Abstract
The increasing impact of climate change and growing consumer interest in healthful foods have forced a reconsideration of indigenous plants as sustainable food resources. Parinari curatellifolia, popularly known as Mobola plum, is a prominent African underutilized plant whose natural habitat stretches from West to Southern Africa. It is an important source of food and ethnomedicines across Africa, a status boosted by the rich content of nutrients and phytochemicals in its different plant parts. Extracts from the different parts of the P. curatellifolia plant, which include leaves, pulp, seed, and whole fruit, have exhibited a broad range of health benefits, promoting its valorization into value-added products that are being marketed globally. This article provides a comprehensive review of the literature on P. curatellifolia, critically discussing its nutritional composition, bioactive phytochemicals, biological activities, safety and allergenicity, application in ethnomedicine, and value-added food and cosmetic products. To incite further research on this plant and its consideration as a vital resource that can be sustainably utilized to improve food and nutrition security and human health, the knowledge gaps and prospects of P. curatellifolia are highlighted.
Keywords: Mobola plum, Nutritional composition, Bioactive phytochemicals, Ethnomedicine, Food and nutrition security, Health
Graphical abstract
Highlights
-
•
P. curatefollia is a vital economic plant rich in nutrients and bioactive phytochemicals with various biological activities.
-
•
Reported pharmacological properties include antioxidant, anti-inflammatory, antidiabetic, anticancer, antiproliferative, and hepatoprotective activities.
-
•
A holistic approach that focuses on different research areas to increase productivity and sustainability is warranted.
1. Introduction
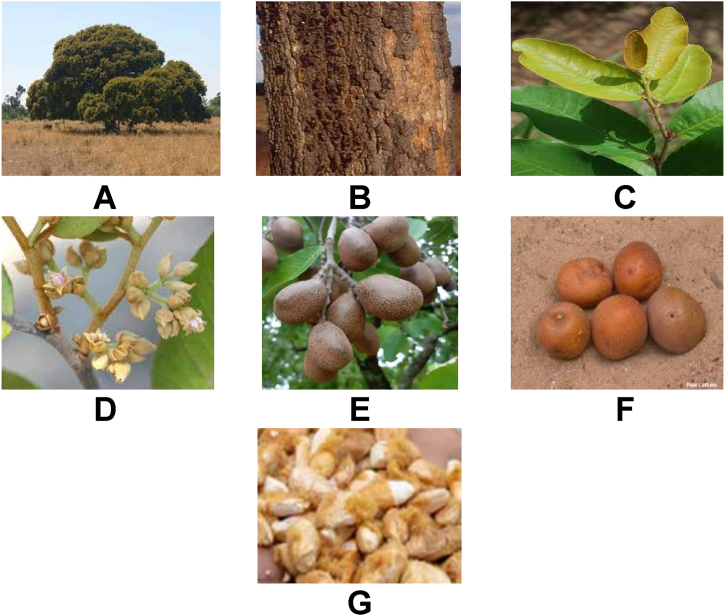
Exploring indigenous crops is vital for enhancing food security as they promote nutritional diversity and support sustainable agriculture. These crops are adapted to local conditions, offer resilience to environmental stresses, and reduce reliance on food imports. In addition, their multifunctional roles, from food to medicinal uses, support local economies and preserve traditional knowledge and biodiversity [1]. P. curatellifolia, commonly known as Mobola plum in Southern Africa, belongs to the Magnoliopsida and Chrysobalancaceae class and family, respectively [2]. The plant grows well in environments characterized by temperatures of 10–30 °C, rainfall ranging from 100 to 2700 mm, and altitudes of 0–1900 m above sea level [2]. The primary distribution zones include West Africa (from Senegal to Chad), East Africa (Kenya), and Southern Africa (Zambia, Zimbabwe, and South Africa) [3]. P. curatellifolia plant exhibits variability in shape and size, with heights ranging from 3 to 20 m. It is characterized by evergreen leaves and a single, bare stem with grey-colored bark [4]. P. curatellifolia bears seasonal ovoid drupe fruit that has a russet-yellow to grey color when unripe and turns orange-yellow when fully ripe (Fig. 1A–D). The fruit contains a light yellow to reddish mealy pulp with a sweet taste and an edible seed.
Fig. 1.
P. curatellifolia tree and its different parts. (a) Whole tree (b) Bark (c) Leaf (d) Flower (e) Unripe fruit and (f) Fully ripe fruit (g) Seed.
The different parts of the P. curatellifolia plant, including roots, stem, leaves, and fruit, have been reported to possess medicinal properties that have been exploited by the traditional medicinal systems to treat different ailments such as cancer, ear problems, sore eyes, fever, pneumonia, and microbial infections [2,4]. Despite the evidence of P. curatellifolia possessing medical properties, the plant remains underutilized. The medicinal properties are attributed to various nutrients and phytochemical compounds found in different parts of the plant.
Compounds with therapeutic functions such as saponins, balsams, alkaloids, tannins, cardiac glycosides, flavonoids, digitalis glycosides, phenols, terpenes, and steroids have been reported from the stem of the plant [5], while the plant leaves have been found to contain compounds such as alkaloids, flavonoids, and saponins [4]. The fruit pulp is a rich source of nutrients such as carbohydrates, protein, fat, fiber, vitamin C, polyphenols, and anthocyanins [6], and its seed is a treasure of bioactive compounds, including phenols, glycosides, alkaloids, flavonoids, anthraquinones, and various fatty acids [[7], [8], [9]]. Based on the biological activities of the highlighted compounds, various in vivo and in vitro animal models, as well as clinical studies, have demonstrated that P. curatellifolia possesses a variety of health benefits such as antioxidant, antimicrobial, anticancer, antiobesity, and antidiabetic properties [2,10].
The edible part of the fruit is used in different parts of Africa to make various food products such as soft porridge, jam, biscuits, and syrup and therefore, P. curatellifolia plays a significant role in contributing to food and nutrition security in rural areas of Africa. The oil from P. curatellifolia seeds has been used to produce value-added cosmetic and detergent products [8]. In light of this, P. curatellifolia holds considerable economic potential and it is imperative to investigate multiple avenues for adding value to the plant involving a variety of sectors from research, agronomy, agro-processing and marketing. This multi-faceted approach can significantly contribute to the continent's economic development, leveraging the plant's potential to enhance agricultural productivity, diversify income sources, and stimulate market growth. The potential benefits of P. curatellifolia go beyond African boundaries, as various value-added products from the seed oil are available in European markets [11]. Therefore, to encourage further research that will reveal more benefits from the plant, this paper critically reviews the nutritional composition, bioactive phytochemicals, and health benefits of P. curatellifolia, highlighting the various industrial applications and value-added products.
2. Methodology
This review was compiled using data from the Google Scholar database and Web of Science, employing Boolean operators 'AND' and 'OR' to enhance the search scope. The keywords P. curatellifolia, Mobola plum, nutritional composition, bioactive phytochemical, health properties, and uses or applications were used to search for information. Attention was given to scientifically indexed articles that were written in English only. In the last decade, the number of publications on P. curatellifolia has fluctuated, suggesting a lack of investment in P. curatellifolia research. The primary focus was on literature published between 2010 and 2024 (Fig. 2), although seminal works predating 2010 were also considered. More than 500 articles were found in the literature, screened into only 115 articles that were finally used to write this review (Fig. 2). These articles were selected based on the presence of specific keywords like ‘P. curatellifolia’, ‘Mobola plum’, ‘nutritional and phytochemical composition’, ‘bioactive and health properties’, ‘volatiles’, and ‘food and non-food application’ in their titles or abstracts. The findings of this review were used to develop suggestions and research prospects for P. curatellifolia.
Fig. 2.
The number of articles on P. curatellifolia searched on the Web of Science and Google Scholar. The articles were analyzed to categorize them into one of the groups of information: nutritional and phytochemical composition, bioactive and health benefits, volatiles, food, and other applications. <2010 refers to articles older than 2010 used to write this review article.
3. Nutritional value and chemical composition
3.1. Proximate composition
P. curatellifolia fruit is a good source of macronutrients such as carbohydrates, proteins, and fat, making it a suitable plant that can be promoted to enhance food nutrition and alleviate nutritional deficiencies. The major source of protein is the seed, which contributes about 12.7–27.1 %, while the pulp and peel contain minor quantities of protein ranging from 2.6 to 5.0 % (Table 1). This presents an opportunity to develop protein-based food products from the seed, given the global shift towards plant-based diets. Different studies have reported varied concentrations of protein in the seeds, pulp, and peel (Table 1), highlighting the influence of variety, growing region, and processing methods on the protein content. Studies on the profiling of amino acids in the different parts of P. curatellifolia are limited. However, Nkosi et al. [6] identified tryptophan, an amino acid with a side chain indole in P. curatellifolia fruit, with a concentration around 185 mg/kg. Apart from being important for the normal growth of infants and for the production and maintenance of the body's proteins, muscles, enzymes, and neurotransmitters, tryptophan has been reported to be an effective antioxidant. The fruit and pulp of P. curatellifolia are rich in carbohydrates, with levels ranging from 86 to 88 %, providing a significant source of energy. The seed also contains carbohydrates, albeit in smaller amounts (Table 1). However, detailed information regarding the specific types and quantities of sugars in P. curatellifolia is limited in literature. However, the pulp and seed may contain 111 and 68 mg/100 g of sugar [12] and some of the identified sugars were galactose, xylose and glucose [13].
Table 1.
The proximate composition of P. curatellifolia fruit.
| Fruit part | Carbohydrate (%) | Protein (%) | Fat (%) | Fiber (%) | Ash (%) | Sugars (mg/100g) | Vitamin C (mg/100g) | Reference |
|---|---|---|---|---|---|---|---|---|
| – | – | 2.0 | 21.4 | 1.6 | – | [12] | ||
| Fruit | 88.2 | 3.0 | 1.5 | 5.5 | 1.8 | – | 75 | [14] |
| 21.2 | 2.6 | 0.3 | 8.9 | 3.7 | – | [76] | ||
| Pulp | 86.2 | 3.9 | 4 | 4.7 | 2.5 | 111 | 531.1 | [12] |
| – | 3.4 | 0.9 | 5.4 | 3.9 | – | 6600 | [13] | |
| Pulp + Peel | – | 5.1 | 2.1 | 6.3 | 2.9 | – | – | [12] |
| – | – | – | – | 3.4–4.0 | – | – | [77] | |
| Peel | – | 4.2 | 3.7 | 19.4 | 5.7 | [12] | ||
| 26 | 15.6 | 46.1 | 1.58 | 2.4 | – | – | [12] | |
| Seed | 73 | 12.7 | 1.8 | 5.45 | 2.7 | 61.8 | – | [78] |
| 26 | 27.1 | 47 | – | – | 61.8 | – | [79] |
Fat content has been analyzed in different parts of P. curatellifolia, with the seed being the primary source of fat. The seed contains up to 47 % fat, which varies with variety and location, presenting a huge potential for its economic exploitation. The fat in the peel and pulp has been reported to vary between 0.3 and 5 % (Table 1). Crude fiber ranged between 1.6 and 5.5 %, 4.7–5.4 %, 6.3–19.4 % and 5.5–21.4 % for the seed, pulp, peel, and fruit, respectively. The fruit is, therefore, a rich source of fiber that can be used to produce affordable high-fiber products to prevent cholesterol accumulation and the risks of cardiovascular diseases and certain types of cancer. The ash content from the different parts of P. curatellifolia fruit is presented in Table 1. There is not much variation in the ash content from different fruit parts, which ranges from 1.6 to 3.7 % (fruit), 0.6–3.9 % (pulp), 2.9–5.6 % (peel), and 2.4–2.7 % (seed) (Table 2).
Table 2.
