Abstract
Pulicaria odora has been traditionally used in northeastern Algeria for treating gastrointestinal disorders, particularly ulcers. This study aimed to assess the gastroprotective, anti-inflammatory, and antioxidant properties of a crude hydroalcoholic extract derived from the leaves of Pulicaria odora, as well as its subsequent fractions. The gastroprotective effect was studied in an ethanol-induced ulcer model in mice. The in vitro antioxidant activity was quantified via the DPPH, and ABTS methods. In vitro anti-inflammatory activity was investigated through the human red blood cell membrane stabilization method and the bovine serum albumin denaturation method. The in vivo anti-inflammatory properties of the crude extract were assessed in mice via a carrageenan-induced acute paw edema model. The crude extract significantly inhibited gastric ulcer formation in a dose-dependent manner, achieving a reduction of up to 99.91 % at a dose of 200 mg/kg. Among the fractions, the aqueous fraction exhibited the most potent gastroprotective effect, with a notable dose-dependent response and high ulcer inhibition rates (95.56 % at 50 mg/kg). Additionally, the crude extract displayed strong in vitro and in vivo anti-inflammatory activity and a high free radical scavenging capacity; with IC50 values comparable to those of ascorbic acid. LC-ESI-MSn analysis led to the identification of nine flavonoid derivatives, which were subsequently subjected to molecular docking techniques to predict their antiulcer activity. These results suggest that both glycosylated and aglycone flavonoids derived from P. odora have the potential to inhibit gastric acid secretion by targeting the proton pump.
Keywords: Pulicaria odora, Gastroprotective effects, Anti-inflammatory properties, Antioxidant activity, Flavonoids, Molecular docking
1. Introduction
A gastric ulcer represents a pathological lesion of the gastric mucosa that is primarily precipitated by an imbalance in the acid-base equilibrium of the stomach [1]. The etiology of gastric ulcers encompasses a complex interplay of factors including excessive gastric acid production, Helicobacter pylori infection, the use of nonsteroidal anti-inflammatory drugs, stress, alcohol, and tobacco exposure [1,2]. Notably, the stomach responds to such pathogenic insults by inducing oxidative stress, which involves reactive oxygen species in the pathogenesis of ulcerative lesions. These lesions can subsequently lead to severe gastric disorders such as gastritis, gastric ulcers, and gastric cancer [3]. Chronic gastric inflammation, predominantly sustained by H. pylori, is a secondary, yet significant, causative factor of ulcers. This bacterium significantly contributes to oxidative stress and inflammation within the gastric milieu [3].
Recent studies have focused on the use of medicinal plants because of their comprehensive therapeutic profiles, which include gastroprotective, antioxidant, anti-inflammatory, antibacterial, and wound-healing properties [4]. The management of gastric ulcers varies according to the underlying cause; therapeutic modalities such as antacids, proton pump inhibitors, and H2 receptor antagonists effectively reduce gastric acid secretion and facilitate ulcer healing [4]. Additionally, a combination of antibiotics and proton pump inhibitors is employed to eradicate H. pylori infections [5].
Pulicaria odora (L.) REICHB., a plant species with yellow blooms belonging to the Asteraceae family, originates from Europe, North Africa, and the Middle East [6]. The roots of this plant are enriched with essential oils comprising approximately 0.6–0.8 % of the dry plant mass [7,8]. These oils are predominantly composed of oxygenated compounds, such as thymol and thymol isobutyrate [7,8]. Furthermore, the phenolic profile of P. odora includes protocatechuic acid, chlorogenic acid, and caffeic acid, alongside a spectrum of flavonoids notably methylated derivatives of 6-hydroxykaempferol, including a 3,5,6,7,4′-pentamethyl ether [9,10]. These compounds bestow the plant with pronounced antimicrobial and antioxidant properties [[7], [8], [9],11]. Traditionally, in northeastern Algeria, P. odora, known locally as "Oudinat Enaadja," has been employed to treat various gastrointestinal disorders (our own investigation). Moreover, P. odora is traditionally used in Moroccan medicine to treat intestinal disorders, back-pain, and menstrual cramps [7].
This study investigated the potential of P. odora for treating gastric ulcer diseases, aiming to advance the development of safe, phytotherapeutic alternatives to conventional pharmaceutical treatments.
2. Materials and methods
2.1. Botanical material
This study used leaves of P. odora, collected in June 2023 from the Taher region in Jijel, which is situated at an elevation of 60 m (36°45′46.0"N, 5°55′10.0"E) in northern Algeria. The samples were identified by the botanist Mohamed Sebti from the Biotechnology, Environment and Health Laboratory, University of Jijel and a voucher sample (PO154) was deposited in the herbarium National Park of Taza. Following collection, the leaves were dried in a shaded, dry area at ambient temperature to preserve their integrity, then finely ground via an electric mill to a fine powder and sieved to <150 μm particle size. The dried plant material was stored in a moisture-controlled environment until further use.
2.2. Animal subjects
The study was conducted on male albino mice, each weighing approximately 30 g, obtained from the Pasteur Institute of Algiers, Algeria. The mice were housed at the University of Jijel's vivarium under controlled conditions with a consistent room temperature of 25–27 °C and a 12-h light/dark cycle. Groups of six mice were housed per cage, with unrestricted access to water and feed, and acclimatized for a period of ten days before the experiments commenced.
2.3. Chemicals and pharmaceuticals
Various reagents including DPPH, ABTS, ascorbic acid, gallic acid, catechin, sodium diclofenac, bovine serum albumin (BSA), and Folin-Ciocalteu's reagent, were used, all of which were procured from Sigma-Aldrich. Omeprazole was sourced from BIOPHARM s.p.a, Algeria. All chemicals used were of analytical grade to ensure experimental consistency.
2.4. Extraction process
The extraction involved macerating 10 g of dried P. odora leaves in 300 ml of an 80/20 v/v methanol/water mixture at room temperature for 24 h, followed by vacuum filtration. The filtrate was then concentrated under reduced pressure at a controlled temperature not exceeding 40 °C and subsequently weighed (yield of crude extract 26 % w/w). Two grams of the concentrated macerate was diluted in 150 ml of distilled water and subjected to successive liquid‒liquid extractions using solvents of increasing polarity: chloroform, ethyl acetate, and n-butanol. Each of the three organic phases, along with the aqueous phase, was concentrated under reduced pressure at temperatures below 40 °C and weighed. The resulting dry fractions and the crude dry extract were stored at 4 °C, ensuring their stability and preservation until further analysis.
2.5. Quantitative analysis of phenolic compounds
2.5.1. Total polyphenols assay
The total polyphenols in the crude extract and fractions of P. odora were quantified via a colorimetric method [12]. Specifically, 200 μl of each methanol-diluted extract was combined with 1 ml of tenfold diluted Folin-Ciocalteu (2N) reagent in distilled water. Following a brief incubation of 3 min, 800 μl of 7 % sodium carbonate (Na2CO3) was added, and the mixtures were immediately agitated. A blue color appeared after a 30-min dark incubation at 40 °C. Absorbance readings were taken at 760 nm using a UV–visible spectrophotometer. The total polyphenol concentration was determined from a linear calibration curve, prepared under identical conditions using gallic acid as the standard. Analyses were conducted in triplicate, and the results are presented as milligrams of gallic acid equivalent per gram of dry extract (mg GAE/g DE).
2.5.2. Total flavonoid assay
The flavonoid content in the crude extract and fractions of P. odora was estimated through a colorimetric method [13]. A quantity of 250 μl of each methanol-diluted extract was added to 1250 μl of distilled water. At time zero, 75 μl of 5 % sodium nitrite (NaNO2) was introduced and the solution was stirred. Six minutes later, 150 μl of 10 % aluminum chloride (AlCl3;6H2O) was added, followed by vigorous stirring. After 5 min at room temperature, 500 μl of 1 M sodium hydroxide (NaOH) was added and the volume was adjusted to 2.5 ml with distilled water. The absorbance was measured directly at 510 nm via a UV–visible spectrophotometer. A calibration curve was established using catechin as the standard under the same operational conditions. Tests were performed in triplicate, and flavonoid concentrations are expressed in milligrams of catechin equivalent per gram of dry extract (mg CE/g DE).
