Abstract
Various lipid and biopolymer-based nanocarriers have been developed to encapsulate food ingredients. The selection of nanocarrier type, preparation techniques, and loading methods should consider the compatibility of nutrient properties, nanocarrier composition, and product requirements. This review focuses on the loading methods for hydrophilic and hydrophobic substances, along with a detailed exploration of nanocarrier categorization, composition, and preparation methods. Both lipid-based and biopolymer-based nanoparticles exhibit the capability to encapsulate hydrophilic or hydrophobic substances. Liposomes and nanoemulsions allow simultaneous encapsulation of hydrophilic and hydrophobic ingredients, while solid lipid nanoparticles and nanostructured lipid carriers are suited for hydrophobic ingredients. The three-dimensional network structure of nanogels can efficiently load hydrophilic substances, while the functional groups in polysaccharides improve the loading capacity of hydrophobic substances through intermolecular interactions. As for protein nanoparticles, the selection of proteins with solubility characteristics analogous to the bioactives is crucial to achieve high encapsulation efficiency.
Keywords: Nanocarrier, Bioactive ingredients, Controlled release, Lipid, Protein, Polysaccharides
Graphical abstract
Highlights
-
•
Nanocarrier preparation techniques and encapsulation approach are discussed.
-
•
Both Lipid and biopolymer nanocarriers can load hydrophilic or hydrophobic compound.
-
•
Liposomes and emulsions can simultaneously load hydrophilic and hydrophobic cargo.
-
•
Improving ingredient-biopolymer interactions can increase encapsulation efficacy.
-
•
Future trends: functional carrier based on composite matrix for protecting bioactive.
1. Introduction
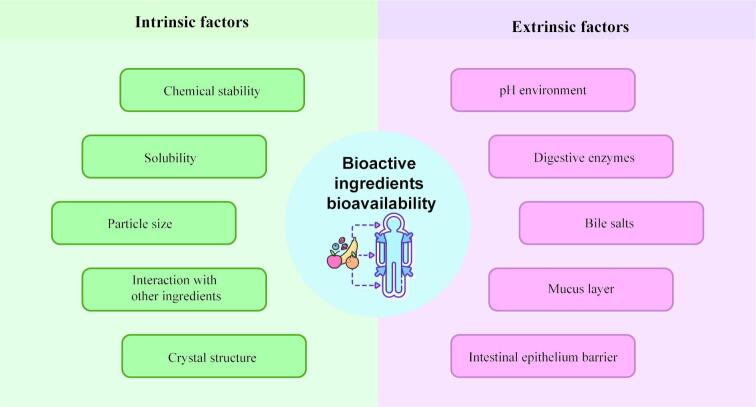
Bioactive compounds (e.g., peptides, carotenoids, fatty acids, polyphenols, etc.) are those nutrient substances extracted from cereal, meat, vegetables, or other natural sources that have physiological benefits and anti-disease activities for humans. Numerous nutritional and clinical studies provided that certain bioactive compounds have antioxidant, anti-inflammatory, antihypertensive, hypotensive, and immunomodulatory activities and also for the prevention of metabolic and degenerative diseases, as well as several forms of cancers (Saifullah, Shishir, Ferdowsi, Rahman, & Vuong, 2019). In the current scenario, there has been a growing interest in adding various bioactive ingredients to food and beverage products to improve the beneficial effects on the human body through diet. However, these nutraceuticals are easily degraded or oxidized due to intrinsic factors and environmental stress (Fig. 1), which leads to the loss of functionality and limits their utilization in the food industry. Furthermore, some bioactive compounds have low solubility in water and easy enzymatic hydrolysis in gastrointestinal fluids, resulting in low bioaccessibility and bioavailability (Rodríguez, Martín, Ruiz, & Clares, 2016). To address these challenges, encapsulation and delivery systems such as lipid-based carriers (e.g., emulsion, liposomes, solid lipid carrier) and biopolymer-based carriers (e.g., polysaccharides hydrogel and protein complex) have been employed to preserve the nutritional quality of bioactive or enhance their applicability in the food industry (Tan, Wang, & Sun, 2021).
Fig. 1.
The factors affecting the bioavaibility of bioactive ingredients.