Mineral content (μg/g) of different components of P. curatellifolia.
| Se | Ca | Mg | K | Zn | P | Fe | Na | Mn | Cr | Cu | Co | Ni | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | 7058.4 | – | 3284.4 | 100.4 | – | – | 42522 | 21.7 | 35.1 | 4.2 | 26.7 | 27 | [16] | |
| Pulp | – | 1000 | 200 | – | 100 | – | – | – | – | – | – | – | – | [13] |
| – | – | 2130.6 | 7126.5 | 175.7 | 4052.3 | 2954.9 | 242.7 | 62.6 | [12] | |||||
| Pulp and peels | – | 3000–5000 | 1000–2000 | 9000-15 000 | – | 2000 | 5000–8000 | – | 1000–2000 | – | 2000–5000 | – | – | [77] |
| – | 2160.8 | 7108.6 | 178.5 | 3705.7 | 3987.8 | 315.3 | 1127.4 | [12] | ||||||
| Peels | – | 2000.1 | 7362.4 | 375.7 | 3826.6 | 4038.1 | 293.3 | 702.3 | [24] | |||||
| Fruit | – | 129 | 830 | 10380 | 399 | – | – | 252 | – | – | – | – | – | [80] |
| – | 3852 | 1564 | 10534 | 20 | 509 | 3.7 | – | ND | – | – | – | – | [17] | |
| Seed | – | 2224.5 | 6802 | 421 | 5406.7 | 3570.2 | 457.9 | 125.9 | [12] | |||||
| 283 | 2753 | 2808 | 4714.3 | 42.5 | 4867 | – | – | 23.3 | ND | 28.8 | – | 2 | [8] |
Limited information has been reported on the content of vitamin C in P. curatellifolia fruit. Benhura et al. [14] and Chatepa et al. [12] reported that the fruit and pulp could contain about 75 and 532.1 mg/100 g of vitamin C. These results significantly differed from the vitamin C content (6600 mg/100g) [13] reported from P. curatellifolia pulp. Given that vitamin C deficiency is prevalent in mothers and infants in some rural communities in African countries [15], value-added vitamin C-rich P. curatellifolia products could contribute to vitamin C deficiency solutions. In addition to being an important physiological antioxidant, vitamin C is required for the biosynthesis of proteins and amino acids such as collagen and L-carnitine, and certain neurotransmitters play a significant role in immune function.
3.2. Mineral content
P. curatellifolia fruit and its by-products are good sources of minerals such as calcium (Ca), magnesium (Mg), potassium (K), zinc (Zn), phosphorus (P), and iron (Fe), with average concentrations of 1660, 1481, 8502, 230, 2819, and 2746 μg/g, respectively, from the different studies found in the literature (Table 2). Minerals such as manganese (Mn) and copper (Cu) are found in high concentrations in the peels, seeds, and pulp, along with notable levels of cobalt (Co) and nickel (Ni). However, there is limited research on the presence of sodium (Na) and chromium (Cr) in these components (Table 2). These minerals are highlighted in the literature as essential for human nutrition and health, playing crucial roles in pH regulation, enzyme systems, hormone synthesis, bone formation, electrolyte balance, blood formation, and immune function [16]. Therefore, P. curatellifolia might significantly contribute to the mineral requirements of the African rural populace. Toxic heavy metals such as lead and aluminium have been reported in P. curatellifolia leaves and fruit but in very low concentrations (7–109 μg/g) [16,17]. These findings underscore the significance of the safety analysis of P. curatellifolia products, which has not been prioritized in many studies.
3.3. Phenolic compounds
Phenolic compounds present a huge potential for use in pharmacology as bioactive agents to promote health due to their antioxidant, cardioprotective, anticancer, and anti-inflammatory effects, as well as their antibacterial properties and their ability to protect the skin from ultraviolet (UV) radiation [2,10]. Different parts of the P. curatellifolia plant have been reported to contain varied concentrations of total phenolic compounds. Large discrepancies in the concentrations of total phenolic content (TPC) in P. curatellifolia leaf extracts (0.3–13.5 mg/g) (Table 3), probably due factors such as the plant-growing region and the extract processing methods. Ibibia et al. [16] reported that P. curatellifolia methanolic leaf extracts contained about 13.5 mg QE/g, while Daouda et al. [18] found that hydroethanolic leaf extracts of the P. curatellifolia plant from Burkina Faso contained about 0.3 mg GAE/g. Although studies investigating TPC in P. curatellifolia remain limited, Mwamatope et al. [19] managed to quantify TPC (725–1016 mg GAE/g fresh weight, FW) in P. curatellifolia fruits from Malawi, while Nkosi et al. [6] both profiled and quantified the different phenolic compounds found in P. curatellifolia fruit from South Africa. The concentrations of phenolic acids ranged from 0.3 to 46.9 mg/kg with the predominant ones being 3-O-p-coumaroylquinic acid, trans-5-caffeoylquinic acid (3-CQA) (neochlorogenic acid), 3-caffeoylquinic acid (5-CQA) (chlorogenic acid), and 3-O-caffeoylshikimic acid [6]. The 3-O-p-coumaroylquinic acid is one of the important antioxidants in plants due to the presence of a p-coumaroyl moiety, which allows it to effectively scavenge free radicals (Fig. 3). The study highlighted that these compounds from P. curatellifolia fruit could serve as functional compounds in dietary supplements and food additives. Given the potential of P. curatellifolia fruit as a fruit that could help alleviate food and nutrition insecurity in African countries, more research is warranted to explore the phytochemicals found in the fruit.
Table 3.
Phytochemicals content (mg/g) in P. curatellifolia.
| Part | Phenols | Flavonoids | Phytates | Oxalates | Alkaloids | Proanthocyanidins | Saponins | Tannins | Terpenoids | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | 13.5 | 58.4 | – | – | – | – | – | – | [16] | |
| 0.3 | – | – | – | – | – | – | – | [18] | ||
| Fruit | 725–1016 | 57–61.3 | – | – | – | – | – | – | [19] | |
| Seed | 9.4 | 1.6 | – | – | – | – | – | – | [81] | |
| 2.2 | – | – | – | – | – | – | – | [10] | ||
| – | – | 65.3 | 169.9 | 102.7 | – | – | – | [24] | ||
| 12.7 | 22.4 | – | – | [82] | ||||||
| 190 | 120.2 | 150 | – | 22 | 330 | 60 | [22] | |||
| Pulp | 12 | – | – | – | – | 0.004 | – | – | [20] | |
| Arial parts | 34.7 | – | – | – | – | 25.8 | – | – | [21] |
Fig. 3.
Chemical structures of the predominant phenolic compounds (phenolic acids and flavonoids) identified in P. curatellifolia fruit and seed oil.
Due to the potential of P. curatellifolia seed oil as an ingredient to formulate various cosmetic and anti-aging products, TPC has been well investigated in this component of the plant with values varying from 2.2 to 190 mg/g, depending on factors such as the plant's growing region, the fruit's maturity, and oil extraction techniques (Table 4). Profiling the individual phenolic compounds from P. curatellifolia seed oil revealed that it contained phenolic acids such as gallic acid, chlorogenic acid, caffeic acid, and ellagic acid, with concentrations ranging from 7.4 to 66.1 mg/kg [7] (Table 4). However, little or no information is available on the maturity indexing of P. curatellifolia fruit, which is valuable data to potential fruit and seed commercial processors. The pulp and arial part of P. curatellifolia were reported to contain 12 and 34.7 mg/g of TPC, respectively [20,21].
Table 4.
Phenolic compounds (mg/kg) in different parts of P. curatellifolia.
| Class | Phenolic compound | Concentration | Extraction solvent | Characterization | References |
|---|---|---|---|---|---|
| Fruit | |||||
| Phenolic acids | Trans-5-Caffeoylquinic acid (3- CQA). (Neochlorogenic acid) | 0.3 | 80 % methanol | UPLC-QTOF/MS | [6] |
| 3-Caffeoylquinic acid (5-CQA) (chlorogenic acid) | 17.8 | 80 % methanol | UPLC-QTOF/MS | ||
| 3-O-p-Coumaroylquinic acid | 46.9 | 80 % methanol | UPLC-QTOF/MS | ||
| 3-O-Caffeoylshikimic acid | 0.8 | 80 % methanol | UPLC-QTOF/MS | ||
| Anthocyanins | Delphinidin 3-galactoside | 0.2 | 80 % methanol | UPLC-QTOF/MS | |
| Delphinidin 3-O-glucoside | 0.1 | 80 % methanol | UPLC-QTOF/MS | ||
| Cyanidin 3,5-O-diglucoside | 2.1 | 80 % methanol | UPLC-QTOF/MS | ||
| Flavonoids | Catechin | 3.36 | 80 % methanol | UPLC-QTOF/MS | |
| Epicatechin | 3.8 | 80 % methanol | UPLC-QTOF/MS | ||
| Myricetin 3-galactoside | 0.9 | 80 % methanol | UPLC-QTOF/MS | ||
| Myricetin 3-arabinoside | nd | 80 % methanol | UPLC-QTOF/MS | ||
| Quercetin 3-galactoside | 0.2 | 80 % methanol | UPLC-QTOF/MS | ||
| Methyl gallate 3-O-beta-D- glucopyranoside | nd | 80 % methanol | UPLC-QTOF/MS | ||
| Gentisic acid 5-O-glucoside | 5.3 | 80 % methanol | UPLC-QTOF/MS | ||
| Procyanidin dimer B1 | 1.8 | 80 % methanol | UPLC-QTOF/MS | ||
| Procyanidin B5 | 0.6 | 80 % methanol | UPLC-QTOF/MS | ||
| Procyanidin B-type dimer | 0.9 | 80 % methanol | UPLC-QTOF/MS | ||
| β-Glucogallin (1-O-Galloyl-β-D- glucopyranose) | 0.1 | 80 % methanol | UPLC-QTOF/MS | ||
| Xanthohumol A Prenylated Flavonoid | nd | 80 % methanol | UPLC-QTOF/MS | ||
| Tryptophan | 185.7 | 80 % methanol | UPLC-QTOF/MS | ||
| Seed | |||||
| Phenolic acids | Gallic acid | 7.4 | 80 % methanol | HPLC | [7] |
| Catechin | 14.9 | 80 % methanol | HPLC | ||
| Chlorogenic acid | 21.7 | 80 % methanol | HPLC | ||
| Caffeic acid | 66.1 | 80 % methanol | HPLC | ||
| Ellagic acid | 32.8 | 80 % methanol | HPLC | ||
| Flavonoids | Epigallocatechin | 13.5 | 80 % methanol | HPLC | |
| Rutin | 52.4 | 80 % methanol | HPLC | ||
| Isoquercitrin | 24.6 | 80 % methanol | HPLC | ||
| Quercitrin | 33.9 | 80 % methanol | HPLC | ||
| Quercitin | 17.7 | 80 % methanol | HPLC | ||
| Kaempferol | 31.3 | 80 % methanol | HPLC | ||
nd = non detected, HPLC= High-performance liquid chromatography, UPLC-QTOF/MS= Ultra-high performance liquid chromatography with quadrupole time-of-flight mass spectrometry.
3.4. Flavonoids
The therapeutic value of P. curatellifolia is dependent on the antioxidant, antimicrobial, antimutagenic and anticarcinogenic properties of the phytochemicals, which include flavonoids. The concentration of total flavonoids in the different parts of P. curatellifolia is presented in Table 3. Flavonoids have been sparsely documented in the leaf and fruit extracts of P. curatellifolia, though their presence has been noted in the seed oil. The flavonoids in the seed oil could range between 1.6 and 120.2 mg/g, with the highest concentration being reported in the P. curatellifolia seed from Nigeria (Table 3) [22]. Total flavonoids of P. curatellifolia seed oil cake was also analyzed (>40 mg CE/100 g dry matter, DM) and found to be fairly comparable to the flavonoids observed in other seed oil cakes such as Entada abyssinica, Brachystegia longifolia, Caesalpinia decapetala, Dodonaea viscosa, Ipomoea involucrata, Myrianthus arboreus, Maesopsis eminii, Sterculia tragacantha, and Uvaria angolensis [23]. Flavonoids including catechin, epicatechin, cyanidin 3,5-O-diglucoside, and gentisic acid 5-O-glucoside were identified in extracts of P. curatellifolia fruit from South Africa [6]. Flavonoids are primary metabolites in P. curatellifolia seed oil, making it a vital resource for pharmaceutical, nutraceutical, and cosmetic applications. Crown et al. [7] reported that P. curatellifolia seed oil contained catechin, epigallocatechin, rutin, isoquercitrin, quercitrin, quercitin, and kaempferol with rutin being the major flavonoid compound (concentration >52.4 mg/kg) (Table 4). Rutin is a citrus flavonoid glycoside (Fig. 3), with various physiological functions in the human body.
3.5. Alkaloids and proanthocyanidins
Alkaloids are bioactive natural nitrogen-containing secondary metabolites with diverse therapeutic functions that include antitumor and antimicrobial activities. They are mostly reported from P. curatellifolia seed oil (Table 3). Chatepa et al. [24] reported that solvent-extracted P. curatellifolia seed oil from Malawi contained about 102.7 mg/g of alkaloids, which was higher than the oil of other indigenous seeds in Malawi, such as Moringa oleifera. Manuwa et al. [22] reported that P. curatellifolia seed oil contained about 150 mg/g of alkaloids. Proanthocyanidins are plant pigments that give the fruit or flowers of many plants their color properties and generate anthocyanins upon heating in acidic media. Thus, these compounds have been studied for their health benefits. Proanthocyanidins were reported in the pulp and aerial parts of P. curatellifolia, with concentrations ranging from 0.004 to 25.8 mg/g (Table 3).