2.6. Evaluation of antioxidant activity
2.6.1. DPPH assay for antioxidant activity
The antioxidant activity of P. odora crude extract and its fractions was assessed via a modified protocol from Brand-Williams et al. [14]. A 2,2-Diphenyl-1-picrylhydrazyl (DPPH) solution with a molecular formula of C18H12N5O6 and a molar mass of 394.33 g/mol, prepared at a concentration of 60 μM in methanol, was stirred for 1 h in the dark at 4 °C. For the assay, 50 μl of each methanol-solubilized extract at different concentrations was added to 1950 μl of the methanolic DPPH solution in dry tubes. Concurrently, a negative control was established by adding 50 μl of methanol to 1950 μl of the DPPH solution. After vigorous shaking for 10 s, the mixtures were incubated for 30 min at ambient temperature in the dark. The absorbance readings were subsequently taken at 515 nm using a UV–visible spectrophotometer. Ascorbic acid served as the positive control, and its absorbance was measured under identical experimental conditions. Each assay was replicated three times, and the antioxidant activity was quantified according to the percentage inhibition of the DPPH radical (% PI), which was calculated as follows:
% PI = [(A negative control- A extract)/A negative control] × 100; where:
-
•
A negative control represents the postreaction absorbance of DPPH.
-
•
A extract represents the absorbance of the extract with DPPH postreaction.
The IC50, which indicates the concentration necessary to reduce 50 % of the DPPH radical, was determined to further quantify the antioxidant efficacy.
2.6.2. ABTS assay for antioxidant activity
The assay was conducted with a modified protocol from Re et al. [15]. The ABTS+• radical cation is generated by reacting a 7 mM aqueous solution of ABTS with potassium persulfate, achieving a final concentration of 2.45 mM. This reaction mixture was incubated in darkness at ambient temperature with agitation for 16 h to produce the ABTS+• master solution. The working solution of ABTS+• was then prepared by diluting the master solution with ethanol until an absorbance of 0.700 (±0.02) was reached at a wavelength of 734 nm. To evaluate the antiradical activity of the crude extract of P. odora and its various fractions, 1950 μL of the diluted ABTS+• solution was combined with 50 μL of each methanol-soluble extract at different concentrations. In addition, a negative control was prepared by adding 50 μL of methanol to 1950 μL of the ABTS+• ethanol solution. After an hour of incubation, the reduction in absorbance was measured at 734 nm via a UV–visible spectrophotometer at each concentration. A positive control using a standard antioxidant solution of ascorbic acid was also set up under conditions identical to those of the extracts. This procedure was repeated three times for each concentration, and the antioxidant activity was quantified as the percentage of inhibition of the ABTS radical (% PI) according to the following formula:
% PI = [(A negative control - A extract)/A negative control] × 100; where:
-
•
A negative control is the absorbance of ABTS after the reaction time.
-
•
A extract is the absorbance of the extract with ABTS after the reaction time.
The IC50 was determined to quantify the effectiveness of the extract at reducing 50 % of the ABTS radical.
2.7. Evaluation of anti-inflammatory activity
2.7.1. Assessment of bovine serum albumin denaturation inhibition
The impact of the crude extract and fractions of P. odora on the heat-induced denaturation of bovine serum albumin (BSA) was assessed via an adapted method described by Williams et al. [16]. The reaction mixtures consisted of 20 μL of varying concentrations of each extract and 2 mL of 0.02 % BSA in Tris saline buffer (pH adjusted to 6.4). Simultaneously, a negative control consisting of 2 mL of BSA was prepared. These mixtures were incubated at 37 °C for 15 min and then heated to 72 °C for an additional 5 min. Upon cooling, the turbidity was measured at 660 nm with a UV–visible spectrophotometer. A positive control was also established with diclofenac sodium, which was measured under conditions identical to those of the extracts. Each concentration was tested three times. The percentage of inhibition of BSA denaturation was calculated via the following formula:
% PI = [(A negative control - A extract)/A negative control] × 100; where:
-
•
A negative control denotes the absorbance of BSA after the reaction period.
-
•
A extract denotes the absorbance of the extract with BSA after the reaction period.
2.7.2. Evaluation of In vitro membrane stabilization activity
The anti-inflammatory efficacy of P. odora crude extract and its fractions was evaluated via an in vitro human red blood cell (HRBC) membrane stabilization method [17]. A blood sample was collected from a healthy volunteer who had been medication-free for two weeks. The sample was mixed with sterilized Alsever's solution, which consisted of 2 % dextrose, 0.8 % sodium citrate, 0.05 % citric acid, and 0.42 % sodium chloride in distilled water. The sample was centrifuged at 3000 rpm, and the packed cells were washed with isosaline and then resuspended in a 10 % (v/v) red blood cell mixture in isosaline. Subsequently, 0.5 ml of various extract concentrations were added to a mixture containing 1 ml of phosphate-buffered saline, 2 ml of hyposaline, and 0.5 ml of red blood cell suspension, which was subsequently incubated at 37 °C for 30 min and then centrifuged. In the negative control group, 2 ml of distilled water was used instead of hyposaline. The hemoglobin content in the supernatant was quantified at 560 nm via a spectrophotometer. The hemolysis percentage was calculated, assuming that the total hemolysis percentage in distilled water was 100 %. Concurrently, a positive control was performed using diclofenac sodium, with the absorbance measured under the same conditions as the extracts. Each concentration was tested in triplicate, and the percentage of hemolysis inhibition was calculated via the following formula:
Percentage of Hemolysis Inhibition = [(A negative control - A extract)/A negative control] × 100; where:
-
•
A negative control: corresponds to the absorbance of the control supernatant.
-
•
A extract: corresponds to the absorbance of the extract supernatant.
2.7.3. Evaluation of In vivo anti-inflammatory activity
The in vivo anti-inflammatory efficacy of P. odora crude extract was assessed by evaluating its capacity to inhibit carrageenan-induced edema in mouse hind paws [18]. Animals (n = 24) that had undergone a 12-h water-fasting period were segregated into four groups (n = 6). The treated groups received aqueous solutions of the crude leaf maceration extract of P. odora at two different dosages (50 and 100 mg/kg). The reference group was administered an aqueous solution of diclofenac at a dosage of 50 mg/kg, whereas the control group received distilled water. Sixty minutes after administration, 100 μl of a 1 % (w/v) carrageenan suspension was injected into the subplantar region of the right hind paw. Paw volumes were measured initially (t0) and subsequently at t1, t3, and t6 postinjection. The percentage of edema inhibition was calculated via the following formula:
Percentage of inhibition = [(Ct-Co) control - (Ct-Co) treated]/[(Ct-Co) control] × 100; where.
-
•
Ct: Linear paw circumference postcarrageenan injection.
-
•
Co: Linear paw circumference before carrageenan injection.
2.8. Evaluation of gastroprotective activity
2.8.1. Ulcer inhibition percentage
This study assessed the gastroprotective efficacy of P. odora crude extract and its fractions, with a specific focus on its ability to mitigate ethanol-induced gastric ulceration. Following the methodology outlined by Robert et al. [19], ninety mice preconditioned with a 24-h water fast were divided into fifteen groups of six. The treatment groups were administered varying concentrations (10, 50, 100, and 200 mg/kg) of an aqueous solution of the crude leaf maceration extract of P. odora or aqueous solution (0.2 % Tween 80 for insoluble extracts) of fractions at two distinct doses (10 and 50 mg/kg). The reference group received an aqueous solution of omeprazole (20 mg/kg), whereas the ulcer control and normal control groups were given 10 ml/kg distilled water. Sixty minutes after administration, all groups except the normal control were subjected to ulcer induction via a 5 ml/kg injection of absolute ethanol. The mice were euthanized 1 h after ethanol administration under chloroform anaesthesia, and their stomachs were harvested through a median ventral dissection. Following an incision along the greater curvature and rinsing with isotonic saline (0.9 % NaCl), stomach images were captured to evaluate the ulcer inhibitory effect of each treatment via ImageJ software (http://rsb.info.nih.gov/ij/).
The degree of gastric mucosal protection conferred by the experimental treatments was quantified via the following formula:
The percentage of ulcer inhibition = [(UA ulcer control - UA extract)/UA ulcer control] × 100, where:
-
•
An ulcer control: represents the ulcer area in the ulcer control group.
-
•
A extract: represents the ulcer area in the treated group.
2.8.2. Total acidity measurement
Following euthanasia, the gastric juice was extracted and centrifuged at 3000 rpm for 10 min. The supernatant was then diluted tenfold with distilled water, and gastric acidity was quantified via the titration method employing 0.01 N NaOH and phenolphthalein as visual indicators [20]. The formula for calculating total acidity is expressed as follows:
2.8.3. Microscopic histological analysis
Stomach tissue samples were sectioned into thin slices and fixed in 10 % formalin. These samples underwent dehydration through successive ethanol baths of increasing concentration, followed by absolute ethanol and xylene to eliminate any residual alcohol. The tissues were then embedded in paraffin, which was subsequently allowed to solidify. Thin 5 μm sections were prepared via a microtome. These sections were deparaffinized in xylene, rehydrated in descending ethanol concentrations, rinsed in water, and stained with hematoxylin and eosin. The staining highlights nuclear (purple) and cytoplasmic (red) components under microscopic examination [21].