Encapsulation is a technology in which various carrier materials coat bioactive ingredients to protect them from unfavorable environments. Concerning the carrier size, encapsulation technology can be categorized into microcarrier (1–1000 μm) and nanocarrier (1–1000 nm) (Rodríguez et al., 2016). There has been a surge of interest in nanocarriers for the protection and delivery of bioactive ingredients due to their unique advantages, such as high encapsulation efficiency, sustained release profile, improved solubility, and bioavailability. Nanocarriers can be classified into lipid-based and biopolymer-based categories according to their composition. Lipid-based nanocarriers, which include liposomes (composed of phospholipids and cholesterol), nanoemulsions (comprising oil, water, and surfactants), solid lipid nanoparticles (constructed from solid lipids and surfactants), and nanostructured lipid carriers (formed from a combination of solid and liquid lipids with surfactants), present numerous advantages such as scalability, high biocompatibility, and low toxicity. These characteristics render them particularly suitable for encapsulating hydrophobic ingredients in the food industry (Kim, Na, & Bae, 2023). Conversely, biopolymer-based nanocarriers encompass polysaccharide nanogels (fabricated from biopolymers such as chitosan, alginate, hyaluronic acid, and cellulose) and protein nanoparticles (comprised of various natural proteins like albumin, gelatin, and fibroin). The abundance of functional groups in biopolymers enhances the interaction with drugs, thereby increasing loading capacity and providing opportunities for surface modification to develop responsive nanocarriers (Machado et al., 2022). Nanocarriers create protective barriers that could mitigate the degradation of gastric juice and facilitate controlled release in the intestine (Li et al., 2022). Moreover, the stimuli-responsive nanocarrier could allow the encapsulated ingredients to be released in specific sections of the intestine, such as the ileum, cecum, and colon, helping bioactive compounds to overcome the intestinal barrier or interact with gut microbiota to achieve increased bioavailability (Chung, Jung, Keum, Kim, & Jon, 2020; Kim et al., 2023). To date, nanocarriers are used in the food industry to coat undesirable flavors, sustain color and texture, and enhance the biological activity of food nutrients (Saifullah et al., 2019).
For decades of development, edible nanocarriers have been created from food-grade materials, such as protein, lipids, polysaccharides, etc., for encapsulating various bioactive ingredients (W. Chen et al., 2019; Q. G. Liu et al., 2022; Zhao et al., 2020). In order to better preserve the active substance, it is necessary to know what kind of carrier material is suitable for encapsulating the bioactive component. For example, most hydrophobic compounds could be loaded in lipid-based carriers, while hydrophilic substances have difficulty entering the lipid cores of solid lipid particles (Mirchandani, Patravale, & S, B., 2021). The selection of nanocarrier types for food or medical applications necessitates understanding the nature of loading ingredients and the requirements of final products. Moreover, it must also be mentioned that the nanocarrier material should be regarded as “generally recognized as safe” material, especially in the food sector.
Recent advancements in nanocarriers for encapsulating food bioactive compounds have garnered significant interest in the food and nutraceutical industry, particularly in enhancing stability and bioavailability. The selection of an appropriate encapsulation strategy and loading method is critical to ensure compatibility between nutrient properties and nanocarrier composition. This review aims to provide a comprehensive overview of the preparation techniques for lipid- and biopolymer-based nanocarriers, focusing on loading techniques for hydrophilic and hydrophobic compounds.
2. Lipid-based nanocarrier for encapsulation of hydrophilic and hydrophobic ingredients
2.1. Liposomes
2.1.1. Brief overview of liposomes
Liposomes are self-assembled nanocarriers characterized by their unique self-assembled structure, consisting of aqueous compartments encapsulated within lipid bilayers. The bilayer membrane is constructed by amphipathic phospholipids, such as soybean lecithin or egg lecithin, which possess both hydrophobic tails and hydrophilic heads. When the phospholipid disperses in an aqueous medium, the tails tend to shield themselves from water exposure, while the hydrophilic heads are prone to interface with water (Chai & Park, 2024). The formation of liposomes is driven by a delicate balance of intermolecular forces. Phospholipid molecules interact through van der Waals forces and hydrogen bonding, ultimately assembling into closed vesicular structures. (Shishir, Karim, Gowd, Zheng, & Chen, 2019). This arrangement gives rise to liposomes featuring an inner aqueous core surrounded by a hydrophobic lipid bilayer (Fig. 2a). This unique architecture endows liposomes with the remarkable ability to encapsulate both hydrophobic and hydrophilic compounds simultaneously, making them versatile carriers for a wide range of bioactive molecules.