3.6. Tocopherols and carotenoids
Epidemiological studies have shown that tocopherols and carotenoids are important bioactive phytochemicals that prevent several chronic diseases [10]. Carotenoids are known to prevent age-related diseases, different forms of cancer, cataracts, cardiovascular diseases, and macular degeneration [8]. Meanwhile, tocopherols are excellent natural antioxidants that prevent cardiovascular, bone, eye, neurological, and nephrological diseases [2]. Tocopherols and carotenoids have been largely studied in P. curatellifolia seed oil, although the concentrations are very low. Frankova et al. [8] quantified the tocopherol, tocotrienol, and carotenoid content of P. curatellifolia seed oil and identified three different types of tocopherols, namely, α-tocopherol (36.4 μg/g), γ-tocopherol (6.6 μg/g), and δ-tocopherol (6.3 μg/g). Their results suggest that α-tocopherol could be the predominant type of tocopherol in P. curatellifolia seed oil. The carotenoid content of 0.7 μg/g was reported, and it was relatively lower than the carotenoid content observed in Ochna serrulate (7.9 μg/g). The study highlighted that the amount of tocopherols and carotenoids observed in their study could have been influenced by environmental factors such as drought, excessive sunlight, genetic variability, and processing methods and storage conditions [8].
3.7. Other phytochemicals
Other phytochemicals, including phytates, oxalates, saponins, tannins, and terpenoids, have been reported mainly in P. curatellifolia seed oil. Despite their biological activities, phytates, oxalates, saponins, tannins have been classified as antinutritional factors that interact with vital nutrients and reduce their biological activities [25]. The studies done by Chatepa et al. [24] and Manuwa et al. [22] revealed that concentrations of these phytochemicals ranged from 22 to 330 mg/g, with tannins and oxalates being found in higher concentrations than phytates, saponins, and terpenoids. This information is presented in Table 3. Given the growing evidence on the medicinal properties of the leaves, flowers, bark, seeds, fruits, stems, and roots of P. curatellifolia, efforts should be focused on phytochemical screening of these various parts of P. curatellifolia to understand the potential physiological effects they have on human health.
3.8. Fatty acids
Studies have reported that P. curatellifolia seeds are a rich source of oil (>70 %), containing various nutritionally essential fatty acids necessary for hormone production in the human body. Frankova et al. [8] studied the fatty acid composition of P. curatellifolia seed oil and compared it with other seed oils indigenous to Africa, such as Ochna serrulate and Schinziophyton rautanenii. Various saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) were identified in the P. curatellifolia seed oil. P. curatellifolia and Schinziophyton rautanenii oils showed comparable fatty acids, which were α-eleostearic, linoleic, and oleic acids, while Ochna serrulata was dominated by palmitic, oleic, and linoleic acids [8]. Seed oils of P. curatellifolia fruit from West Africa were found to contain low amounts of SFA (13 %), significant amounts of MUFA (37 %), and PUFA (48 %). The individual fatty acids identified were palmitic, stearic, oleic, linoleic, linolenic, arachidic, and eicosenoic acids, which accounted for 6.7, 6.3, 36.2, 10.2, 38.2, 0.5, and 1.2 %, respectively [9]. As a result of the diversified fatty acids, P. curatellifolia seed oil has been a target as a raw material for producing various cosmetic products. A principal component analysis (PCA) of the composition and content of fatty acids in P. curatellifolia seed oil with Adansonia digitata L, Schinziophyton rautanenii, Mimusops caffra, and Ochnaserrula seed oils commonly found in Africa revealed that P. curatellifolia and Schinziophyton rautanenii contained comparable fatty acids in the form of eicosanoic acid, α-eleostearic acid, and linoleidic acid (Fig. 4) [8,26,27]. The pharmacological properties of α-eleostearic acid and eicosanoic acid have been widely reported, therefore, a great potential exists for P. curatellifolia seed oil to be used in formulating functional foods.
Fig. 4.
Principal component analysis showing a comparison of fatty acids in oils from P. curatellifolia, Adansonia digitata L, Mimusops caffra, Ochnaserrulata and Schinziophyton rautanenii seeds. Adapted from Chivandi et al. [27]; Frankova et al. [8]; Idris et al. [26].
3.9. Volatiles
Evaluating the volatiles in food is a crucial part of the food production process since it provides information about the quality of the food and how it influences consumer decisions. Factors such as developmental stages, genetic and environmental factors, postharvest treatment, and storage are implicated in the concentration and composition of volatile chemicals in plants [28]. Studies on the volatile compounds from P. curatellifolia are limited in the literature. Considering the potential of P. curatellifolia application in the production of value-added food products, there is a need for more studies that profile and quantify the volatiles in various parts of P. curatellifolia. However, Joulain et al. [29] and Shoko et al. [28] have studied the volatile compounds in the pulp of P. curatellifolia fruit. In their study, Joulain et al. [29] reported 88 volatile compounds, classified as esters, alcohols, phenols, carbonyls, and other compounds containing nitrogen and sulfur. The authors highlighted that the flavor profile exhibited a diverse range of compounds, suggesting their potential application in flavor compounding [29]. In a similar study, Shoko et al. [28] explored the potential of headspace extraction of volatiles from the edible pulp of P. curatellifolia ripe fruit. Their study revealed that the pulp contained volatile compounds, including ethyl butyrate, ethyl isovalerate, ethyl valerate, ethyl hexanoate, ethyl benzoate, isoamyl isovalerate, phenol, α-bergamotene, β-farnesene, 2,6-diterbutyl-4-methylphenol, and phenylacetonitrile, which varied from 1.1 to 28.7 %. The influence of environmental conditions on the formation of volatiles was emphasized [28].
4. Biological activities
4.1. Antioxidant activity
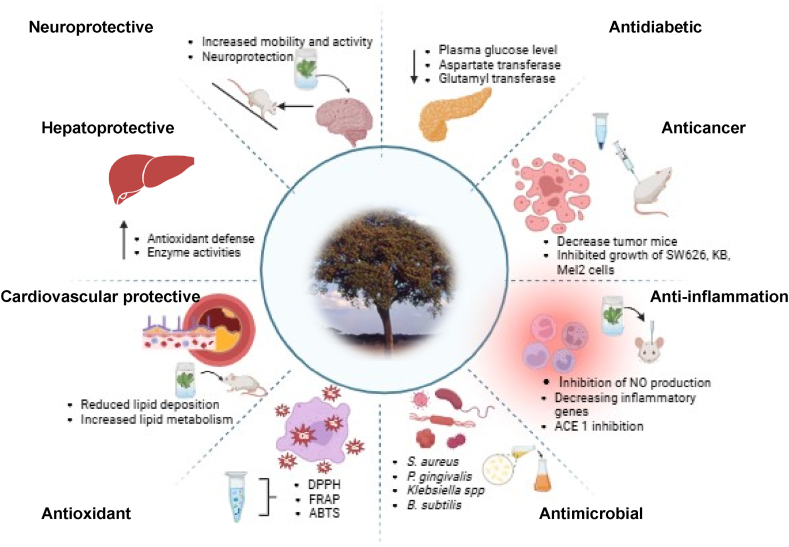
Research on the in vitro antioxidant activity of extracts from different P. curatellifolia tree parts support its wide application in disease management through its various biological activities (Fig. 5). In addition, the observed demonstration of antioxidant activity by all the plant parts evaluated may indicate that the bioactive benefits of P. curatellifolia are not localized in one part of the plant. Studies also showed the various antioxidant mechanisms of the reaction of the polyphenols from P. curatellifolia. Nkosi et al. [6] observed higher ferric-reducing antioxidant power (FRAP) in P. curatellifolia pulp than in other indigenous fruits as well as commercial fruits such as mango, orange, and pineapple [6]. According to Nkosi et al. [6], the reducing activity was attributed to its content of glucogallin, procyanidin B5, and procyanidin dimer B1, based on the high correlation between the content of individual phenolics and reducing activity. The proanthocyanidin structure has hydroquinone moieties (B-ring), which enable efficient electron donation properties. This property of proanthocyanidins increases with increasing polymerization, making proanthocyanidins effective reducing agents.
Fig. 5.
A summary of the biological activities of phytochemical compounds from different parts of P. curatellifolia.
Extracts from P. curatellifolia also showed high radical scavenging activity based on DPPH, nitrite radical scavenging, H2O2 radical scavenging, and OH radical scavenging activity (Table 5). The observed radical scavenging relies on the H-atom transfer ability of the polyphenols [30]. The catechol structure of the B-ring, the 2,3-double bond with 4-oxo function, as well as the presence of the 3- and 5- OH groups on the quercetin glycosides and procyanidins identified in P. curatelifolia extracts, support the suggested mode of action [19]. In other research, Ademosun et al. [31], as well as Perron and Brumaghim [32] showed that the chelating ability of polyphenols allows them to inhibit metal-catalyzed reactions that form deleterious products with pathological implications. One such by-product is malondialdehyde, a biomarker for lipid peroxidation of polyunsaturated fatty acids that can contribute to in vivo DNA damage and mutation.
Table 5.
Antioxidant activity of different tree parts of P. curatellifolia.
| Plant part | Extraction solvent | Observed Effects | References |
|---|---|---|---|
| Stem bark | 20 % Ethanol-Diethyl ether-n Butanol |
|
[5] |
| Fruit pulp | 80 % Methanol |
|
[6] |
| Aqueous, 80 % Methanol |
|
[19] | |
| Aqueous |
|
||
| Peel | – |
|
[70] |
| Leaf | Ethanol | [44] | |
| Ethanol |
|
[43] | |
| Aerial parts | 70 % Methanol |
|
[21] |
| Seed | 80 % Methanol |
|
[7] |
| Ethanol |
|
[82] | |
| Aqueous |
|
[31] |
- 2,2-diphenyl-1-picrylhydrazyl inhibition assay.
- Ferric ion reducing antioxidant power assay.
– Total Antioxidant Activity assay.
-Tetramethoxy azobismethylene quinone free-radical scavenging.
– Malondialdehyde.
- concentration that quenched 100 % of radicals.
4.2. Antimicrobial activity
P. curatellifolia is one of the medicinal plants used to treat infectious diseases, which points to its potential as a source of natural antimicrobials (Fig. 5; Table 6). This characteristic is particularly important in the advent of antibiotic-resistant pathogens. In that regard, the antimicrobial efficacy of P. curatellifolia stem extracts against Pseudomonas aeruginosa, Salmonella typhi, Klebsiella spp. Bacillus subtilis, and Staphylococcus aureus using the agar diffusion method was investigated by Peni et al. [33]. Aqueous stem extracts were shown to have the most antimicrobial activity, with S. aureus and Klebsiella spp. being the most susceptible among the gram-positive and gram-negative bacteria, respectively. The aqueous solvent resulted in an extract with a more varied phytochemical profile than the other solvents, and most importantly, it extracted alkaloids, which have high antimicrobial efficacy [33]. Halilu et al. [34] evaluated the antibacterial activity of stem bark extracts against B. subtilis, S. aureus, Escherichia coli, and P. aeruginosa using the agar diffusion method. The ethyl acetate fraction was the most active, particularly against the gram-positive S. aureus and B. subtilis. This trend was the same in the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the ethyl acetate fraction, with S. aureus having the lowest values of 0.8 and 6.3 mg/mL [35].
Table 6.