2.9. Characterization of flavonoids by HPLC-MSn
A high-performance liquid chromatography (HPLC) system (Agilent Technologies) interfaced with an LCQ Deca ion trap mass spectrometer (Thermo Finnigan) was used for the comprehensive analysis and characterization of flavonoids present in P. odora. HPLC‒MS analysis was performed directly on acidified methanolic leaf extracts. Specifically, 10 mg of plant material was subjected to ultrasonic-assisted extraction at a frequency of 59 kHz and a temperature of 25 °C in 2 mL of methanol containing 1 % acetic acid for 30 min. This extraction protocol was repeated twice with solvent replenishment. An aliquot of 5 μL of the resulting extract was then introduced into the HPLC system via an automated sample injector (Thermo Finnigan).
Chromatographic separation was achieved on a reversed-phase C18 column (150 mm × 2.1 mm, 3.5 μm particle size; Zorbax Eclipse XDB-C18) integrated into an SCM1000 degasser (ThermoQuest). The mobile phase was composed of solvent A (0.1 % formic acid in ultrapure water) and solvent B (0.1 % formic acid in acetonitrile), which were delivered by a binary pump (1100 Series, Agilent Technologies). The gradient elution profile is delineated in Table 1, with the flow rate set at 0.2 mL/min. The column temperature was maintained at 30 °C throughout the analysis. Detection was conducted via a diode array detector (Spectra System UV6000LP, Thermo Finnigan).
Table 1.
Gradient elution program for HPLC-MSn analysis.
| Time (min) | % Solvent A (0.1 % formic acid in water) | % Solvent B (0.1 % formic acid in acetonitrile) |
|---|---|---|
| 0 | 97 % | 3 % |
| 0–5 | 91 % | 9 % |
| 5–15 | 84 % | 16 % |
| 15–45 | 50 % | 50 % |
The full effluent from the diode array detector was directed to the mass spectrometer equipped with an electrospray ionization (ESI) source operating in negative ion mode at an ionization voltage of 5 kV. Nitrogen (N2) was employed as both the nebulizing gas and auxiliary gas, while the capillary temperature was maintained at 240 °C. Helium (He) was used as the stabilization, collision and focusing gas. The mass spectrometric analysis covered a mass-to-charge (m/z) range from 50 to 2000 atomic mass units (amu). Data acquisition and analysis were performed via Xcalibur™ software (version 1.2).
2.10. Molecular docking
This molecular docking investigation aimed to predict the antiulcer activity of key compounds identified in P. odora through HPLC‒MS analysis. The molecular structures of the studied compounds were retrieved from the PubChem database [22]: Kaempferol-3-O-glucoside-7-O-rhamnoside (C₂₇H₃₀O₁₅, CID 14035324); Rutin (C₂₇H₃₀O₁₆, CID 5280805); Rhoifolin (C₂₇H₃₀O₁₄, CID 5282150); Chrysoeriol 7-neohesperidoside (C₂₈H₃₂O₁₅, CID 44593486); Isorhamnetin 3-O-glucoside (C₂₂H₂₂O₁₂, CID 5318645); Thermopsoside (C₂₂H₂₂O₁₁, CID 11294177); Quercetin (C₁₅H₁₀O₇, CID 5280343); Luteolin (C₁₅H₁₀O₆, CID 5280445); and Isorhamnetin (C₁₆H₁₂O₇, CID 5281654). Each compound was preoptimized via the MMFF94 force field in Avogadro software [23].
The crystallographic structure of the gastric proton pump bound to vonoprazan (PDB ID: 5YLU) [24], acquired from the Protein Data Bank (PDB), was selected as the molecular target for evaluating antiulcer activity. Structural refinement was conducted via Swiss-Pdb Viewer [25], and structural preparation was performed via Discovery Studio [26] and AutoDock Tools [27], which included the addition of hydrogen atoms, Kollman charges, and the removal of water molecules, cocrystallized ligands, and other irrelevant protein conformations. The active site was delineated on the basis of the original position of the vonoprazan ligand (HKT).
Docking simulations for each compound against the target structure (PDB ID: 5YLU) were executed via AutoDock Vina, as implemented in PyRx [28]. The search center (SC) coordinates and box dimensions (BD) were set as follows: (SC: X = 49.00, Y = −16.42, Z = −3.96; BD: X = 25, Y = 25, Z = 25). Visualization and analysis of the docking results were conducted via Discovery Studio Visualizer [26].
2.11. ADMET analysis
The pharmacokinetic properties—including absorption, distribution, metabolism, and excretion (ADME)—as well as the toxicological profiles of the flavonoid compounds identified in P. odora via HPLC/MS were predicted via in silico tools such as SwissADME [29] and ADMETlab 3.0 [30].
2.12. Statistical analysis
The data are reported as the means ± standard errors of the means (SEMs). Differences between groups were statistically analysed via analysis of variance (ANOVA), followed by Tukey's post hoc test for specific comparisons.
3. Results and discussion
3.1. Total phenolic compounds and antioxidant efficacy
The concentrations of total polyphenols and flavonoids, as well as the antioxidant activities expressed in terms of the IC50 values of the crude extract and its fractions, are detailed in Table 2. The crude leaf extract of P. odora is characterized by a significant phenolic content, quantified at 594.49 mg GAE/g DE, and flavonoid levels reaching 184.54 mg CE/g DE. These results are consistent with those reported by Touati et al. [11], who reported high concentrations of polyphenols (90 μg CE/g DM) and flavonoids (11.34 μg QE/g DM) in the methanolic extract of P. odora leaves.
Table 2.
Total phenolic content, total flavonoid content and antioxidant activity of Pulicaria odora crude extract and its fractions.
| Sample | Phenolic compounds |
Antioxidant activity |
||
|---|---|---|---|---|
| Total phenolic content (mg GAE/g DE) | Total flavonoid content (mg CE/g DE) | DPPH |
ABTS |
|
| IC50 (mg/ml) | IC50 (mg/ml) | |||
| Crude extract | 594.5 ± 59.6ab | 184.5 ± 18.7a | 0.067 ± 0.006c | 0.25 ± 0.007cd |
| Chloroform fraction | 418.7 ± 48.2c | 43.91 ± 3.59c | 3.42 ± 0.35a | 2.19 ± 0.19a |
| Ethyl acetate fraction | 619.9 ± 51.3a | 164 ± 8.12a | 0.26 ± 0.02c | 0.45 ± 0.03bc |
| n-butanol fraction | 586.66 ± 14.29ab | 189.06 ± 5.43a | 0.32 ± 0.01c | 0.61 ± 0.01b |
| Aqueous fraction | 489.96 ± 7.64bc | 70.54 ± 3.01b | 1.42 ± 0.21b | 0.24 ± 0.03cd |
| Ascorbic acid | NA | NA | 0.049 ± 0.0008c | 0.041 ± 0.0004d |
Data were expressed as mean ± SEM (n = 3).
a-e Values bearing the same letters show no significant differences (P < 0.05).
NA, Not Applicable.
Our study demonstrated that the crude extract of P. odora leaves has strong scavenging activity against DPPH and ABTS radicals, with IC50 values of 0.067 mg/ml and 0.25 mg/ml, respectively. The extract's effectiveness in neutralizing DPPH radicals is on par with that of ascorbic acid, which has an IC50 of 0.049 mg/ml.
Investigating the correlation between antioxidant activity and the concentrations of total polyphenols and flavonoids across different fractions revealed distinct profiles. Notably, the ethyl acetate and n-butanol fractions exhibited the most potent antiradical activity in both DPPH assays, alongside significantly higher levels of phenolic compounds than the other fractions did. Additionally, as the concentrations of total polyphenols and flavonoids decrease, there is a corresponding reduction in antiradical efficacy within the DPPH assay across the fractions. The aqueous fraction, while displaying the highest antiradical power in the ABTS assay, contains relatively lower phenolic content than the ethyl acetate and n-butanol fractions. These latter fractions demonstrate moderate antiradical potential but are distinguished by relatively high concentrations of both total polyphenols and flavonoids. Furthermore, the chloroform fraction exhibited significantly lower antiradical activity across the DPPH and ABTS assays. This fraction also contains the lowest concentrations of total polyphenols and flavonoids.
To account for the observed differences in antioxidant capacity among diverse phytochemical systems, two hypotheses have been formulated. The first posits that the spectrum of antioxidant efficacy transcends the traditional confines attributed to phenolic constituents. It postulates that myriad compounds, which are both structurally and functionally distinct from polyphenols, manifest robust antioxidant activities [31]. The second hypothesis elaborates on the nuanced interaction between polyphenol structure and antioxidant activity. These findings suggest that while certain botanical species demonstrate a direct correlation between polyphenol concentration and antioxidant effectiveness, others are dependent on the specific molecular configuration and chemical composition of these compounds [32].