Fig. 2.
The schematic diagram of lipid based nanocarriers preparation methods and lipid-based nanocarriers loading with hydrophilic and hydrophobic bioactives: liposomes (A, a), nanoemulsions (B, b), solid lipid nanoparticles and nanostructured lipid carriers (C, c).
Various liposomal formulations have been developed to date, with categorization dependent on distinct parameters such as surface charge, particle size, and the number of membrane layers. Specifically, liposomes can be classified as negative, neutral, and positive based on the surface charge type. In terms of bilayer numbers, liposomes can be categorized as unilamellar vesicles with a single lipid bilayer or multilamellar vesicles featuring multiple bilayer membranes (Fig. 2a). Additionally, based on vesicle size, unilamellar vesicles can be further classified into large unilamellar vesicles with dimensions exceeding 100 nm, or small unilamellar vesicles with sizes below 100 nm (W. L. Liu et al., 2020). Overall, liposomes are extensively utilized to protect various bioactive, such as vitamins, polyphenols, proteins, and peptides, from harsh environments by offering the following advantages (Ajeeshkumar et al., 2021).
-
•
Lipid membranes and water cores grant simultaneous loading of hydrophilic and lipophilic bioactive molecules
-
•
Inexpensive and easy to manufacture
-
•
Toxicity worries could be avoided by preparing with food-grade phospholipids
-
•
Scale up methods in industry have been established
2.1.2. Production methods of liposomes
The commonly employed liposome preparation techniques encompass thin film hydration, injection, and, reverse-phase evaporation methods. These approaches can be outlined in a series of steps: (i) dissolution of phospholipid in an organic solvent; (ii) evaporation of the organic solvent; (iii) dispersion of lipids in an aqueous medium to form a liposomal solution; (iv) purification and modification of the liposomes.
The thin film hydration involves dissolving lipids in organic solvents, evaporating the solvent to form a thin lipid film, and subsequently hydrating this film with an aqueous medium under agitation (Fig. 2A). There are two theories of liposome formation through thin film hydration: i) budding-off/fission model and ii) open bilayer fragment re-closing models (Y. Zhang et al., 2024). As for the first model, due to asymmetric interactions across the lipid bilayers, the domain in the near aqueous layer swells with respect to the other side, leading to the bending of the lipid membrane to form a bulge. The blended lipid eventually detaches from the lipid layer and forms liposomes. These processes can occur spontaneously without any energy input. However, mechanical energy, such as sonication and agitation, is usually employed to accelerate liposome formation. For open bilayer fragment re-closing models, since a number of open bilayers are usually already present in suspensions based on the first mechanism, the strong forces can break large or bilayer vesicles into small fragments or sheets. If the open bilayers are large enough, they can reclose to produce a new generation of liposomes. The too small lipid pieces cannot be reclosed, and only if the aggregate first into larger sheets large enough to bend can they be reclosed to form a liposome (Andra, Pammi, Bhatraju, & Ruddaraju, 2022; Has & Sunthar, 2020). For reverse-phase evaporation, phospholipids are dissolved in an organic solvent and form inverted micelles (Fig. 2A). Adding water and applying sonication, the lipids are rearranged at the interface between water and oil to form liposomes (Lombardo & Kiselev, 2022). The injection method, the rapid dilution of ethanol in the aqueous phase, leads to the precipitation of lipid molecules and the successive formation of bilayer sheets encapsulating the aqueous phase. Afterward, the bilayer sheets are formed into liposomes by evaporation of ethanol (Gouda, Sakr, Nasr, & Sammour, 2021). While these conventional methods have been widely adopted in scientific investigations and industrial manufacturing for their simplicity and cost-effective equipment, these preparation methods face limitations in the food industry. The primary concerns include the incomplete removal of organic solvents and the potential degradation of sensitive bioactive compounds due to exposure to extreme pH conditions or mechanical stresses during the formation process.