In vitro antimicrobial activity of P. curatellifolia.
| Plant part | Solvent | Experimental model | Key findings | References |
|---|---|---|---|---|
| Stem | Aqueous, Diethyl ether, Methanol, Hexane |
Extract concentration – 2.8 g/100 mL Microorganisms tested: Pseudomonas aeruginosa, Salmonella typhi, Klebsella spp, Bacillus aureus, Escherichia coli, Bacillus subtilis and Staphylococcus aureus |
Aqueous extracts inhibited microbial growth and most effective against S. aureus and Klebsiella spp. methanolic fraction was effective against B. subtilis and P. aeruginosa while diethyl ether and n-hexane had no antimicrobial activity. | [33] |
| Leaves | Dichloromethane-methanol mixture (50 % v/v), hexane, DCM, acetone, ethyl acetate, ethanol, methanol, and water |
Extract concentration: 0.4–200 μg/mL Microorganisms tested: Broth microdilution with nosocomial pathogens including Mycobacterium smegmatis 155 mc2, Candida krusei, Klebsiella pneumoniae (ATCC 700603) and Staphylococcus aureus (ATCC 9144) |
Mycobacterium smegmatis was the most susceptible to most extracts. The methanol and ethanol extracts were the most active against M. smegmatis with an MIC of 25 μg/mL. Klebsiella pneumoniae and Staphylococcus aureus showed resistance against all extracts tested. Antifungal properties of P. curatellifolia extracts were attributed to β-sitosterol. | [36] |
| Dichloromethane-methanol mixture (50 % v/v), hexane, DCM, acetone, ethyl acetate, ethanol, methanol, and water |
Extract concentration: 0–1000 μg/mL Microorganisms used: Broth microdilution with Mycobacterium smegmantis |
Acetonic extracts had the lowest MIC at 6.2 μg/mL, followed by ethanol (12.5 μg/mL), with methanol and ethyl acetate at 50 μg/mL biofilm formation by M. smegmantis was inhibited by ethanol, dichloromethane, and water extracts. | [38] | |
| Stem bark, root, and leaves | Water, ethanol, and methanol |
Extract concentration: 50–500 mg/mL Microorganisms used: Staphylococcus aureus, Streptococcus mutans and Lactobacillus spp. |
Methanolic extracts had high inhibitory activity against all three microbes. | [83] |
| Root bark | Ethyl acetate | Microorganisms used:Candida albicans (ATCC 90028), Cryptococcus neoformans (ATCC 90112), Aspergillus niger (AZN 8240) and Candida albicans clinical isolates | Crude extracts showed lower MIC against C. neoformans and A. niger compared to control (clotrimazole) | [40] |
| Trunk bark | Ethanol |
Extract concentrations: 0.25–2.5 mg/mL Microorganisms used: Trichophyton rubrum, Trichophyton schoenleinii, and Microsporum canis |
The extracts were most effective against T. schoenleinii (MIC – 0.75 mg/mL), while the MIC for T. rubrum and M. canis was 1.5 mg/mL | [41] |
| Stem bark | Methanol, ethyl acetate and n-butanol |
Extract concentration: 50 mg/mL Microorganisms used: Cup plate method with Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus subtilis |
The ethyl acetate fraction was most effective, with its activity against S. aureus comparable to 0.01 mg/mL ampicillin. Additionally, the same fraction sowed a MIC and MBC of 0.78 mg/mL and 6.25 mg/mL against S. aureus. | [35] |
| Bark | Ethanol |
Extract concentration: 0.8–25 mg/mL Microorganisms used: Microdilution assay with oral pathogens Actinomyces naeslundii, Actinomyces israelii, Streptococcus mutans (Gram positive); Actinobacillus actinomycetemcomitans, Privotella intermedia, Porphyromonus gingivalis (Gram negative) |
Extracts were active against Gram negative oral pathogens with MIC and MBC in the range 1.6–6.3 mg/mL: MIC of 3.1–12.5 mg/mL for Gram negative pathogens with MBC of 25 mg/mL against Gram positive P. gingivalis | [84] |
MIC = minimum inhibitory concentration, MBC = minimum bactericidal concentration.
In another study, Mawire et al. [36] evaluated the antimicrobial activities of leaf extracts on four microbial isolates, including Klebsiella pneumoniae, Mycobacterium smegmatis, Candida krusei, and S. aureus. Serial exhaustive extraction had been applied to produce seven leaf extracts, including dichloromethane (DCM)-methanol (50 % v/v), followed by hexane, DCM, acetone, ethyl acetate, ethanol, methanol, and water. Klebsiella pneumoniae and S. aureus were resistant to all the extracts, with M. smegmantis being the most susceptible and C. krusei being the most sensitive to the hexane extracts [36]. Further evaluation by Mawire et al. [36] showed that the active compound was β-sitosterol, which achieved an 83 % reduction of C. krusei growth on Mendy fabric. According to Evangelina et al. [37], β-sitosterol inhibits the enzymes involved in the biosynthesis of peptidoglycan necessary for bacterial cell wall formation.
Leaf extracts of P. curatellifolia were also evaluated for compounds with anti-biofilm formation properties against Mycobacterium tuberculosis [38]. This bacterium is the causative agent for tuberculosis and has developed a drug-tolerant pellicle with serious public health consequences [38]. Acetone and ethanol extracts produced the least minimum inhibitory concentrations of 6.2 and 12.5 μg/mL, respectively. Ethanol, DCM, and aqueous extracts effectively inhibited M. tuberculosis biofilm formation (Table 6). The anti-biofilm properties can be attributed to β-sitosterol, which was identified in P. curatellifolia leaf by Mawire et al. [36]. Evidence from Vikram et al. [39] shows that β-sitosterol modulates biofilm formation via downregulation of the flhD gene, which contributes to biofilm and motility.
Research by Mbunde et al. [40] demonstrated that the bioactivity of P. curatellifolia extracts includes antifungal properties. In their work, Mbunde et al. [40] isolated two compounds from root bark ethyl acetate extracts, namely toddalolactone and 13-methoxy-15-oxozoapatlin. However, crude extracts showed greater antifungal efficacy compared to the isolates against all four fungi, including Candida albicans (ATCC 90028), Cryptococcus neoformans, Aspergillus niger, and a clinical isolate of Candida albicans. Mbunde et al. [40] argued that the increased antifungal properties of the crude compared to pure compounds may underscore the synergistic relationship between compounds that allow the crude extract to exert a greater effect. The ethnomedicinal use of P. curatellifolia in treating microbial diseases motivated Issakou et al. [41] to investigate the antidermatophyte properties of ethanolic stem-bark extracts. Three dermatophyte strains, including Trichophyton rubrum, Trichophyton schoenleinii, and Microsporum canis, were evaluated using the poisoned food method. The stem bark extracts inhibited dematophyte growth with a lower MIC of 0.8–1.8 mg/mL than fluconazole (MIC = 2 mg/mL). The antifungal activity was attributed to the phytochemical profile of the stem-bark extracts, including flavonoids, sterol terpenoids, tannins, quinones, alkaloids, heterosides, anthocyanins, and saponosides [41].
4.3. Antiinflammatory and anti-hypertensive activity
In their research, Gororo et al. [42] investigated the use of P. curatellifolia leaf extracts in the management of pain, inflammatory, and neoplastic conditions based on the traditional uses of the leaf extracts (Table 7). Aqueous leaf extracts of P. curatellifolia were found to scavenge nitric oxide (NO) radicals, in agreement with earlier research by Boora et al. [43]. Although NO has been noted to have limited chemical reactivity and direct toxicity, its bioavailability and downstream actions are modulated by the superoxide radical, which reacts with it to produce the highly toxic peroxinitrite ONOO•− radical. As described by Gororo et al. [42], prolonged cellular exposure to NO radicals and their by-products is implicated in pathological conditions, including juvenile diabetes, multiple sclerosis, arthritis, and ulcerative colitis. Under the conditions described by Gororo et al. [42], the P. curatellifolia polyphenols are likely to have scavenged the free radicals employing a single proton loss-electron transfer (SPLET) mechanism [30].
Table 7.
Anti-inflammatory effects and antihypertensive activity of P. curatellifolia.
| Plant part | Solvent | Type of study | Experimental model | Key findings | References |
|---|---|---|---|---|---|
| Leaf | Methanol, water, acetone, and ethanol | In vitro | Xanthine oxidase (XO) inhibition assay with extract concentration of 3.9–250 μg/mL Nitric oxide production in lipopolysaccharide (LPS), menadione and hydrogen peroxide-activated RAW 264.7 cells with ethanolic extract concentration of 25 μg/mL |
Ethanol and methanol extracts inhibited XO activity with IC50 of 1.38 μg/mL and 2.19 μg/mL, respectively Aqueous extracts reduced NO production in LPS activated RAW cells. |
[42] |
| Ethanol | In vitro | Cyclooxygenase (COX) enzyme inhibition with 20 μL of extract Sheep erythrocyte membrane stabilization activity |
Inhibition of COX-1 and activation of COX-2 Higher stabilization of membrane at 250 μg/mL than control (indomethacin) |
[44] | |
| Ethanol | In vitro | Hematopoietic prostaglandin D2 synthase (H-PGDS) inhibition assay | Mixed type reversible inhibition of H-PGDS, IC50 was 3.8 μg/mL | [45] | |
| Ethanol | In vivo | Adult male Sprague-Dawley rats (90–160 g) (n = 18). Dosage of 0, 500 and 1000 mg/kg body weight; orally once every 24 h for 96 h | Ethanolic leaf extracts reduced in vivo glutathione transferase activity (IC50 – 12 μg/mL) | [46] | |
| Seed | Ethanol | In vivo | Wister albino rats (150–200 g) (n = 24). Dosage of 200–800 mg/kg body weight for 2 weeks | Reduced contraction force and heart rate; caused a dose-dependent ↓ in systolic diastolic blood pressure and mean arterial blood pressure; an ↑ in the percentage change in mean arterial blood pressure, ↑ thiobarbituric acid reactive substance production and catalase, superoxide dismutase and glutathione peroxidase activities. | [82] |
| 80 % Methanol | In vivo | Adult male Wistar rats (160 ± 10g) infected with sodium nitroprusside (5 mg/kg). Dosage of 400, 500, and 600 mg/kg bwt, orally for 7 days | Extracts ↓ sodium nitroprusside (SNP) toxicity on the heart and artery tissue in rats | [47] | |
| 80 % Methanol | In vitro | Angiotensin-I-converting enzyme (ACE I) inhibition assay | Crude extracts showed superior ACE I inhibition (IC50 – 13.5 mg/mL) | [7] |
Anti-inflammatory properties can also be affected by inhibiting cyclooxygenase enzymes involved in the production of intermediates [44]. These enzymes, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) are involved in the formation of pro-inflammatory prostaglandins [44]. In their research, Chirisa and Mukanganyama [44] investigated the potential of P. curatellifolia extracts to selectively inhibit the COX-2 enzyme and potentially replace current synthetic inhibitors with negative physiological side effects. While the extracts had antioxidant activity via DPPH and tetramethoxy azobismethylene quinone (TMAMQ) free-radical scavenging assays, no selective inhibition of COX-2 was observed. As such, the use of P. curatellifolia leaf extracts in the management of inflammation points to other mechanisms than the inhibition of cyclooxygenases. The synthesis of prostaglandins in peripheral tissue can also be mediated by the hematopoietic prostaglandin D synthase (H-PGDS) enzyme [45]. According to Gweshelo et al. [46], leaf extracts inhibited H-PGDS activity as effectively as the standard inhibitor, Cibacron blue. The extracts were able to inhibit the free enzyme and bind the enzyme-substrate complex, thus demonstrating mixed-type inhibition.
Ethnomedicinal applications of P. curatellifolia in managing hypertension have also led Crown et al. [7] to investigate its effects on important mediators (Table 7). One such important therapeutic target is angiotensin-1-converting enzyme (ACE 1), whose inhibition has been established as an approach in the treatment of hypertension [7]. In that regard, crude methanolic seed extracts showed mixed-type inhibition of ACE 1 and the enzyme/substrate complex. From their observations, Crown et al. [7] argued that the inhibition of the metallopeptidase ACE 1 enzyme occurred via chelation of the zinc atom at its active center or through hydrogen bonding between polyphenols and amino acids at the active center. In vivo evaluation by Josiah et al. [47] showed that P. curatellifolia seed extracts had protective effects against sodium nitroprusside-induced toxicity on the heart and artery. While they alluded to the lack of mechanistic evidence to support their findings, earlier assertions by Crown et al. [7] provide ground to support the findings.
4.4. Anti-cancer and anti-proliferative activity
According to Sung et al. [48], cancer ranks as one of the leading causes of death and reduced life expectancy, with an estimated 19.3 million cases as of 2020 and a projected increase of 47 % to 28.4 million cases in 2040. Global cancer control is therefore calling for sustainable approaches to cancer prevention measures and care. As such, ethnomedicinal evidence has generated interest in natural remedies for combating cancer, including P. curatellifolia. In their research, Mukanganyama et al. [49] demonstrated the inhibitory activity of methanolic leaf extracts against breast cancer cell lines (Table 8). Earlier research by Shamon et al. [50] with root bark extracts led to the isolation of two ent-kaurene diterpenoids, demonstrating broad-spectrum cytotoxic activity. The compounds 13-methoxy-15-oxozoapatlin and 13-hydroxy-15-oxozoapatlin were attributed for their anticancer activity against human breast cancer cells (ZR-75-1). A follow-up study by Mi et al. [51] showed that 13-methoxy-15-oxozoapatlin had broad-spectrum cytotoxicity against a panel of human cancer cell lines (Table 9), further highlighting its chemotherapeutic efficacy.