In delving into the mechanistic foundations of each employed assay, distinct variations in the underlying chemical reactions become apparent. The DPPH assay is governed by electron transfer mechanisms, whereas the ABTS assay involves both electron and proton transfers, highlighting its versatility. Notably, the solubility of the ABTS cation radical in both aqueous and organic solvents renders it exceptionally suitable for assessing the antioxidant capabilities of substances across a diverse spectrum of hydrophilic and lipophilic environments over an extensive pH range. This adaptability is particularly beneficial for comprehensive antioxidant profiling. Moreover, steric accessibility is a critical determinant of DPPH reaction dynamics, where molecular size and access to the radical site significantly influence antioxidant efficacy. Owing to their enhanced access to the radical site, smaller molecules exhibit superior antioxidant properties in the DPPH assay [33].
Consistent with these findings, the findings from this study align with those reported by Saidani et al. [9], which substantiate that extracts of Pulicaria odora are characterized by a rich composition of phenolic compounds, which are essential for their marked antioxidant activity.
3.2. Anti-inflammatory activity
The analysis of protein denaturation inhibition (Fig. 1) revealed that the crude maceration extract of P. odora leaves exhibited substantial anti-denaturation activity, with a maximum inhibition rate of 44.60 % at a concentration of 0.1 μg/ml. In contrast, at higher concentrations (10 μg/ml), the inhibition rate significantly decreased to −41.06 %, suggesting a denaturing effect at these concentrations. The efficacy of the crude extract was notably similar to that of diclofenac, with an inhibition percentage of 30.91 % at 1 μg/ml, which was comparable to that of diclofenac at 100 μg/ml. Fig. 1 shows that the chloroform fraction, in particular, significantly inhibited protein denaturation, with percentages of 42.72 % and 38.67 % at 10 μg/ml and 1 μg/ml, respectively, which are comparable to diclofenac at 250 μg/ml. The aqueous fraction displayed moderate efficacy, with peak inhibition of 24.83 % at 0.01 μg/ml, whereas the ethyl acetate and n-butanol fractions demonstrated lower efficacy, peaking at 17.22 % and 10.47 % at 1 μg/ml and 0.01 μg/ml, respectively.
Fig. 1.
Effect of Pulicaria odora crude extract and its fractions on denaturation inhibition of bovine serum albumin.
a-k Values bearing the same letters show no significant differences (P < 0,05).
Vertical bars represent standard deviation.
The hemolytic inhibition data (Fig. 2) revealed that the crude extract of P. odora exhibited robust antihemolytic activity, reaching 91.94 % and 88.25 % at concentrations of 0.1 μg/ml and 1 μg/ml, respectively, with activities comparable to those of high-concentration diclofenac (250 μg/ml). However, the effectiveness diminished sharply at 10 μg/ml, with only 40.64 % hemolytic inhibition observed. In our exploration of erythrocyte membrane stabilization by extract fractions, the results highlight the distinct efficacies among the solvent fractions tested. The chloroform and ethyl acetate fractions demonstrated exceptionally robust hemolysis inhibition capabilities, with the chloroform fraction achieving inhibition rates of 87.95 %, 92.88 %, and 93.47 % and the ethyl acetate fraction showing rates of 87.19 %, 92.27 %, and 93.33 % at increasing concentrations of 0.01 μg/ml, 0.1 μg/ml, and 1 μg/ml, respectively. Concurrently, the aqueous and n-butanol fractions also displayed significant anti-hemolytic activity, although slightly less pronounced, with inhibition percentages of 87.13 % and 85.30 %, respectively, at a concentration of 1 μg/ml.
Fig. 2.
Effect of Pulicaria odora crude extract and its fractions on HRBC membrane stabilization.
a-i Values bearing the same letters show no significant differences (P < 0,05).
Vertical bars represent standard deviation.
The anti-inflammatory properties of the crude P. odora leaf extract were assessed quantitatively through its effect on carrageenan-induced edema in mouse hind paws (Fig. 3). The study employed two concentrations of the extract, 50 and 100 mg/kg, alongside a reference treatment with diclofenac, a well-established anti-inflammatory agent administered at 50 mg/kg. Upon intraplantar injection of carrageenan, notable induction of localized edema occurs. The oral administration of the crude extract of P. odora leaf maceration at doses of 50 and 100 mg/kg significantly decreased the edematous response 1 h after carrageenan injection. However, this effect is inferior to that of diclofenac, a standard medication administered at a dose of 50 mg/kg, which also significantly reduces the volume of carrageenan-induced paw edema within 1 h. After 3 h, diclofenac at 50 mg/kg and the lower dose of the crude extract of leaf maceration at 50 mg/kg had similar inhibitory effects on paw edema, whereas the crude extract of leaf maceration at 100 mg/kg had significantly greater anti-inflammatory potency. Six hours after carrageenan injection, superior and significant inhibition of paw edema was observed with diclofenac compared with the crude extract of P. odora leaf maceration at doses of 50 and 100 mg/kg.
Fig. 3.
Effect of Pulicaria odora crude extract and diclofenac on the carrageenan-induced hind paw edema.
a-c Values bearing the same letters show no significant differences (P < 0,05).
Vertical bars represent standard deviation.
Edema development is characterized by two distinct phases: an initial phase triggered by inflammatory mediators from damaged tissues occurring approximately 2.5 h after carrageenan administration, followed by a secondary phase driven by prostaglandin release between three and 6 h [34]. This study uniquely highlights that the temporal dynamics of the anti-inflammatory effects of the P. odora extracts differ significantly from those observed with diclofenac, as illustrated in Fig. 4. The efficacy of diclofenac peaks in the later phase, likely due to its role as a nonselective COX inhibitor, which affects both the COX-1 and COX-2 pathways [35]. In contrast, the crude extract of P. odora significantly inhibited all three phases of carrageenan-induced inflammation. These observations suggest that the active constituents present in P. odora act by reducing the synthesis, release, and action of inflammatory mediators.
Fig. 4.
Temporal dynamics of inhibition of the inflammation induced by carrageenan of Pulicaria odora crude extract and diclofenac.
Furthermore, several plants of the Pulicaria genus, which are traditionally used as remedies for inflammation, have demonstrated potential anti-inflammatory effects [[36], [37], [38]]. For example, Mohammed et al. [37] reported that the nonpolar n-hexane extract of P. jaubertii demonstrated the most potent anti-inflammatory effects in vitro, as evidenced by a membrane stabilization IC50 of 60.8 μg/ml. Additionally, the extract of P. crispa was shown to significantly counteract carrageenan-induced inflammation in rat models, with high-dosage treatments (500 mg/kg) achieving up to a 32 % reduction in paw edema over 3 h [38], highlighting the pharmacological potential of these plant extracts in clinical settings.
3.3. Gastroprotective activity
This study evaluated various gastric parameters, including macroscopic measures such as ulcer surface calculations expressed as percentages of ulcer inhibition and total acidity assessments, complemented by microscopic histological analyses.
Macroscopic assessments and the classification of gastric lesions observed in mice subjected to the ulcerogenic agent ethanol, along with those pretreated with crude extracts, their fractions, or the reference medication omeprazole, are depicted in Fig. 5. The results of the quantitative evaluation of gastric mucosa ulceration inhibition are presented in Table 3.
Fig. 5.
Macroscopic appearance of the gastric mucosa of the mice subjected to the ulcerogenic agent, ethanol (B), along with those pre-treated with Pulicaria odora crude extracts at doses 10, 50, 100, 200 mg/kg (D,E,F,G), their fractions; chloroform fraction at doses 10,50 mg/kg (H,I), ethyl acetate fraction at doses 10,50 mg/kg (J,K), n-butanol fraction at doses 10,50 mg/kg (L,M), aqueous fraction at doses 10,50 mg/kg (N,O), or the reference medication omeprazole at dose 20 mg/kg (C). (A) Showed normal macroscopic appearance of the gastric mucosa from normal group.
Table 3.