To address the shortcomings of conventional techniques, the supercritical fluid method has emerged as a promising alternative. This approach significantly reduces or entirely eliminates the use of organic solvents, making it particularly attractive for food-grade liposome production (William, Noémie, Brigitte, & Géraldine, 2020). Among the various supercritical fluids, supercritical carbon dioxide is the most frequently used in supercritical fluid methods due to its non-toxicity, non-flammability, and low critical temperature and pressure (Chai & Park, 2024). At high-pressure conditions, supercritical carbon dioxide fluid combines the characteristics of both gases and liquids—it can enter solids through porous materials like gases and dissolve liposomal formulations like liquids. The preparation process can be divided into two stages. (i) mixing phospholipids and supercritical carbon dioxide. (ii) the high pressure is rapidly reduced, causing the supersaturation and the phospholipids to desolvate from carbon dioxide and form liposomes. A key advantage of this method is the complete removal of carbon dioxide from the liposomal formulation upon pressure reduction, eliminating concerns of residual solvent contamination. Moreover, supercritical preparation methods can be further categorized based on the timing of liposome hydration: the liposome hydration required before the depressurization process and the liposome hydration during the depressurization process. Despite the advantages of supercritical state fluids, including being free of organic solvents, providing a reproducible process, and offering higher encapsulation efficiency, there remains a need to reduce preparation costs and further enhance encapsulation efficiencies to achieve commercial viability.
2.1.3. Encapsulation strategies for hydrophilic and hydrophobic ingredients in liposomes
Liposome preparation techniques have evolved significantly to address the challenges of encapsulating diverse bioactive compounds. Traditional methods, such as thin film dispersion, ethanol injection, and reverse-phase evaporation, incorporate bioactive ingredients concurrently with liposome formation. These techniques exploit the amphiphilic nature of liposomes, allowing for the encapsulation of both hydrophilic and hydrophobic compounds. Hydrophilic compounds are dissolved in the aqueous phase and subsequently encapsulated within the liposomal core during hydration. In contrast, hydrophobic ingredients usually dissolve in organic solvents accompanied by phospholipids and fat-soluble additives. Upon evaporation of the organic solution, these components spontaneously form liposomes when introduced to an aqueous environment (Table 1). The approach is particularly effective for hydrophobic compounds that can interact with lipid membranes through hydrophobic interactions, facilitating their incorporation into the liposomal bilayer. However, the encapsulation of hydrophilic substances presents a significant challenge due to their limited interaction with lipid components. This often results in the dispersion of hydrophilic compounds in the external aqueous phase, ultimately leading to lower encapsulation efficiency (Favarin et al., 2024).
Table 1.
Summary of fabrication methods and encapsulation of hydrophilic and hydrophobic bioactives by liposomes.
| Bioactive encapsulant and encapsulant type | Fabrication methods | Liposomes composition | Encapsulation efficiency (%) | Loading process detail | Reference |
|---|---|---|---|---|---|
| Curcumin (Hydrophobic) |
Ethanol injection | Tilapia head glycolipids, tilapia head phospholipids, and cholesterol | 60–90 | Curcumin was added to an ethanol solution containing the liposome component. The ethanol mixture was subsequently injected into PBS and ethanol was evaporated to obtain curcumin loaded liposomes. | (Yi, Gao, Zhang, Xia, & Shen, 2023) |
| Vitamins (A, D, E and K) (Hydrophobic) |
Ethanol injection | Egg yolk phosphatidylcholine, cholesterol and tween 80 | – | Vitamins were dissolved in ethanol along with liposomal formation ingredients, followed by injecting the mixture into PBS to form milky liposomes. Then, using rotary evaporation to remove the ethanol and homogenizing the resulted liposome suspension to form final liposomes. | (Fan, Feng, Wang, Xia, & Swing, 2023) |
| Curcumin (Hydrophobic) |
Thin film hydration | Hydrogenated soy phosphatidylcholine, Distearoyl-Glycero-3-Phosphoethanolamine- methoxypolyethylene glycol2000, cholesterol, dipalmitoyl-phosphatidylglycerol | 82–89 | Curcumin powder was dissolved in DMSO and incubated with empty liposome solution | (Karimi et al., 2020) |
| Rutin (Hydrophobic) |
Thin film hydration | Soybean phospholipids | 73–88 | Rutin and soybean phospholipids with different drug-to-lipid ratios were dissolved in ethanol, and then the ethanol was evaporated to form a lipid film. This film was hydrated by PBS to form rutin-loaded liposomes. | (Hamadou, Zhang, Chao, & Xu, 2022) |
| Quercetin (Hydrophobic) |
Thin film hydration | Lecithin, cholesterol | 63–67 | Lecithin, cholesterol, and quercetin were dissolved in ethanol, followed by evaporation, hydration and sonication to form liposomes. | (Cong et al., 2023) |
