciprofloxacin in uncomplicated Salmonella Typhi infection: a comparison of clinical and microbiological data from a controlled human infection model. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UpToDate. Enteric (typhoid and paratyphoid) fever: treatment and prevention, https://www.uptodate.com/contents/enteric-typhoid-and-paratyphoid-fever-treatment-and-prevention#H1671911949; 2024 [accessed 24 January 2024].
- 20.Arumugham VB, Gujarathi R, Cascella M. StatPearls Publishing; Treasure Island: 2024. Third-generation cephalosporins. [PubMed] [Google Scholar]
- 21.Islam A, Butler T, Kabir I, Alam NH. Treatment of typhoid fever with ceftriaxone for 5 days or chloramphenicol for 14 days: a randomized clinical trial. Antimicrob Agents Chemother. 1993;37:1572–1575. doi: 10.1128/AAC.37.8.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Li H, Zhang T, Chu Y, Zuo J, Chen D. Study on antibiotic susceptibility of Salmonella typhimurium L forms to the third and forth generation cephalosporins. Sci Rep. 2020;10:3042. doi: 10.1038/s41598-020-59456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasopang ES, Hasanah F, Bakri TK, Isma M. Comparative effectiveness study of chloramphenicol and ceftriaxone in the treatment of typhoid fever in children admitted to Putri Hijau Kesdam I/Bb hospital Medan. Open Access Maced J Med Sci. 2019;7:3847–3851. doi: 10.3889/oamjms.2019.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Q, Ye Y, Lan P, Han X, Quan J, Zhou M, et al. Prevalence and characteristics of ceftriaxone-resistant Salmonella in children's hospital in Hangzhou. China. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.764787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baggio D, MR Ananda-Rajah. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161–164. doi: 10.18773/austprescr.2021.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shariati A, Arshadi M, Khosrojerdi MA, Abedinzadeh M, Ganjalishahi M, Maleki A, et al. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.1025633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhavan BJ, Khanna NR, Vijhani P. StatPearls Publishing; Treasure Island: 2024. Amoxicillin. [PubMed] [Google Scholar]
- 28.Nelson JD, Kusmiesz H, Jackson LH, Woodman E. Treatment of Salmonella gastroenteritis with ampicillin, amoxicillin, or placebo. Pediatrics. 1980;65:1125–1130. doi: 10.1542/peds.65.6.1125. PMID: 7375236. [DOI] [PubMed] [Google Scholar]
- 29.Giri A, Karkey A, Dangol S, Arjyal A, Pokharel S, Rijal S, et al. Trimethoprim-sulfamethoxazole versus azithromycin for the treatment of undifferentiated febrile illness in Nepal: a double-blind, randomized, placebo-controlled trial. Clin Infect Dis. 2021;73:e1478–e1486. doi: 10.1093/cid/ciaa1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khadka S, Shrestha B, Pokhrel A, Khadka S, Joshi RD, Banjara MR. Antimicrobial resistance in Salmonella typhi isolated from a referral hospital of Kathmandu. Nepal. Microbiol Insights. 2021;14 doi: 10.1177/11786361211056350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Palanisamy Sivanandy, upon reasonable request.