Gastric mucosa ulceration inhibition and effect in gastric acidity of Pulicaria odora crude extract and its fractions.
| Pre-treatment | Dose | Inhibition (%) | Total acidity (meq/l per 100 g) |
|---|---|---|---|
| Normal control | 10 ml/kg | 00.00 | 1.6 ± 0.04g |
| Ulcer control | 5 ml/kg | NA | 3.06 ± 0.04a |
| Omeprazole | 20 mg/kg | 67.31 ± 1.78e | 1.27 ± 0.06i |
| Crude extract | 10 mg/kg | 42.93 ± 0.81gh | 2.77 ± 0.06b |
| 50 mg/kg | 82.37 ± 0.80d | 1.8 ± 0.04e | |
| 100 mg/kg | 99.43 ± 0.77ab | 0.82 ± 0.02k | |
| 200 mg/kg | 99.91 ± 0.16a | 0.77 ± 0.02k | |
| Chloroform fraction | 10 mg/kg | 56.84 ± 1.21f | 2.57 ± 0.06c |
| 50 mg/kg | 89.83 ± 0.32c | 1.75 ± 0.04ef | |
| Ethyl acetate fraction | 10 mg/kg | 30.33 ± 2.1i | 3 ± 0.04a |
| 50 mg/kg | 47.51 ± 2.89g | 2.25 ± 0.04d | |
| n-butanol fraction | 10 mg/kg | 46.96 ± 1.94h | 1.63 ± 0.04fg |
| 50 mg/kg | 65.26 ± 2.33e | 1.43 ± 0.04h | |
| Aqueous fraction | 10 mg/kg | 83.98 ± 1.24d | 1.23 ± 0.02i |
| 50 mg/kg | 95.56 ± 2.2b | 1.03 ± 0.04j |
Data were expressed as mean ± SEM (n = 6).
a-k Values bearing the same letters show no significant differences (P < 0.05).
NA, Not Applicable.
Macroscopically, extensive deep lesions and hemorrhages covering the entire stomach surface were observed in mice administered ethanol at a dosage of 5 ml/kg. In stark contrast, the mice that received distilled water presented normal and healthy gastric mucosa, highlighting the pronounced ulcerogenic impact of ethanol. Ethanol is well documented as a potent ulcerogenic agent that causes severe damage to gastric tissues through complex, multifactorial mechanisms and has been extensively studied and corroborated by numerous researchers [39,40]. Mice pretreated with 20 mg/kg omeprazole exhibited substantial ulcer protection, characterized by localized and well-demarcated lesions, with a significant 67.31 % reduction in ulceration. Our study on the gastroprotective effects of the crude leaf extract of P. odora revealed a marked dose-dependent protective effect against ethanol-induced lesions. Specifically, less severe lesions were noted in the groups pretreated with the extract at concentrations of 10 mg/kg and 50 mg/kg, with significant ulceration inhibition percentages of 42.65 % and 82.37 %, respectively. Remarkably, the gastric mucosa of the group pretreated with 100 mg/kg crude extract almost completely mirrored the healthy appearance of the control group, with an ulceration inhibition rate of 99.43 %. At the highest tested dose of 200 mg/kg, the extract effectively restored the appearance of normal gastric mucosa after ethanol challenge, resulting in an inhibition rate of 99.91 %, indicating no observable lesions.
To determine the nature of the molecule(s) responsible for the gastroprotective effect of P. odora leaves, a detailed assessment was conducted on various fractions derived from the crude leaf extract. The study evaluated two dosages, 10 mg/kg and 50 mg/kg, for each fraction to determine their relative efficacies. The findings distinctly illustrated a pronounced dose‒dependent relationship.
The aqueous fraction was notably effective at 10 mg/kg, achieving 83.98 % inhibition of ulceration. However, the impact was markedly enhanced at 50 mg/kg, where a significant reduction in gastric lesions was observed, reducing the lesion severity to superficial levels and achieving an ulceration inhibition of 95.56 %. This response underscores a potent dose-dependent efficacy, bolstering the promising gastroprotective potential of this fraction. These findings suggest that the aqueous fraction of the crude leaf extract of P. odora possesses a superior pharmacological profile compared with omeprazole, likely due to the action of specific molecules that mitigate ulceration.
The chloroform fraction also displayed a gastroprotective effect, albeit slightly less potent than the aqueous fraction. At 10 mg/kg, a significant reduction in ulceration was noted, with an inhibition rate of 56.84 %. Increasing the dose to 50 mg/kg significantly enhanced its protective effects, with an ulceration inhibition rate of 89.83 %, demonstrating effective gastric protection despite minimal lesion presence. This finding indicates that the chloroform fraction maintains a beneficial gastroprotective role, increasing in efficacy with increasing dosage.
Conversely, the n-butanol fraction had moderate gastroprotective effects. At dosages of 10 mg/kg and 50 mg/kg, it provided noticeable protection, with ulceration inhibition rates of 46.96 % and 65.26 %, respectively. Although its overall impact was less effective than that of the previous fractions, it still contributed to the collective gastroprotective effect observed, highlighting a significant variance between the administered dosages.
The ethyl acetate fraction had a relatively minimal gastroprotective effect. At 10 mg/kg, the impact was minimal, with a mere 30.33 % inhibition of ulceration observed. Even at a higher dose of 50 mg/kg, the effect remained subdued, resulting in a 47.51 % inhibition rate. This fraction thus demonstrated a weak but still dose-dependent response, suggesting its limited gastroprotective capability compared with the other fractions examined.
These results underscore the critical role of dosage in determining the gastroprotective efficacy of the different fractions of the crude leaf extract of P. odora. These findings highlight how the therapeutic effect varies with the administered dose, facilitating the identification of the most promising fractions for further detailed pharmacological investigations.
Gastric acidity primarily arises from hydrochloric acid secretion by stomach epithelial cells [41]. Under ulcerative conditions, a significant reduction in gastric pH occurs, which increases acidity levels, thereby intensifying mucosal lesions [42]. In our study, as detailed in Table 3, a marked increase in gastric acidity was observed in the ethanol-treated mice, with an acidity level of 3.06 meq/l per 100 g, in stark contrast to that of the control group, which was 1.6 meq/l per 100 g. These findings substantiate the deleterious effects of ethanol on the gastric mucosa, notably through a reduction in pH and an increase in gastric acidity, as corroborated by several researchers [43].
Pretreatment with the crude leaf extract of P. odora or its distinct fractions significantly mitigated acidity. The acidity progressively decreased with increasing dose, reaching 0.77 meq/l per 100 g at the highest dose (200 mg/kg), whereas the acidity of the standard drug omeprazole was 1.27 meq/l, attributable to its mechanism of proton pump inhibition [44]. Among the fractions, the aqueous fraction was the most effective, reducing the acidity to 1.03 meq/l per 100 g, whereas the chloroform fraction had a lesser effect, with an acidity of 1.75 meq/l. This differential response among the fractions highlights the diverse biochemical pathways and active compounds inherent to each fraction. The possible mechanisms underlying the gastroprotective effect still require further investigation through additional models.
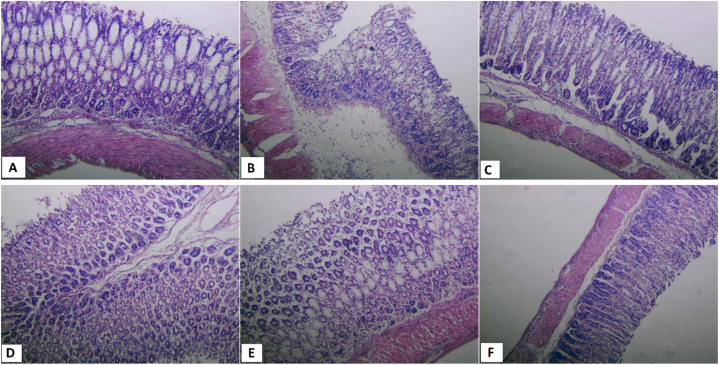
The histological analyses of the gastric mucosa, as illustrated in Fig. 6, demonstrated distinct responses depending on the treatment administered. The mice that received distilled water displayed a normal mucosal condition, indicative of an undisturbed gastric environment. In stark contrast, the application of the ulcer-inducing agent resulted in marked ulcerative changes within the mucosa. These changes are characterized by evident disruptions in mucosal continuity, significant inflammatory sites, edema, and necrosis.
Fig. 6.
Histological appearance of the gastric mucosa of the mice subjected to the ulcerogenic agent, ethanol (B), along with those pre-treated with Pulicaria odora crude extracts at dose 200 mg/kg (D), their fractions; aqueous fraction at dose 50 mg/kg (E), chloroform fraction at dose 50 mg/kg (F), or the reference medication omeprazole at dose 20 mg/kg (C). (A) Showed normal histological appearance of the gastric mucosa from normal group ( × 10).
Histopathological examination of the stomachs of the mice treated with the crude P. odora leaf extract revealed a substantial reduction in the number of gastric lesions. The protective effect at 50 mg/kg was comparable to that achieved with omeprazole, underscoring the extract's potent gastroprotective efficacy. Further increasing the dose to 100 mg/kg and 200 mg/kg resulted in an even more pronounced protective effect, with the mucosal tissue maintaining a histologically intact and healthy appearance akin to that observed in the control group.