| Ascorbic acid (hydrophilic) and ascorbyl palmitate (hydrophobic) | Reverse-phase evaporation | Phospholipid, polysorbate 80 and cholesterol | 37 (ascorbic acid); 79 (ascorbyl palmitate) |
Dissolve phospholipids and cholesterol in ethanol via ultrasound to create a lipid solution. Evaporate the organic solvent to create an organogel, which is converted into vesicles by adding the aqueous phase. | (Favarin et al., 2024) |
| Carotenoids extracted (hydrophobic) |
Thin film hydration | Phospholipids, cholesterol, Tween80 | 70–95 | Phospholipids and cholesterol were dissolved in ethanol, followed by incorporating Tween80 to enhance stability and encapsulation efficiency. Forming a thin film through solvent evaporation, followed by rehydration to get liposome | (X. Z. Liu et al., 2024b) |
| EGCG (Hydrophilic) and quercetin (Hydrophobic) | Thin film hydration | Lecithin, cholesterol, tween80 | 20–64 (EGCG), 30–61 (quercetin) |
EGCG was dissolved in water, while quercetin and lipid ingredients were dissolved in organic solvents. The lipid film containing quercetin was then hydrated using the aqueous EGCG solution. | (W. Chen et al., 2019) |
| Egg yolk immunoglobulin (Hydrophilic) |
Supercritical fluid | Egg yolk phosphatidylcholine, soybean phosphatidylcholine and cholesterol | 76.9 | Lipid ingredients were dispersed in PBS followed by mixing with IgY solution. Then, the mixture was put into the autoclave of the supercritical fluid device to prepare liposomes. | (Dong, Tang, Xia, Sheng, & Cai, 2022) |
| Soy hydrolysate peptides (Hydrophilic) |
Thin film hydration | Phospholipon®90G, lysophosphatidylcholine, tocopherol, cholesterol and Tween-80 | 60.5 | The preheated soy peptides solution was used to hydrate lipid thin film and followed by a sonication process to form liposomes | (Pavlovic et al., 2022) |
| β-cyclodextrin/vitamin E inclusion complex (Hydrophilic) |
Thin film hydration | Lecithin | 83.8 | Lipid film was hydrated by deionized water containing β-CD/Vitamincomplex | (Souri, Almasi, Hamishehkar, & Amjadi, 2021) |
| β-lactoglobulin (Hydrophilic) |
Reverse-phase evaporation | Phosphatidylcholine and cholesterol | 10–67 | The β-Lg was dissolved in a phosphate buffer solution and then mixed with the organic phase containing the liposomal component. The mixture was subsequently ultrasonicated and evaporated to obtain the liposomes. | (Ma et al., 2014) |
| Anthocyanin (Hydrophilic) |
Thin film hydration | L-α-Phosphatidylcholine, hydrogenated soy, cholesterol, Tween 80 | 71 | The liposome solution (pH 3) was dialyzed in deionized water to form an H ion gradient. Then, the liposome solution was mixed with the anthocyanin solution to prepare anthocyanin-loaded liposomes | (Lee et al., 2019) |
| Caffeine and anthocyanin (Hydrophilic) |
Thin film hydration | Lecithin, cholesterol | – | Commence by mixing hydrated lecithin and cholesterol solutions to form the lipid phase. Subsequently, dissolve caffeine and roselle ANC powders in a phosphate buffer and gradually inject this buffer into the lipid phase while maintaining agitation. | (Javadi, Farahmand, Soltani-Gorde-Faramarzi, & Hesarinejad, 2024) |
Despite the advantages of simplicity and convenience offered by traditional loading techniques, the imperative to enhance loading efficiency persists. Guldiken and coworkers showed a positive correlation between phospholipid concentration and the encapsulation rate of liposomes with the two-stage homogenization preparation method (Guldiken, Gibis, Boyacioglu, Capanoglu, & Weiss, 2021). Similarly, investigations have identified that ratios of lecithin to polyphenols, lecithin to cholesterol, lecithin to Tween, and ultrasonic exposure during the film dispersion method exert differing effects on the encapsulation rate of compounds like epigallocatechin gallate (EGCG) and quercetin(W. Chen et al., 2019). Tripathy found that the addition of stigmasterol could increase the encapsulation efficiency of centella asiatica leaf extract (Tripathy & Srivastav, 2023). Moreover, response surface methodology has also been employed to optimize the preparation of liposomes, and caffeine achieved a maximum encapsulation efficiency of approximately 95 %. The optimization process involved adjusting parameters such as sonication power, chitosan concentration, and caffeine concentration, and it was observed that ultrasonication power and caffeine content significantly affected encapsulation efficiency (Seyedabadi, Rostami, Jafari, & Fathi, 2021). Wang et al. unitized the spectrophotometry combined with response surface methodology to get the highest encapsulation rate of over 95 % for lycopene and β-carotene by alerting temperature, phospholipid to cholesterol, and aqueous phase to lipid phase ratios (X. Z. Liu et al., 2024a). However, establishing a definitive relationship between encapsulation efficiency and the preparation parameters remains complex. Researchers must carefully select preparation methods based on the physicochemical properties of the target ingredients and judiciously modulate the drug-lipid ratio and dispersion conditions to maximize loading efficiency.