Table 8.
Anticancer and antiproliferative effects of P. curatellifolia.
| Plant part | Solvent | Type of study | Experimental model | Key findings | References |
|---|---|---|---|---|---|
| Leaf | Ethanol | In vitro | Jurkat cells E 6.1 human leukemic T cell lymphocytes pretreated with extract (0–100 μg/mL). | Extracts induced apoptosis in Jukat T cell resulting in anti-proliferative effects. | [42] |
| Methanol | In vitro | ECACC strain Jurkat E6 (T-cell lymphocytic cell line) and ATCC strain Wil 2 (B cell lymphocytic cell line) with extract at concentrations of 0, 50, 100, 250, 500 and 1000 μg/mL. | The IG50 (the concentration for 50 % growth inhibition) against Wil 2 cell line was 93 μg/mL but was cytotoxic at > 500 μg/mL. Extract at 10 μg/mL reduced cell proliferation of Jurkat T cells by 70 % after 48 h of incubation. | [49] | |
| Root bark | Methanol | In vivo | Hollow fibers filled with each of the Lu1 human lung carcinoma cell, Mel2 human melanoma cell line, human oral epidermoid carcinoma KB cells, human prostate carcinoma LNCaP cells, human breast carcinoma MCF-7, mouse lymphoid neoplasm P-388 cells, and human ovarian adenocarcinoma SW626 cells, were implanted at the intraperitoneal (i.p.) and subcutaneous (s.c.) compartments of athymic mice (n = 9). Test compounds at doses of 72.6, 145.3, and 290.7 μmol/kg administrated once daily by intraperitoneal injection from day 3–6 after implantation. | 13-Methoxy-15-oxozoapatlin isolated from P. curatellifolia inhibited growth of cells at the i.p. compartment (except Lu1). The compound also inhibited the growth of SW626, Mel2, and KB cells implanted at the s.c. site. | [51] |
| Methanol | In vitro and in vivo | Cytotoxic evaluation against human cancer cell lines including A431, epidermoid carcinoma; BCI, breast cancer; Col2, colon cancer; HT. fibrosarcoma; LNCaP, prostate cancer; Lu 1, lung cancer; Me12, melanoma; U373, ghoma; KB, oral epidermal carcinoma; ZR75- I, breast cancer. Athymic mice implanted with human epidermoid carcinoma KB cells subcutaneously and administered intraperitoneally with 13-methoxy-15-oxozoapatlin (90 mg/kg) on days 1, 5 and 9. Breast cancer ZR-75-1 cells treated with 13-methoxy-15-oxozoapatlin (0–3 μM). |
Isolates of P. curatellifolia were cytotoxic against cancer cell lines (ED50 range 0.3–16.5 μM) via Michael-type addition at the β-position of the α,β-unsaturated carbonyl. Treated athymic mice carrying KB cells had no antitumor activity. Treated ZR-75-1 had reduced biosynthesis of DNA, RNA and protein, accumulation at the G2/M phase of the cell cycle. |
[85] |
Table 9.
Antidiabetic activity effect of P. curatellifolia.
| Plant part | Solvent | Type of study | Experimental model | Key findings | References |
|---|---|---|---|---|---|
| Root | Methanol | In vivo | Male albino rats with CCl4 (n = 30) Single (0.6 mL/kg) and repeated doses of CCl4 (0.3 mL/kg) |
Extracts reduced malondialdehyde, aspartate aminotransferase, alanine aminotransferase levels. Increased catalase and superoxide dismutase enzyme activity. |
[86] |
| Seed | 80 % Methanol | In vivo | Adult Wistar male rats (180–220 g); prooxidant – acetaminophen (25 mg/kg). Extract (10-, 20- or 30 mg/kg body weight) or silymarin (25 mg/kg), dissolved in corn oil; orally for 14 days. |
Reduced activity of hepatotoxicity markers including alanine aminotransferase (ALT), aspartate aminotransferase (AST), c- glutamyl transferase (GGT) and lactate dehydrogenase (LDH) and prothrombin time (PT). | [72] |
| 80 % Ethanol | In vivo | Wistar strain albino rats weighing 160 ± 20 g (n = 20); Single dose of P. curatellifolia and Aristolochia vogelli mixture (1:1) (500 mg/kg bwt); P. curatellifolia seed extract (500 mg/kg bwt) with oral administration of 40 % (w/v) glucose at a dose of 1 mL/100 g bwt after 30 min. Wistar strain albino rats weighing 160 ± 20 g (n = 30); alloxan monohydrate (150 mg/kg bwt) induced diabetes; Single dose of P. curatellifolia and Aristolochia vogelli mixture (1:1) (500 mg/kg bwt); P. curatellifolia seed extract (500 mg/kg bwt). |
Reduction in postprandial sugar levels after 30 min; reduction in plasma glucose levels in rats; reduction in low density lipoprotein (LDL)-cholesterol levels and increase in high density lipoprotein (HDL)–cholesterol in the treated group. Reductions in aspartate aminotransferases (AST) and alanine aminotransferases (ALT) in extracts treated diabetic rats; reduction in the creatinine and increase in the protein levels,respectively, in treated diabetic groups. |
[69] | |
| Aqueous | In vitro | Inhibition of α-amylase and α-glucosidase from pancreatic tissue homogenates of Wistar rats. | The EC50 for α-amylase and α-glucosidase inhibition were 0.7 and 0.6 mg/mL, respectively. | [31] |
Nevertheless, the interest of Shamon et al. [50] was the mode of action of these chemotherapeutic products. According to Shamon et al. [50], the α,β-unsaturated carbonyl group present in these compounds is believed to contribute to the cytotoxic effects as it participates in Michael-type addition covalent reactions with cellular components necessary for mitotic progression. In addition, Shamon et al. [50] opined that the activity was specific to the G2/M phase, thereby inducing cell cycle arrest. The G2 phase is important as macromolecules, proteins, and RNA, which are important for cellular multiplication, are organized at this phase. As such, any damage to DNA or sequestration of cellular components that ensure genetic stability at this stage triggers apoptosis [52]. An investigation by Mi et al. [51] further revealed the chemotherapeutic efficacy of 13-methoxy-15-oxozoapatlin at the subcutaneous site when tested using the hollow fiber model and xenograft. It is also important to note that these compounds present tumor-selective therapy with minimal toxicity, as supported by Kundishora et al. [4]. Leaf extracts, according to Kundishora et al. [4] were not toxic in vitro and showed immunostimulatory potential.
Research by Gororo et al. [42] also showed that leaf extracts had antiproliferative effects against Jurkat cells E 6.1 human leukemic T cell lymphocytes. The anti-cancer activity of the leaf extracts was shown to increase in the presence of glutathione. Moskaug et al. [53] provided the context for this observation, and they argued that intracellular antioxidant activity is mediated by glutathione, while dietary polyphenols modulate its activity and possible synthesis. In addition, Carreon-Gonzalez and Alvarez-Idaboy [54] gave insight into the synergistic cycle relationship between polyphenols and glutathione. Their study showed that within aqueous media, polyphenols regenerate glutathione via SPLET reactions, while a large lipid media promotes regeneration via proton-coupled electron transfer (PCET). Therefore, the regeneration allows glutathione to participate in more antioxidant reactions. However, there is still debate on the clinical anticancer interventions that include polyphenols, with more development needed to understand delivery systems and effects in combination with other agents [55].
4.5. Antidiabetic activity
Type 2 diabetes arises from a cluster of conditions collectively termed metabolic syndrome [56]. Along with physical activity and weight management, diet improvements are one of the most important interventions to reduce risk factors associated with type 2 diabetes. Increasing research evidence indicates that dietary bioactive compounds are key in preventing and ameliorating the progression of important risk factors associated with metabolic syndrome [56]. P. curatellifolia is among various ethnomedicinal remedies applied in diabetes management (Fig. 5). Administration of seed extracts led to a significant reduction in postprandial blood glucose levels in rats 30 min after ingestion of glucose [57]. Postprandial glucose level is important in determining overall control of glucose levels. Conditions associated with type 2 diabetes, including reduced insulin production due to β-cell dysfunction and insulin resistance, ultimately lead to hyperglycaemia [58]. In agreement, research by Omale et al. [59] showed that the administration of P. curatellifolia stembark extracts significantly decreased glucose levels in diabetic Drosophila melanogaster flies.
Polyphenols from P. curatellifolia can inhibit α-amylase and α-glucosidase activity, thus providing management of blood glucose levels [31]. Besides inhibiting the activity of digestive enzymes responsible for glucose production, dietary polyphenols also target transporters responsible for glucose uptake in the gut [60]. Research has shown that polyphenols modulate the function of sodium glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2) glucose transporters, thereby lowering plasma glucose levels [58]. Further evidence shows that while mRNA expression of glucose transporters is upregulated in the intestines of diabetic individuals [61]. This aligns with studied indicating that 16 h exposure of Caco-2 cells to berry extract polyphenol (0–0.25 %, w/v), significantly reduced the long-term expression of these glucose transporters in the gut [62].
5. Industrial application
Limited information on the industrial processing of indigenous fruits like P. curatellifolia and its by-products has contributed to their underutilization as well as postharvest losses. In sub-Saharan Africa, indigenous plants have a huge potential to alleviate food and nutrition insecurity and generate income for rural households. In addition, the by-products from indigenous fruits, such as peels and seeds, are sources of bioactive compounds that have contributed to their use in the treatment of various ailments. However, information regarding the processing of indigenous fruits and their by-products into value-added products that can be used in foods, cosmetics, pharmaceuticals, and nutraceuticals is poorly documented or not acknowledged as strategies that can reduce poverty or generate income [63]. Given the seasonality and perishability of indigenous fruits, their value addition into shelf-stable products is essential, as there are no controlled environmental facilities to store them in rural communities.
Despite their use as traditional medicine, P. curatellifolia fruit and its by-products have been underutilized, with little attention given to various products that can be developed from either the peel, pulp, or seeds [64]. P. curatellifolia fruit is regarded as one of the most nutritious indigenous fruits and is mostly consumed as a snack, except in times of famine, when the fruit becomes the principal source of food [65]. Nonetheless, various value-added products can be produced from fruit peel, pulp, and seeds, thus diversifying their use from food to pharmaceutical and cosmetic products [28]. Exploring alternative uses of P. curatellifolia fruit peel, pulp, and seeds will not only minimize postharvest losses but will reduce food and nutrition insecurity and greenhouse gas emissions and promote diversity in diets. Further, the value addition of indigenous fruits and their by-products will create competition with exotic fruit products, creating a competitive market that may force a decrease in the prices of current fruit products [12].
5.1. Food
The pulp, peel, and seeds of P. curatellifolia can be valorized into various food products with excellent nutritional, physical, and antioxidant properties. The pulp from P. curatellifolia fruit constitutes over 50 % of the intact fruit, thus making it a useful raw material for processing into various products [66]. In Malawi, the pulp has been used to make porridge, alcoholic beverages, jam, juice, and fritters that are used to feed infants [67]. The pulp can be sun-dried and consumed as a snack or ground into powder for use in different recipes. Benhura et al. [66] reported that Mrewa and Mutoko communities of Mashonaland East province in Zimbabwe utilize the pulp to prepare traditional biscuits popularly known in the area as zvambwa. To prepare the product, the pulp is converted into syrup, which is then mixed with finger millet (Eleusine coracana) meal and then baked. Benhura and colleagues managed to study the drying kinetics of this product at temperatures between 30 and 80 °C and established Modified Page model as the best model to describe the drying behaviour of this product [66]. A huge potential exists for the P. curatellifolia fruit pulp-based biscuit, syrup, juice, and jam to be developed into commercial products (Table 10). Despite these efforts in food product development from the pulp of P. curatellifolia, comprehensive studies on quality control of these products, specifically changes in physical properties, chemical composition, and sensory evaluation are needed.
Table 10.