Histopathological evaluations of gastric mucosa samples treated with four distinct fractions of P. odora crude leaf extract revealed a dose-dependent gastroprotective effect. Enhanced protection was particularly notable at elevated doses. The aqueous fraction provided the best overall protection among the tested fractions. This treatment improved the tissue condition of the stomach by reducing ulceration and infiltration. The effect of the aqueous fraction was superior to that of omeprazole.
Emerging research underscores the gastroprotective qualities of two species within the Pulicaria genus—Pulicaria crispa and Pulicaria undulata [45,46]. The present study highlights the initial exploration of the gastroprotective effects of P. odora leaves, revealing encouraging outcomes that advocate further exploration of the Pulicaria genus as a source of novel therapeutic agents for gastrointestinal ailments. Moreover, the propensity of the Pulicaria genus to accumulate gastroprotective compounds merits additional study.
3.4. Identification of flavonoids via HPLC-MSn
The identification of flavonoids in P. odora was accomplished through high-performance liquid chromatography coupled with mass spectrometry (HPLC-MSn). The separation and characterization were based on retention times, ultraviolet (UV) spectra, MS, and MSn spectra and were further validated against established standards and the literature. This analytical process enabled the tentative identification of nine phenolic compounds, all of which were classified as flavonoids (Table 4).
Table 4.
Tentative identification of flavonoids in Pulicaria odora.
| Peak | Tentative identification | RT (min) | λ max (nm) | [M − H]- (m/z) | m/z fragments |
|---|---|---|---|---|---|
| 1 | Kaempferol 3-O-glucoside 7-O-rhamnoside | 13.35 | 266; 355 | 593 | 447, 431, 285 |
| 2 | Quercetin 3-O-rutinoside (rutin) | 14.90 | 254; 360 | 609 | 301, 271, 179, 151 |
| 3 | Apigenin 7-O-neohesperidoside (Rhoifolin) | 16.70 | 270; 350 | 577 | 413, 269 |
| 4 | Chrysoeriol 7-neohesperidoside | 18.15 | 261; 352 | 607 | 299, 284 |
| 5 | Isorhamnetin 3-O-glucoside | 19.91 | 245; 364 | 477 | 357, 315, 314, 285, 271, 243 |
| 6 | Chrysoeriol 7-O-glucoside (Thermopsoside) | 24.11 | 269; 356 | 461 | 299 |
| 7 | Quercetin | 26.71 | 254; 365 | 301 | 273, 257, 179, 151 |
| 8 | Luteolin | 33.89 | 263; 359 | 285 | 241, 151, 133, 107 |
| 9 | Isorhamnetin | 35.26 | 253; 345 | 315 | 300, 271, 251 |
Among the identified compounds were five flavonols, three of which were glycosylated: quercetin, rutin, isorhamnetin, isorhamnetin-3-O-glucoside, and kaempferol 3-O-glucoside 7-O-rhamnoside. Additionally, luteolin and three glycosylated flavones—rhoifolin, chrysoeriol-7-O-glucoside, and chrysoeriol-7-neohesperidoside—were detected.
Compounds corresponding to peaks 1, 2, 3, and 4 (Fig. 7) exhibited deprotonated ions [M − H] ⁻ at m/z 593, 609, 577, and 607, respectively. The early elution profiles of these compounds indicated high polarity, which is consistent with glycosylated flavonoid structures. UV spectral analysis further corroborated their classification as flavonoids. MS/MS fragmentation patterns revealed the characteristic breakdown of glycosidic bonds, confirming their aglycone forms through comparison with reference standards. These compounds were identified as kaempferol 3-O-glucoside 7-O-rhamnoside, rutin, rhoifolin, and chrysoeriol-7-neohesperidoside, in accordance with previously reported findings [[47], [48], [49], [50]].
Fig. 7.
RP-LC-ESI-MS Base peak chromatogram of the Pulicaria odora extract. RT: Retention Time.
Peaks 5 and 6 were partially identified as isorhamnetin-3-O-glucoside ([M − H]⁻ = 477) and chrysoeriol-7-O-glucoside ([M − H]⁻ = 461). The identities of these flavonoids were confirmed through strong MS/MS spectral matches to standards of isorhamnetin and chrysoeriol, as described in earlier studies by Du et al. and Khallouki et al. [48,51].
Peaks 7 to 9 (Fig. 7) were attributed to quercetin ([M − H]⁻ = 301), luteolin ([M − H]⁻ = 285), and isorhamnetin ([M − H]⁻ = 315), respectively. Their identification was based on a combination of chromatographic behavior, UV–Vis spectral characteristics, and mass spectrometric data, which were consistent with authenticated standards.
3.5. Molecular docking
The binding energies derived from molecular docking simulations of the nine identified compounds in conjunction with the native ligand are detailed in Table 5.
Table 5.
Binding score (kcal/mol) for Pulicaria odora flavonoids in comparison with vonoprazan responsible for proton pump inhibition.
| Protein | Compound | Binding scores (kcal/mol) |
|---|---|---|
| Gastric proton pump (PDB ID: 5 YLU) | Vonoprazan | −9.5 |
| M1: Kaempferol 3-O-glucoside 7-O-rhamnoside | −8.2 | |
| M2: Quercetin 3-O-rutinoside (rutin) | −10.2 | |
| M3: Apigenin 7-O-neohesperidoside (Rhoifolin) | −9.5 | |
| M4: Chrysoeriol 7-neohesperidoside | −9.5 | |
| M5: Isorhamnetin 3-O-glucoside | −7.9 | |
| M6: Chrysoeriol 7-O-glucoside (Thermopsoside) | −9.5 | |
| M7: Quercetin | −9.0 | |
| M8: Luteolin | −9.3 | |
| M9: Isorhamnetin | −8.4 |
The docking results indicate that complexes M2/5YLU (−10.2 kcal/mol), M3/5YLU (−9.5 kcal/mol), M4/5YLU (−9.5 kcal/mol), and M6/5YLU (−9.5 kcal/mol) exhibit superior binding affinities relative to the native ligand vonoprazan (−9.5 kcal/mol). Fig. 8 shows that the M2/5YLU complex forms multiple hydrogen bonds with critical residues, including ARG328 (2.12 Å), GLN127 (2.68 Å), ASN138 (2.55 Å), ASN792 (2.7 Å), and CYS813 (2.65 Å), along with hydrophobic interactions involving residues such as LEU141 (3.44 Å), ALA335 (5.44 Å), ALA339 (3.49 Å and 4.19 Å), and ILE816 (4.48 Å and 4.81 Å). Other types of interactions were also observed with residue CYS813 (4.98 Å).
Fig. 8.
3D (a) and 2D (b) receptor-ligand interaction between gastric proton pump (PDB ID: 5 YLU) and rutin.
These findings suggest that the gastroprotective effect of P. odora leaves may be partially attributed to the presence of flavonoids capable of inhibiting gastric acid secretion through targeted interactions with proton pumps. Moreover, certain flavonoids identified in this study are well documented for their ability to protect the gastrointestinal mucosa against ulcerative damage in various experimental models [52]. Prior investigations further substantiated the antisecretory activity of prominent flavonoids such as rutin and quercetin [53,54].
3.6. ADMET analysis
The ADMET (absorption, distribution, metabolism, excretion, and toxicity) profiles of the flavonoids identified from P. odora via HPLC/MS were computationally predicted via the SwissADME and ADMETlab 3.0 tools. The results are summarized in Table 6.
Table 6.
ADMET properties of the identified flavonoids of Pulicaria odora, (1) kaempferol 3-O-glucoside 7-O-rhamnoside, (2) rutin, (3) rhoifolin, (4) chrysoeriol 7-neohesperidoside, (5) isorhamnetin 3-O-glucoside, (6) thermopsoside, (7) quercetin, (8) luteolin, (9) isorhamnetin.
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Druglikeness | Lipinski | No | No | No | No | No | No | Yes | Yes | Yes |
| Bioavailability Score | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.55 | 0.55 | 0.55 | |
| Pharmacokinetics | GI absorption | Low | Low | Low | Low | Low | Low | High | High | High |
| BBB permeant | No | No | No | No | No | No | No | No | No | |
| P-gp substrate | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | |
| P-gp inhibitor | No | No | No | No | No | No | No | No | Yes | |
| CYP3A4 inhibitor | No | No | No | No | No | Yes | Yes | Yes | Yes | |
| CL plasma | Low | Low | Low | Low | Low | Low | Moderate | Moderate | Low | |
| Toxicity | AMES Toxicity | No | Yes | No | No | Yes | Yes | Yes | No | No |
| Hepatotoxicity | No | No | No | No | No | No | No | No | No | |
The bioavailability radar plots shown in Fig. 9 indicate that the three aglycone flavonoids (compounds 7–9) comply with Lipinski's Rule of Five, yielding a bioavailability score of 0.55. In contrast, diglycosylated flavonoids (compounds 1–4) violate three parameters by exceeding the thresholds for molecular size and polarity, whereas monoglycosylated flavonoids (compounds 5–6) violate two parameters owing to increased polarity.