In recent years, novel active loading methods have been introduced to enhance the loading efficiency of liposomes. An early study by Modi et al. demonstrated that establishing a transmembrane proton gradient across liposomal membranes contributes to the accumulation of amphiphilic amines within liposomes (Modi, Xiang, & Anderson, 2012). Lee et al. utilized the pH-gradient loading approach to encapsulate anthocyanins within liposomes, achieving significantly higher loading efficiency and more excellent anthocyanin stability compared to passive loading methods (Lee & Na, 2019). In addition, an ionizable group in the encapsulated molecule structure could facilitate the active loading of cargo inside liposomes. However, the active loading methods are only suitable for amphiphilic soluble ingredients.
The solvent-assisted active loading method has been proposed to address the challenge of incorporating poorly soluble ingredients. This approach facilitates the introduction of insoluble compounds into the liposomal aqueous core. This method dissolves insoluble compounds in water-miscible organic solvents, such as ethanol, dimethyl sulfoxide (DMSO), or acetonitrile. The solution with hydrophobic ingredients is introduced into preformed blank liposomes possessing a proton/ion gradient, enabling efficient loading of the compound into the liposomal inner aqueous core at a high drug-lipid ratio (Karimi et al., 2020). In the current scenario, the active loading methods are only applied in the experimental stage due to the requirements for the physicochemical properties of ingredients and the introduction of more organic solvents.
Overall, despite the versatility of liposomes in simultaneously encapsulating hydrophobic and hydrophilic actives, their loading capacity is inherently limited by the finite volume of the lipid bilayer and aqueous core. Some researchers have proposed using giant unilamellar liposomes to increase the loading capacity to encapsulate large amounts of active compounds (Hanley, Ghazani, & Marangoni, 2024; Michelon, Huang, de la Torre, Weitz, & Cunha, 2019). Yet, the increased volume may result in vesicle fusion and aggregation, potentially compromising the liposomes' ability to protect the active substances. In conclusion, while significant progress has been made in optimizing liposome loading techniques, further research is needed to maximize the encapsulation of bioactive ingredients within the limited volume of liposomes. Future studies should focus on developing innovative strategies to enhance loading capacity without compromising liposome stability and functionality.
2.2. Nanoemulsions
2.2.1. Brief overview of nanoemulsions
Nanoemulsions is colloidal dispersions formed by mixing two immiscible liquids into a uniform homogeneous. In contrast to conventional emulsions with diameters exceeding 200 nm, the nano-scale droplet size imparts nanoemulsions with several advantages in comparison to traditional emulsions, including optically transparent, stable against gravitational separation, high surface area-to-volume ratio, kinetically stable during storage, and lower surfactant requirement (Sharma, Cheng, Bhattacharya, & Chakkaravarthi, 2019).
Nanoemulsions have three essential components: oil phase, aqueous phase, and emulsifier. Categorized based on the matrix of dispersed and aqueous phases, there are four types of nanoemulsions (Fig. 2b): oil in water(O/W), water in oil (W/O), oil in water in oil (O1/W/O2) and water in oil in water (W1/O/W2). The oil phase typically comprises natural triacylglycerols extracted from plants and animals, which are preferred due to their low toxicity, nutritional benefits, and consumer acceptance (Dammak, Sobral, Aquino, Neves, & Conte-Junior, 2020). Fatty acid chain length in triacylglycerols is considered a key factor affecting nanoemulsion preparation and stability. The nanoemulsions prepared from long-chain triacylglycerols have high physical stability, whereas characteristics of medium- and short-chain triacylglycerols present challenges due to their low polarity, high interfacial tension, and viscosity (Inapurapu et al., 2020).