Value-added products from P. curatellifolia fruit and by-products.
| Component | Quantity used | Type of product | Key parameters evaluated | Key findings | References |
|---|---|---|---|---|---|
| Pulp and peel | 10–40 % | Fortified finger millet flour | Color, proximate composition, thermal properties, bulk density, oil absorption capacity, water absorption capacity, swelling capacity and dispersibility | Addition of P. curatellifolia flour decreased the lightness but increased yellowness, redness, oil absorption capacity, bulk density, and dispersibility of the flour. P. curatellifolia flour also increased viscosity, protein, minerals, and carbohydrates on the flour. | [3] |
| Seed | 1–6% P. curatellifolia seed oil | Biodiesel | Viscosity, cloud, density and pour points, and acid value | Viscosity, cloud and pour points, and acid value were within acceptable range as prescribed in ASTM D6751 while density was slightly higher than the limit. | [87] |
| Pulp | 62 % of P. curatellifolia syrup | Biscuit | Drying kinetics, moisture diffusivity, and activation energy | The moisture content and drying time of the syrup decreased with an increase in drying temperature. Thin layer drying processes for the syrup were best described by the Modified Page model. Effective moisture diffusivity for drying of syrup and the activation energies for drying of syrup was 21.0 ± 2.0 kJ/mol. | [66] |
| 100 % P. curatellifolia pulp | Syrup | Reducing sugars | The highest concentration (23.6 mg/100 mg) of reducing sugars was obtained by drying the syrup at 40 °C. | [88] | |
| 90 % pulp | Juice | Total phenolics and radical scavenging activity | Juice obtained had phenolics and antioxidant activities comparable to oranges. | [20] | |
| 100 % P. curatellifolia pulp | Jam | Protein, vitamin C, fat, and ash minerals (calcium, iron, magnesium, and zinc) and monosaccharides. | The jam produced was rich in minerals and vitamin C. | [13] | |
| Peel | 5 %, 10 %, 15 % and 20 % of P. curatellifolia peel flour | Biscuit | Thermal properties, gelatinisation, viscosity, pH, nutritional analysis, polyphenolic compounds, and antioxidant activity. | P. curatellifolia peel flour improved the nutritional, physical and antioxidant properties of the baked biscuits. | [70] |
The P. curatellifolia seed is rich in edible oil, which can be used in food applications exploiting their diverse fatty acids, which are beneficial for human health and nutrition [68,69]. Currently, the seeds are consumed as raw or semi-processed nuts, and they form an important diet for rural people [14]. Powdered P. curatellifolia seeds are traditionally used as a substitute for groundnut flour, while its extracted oil is used for cooking [65]. With respect to the application of oil in foods, detailed investigations on the properties of the oil such as thermal and oxidative stability are necessary.
Despite being a rich source of polyphenolic compounds and antioxidants, P. curatellifolia fruit peels are normally discarded, used as livestock feed, or to enrich soils. However, Ramashia et al. [70] studied the effects of enriching biscuits with 5 %, 10 %, 15 %, and 20 % P. curatellifolia peel flour on their nutritional, physical, and antioxidant properties (Table 10). The authors established that P. curatellifolia peel flour enhanced the nutritional value, polyphenolic compounds, and antioxidant activity of baked biscuits. The constituent of polyphenolic compounds and other essential phytochemicals indicated that the produced P. curatellifolia peel flour has biological activities such as antidiabetic, anticancer, and antiinflammation, which can be utilized to produce healthy confectionery products such as bread, biscuits, and cakes.
Overall, insufficient knowledge and information on products that can be commercially produced from the peel, pulp, and seeds of P. curatellifolia remain a prominent research gap. Therefore, scientific studies are warranted to explore commercial products derived from the peel, pulp, and seeds of the fruit to satisfy consumers’ demand for products with nutraceutical and functional properties in addition to providing basic nutrition.
5.2. Cosmetics
The P. curatellifolia seed oil is desired for its skin-conditioning and emollient properties, which assist in softening and smoothening the skin. Typically, P. curatellifolia seed oil is extracted by mechanical pressing; however, the oil can also be extracted mechanically without the application of heat to produce cold-pressed oil marketed as a skin-antiaging product due to the preservation of the antioxidants during pressing. According to the COSMILE [11], P. curatellifolia seed oil has been used to produce olyglyceryl-6 esters containing hydrophilic polyglycerol group and a hydrophobic fatty acid component, making them suitable ingredients for making emulsifying agents, surfactants, and skin care products (https://cosmileeurope.eu/inci/detail/10270/parinari-curatellifolia-oil-polyglyceryl-6-esters/). The online markets are awash with P. curatellifolia seed oil-based cosmetic products that are sold at exorbitant prices, with the prices varying depending on the supplier and the specific product.
5.3. Ethnomedicine
In several African countries, traditional medicine is still relied on for primary healthcare. P. curatellifolia has been reported to be a valuable medicinal plant that has been exploited by traditional herbalists, especially in Africa, to treat various ailments. Through oral or topical administration, the stem and root of P. curatellifolia have been used to treat constipation, toothache, snake bites and pain disorders, wounds, and chronic tumors indicating its versatility in therapeutic applications (Table 11). Chitemerere and Mukanganyama [71] and Gweshelo et al. [46] have reported that the stem and twigs could treat skin rash, tuberculosis, chronic diarrhea, herpes zoster, typhoid, and herpes simplex. The whole fruit has been used to treat hypertension, diabetes, and liver-related illnesses, as well as a sedative (Table 11). The seed has been used to manage various diseases, including hypertension, diabetes, and liver-related illnesses [72]. According to Janick and Paull [73], crushed or pulped leaves can be used as a poultice to treat cuts, wounds, sores, and bone fractures. Other reported medicinal applications of P. curatellifolia are presented in Table 11. Despite this important information, there is a dearth of knowledge about quantities and preparation methods, which has made it difficult to integrate P. curatellifolia with modern medicine. In addition, comprehensive pharmacological studies are still needed to validate and expand on these traditional uses of P. curatellifolia.
Table 11.
Traditional medicine applications of the different parts of P. curatellifolia.
| Medical application | Plant part | Method of administration | References |
|---|---|---|---|
| Hypertension diabetes and liver-related illnesses | Seed, fruit | Oral | [33,82] |
| Wounds and chronic tumors | Leaf, root, and root bark | Oral and topical | [38] |
| Constipation and tooth ache | Root | Oral | [42] |
| Dental hygiene | Bark | Oral | [42,89] |
| Vaginal douches, itchy scalp, dandruff and, cough | Bark | Topical and oral | [33,89] |
| Sedative | Fruit | Oral | [33] |
| Fever, diabetes mellitus, body aches, and piles | – | Oral and topical | [2] |
| Bacterial infections and dressing of fractures, dislocations, fever, typhoid, and malaria. | – | Oral and topical | [4,38] |
| Skin rash, tuberculosis, chronic diarrhea, herpes zoster, typhoid and herpes simplex | Stems and twigs | Oral and topical | [46,71] |
| Snake bites and pain disorders | Root and bark | Topical | [90,91] |
-, Information not provided.
6. Potential toxicity and allergenicity
Toxicity studies are required to determine the safety profile of P. curatellifolia in consideration of its potential pharmaceutical benefits. Among the toxicities associated with the use of medicinal plants are allergic reactions, gastrointestinal tract irritation, hemolysis, damage to key organs like the liver, kidneys, and heart, as well as carcinogenicity, which could be caused by the presence of toxic phytochemicals [4]. Ogbonnia et al. [74] evaluated the acute and subchronic toxicity of a single dose of P. curatellifolia seed hydroethanolic extract in albino mice. The results showed that an LD50 of 7.3 g/kg body weight indicated its potential safety; however, the potential for kidney damage at higher doses resulted in renal failure and had toxic effects on the heart during long-term treatment and potentially damaging the kidneys and the heart tissue. Meanwhile, a study by Kundishora et al. [4] showed that P. curatellifolia leaf extracts were not toxic to erythrocytes and immune cells. Tsatsakis et al. [75] reported that the homeostasis and defense mechanisms of people are affected by the delayed elimination of toxic chemicals in natural products, as well as long-term exposure to less harmful substances, even at low doses. Documented evidence on the allergic effects of P. curatellifolia is not available. Due to the limited information in the literature on the toxicity and allergenicity of P. curatellifolia, there is not enough evidence to make conclusive decisions on its toxic and allergen effects on humans.
7. Conclusions and prospects
7.1. Conclusions
P. curatellifolia is potentially an essential economic plant that is rich in a variety of nutrients and phytochemicals from its different components. The different components of the plant, which include the leaves, pulp, peel, and seed, are rich sources of macro- and micronutrients, essential fatty acids, and bioactive phytochemicals, including polyphenols, flavonoids, phytates, oxalates, alkaloids, proanthocyanidins, saponins, tannins, terpenoids, tocopherols, and carotenoids. Therapeutic properties such as antioxidant, anti-inflammatory, antidiabetic, anticancer, antiproliferative, and hepatoprotective activity have been reported from various parts of P. curatellifolia. This showcases myriads of benefits from P. curatellifolia that can be exploited to develop nutritious and functional foods, cosmetics, nutraceuticals, and medicine. Although more studies are required, various value-added foods, cosmetics, and medicinal products have been produced from various parts of the plant. These are desirable developments, given the plant's potential to contribute to food and nutrition security, health, and income generation. P. curatellifolia has a promising future due to its various uses in ethnomedicine, particularly in treating ailments like cancer and as a valuable food source. Its medicinal properties and adaptability to climactic changes make it a vital resource requiring further research.
7.2. Prospects
The future of P. curatellifolia may depend on several key issues that need to be addressed to realize and promote its value in various sectors. Although there is strong evidence supporting the medicinal use of the plant, no documented procedures specify the quantities used in preparing traditional medicine. This is particularly relevant given that safety issues must be prioritized before any recommendation for public use can be made. This, therefore, has made it difficult to integrate not only P. curatefollia but also traditional medicinal systems with modern medicine. Considering that the fruit is seasonal and perishable, there is a need for technology and innovation to develop advanced methods for processing, preservation, and packaging of P. curatellifolia to enhance its shelf life and nutritional value.
P. curatefollia research efforts could be hindered by the lack of support systems in African countries that promote research on native plants due to inadequate knowledge of the potential of these plants in improving the health, well-being, and livelihoods of people. As such, there is a need for a collaborative approach from various research sectors to promote indigenous plants and lobby various stakeholders to support these efforts. Given the increasing global demand and fast-growing market for medicinal plants and other herbal healthcare products, there is a huge prospect for P. curatefollia, as a cosmetic product ingredient and as herbal medicine. In addition, innovative food product development using P. curatellifolia should be explored to diversify its use in the food industry. This includes creating new recipes, processed foods, and beverages that appeal to local and international markets. To realize economic benefits from P. curatefollia research should focus on optimizing agronomic practices, including breeding programs to develop high-yielding and disease-resistant varieties, suitable planting density, irrigation methods, and integrated pest management strategies to enhance productivity and sustainability. This heterogenous approach will, therefore, increase production output, lower postharvest losses, promote rural development and economic expansion, enhance food security for the expanding population, and ultimately accomplish several of the United Nations' sustainable development goals, including SDGs 2, 3 and 8.
CRediT authorship contribution statement
Tafadzwa Kaseke: Writing – original draft, Visualization, Validation, Data curation, Conceptualization. Trust Mukudzei Pfukwa: Writing – original draft, Methodology, Investigation. Kwanele Andy Nxumalo: Writing – original draft, Investigation. Mawande Hugh Shinga: Writing – original draft. Umezuruike Linus Opara: Writing – review & editing. Olaniyi Amos Fawole: Writing – review & editing, Supervision, Resources, Conceptualization.
Ethics declaration
This study did not require informed consent or review and approval by an ethical committee because it was a literature analysis that solely used data from published studies and did not involve any direct experimentation or studies on living beings.
Data availability statement
This research did not utilize any data. This article's accompanying data has not been added to any publicly accessible databases.
Funding
The authors gratefully acknowledge the financial support provided by the National Research Foundation of South Africa (Grant No. SPAR231013155231) and the University Research Committee at the University of Johannesburg, which contributed to covering the article publication costs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
All the authors are appreciated for the time they devoted to writing this review article.
Contributor Information
Tafadzwa Kaseke, Email: tafakaseqe@gmail.com.
Olaniyi Amos Fawole, Email: olaniyif@uj.ac.za.