Fig. 9.
Bioavailability radars of the identified flavonoids of Pulicaria odora, (1) kaempferol 3-O-glucoside 7-O-rhamnoside, (2) rutin, (3) rhoifolin, (4) chrysoeriol 7-neohesperidoside, (5) isorhamnetin 3-O-glucoside, (6) thermopsoside, (7) quercetin, (8) luteolin, (9) isorhamnetin.
All aglycone flavonoids demonstrated high human intestinal absorption rates, as predicted by the BOILED-Egg model (Fig. 10). Additionally, none of the aglycone compounds are substrates for P-glycoprotein. With respect to metabolic processing, none of the glycosylated flavonoids inhibited CYP3A4, with the exception of chrysoeriol-7-O-glucoside. The renal excretion rates for most flavonoids are low, except for those of quercetin and luteolin, which exhibit moderate excretion levels. Moreover, quercetin, isorhamnetin-3-O-glucoside, chrysoeriol-7-O-glucoside, and rutin tested positive in the AMES mutagenicity assay, whereas the other flavonoids tested negative. Importantly, none of the flavonoids appear to compromise hepatic function in humans.
Fig. 10.
BOILED-Egg model of Pulicaria odora flavonoids using SwissADME, (5) isorhamnetin 3-O-glucoside, (6) thermopsoside, (7) quercetin, (8) luteolin, (9) isorhamnetin ((1) kaempferol 3-O-glucoside 7-O-rhamnoside, (2) rutin, (3) rhoifolin and (4) chrysoeriol 7-neohesperidoside out of rangel).
4. Conclusion
In conclusion, the results of this study suggest that the crude extract of P. odora leaves possesses significant gastroprotective properties. The various fractions obtained from this extract, namely, the aqueous fraction, chloroform fraction, n-butanol fraction, and ethyl acetate fraction, exhibited varying degrees of gastroprotective effects. The aqueous fraction was found to be the most effective, demonstrating marked inhibition of gastric ulceration. The chloroform fraction also had a notable gastroprotective effect. These findings support the traditional use of P. odora leaves in the treatment of gastric ulcers. LC-ESI-MS analysis of the crude extract led to the identification of nine distinct flavonoid structures, which may have the strongest affinity for the target of interest, the gastric proton pump (PDB: 5YLU). The molecular docking results suggest that the gastroprotective properties of P. odora leaves may be partially attributed to the presence of flavonoids, which have an antisecretory mechanism of action. Further studies are needed to isolate and characterize the active compounds present in this medicinal plant, as well as to evaluate their efficacy in additional animal models. Overall, this study provides encouraging evidence for the gastroprotective activity of the crude leaf extract. The distinct gastroprotective effects highlight the diversity of bioactive compounds present in the different fractions of the crude extract. These findings are valuable for guiding future research, with an emphasis on the most effective fractions to identify the molecules responsible for the gastroprotective effect. A deeper exploration of these fractions could facilitate the isolation and characterization of active compounds, thereby paving the way for potential therapeutic applications in the gastroprotective field.
CRediT authorship contribution statement
Khadidja Boudebbaz: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Manel Brouk: Methodology. Roumaissa Laalem: Methodology. Nada Zabaiou: Methodology.
Ethics statement
All experiments were conducted in accordance with international guidelines for the care and use of laboratory animals and the use of human blood. Approval number of the animal experiment (CP00009) was given also by Algerian Pasteur Institute. The blood sample from a healthy volunteer was collected at Medjoub Said Hospital in Taher (Algeria). Approval number of the human red blood cells experiment (3707) was given by Medjoub Said Hospital of Taher (Algeria) after examining the obtention of informed consent from the donor. The Informed consent was also obtained from the donor for the publication of any images, clinical data, and other data included in the manuscript.
Data availability statement
Data will be made available on request.
Funding
This research did not receive any specific grant from funding.
Declaration of competing interest
The authors declare that they have no conflicts of interest related to the publication of this manuscript.
No financial support or sponsorship was received for this study. All authors have disclosed any potential conflicts of interest, and there are no competing interests to report.
The research presented in this manuscript was conducted with full adherence to ethical guidelines, and the authors have no financial or personal relationships that could be perceived as influencing the work reported in this article.
References
- 1.Calam J., Baron J.H. Pathophysiology of duodenal and gastric ulcer and gastric cancer. BMJ. 2001;323:980–982. doi: 10.1136/bmj.323.7319.980. 10.1136%2Fbmj.323.7319.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones M.P. The role of psychosocial factors in peptic ulcer disease: beyond Helicobacter pylori and NSAIDs. J. Psychosom. Res. 2006;60:407–412. doi: 10.1016/j.jpsychores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.J., Kim E.H., Hahm K.B. Oxidative stress in inflammation‐based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012;27:1004–1010. doi: 10.1111/j.1440-1746.2012.07108.x. [DOI] [PubMed] [Google Scholar]
- 4.Sharifi-Rad M., Fokou P.V.T., Sharopov F., Martorell M., Ademiluyi A.O., Rajkovic J., Salehi B., Martins N., Iriti M., Sharifi-Rad J. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018;23:1751. doi: 10.3390/molecules23071751. 10.3390%2Fmolecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.M., Breslin N.P., Hyde D.K., Buckley M.J., O'morain C.A. Treatment options for Helicobacter pylori infection when proton pump inhibitor‐based triple therapy fails in clinical practice. Aliment Pharmacol. Therapeut. 1999;13:489–496. doi: 10.1046/j.1365-2036.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderberg A.A. Taxonomy and phylogeny of the tribe inuleae (Asteraceae) Plant Systemat. Evol. 1991;176:75–123. doi: 10.1007/BF00937905. [DOI] [Google Scholar]
- 7.Ezoubeiri A., Gadhi C.A., Fdil N., Benharref A., Jana M., Vanhaelen M. Isolation and antimicrobial activity of two phenolic compounds from Pulicaria odora L. J. Ethnopharmacol. 2005;99:287–292. doi: 10.1016/j.jep.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Hanbali F.E., Akssira M., Ezoubeiri A., Mellouki F., Benherraf A., Blazquez A.M., Boira H. Chemical composition and antibacterial activity of essential oil of Pulicaria odora L. J. Ethnopharmacol. 2005;99:399–401. doi: 10.1016/j.jep.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Saidani K., Touati N., Merzouk H., Boussaa H., Bedjou F., Oomah B.D. Phenolic compounds and antioxidant and antimicrobial activities of Pulicaria odora extract. Curr. Bioact. Compd. 2023;19:11–20. doi: 10.2174/1573407218666220404094002. [DOI] [Google Scholar]
- 10.Williams C.A., Harborne J.B., Greenham J.R., Grayer R.J., Kite G.C., Eagles J. Variations in lipophilic and vacuolar flavonoids among European Pulicaria species. Phytochemistry. 2003;64:275–283. doi: 10.1016/s0031-9422(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 11.Touati N., Saidani K., Boudries H., Hammiche H., Ouazene N., Bedjou F. Antibacterial activity of phenolic compounds of Pulicaria odora, wild plant in northern Algeria. Int. Food Res. J. 2018;25:2021–2030. doi: 10.2174/2211352520666220501163744. [DOI] [Google Scholar]
- 12.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 13.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radical. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 14.Brand-Williams W., Cuvelier M.E., Berset C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT--Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 15.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 16.Williams L.A.D., O'connar A., Latore L., Dennis O., Ringer S., Whittaker J.A., Conrad J., Vogler B., Rosner H., Kraus W. The in vitro anti-denaturation effects induced by natural products and nonsteroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. W. Indian Med. J. 2008;57:327–331. doi: 10.1215/9780822388630-010. [DOI] [PubMed] [Google Scholar]