The aqueous phase of nanoemulsion predominantly consists of water. It should be noted that the inclusion of various compounds in water, such as co-solvents, proteins, acids, and gases, can alter the aqueous medium's properties, including polarity, interfacial tension, and ionic strength. These alterations have the potential to influence the physicochemical properties and stability of the nanoemulsions, necessitating careful consideration during preparation.
Emulsifiers play a crucial role in nanoemulsion formation and stability by adsorbing the oil-water interface and reducing interfacial tension. This action prevents the breakdown of the nanoemulsions system through various mechanisms such as droplet flocculation, coalescence, Ostwald ripening, and gravitational separation (Marhamati, Ranjbar, & Rezaie, 2021). Common emulsifiers used in the food industry include proteins, phospholipids, saponins, polysaccharides, and small molecule surfactants. The choice of emulsifier significantly influences preparation techniques, stability, rheological properties, and ultimate applications of nanoemulsions. For instance, proteins and polysaccharides are generally unsuitable for low-energy nanoemulsion preparation methods, while small molecule surfactants can rapidly adsorb to oil-water interfaces, facilitating the formation of stable nanoemulsions during low-energy homogenization(Ozogul et al., 2022). The ideal emulsifier should exhibit the most significant initial decrease in interfacial tension with increasing emulsifier concentration while maintaining the lowest interfacial tension at higher emulsifier levels (Dammak et al., 2020). This characteristic ensures optimal stabilization of the nanoemulsion system and contributes to its long-term stability. The careful selection and optimization of nanoemulsion components, particularly the emulsifier, are crucial for developing stable and effective nanoemulsion systems for various applications in the food and related industries. Some advantages of nanoemulsions in the encapsulation of actives are as follows (Ozogul et al., 2022; Sneha & Kumar, 2022):
-
•
Capable of encapsulating hydrophilic and hydrophobic compounds simultaneously
-
•
Simple fabrication method
-
•
Avoiding the organic solvent involved in the preparation
-
•
High encapsulation efficiency
-
•
Various preparation methods are available for different applications
2.2.2. Production methods of nanoemulsions
Nanoemulsion preparation methods can be categorized into two groups based on their energy consumption: high-energy methods and low-energy methods. Each approach offers distinct advantages and limitations, influencing their applicability in various industrial and research contexts.
High-energy methods involve the application of intense and disruptive forces generated by mechanical equipment, such as high-pressure homogenizer and ultrasonicator, to reduce emulsion droplet size to the nanoscale. This process typically consists of two steps: (i) the initial mixing of the oil and aqueous phases to form a macroemulsion, (ii) the reduction of droplet diameter through mechanical force (Fig. 2B). In a homogenizer, the macroemulsion was passed through a narrow gap to break the large droplets into smaller droplets through extreme elongational and shear stress. Conversely, an ultrasonicator generates high-energy shock waves that create turbulence through cavitation, effectively transforming the macroemulsion into a nanoemulsion. Whether a homogenizer or ultrasonicator is employed, these processes should be repeated multiple times or continued until the droplet size becomes constant (Gupta, Eral, Hatton, & Doyle, 2016). The High-energy methods are particularly advantageous due to their applicability to a broad spectrum of raw materials and their ability to produce nanoemulsions without requiring excessive emulsifiers, making them suitable for large-scale production in the food industry (Huang et al., 2021). However, it is imperative to notice that high-energy methods can produce “by-products” such as increased pressure, temperature, and shearing forces, which may disrupt the molecular structure of bioactive compounds and diminish the functional properties of the bioactive compounds, especially concerning fragile molecules like peptides and proteins.