References
- 1.Kunene T., Hlophe-Ginidza S., Chimonyo V.G.P., Modi A.T., Mpandeli S., Nhamo L., Mabhaudhi T. Food Security for African Smallholder Farmers. Springer; 2022. Contribution of underutilised indigenous crops to enhanced food and nutrition security in the advent of climate change; pp. 295–310. [Google Scholar]
- 2.Fakudze N.T., Sarbadhikary P., George B.P., Abrahamse H. Ethnomedicinal uses, phytochemistry, and anticancer potentials of african medicinal fruits: a comprehensive review. Pharmaceuticals. 2023;16(8):1117. doi: 10.3390/ph16081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onipe O.O., Matshisevhe M.M., Ramashia S.E., Mashau M.E. Physicochemical and functional properties of finger millet (Eleusine coracana) flour supplemented with Parinari curatellifolia flour. Scientific African. 2024;23 [Google Scholar]
- 4.Kundishora A., Sithole S., Mukanganyama S. Determination of the cytotoxic effect of different leaf extracts from Parinari curatellifolia (Chrysobalanaceae) J. Toxicol. 2020;2020(1) doi: 10.1155/2020/8831545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halilu E.M. Characterization of crude saponins from stem bark extract of Parinari curatellifolia and evaluation of its antioxidant and antibacterial activities. Physical Sciences Reviews. 2024;9(5):2077–2095. [Google Scholar]
- 6.Nkosi N.J., Shoko T., Manhivi V.E., Slabbert R.M., Sultanbawa Y., Sivakumar D. Metabolomic and chemometric profiles of ten southern African indigenous fruits. Food Chem. 2022;381 doi: 10.1016/j.foodchem.2022.132244. [DOI] [PubMed] [Google Scholar]
- 7.Crown O.O., Olayeriju O.S., Kolawole A.O., Akinmoladun A.C., Olaleye M.T., Akindahunsi A.A. Mobola plum seed methanolic extracts exhibit mixed type inhibition of angiotensin Ⅰ-converting enzyme in vitro. Asian Pac. J. Trop. Biomed. 2017;7(12):1079–1084. [Google Scholar]
- 8.Frankova A., Manourova A., Kotikova Z., Vejvodova K., Drabek O., Riljakova B., Gamera O., Ngula M., Ndiyoi M., Polesny Z., Verner V., Tauchen J. The chemical composition of oils and cakes of Ochna serrulata (Ochnaceae) and other underutilized traditional oil trees from Western Zambia. Molecules. 2021;26(17):5210. doi: 10.3390/molecules26175210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lykke A.M., Gregersen S.B., Padonou E.A., Bassolé I.H.N., Dalsgaard T.K. Potential of unconventional seed oils and fats from West African trees: a review of fatty acid composition and perspectives. Lipids. 2021;56(4):357–390. doi: 10.1002/lipd.12305. [DOI] [PubMed] [Google Scholar]
- 10.Niyukuri J., Raiti J., Ntakarutimana V., Hafidi A. Lipid composition and antioxidant activities of some underused wild plants seeds from Burundi. Food Sci. Nutr. 2021;9(1):111–122. doi: 10.1002/fsn3.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosmile europe [online] https://cosmileeurope.eu/inci/detail/10270/parinari-curatellifolia-oil-polyglyceryl-6-esters/
- 12.Chatepa L.E.C., Masamba K., Jose M. Proximate composition, physical characteristics and mineral content of fruit, pulp and seeds of Parinari curatellifolia (Maula) from Central Malawi. Afr. J. Food Sci. 2018;12(9):238–245. [Google Scholar]
- 13.Muchuweti M., Matongo N., Benhura M., Bhebhe M., Kasiyamhuru A., Chipurura B. Nutritional composition of Parinari curatellifolia fruit and a jam made from the pulp of the fruit: an untapped resource. II International Symposium on Underutilized Plant Species: Crops for the Future-Beyond Food Security. 2011;979:621–624. [Google Scholar]
- 14.Benhura C., Benhura M., Muchuweti M., Nyagura S., Gombiro P. Proximate analysis of Parinari curatellifolia fruit pulp of fruit from parts of Harare and a rural area in Zimbabwe. Pakistan J. Nutr. 2012;11(7):541. [Google Scholar]
- 15.Moya‐Alvarez V., J Koyembi J.C., Kayé L.M., Mbecko J.R., Sanke‐Waîgana H., Djorie S.G., Nyasenu Y.T., Mad‐Bondo D., Kongoma J.B., Nakib S., Madec Y., Ulmann G., Neveux N., Sansonetti P.J., Vray M., Marteyn B. Vitamin C levels in a Central‐African mother–infant cohort: does hypovitaminosis C increase the risk of enteric infections? Matern. Child Nutr. 2021;17(4) doi: 10.1111/mcn.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibibia E.T., Obaloluwa A.P., Olasunkanmi A.M., Ijeoma M.A., Olugbemiga O.S. "Total phenolic, flavonoid and mineral contents of the methanolic leaf extract of parinari curatellifolia planch. Int. J. Chem. Res. 2023:13–18. ex benth". [Google Scholar]
- 17.Sibiya N., Kayitesi E., Moteetee A. Mineral composition of selected indigenous wild southern African fruits. South Afr. J. Bot. 2020;132:87–94. [Google Scholar]
- 18.Daouda Z., Lassina O., Bienvenu S.M., Abdoul-Aziz Z., Daniel P.F., Yacouba S., Justin O.R., Pawende K., Kadougoudiou K.A., Anicet O.G. "Antiradical and α-amylase inhibitory effect of hydroethanolic extract of Parinari curatellifolia planch. Ex. Benth.(Chrysobalanaceae) and Leptadenia Lanceolata (Poir.) Goyder Ex Leptadenia Hastata Pers (Decne)(Apocinaceae)," International Journal of Medicinal Plants and Natural Products (IJMPNP) 2022;8(3):1–8. doi: 10.20431/2454-7999.0803001. [DOI] [Google Scholar]
- 19.Mwamatope B., Chikowe I., Tembo D.T., Kamanula J.F., Masumbu F.F., Kumwenda F.D. Phytochemical composition and antioxidant activity of edible wild fruits from Malawi. BioMed Res. Int. 2023;2023(1) [Google Scholar]
- 20.Nhukarume L., Chikwambi Z., Muchuweti M., Chipurura B. Phenolic content and antioxidant capacities of Parinari curatelifolia, Strychnos spinosa and Adansonia digitata. J. Food Biochem. 2010;34:207–221. [Google Scholar]
- 21.Karou S.D., Tchacondo T., Outtara L., Anani K., Savadogo A., Agbonon A., Attaia M.B., de Souza C., Sakly M., Simpore J. Antimicrobial, antiplasmodial, haemolytic and antioxidant activities of crude extracts from three selected Togolese medicinal plants. Asian Pac. J. Tropical Med. 2011;4(10):808–813. doi: 10.1016/S1995-7645(11)60199-5. [DOI] [PubMed] [Google Scholar]
- 22.Manuwa T.R., Akinmoladun A.C., Crown O.O., Komolafe K., Olaleye M.T. Toxicological assessment and ameliorative effects of Parinari curatellifolia alkaloids on triton-induced hyperlipidemia and atherogenicity in rats. Proc. Natl. Acad. Sci. India B Biol. Sci. 2017;87:611–623. [Google Scholar]
- 23.Niyukuri J., Raiti J., El Qarnifa S., El Abbassi A., Hafidi A. Potential of some autochthonous wild plants of Burundi for vegetable oil and valuable compounds production. Braz. J. Biol. 2019;80(4):860–871. doi: 10.1590/1519-6984.223481. [DOI] [PubMed] [Google Scholar]
- 24.Chatepa L.E.C., Uluko H., Masamba K. Comparison of oil quality extracted from selected conventional and non conventional sources of vegetable oil from Malawi. Afr. J. Biotechnol. 2019;18(8):171–180. [Google Scholar]
- 25.Singh P., Pandey V.K., Sultan Z., Singh R., Dar A.H. Classification, benefits, and applications of various anti-nutritional factors present in edible crops. Journal of Agriculture and Food Research. 2023;14 [Google Scholar]
- 26.Idris A.A., Nour A.H., Ali M.M., Erwa I.Y., Ishag O.A.O. "Physicochemical properties and fatty acids composition of Sudanese baobab (Adansonia digitata L.) seed oil. Int. J. Pharma Bio Sci. 2020;11(1):34–42. 2020. [Google Scholar]
- 27.Chivandi E., Mukonowenzou N., Berliner D. The coastal red-milkwood (Mimusops caffra) seed: proximate, mineral, amino acid and fatty acid composition. South Afr. J. Bot. 2016;102:137–141. [Google Scholar]
- 28.Shoko T., Saka J.K., Apostolides Z. Headspace volatiles of the edible fruit pulp of Parinari curatellifolia growing in Malawi using solid phase microextraction. South Afr. J. Bot. 2014;90:128–130. [Google Scholar]
- 29.Joulain D., Casazza A., Laurent R., Portier D., Guillamon N., Pandya R., Le M., Viljoen A. Volatile flavor constituents of fruits from Southern Africa: mobola plum (Parinari curatellifolia) J. Agric. Food Chem. 2004;52(8):2322–2325. doi: 10.1021/jf030702i. [DOI] [PubMed] [Google Scholar]
- 30.Di Meo F., Lemaur V., Cornil J., Lazzaroni R., Duroux J., Olivier Y., Trouillas P. Free radical scavenging by natural polyphenols: atom versus electron transfer. J. Phys. Chem. 2013;117(10):2082–2092. doi: 10.1021/jp3116319. [DOI] [PubMed] [Google Scholar]
- 31.Ademosun A., Oboh G. Inhibition of carbohydrate hydrolyzing enzymes associated with type 2 diabetes and antioxidative properties of some edible seeds in vitro. Int. J. Diabetes Dev. Ctries. 2015;35:516–521. [Google Scholar]
- 32.Perron N.R., Brumaghim J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 33.Peni I.J., Elinge C.M., Yusuf H., Adams I.U., Agaie B.M., Mbongo A., Chogo E. Phytochemical screening and antibacterial activity of Parinari curatellifolia stem extract. J. Med. Plants Res. 2010;4(20):2099–2102. [Google Scholar]
- 34.Halilu M., Akpulu I., Agunu A., Ahmed A., Abdurahman E. Phytochemical and antibacterial evaluation of Parinari curatetellifolia Planch ex Benth (Chrysobalanaceae) Nigerian Journal of Basic and Applied Sciences. 2008;16(2):281–285. [Google Scholar]
- 35.Halilu M., Agunu A., Ibrahim H., Abdurahman E. Pharmacognostic evaluation of the stem bark of Parinari curatellifolia planch. Ex benth (Chrysobalanaceae) Niger. J. Pharm. Sci. 2008;7(1):79–85. [Google Scholar]
- 36.Mawire P., Mozirandi W., Heydenreich M., Chi G.F., Mukanganyama S. Isolation and antimicrobial activities of phytochemicals from Parinari curatellifolia (Chrysobalanaceae) Advances in Pharmacological and Pharmaceutical Sciences. 2021;2021(1) doi: 10.1155/2021/8842629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evangelina I.A., Hediyati Y., Laviana A., Rikmasari R., Zubaedah C., Anisah, Kurnia D. Bio-Mechanism inhibitory prediction of β-sitosterol from Kemangi (Ocimum basilicum L.) as an inhibitor of MurA enzyme of oral bacteria: In vitro and in silico Study. Comput. Biol. Chem. Adv. Appl. 2021:103–115. doi: 10.2147/AABC.S301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhunu B., Mautsa R., Mukanganyama S. Inhibition of biofilm formation in Mycobacterium smegmatis by Parinari curatellifolia leaf extracts. BMC Compl. Alternative Med. 2017;17:1–10. doi: 10.1186/s12906-017-1801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vikram A., Jayaprakasha G., Uckoo R.M., Patil B.S. Inhibition of Escherichia coli O157: H7 motility and biofilm by β-sitosterol glucoside. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013;1830(11):5219–5228. doi: 10.1016/j.bbagen.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Mbunde M.V.N., Innocent E., Mabiki F., Andersson P.G. In vitro study for antifungal compounds from Parinari curatellifolia (Chrysobalanaceae) and Terminalia sericea (Combretaceae) International Journal of Biological and Chemical Sciences. 2021;15(1):367–378. [Google Scholar]
- 41.Issakou B.-V., Payang Y.P., Christophe D., Alio H.M., Otchom B.B., Valery M.B., Tidjani A. Antidermatophyte activity of ethanolic extracts of Daniellia oliveri (Fabaceae) and Parinari curatellifolia (Chrysobalanaceae) in Chad. GSC Advanced Research and Reviews. 2022;13(2):170–179. [Google Scholar]
- 42.Gororo M., Chimponda T., Chirisa E., Mukanganyama S. Multiple cellular effects of leaf extracts from Parinari curatellifolia. BMC Compl. Alternative Med. 2016;16:1–14. doi: 10.1186/s12906-016-1287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boora F., Chirisa E., Mukanganyama S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. Journal of Food Processing. 2014;2014(1) [Google Scholar]