- 17.Hossain M.M., Kabir M.S.H., Hasanat A., Kabir M.I., Chowdhury T.A., Kibria A.S.M.G. Investigation of in vitro anti-arthritic and membrane stabilizing activity of ethanol extracts of three Bangladeshi plants. Rasayan J. Chem. 2015;4:457–460. [Google Scholar]
- 18.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 19.Robert A., Nezamis J.E., Lancaster C., Hanchar A.J. Cytoprotection by prostaglandins in rats: prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979;77:433–443. doi: 10.1016/0016-5085(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 20.Anoop A., Jegadeesan M. Biochemical studies on the anti-ulcerogenic potential of Hemidesmus indicus R. Br. var. indicus. J. Ethnopharmacol. 2003;84:149–156. doi: 10.1016/S0378-8741(02)00291-X. [DOI] [PubMed] [Google Scholar]
- 21.Sidahmed H.M.A., Azizan A.H.S., Mohan S., Abdulla M.A., Abdelwahab S.I., Taha M.M.E., Hadi A.H.A., Ketuly K.A., Hashim N.M., Loke M.F. Gastroprotective effect of desmosdumotin C isolated from Mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-Helicobacter pylori activity. BMC Compl. Alternative Med. 2013;13:1–15. doi: 10.1186/1472-6882-13-183. 10.1186%2F1472-6882-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.PubChem. https://pubchem.ncbi.nlm.nih.gov/
- 23.Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeersch T., Zurek E., Hutchison G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012;4:1–17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe K., Irie K., Nakanishi H., Suzuki H., Fujiyoshi Y. Crystal structures of the gastric proton pump. Nature. 2018;556:214–218. doi: 10.1038/s41586-018-0003-8. [DOI] [PubMed] [Google Scholar]
- 25.Guex N., Peitsch M.C. SWISS‐MODEL and the Swiss‐Pdb Viewer: an environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 26.Biovia D.S. 2024. Discovery Studio Visualizer. [software], San Diego, CA, USA. [Google Scholar]
- 27.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dallakyan S., Olson A.J. Small-molecule library screening by docking with PyRx. Chem. Biol.: methods and protocols. 2015:243–250. doi: 10.1007/978-1-4939-2269-7_19. [DOI] [PubMed] [Google Scholar]
- 29.SwissADME. http://www.swissadme.ch/
- 30.ADMETlab 3.0. https://admetlab3.scbdd.com/
- 31.Seifu D., Assefa F., Abay S.M. In: Medicinal Plants as Antioxidant Agents: Understanding Their Mechanism of Action and Therapeutic Efficacy. Capasso Anna., editor. Research Signpost; Kerala, India: 2012. Medicinal plants as antioxidant agents: understanding their mechanism of action and therapeutic efficacy; pp. 97–145. [Google Scholar]
- 32.Shahidi F., Naczk M. CRC press; 2003. Phenolics in Food and Nutraceuticals. [Google Scholar]
- 33.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 34.Rosa M.D. Biological properties of carrageenan. J. Pharm. Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 35.Euchenhofer C., Maihöfner C., Brune K., Tegeder I., Geisslinger G. Differential effect of selective cyclooxygenase-2 (COX-2) inhibitor NS 398 and diclofenac on formalin-induced nociception in the rat. Neurosci. Lett. 1998;248:25–28. doi: 10.1016/S0304-3940(98)00325-5. [DOI] [PubMed] [Google Scholar]
- 36.Kasote D.M., Nawaz M.A., Usman K., Ullah N., Alsafran M. A critical review on Pulicaria species occurring in Qatar: traditional uses, phytochemistry and biological activities. Phytochemistry Rev. 2024;23:1–52. doi: 10.1007/s11101-024-09932-0. [DOI] [Google Scholar]
- 37.Mohammed H.A., Abdelwahab M.F., El-Ghaly E.S.M., Ragab E.A. Phytochemical characterization, in vitro anti-inflammatory, anti-diabetic, and cytotoxic activities of the edible aromatic plant; Pulicaria jaubertii. Molecules. 2021;26:203. doi: 10.3390/molecules26010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soliman G.A., Ansari M.N., Alqarni M.H., Foudah A.I., Alam A., Salkini M.A., Yusufoglu H.S. Analgesic, antipyretic, anti-inflammatory, and hepatoprotective activities of Pulicaria crispa (Forssk.) Oliv. (Asteraceae) Brazil. J. Pharma. Sci. 2022;58 doi: 10.1590/s2175-97902022e18851. [DOI] [Google Scholar]
- 39.Liu E.S., Cho C.H. Relationship between ethanol-induced gastritis and gastric ulcer formation in rats. Digestion. 2000;62:232–239. doi: 10.1159/000007821. [DOI] [PubMed] [Google Scholar]
- 40.Piotrowski J., Piotrowski E., Skrodzka D., Slomiany A., Slomiany B.L. Gastric mucosal apoptosis induced by ethanol: effect of antiulcer agents. IUBMB Life. 1997;42:247–254. doi: 10.1080/15216549700202631. [DOI] [PubMed] [Google Scholar]
- 41.Heinz E., Öbrink K.J. Acid formation and acidity control in the stomach. Physiol. Rev. 1954;34:643–673. doi: 10.1152/physrev.1954.34.4.643. [DOI] [PubMed] [Google Scholar]
- 42.Ravisankar P., Koushik O., Reddy A., Kumaru A.P., Pragna P. A detailed analysis on acidity and ulcers in esophagus, gastric and duodenal ulcers and management. IOSR J. Dent. Med. Sci. 2016;15:94–114. doi: 10.9790/0853-1511094114. [DOI] [Google Scholar]
- 43.Morris G.P., Wallace J.L. The roles of ethanol and of acid in the production of gastric mucosal erosions in rats. Virchows Arch. B. 1981;38:23–38. doi: 10.1007/BF02892800. [DOI] [PubMed] [Google Scholar]
- 44.Lorentzon P., Eklundh B., Brändström A., Wallmark B. The mechanism for inhibition of gastric (H++ K+)-ATPase by omeprazole. Biochim. Biophys. Acta Biomembr. 1985;817:25–32. doi: 10.1016/0005-2736(85)90064-1. [DOI] [PubMed] [Google Scholar]
- 45.Fahmi A.A., Abdur-Rahman M., Naser A.F.A., Hamed M.A., Abd-Alla H.I., Nasr M.I. Pulicaria crispa mitigates gastric ulcer induced by ethanol in rats: role of treatment and auto healing. Biomarkers. 2019;24:286–294. doi: 10.1080/1354750x.2018.1556340. [DOI] [PubMed] [Google Scholar]
- 46.Fahmi A.A., Abdur-Rahman M., Naser A.F.A., Hamed M.A., Abd-Alla H.I., Shalaby N.M., Nasr M.I. Chemical composition and protective role of Pulicaria undulata (L.) CA Mey. subsp. undulata against gastric ulcer induced by ethanol in rats. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01359. 10.1016%2Fj.heliyon.2019.e01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali A., Bashmil Y.M., Cottrell J.J., Suleria H.A., Dunshea F.R. Lc-ms/ms-qtof screening and identification of phenolic compounds from australian grown herbs and their antioxidant potential. Antioxidants. 2021;10:1770. doi: 10.3390/antiox10111770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khallouki F., Ricarte I., Breuer A., Owen R.W. Characterization of phenolic compounds in mature Moroccan Medjool date palm fruits (Phoenix dactylifera) by HPLC-DAD-ESI-MS. J. Food Compos. Anal. 2018;70:63–71. doi: 10.1016/j.jfca.2018.03.005. [DOI] [Google Scholar]
- 49.Jaiswal R., Karar M.G.E., Gadir H.A., Kuhnert N. Identification and characterization of phenolics from Ixora coccinea L.(Rubiaceae) by liquid chromatography multi‐stage mass spectrometry. Phytochem. Anal. 2014;25:567–576. doi: 10.1002/pca.2530. [DOI] [PubMed] [Google Scholar]
- 50.Rösch D., Krumbein A., Mügge C., Kroh L.W. Structural investigations of flavonol glycosides from sea buckthorn (Hippophaë rhamnoides) pomace by NMR spectroscopy and HPLC-ESI-MSn. J. Agric. Food Chem. 2004;52:4039–4046. doi: 10.1021/jf0306791. [DOI] [PubMed] [Google Scholar]
- 51.Du L.Y., Zhao M., Xu J., Qian D.W., Jiang S., Shang E.X., Guo J.M., Duan J.A. Analysis of the metabolites of isorhamnetin 3-O-glucoside produced by human intestinal flora in vitro by applying ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2014;62:2489–2495. doi: 10.1021/jf405261a. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W., Lian Y., Li Q., Sun L., Chen R., Lai X., Lai Z., Erdong Y., Sun S. Preventative and therapeutic potential of flavonoids in peptic ulcers. Molecules. 2020;25:4626. doi: 10.3390/molecules25204626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubey S., Ganeshpurkar A., Shrivastava A., Bansal D., Dubey N. Rutin exerts antiulcer effect by inhibiting the gastric proton pump. Indian J. Pharmacol. 2013;45:415–417. doi: 10.4103/0253-7613.115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mota K.S., Dias G.E.E., Pinto M.E.F., Luiz-Ferreira Â., Monteiro Souza-Brito A.R., Hiruma-Lima C.A., Barbosa-Filho J.M., Batista L.M. Flavonoids with gastroprotective activity. Molecules. 2009;14:979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.