- 44.Chirisa E., Mukanganyama S. Evaluation of in vitro anti-inflammatory and antioxidant activity of selected Zimbabwean plant extracts. J. Herbs, Spices, Med. Plants. 2016;22(2):157–172. [Google Scholar]
- 45.Chimponda T., Mukanganyama S. Evaluation of selected Zimbabwean plant extracts as inhibitors of hematopoietic prostaglandin D2 synthase. J. Herbs, Spices, Med. Plants. 2015;21(3):243–258. [Google Scholar]
- 46.Gweshelo D., Muswe R., Mukanganyama S. In vivo and in vitro inhibition of rat liver glutathione transferases activity by extracts from Combretum zeyheri (Combretaceae) and Parinari curatellifolia (Chrysobalanaceae) BMC Compl. Alternative Med. 2016;16:1–15. doi: 10.1186/s12906-016-1235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josiah S.S., Oyeleye S.I., Crown O.O., Olaleye M.T. Ameliorative effect of Parinari curatellifolia seed extracts on sodium nitroprusside–induced cardiovascular toxicity in rats. Comp. Clin. Pathol. 2020;29(1):239–246. [Google Scholar]
- 48.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 49.Mukanganyama S., Dumbura S.C., Mampuru L. Anti-proliferative effects of plant extracts from Zimbabwean medicinal plants against human leukaemic cell lines. Afr. J. Plant Sci. Biotechnol. 2012;6(1):14–20. [Google Scholar]
- 50.Lee I.S., Shamon L.A., Chai H.B., Chagwedera T.E., Besterman J.M., Farnsworth N.R., Cordell G.A., Pezzuto J.M., Kinghorn A.D. Cell-cycle specific cytotoxicity mediated by rearranged ent-kaurene diterpenoids isolated from Parinari curatellifolia. Chem. Biol. Interact. 1996;99(1–3):193–204. doi: 10.1016/0009-2797(95)03669-5. [DOI] [PubMed] [Google Scholar]
- 51.Mi Q., Lantvit D., Reyes-Lim E., Chai H., Zhao W., Lee I.-S., Peraza-Sanchez S., Ngassapa O., Kardono L.B.S., Riswan S., Hollingshead M.G., Mayo J.G., Farnsworth N.R., Cordell G.A., Kinghorn A.D., Pezzuto J.M. Evaluation of the potential cancer chemotherapeutic efficacy of natural product isolates employing in vivo hollow fiber tests. Journal of natural products. 2002;65(6):842–850. doi: 10.1021/np010322w. [DOI] [PubMed] [Google Scholar]
- 52.Rattanapornsompong K., Khattiya J., Phannasil P., Phaonakrop N., Roytrakul S., Jitrapakdee S., Akekawatchai C. Impaired G2/M cell cycle arrest induces apoptosis in pyruvate carboxylase knockdown MDA-MB-231 cells. Biochemistry and Biophysics Reports. 2021;25 doi: 10.1016/j.bbrep.2020.100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moskaug J.Ø., Carlsen H., Myhrstad M.C., Blomhoff R. Polyphenols and glutathione synthesis regulation. The American journal of clinical nutrition. 2005;81(1):277S–283S. doi: 10.1093/ajcn/81.1.277S. [DOI] [PubMed] [Google Scholar]
- 54.Carreon-Gonzalez M., Alvarez-Idaboy J.R. The synergy between glutathione and phenols—phenolic antioxidants repair glutathione: closing the virtuous circle—a theoretical insight. Antioxidants. 2023;12(5):1125. doi: 10.3390/antiox12051125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L., Jin P., Guan Y., Luo M., Wang Y., He B., Li B., He K., Cao J., Huang C., Li J., Shen Z. Exploiting polyphenol-mediated redox reorientation in cancer therapy. Pharmaceuticals. 2022;15(12):1540. doi: 10.3390/ph15121540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Filippis A., Ullah H., Baldi A., Dacrema M., Esposito C., Garzarella E.U., Santarcangelo C., Tantipongpiradet A., Daglia M. Gastrointestinal disorders and metabolic syndrome: dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int. J. Mol. Sci. 2020;21(14):4929. doi: 10.3390/ijms21144929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogbonnia S. Nuts and Seeds in Health and Disease Prevention. Elsevier; 2011. Extracts of Mobola plum (Parinari curatellifolia Planch ex Benth, Chrysobalanaceae) seeds and multiple therapeutic activities; pp. 767–774. [Google Scholar]
- 58.Hershon K.S., Hirsch B.R., Odugbesan O. Importance of postprandial glucose in relation to A1C and cardiovascular disease. Clin. Diabetes. 2019;37(3):250–259. doi: 10.2337/cd18-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omale S., Aguiyi J., Adekunle O., Ochala S., Etuh M., Eze M.C. Evaluation of the antidiabetic effects of the stem bark extract of Parinari curatellifolia (planch. Ex benth.) in Drosophila melanogaster. J. Pharmacol. Toxicol. 2020 doi: 10.3923/jpt.2021.9.21. [DOI] [Google Scholar]
- 60.Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013;57(1):48–57. doi: 10.1002/mnfr.201200511. [DOI] [PubMed] [Google Scholar]
- 61.Dyer J., Wood I., Palejwala A., Ellis A., Shirazi-Beechey S. Expression of monosaccharide transporters in intestine of diabetic humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282(2):G241–G248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 62.Alzaid F., Cheung H.-M., Preedy V.R., Sharp P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tata N., Precillia I., Shackleton C., Degrande A., Tieguhong J.C. Addressing constraints in promoting wild edible plants' utilization in household nutrition: case of the Congo Basin forest area. Agric. Food Secur. 2017;6:1–10. [Google Scholar]
- 64.Salih N.-E., Yahia E. Nutritional value and antioxidant properties of four wild fruits commonly consumed in Sudan. Int. Food Res. J. 2015;22(6) [Google Scholar]
- 65.Lost crops of Africa. Fruits. 2008;III [Google Scholar]
- 66.Benhura C., Kugara J., Muchuweti M., Nyagura S.F., Matarise F., Gombiro P.E., Nyandoro G. Drying kinetics of syrup of Parinari curatellifolia fruit and cereal based product, zvambwa. Journal of food science and technology. 2015;52:4965–4974. doi: 10.1007/s13197-014-1616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saka J.K., Swai R., Mkonda A., Schomburg A., Kwesiga F., Akinnifesi F.K. Agroforestry Impacts on Livelihoods in Southern Africa: Putting Research into Practice. Proceedings of the Regional Agroforestry Conference Held in Warmbaths. 2002. Processing and utilisation of indigenous fruits of the miombo in southern Africa; pp. 20–24. South Africa. [Google Scholar]
- 68.Swai R., Kimata B. Utilisation and Commercialisation of Drylands Indigenous Fruit Tree Species to Improve Livelihood in ECA. Regional workshop held in Kitui June; 2005. Processing and value adding of indigenous fruit tree products of drylands of East and Central Africa; pp. 20–24. [Google Scholar]
- 69.Ogbonnia S., Mbaka G., Anyika E., Lediju O., Ota D. Evaluation of the effects of Parinari curatellifolia seed and Anthocleista vogelli root extracts individually and in combination on postprandial and alloxan-induced diabetes in animals. Planta Med. 2011;77(12):PF45. [Google Scholar]
- 70.Ramashia S.E., Mamadisa F.M., Mashau M.E. Effect of Parinari curatellifolia peel flour on the nutritional, physical and antioxidant properties of biscuits. Processes. 2021;9(8):1262. [Google Scholar]
- 71.Chitemerere T.A., Mukanganyama S. In vitro antibacterial activity of selected medicinal plants from Zimbabwe. Afr. J. Plant Sci. Biotechnol. 2011;5(1):1–7. [Google Scholar]
- 72.Olaleye M.T., Amobonye A.E., Komolafe K., Akinmoladun A.C. Protective effects of Parinari curatellifolia flavonoids against acetaminophen-induced hepatic necrosis in rats. Saudi J. Biol. Sci. 2014;21(5):486–492. doi: 10.1016/j.sjbs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janick J., Paull R.E. CABI; 2008. The Encyclopedia of Fruit & Nuts. [Google Scholar]
- 74.Ogbonnia S.O., Olayemi S.O., Anyika E.N., Enwuru V.N., Poluyi O.O. Evaluation of acute toxicity in mice and subchronic toxicity of hydroethanolic extract of Parinari curatellifolia Planch (Chrysobalanaceae) seeds in rats. Afr. J. Biotechnol. 2009;8(9) [Google Scholar]
- 75.Tsatsakis A., Docea A.O., Constantin C., Calin D., Zlatian O., Nikolouzakis T.K., Stivaktakis P.D., Kalogeraki A., Liesivuori J., Tzanakakis G., Neagu M. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicology letters. 2019;316:154–170. doi: 10.1016/j.toxlet.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Sibiya N.P., Kayitesi E., Moteetee A.N. Proximate analyses and amino acid composition of selected wild indigenous fruits of Southern Africa. Plants. 2021;10(4):721. doi: 10.3390/plants10040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benhura M., Muchuweti M., Gombiro P., Benhura C. Properties of (Parinari curatellifolia)(hacha or chakata) fruit from different parts of harare, Zimbabwe. Afr. J. Food Nutr. Sci. 2013;13(4):8004–8018. [Google Scholar]
- 78.Ogungbenle H., Atere A. The chemical, fatty acid and sensory evaluation of Parinari curatellifolia seeds. Br. Biotechnol. J. 2014;4 doi: 10.9734/BBJ/2014/7712. [DOI] [Google Scholar]
- 79.Ndabikunze B., Mugasha A., Chcimshama S., Tiisekwa B. Nutritive, value of selected forest/woodland edible fruits, seeds and nuts in Tanzania. Tanzania Journal of Agricultural Sciences. 2006;7(1) [Google Scholar]
- 80.Saka J.K., Msonthi J.D. Nutritional value of edible fruits of indigenous wild trees in Malawi. For. Ecol. Manag. 1994;64(2–3):245–248. [Google Scholar]
- 81.Ogunbolude Y., Ajayi M., Ajagbawa T., Igbakin A., Rocha J., Kade I. Ethanolic extracts of seeds of Parinari curatellifolia exhibit potent antioxidant properties: a possible mechanism of its antidiabetic action. J. Pharmacogn. Phytotherapy. 2009;1(6):67–75. [Google Scholar]
- 82.Olaleye M., Adegboye O., Akindahunsi A. 2010. Antioxidant and Antihypertensive Investigation of Seed Extract of Parinari Curatellifolia. [Google Scholar]
- 83.Sylvanus U., Olakunle F., Amos J., Olutayo O. Antibacterial activity and phytochemical evaluation of the leaf root and stem bark extracts of parinari curatellifolia (planch. ex benth) Int. J. Adv. Chem. 2014;2:178–181. [Google Scholar]
- 84.More G., Tshikalange T.E., Lall N., Botha F., Meyer J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 2008;119(3):473–477. doi: 10.1016/j.jep.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Lee D.S., Yam K.L., Piergiovanni L. CRC press; 2008. Food Packaging Science and Technology. [Google Scholar]
- 86.Atawodi S., Yakubu O., Umar I. 2013. "Antioxidant and Hepatoprotective Effects of Parinari Curatellifolia Root". [Google Scholar]
- 87.Nabora C.S., Kingondu C.K., Kivevele T.T. Tamarindus Indica fruit shell ash: a low cost and effective catalyst for biodiesel production from Parinari curatellifolia seeds oil. SN Appl. Sci. 2019;1:1–9. [Google Scholar]
- 88.Benhura C., Kugara J., Muchuweti M., Nyagura S., Gombiro P., Dotito P. Ex Benth. Fruit and Cereal Based Products, Zvambwa". 2016. "Effect of drying temperature on the content of reducing sugars in syrup of Parinari curatellifolia Planch. [Google Scholar]
- 89.Oshomoh E., Idu M. Antimicrobial activity of ethanol and aqueous extracts of Parinari curatellifolia (Stem) on dental caries causing microbes. Int. J. Pharmaceut. Sci. Res. 2012;3(7):2113–2118. [Google Scholar]
- 90.Halilu E.M., October N., Ugwah-Oguejiofor C.J., Jega A.Y., Nefai M.S. Anti-snake venom and analgesic activities of extracts and betulinic and oleanolic acids isolated from Parinari curatellifolia. Journal of medicinal plants for economic development. 2020;4(1):1–8. [Google Scholar]
- 91.Malengue A., Lourenço A., Patrício A.H., Costa R.A., Quilhó T., Gominho J. Tropical mobola plum (Parinari Curatellifolia): a full characterization of wood and bark within the scope of biorefineries. European Journal of Wood and Wood Products. 2024;82:1–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This research did not utilize any data. This article's accompanying data has not been added to any publicly accessible databases.