In contrast to the formation of nanoemulsions via high-energy equipment, which necessitates significant external energy input, the primary driving force for the formation of “low-energy nanoemulsions” is the internal energy of the mixture out of chemical equilibrium (Silva & Fabi, 2022). The low-energy preparation methods could be divided into spontaneous emulsification methods (or self-emulsification) and phase inversion methods (Fig. 2B). Spontaneous emulsification methods utilize the movement of water miscible ingredients (such as solvent, surfactant, and co-surfactant) from the organic phase into the aqueous phase, typically under constant temperature and gentle stirring conditions (Safaya & Rotliwala, 2019). This process involves the dropping organic phase into an aqueous phase containing surfactants to prepare O/W nanoemulsion. The rapid migration of water-miscible components into the aqueous phase generates turbulence at the interface of the two phases, which consequently leads to the spontaneous formation of nanoemulsion upon disrupting the bicontinuous microemulsion phase. Moreover, the reverse process — the addition of aqueous into the oil phase— to form W/O nanoemulsion is equally feasible.
In contrast to spontaneous emulsification methods, phase inversion methods utilize the chemical energy released by phase transitions, often associated with changes in system composition or temperature, to form nanoemulsions and control droplet diameters. The phase inversion method could be further divided into the phase inversion temperature method (PIT) and phase inversion composition methods (PIC). In the PIT process (Fig. 2B), a W/O macroemulsion is prepared at a temperature higher than the phase inversion temperature of the mixture. When the temperature is cooled below the inversion temperature point, the crude emulsion transforms from W/O to O/W emulsion. The interfacial tension of the oil-water interface is very low at the inversion point, so the macroemulsion converts to a nanoemulsion without high energy input. For the PIC procedure (Fig. 2B), slowly dilute the pre-prepared W/O macroemulsion with water, and the transition from W/O to O/W nanoemulsion occurs as the system passes through an inversion point (Komaiko & McClements, 2016; Marhamati et al., 2021). Both PIT and PIC involve reversing the curvature of the surfactant film from positive to negative or vice versa. Consequently, the selection of appropriate surfactants and a thorough understanding of surfactant phase behavior are critical in preparing nanoemulsions using phase inversion methods. The PIT method is exclusively applicable to temperature-sensitive surfactants, such as polyoxyethylene-type nonionic surfactants, wherein temperature-induced changes in poly(oxyethylene) chain hydration lead to consequential changes in surfactant curvature (Koroleva, Nagovitsina, & Yurtov, 2018). Conversely, the PIC method initiates phase transitions through compositional changes during emulsification at a constant temperature, thereby extending its applicability to a broader spectrum of surfactants beyond ethoxylated types (Mushtaq et al., 2023). Despite the advantage of low energy consumption, the preparation of nanoemulsions by the low-energy method is still in the laboratory stage since it requires a high concentration of emulsifiers, negatively affecting both taste and safety.
2.2.3. Encapsulation strategies for hydrophilic and hydrophobic ingredients in nanoemulsions
Nanoemulsions consist of both nonpolar (oil phase) and polar (aqueous phase) regions, allowing for the encapsulation of hydrophobic and hydrophilic food ingredients within the system. During nanoemulsion preparation, the encapsulation process typically involves dissolving hydrophilic compounds in the aqueous phase and hydrophobic compounds in the oil phase. Rabelo et al. (2018a) demonstrated the efficacy of nanoemulsions in encapsulating hydrophilic compounds by dispersing berry anthocyanin in the aqueous phase prior to emulsification with medium-chain triglyceride (MCT) oil containing nitroglycerin monolaurate condensed ricinoleic acid esters as lipid phase. The fabricated W/O nanoemulsions exhibited antioxidant activity and provided high anthocyanin protection against 30 days of storage (Rabelo et al., 2018).
As for encapsulation of hydrophobic compounds, nanoemulsions have found extensive application in enhancing the water solubility and stability of various hydrophobic compounds, such as curcumin, quercetin, and coenzyme Q10 (Niu et al., 2020). Take a typical example: Das and colleagues fabricated nanoemulsions loaded with quercetin using a high-energy method. The authors initially evaluated the solubility and compatibility of quercetin with different lipid phases, followed by the incorporation of quercetin into the selected oil phase and subsequent addition of aqueous droplets. The mixture was then homogenized to produce the quercetin-loaded nanoemulsions (S. S. Das, Verma, & Singh, 2020). Furthermore, enhancing the solubility of the active molecules in the oil phase can improve the encapsulation efficiency of nanoemulsions. McClements et al. demonstrated that adjusting the pH of the system and increasing the temperature can increase the solubility of curcumin in the oil phase. Their study revealed that nanoemulsions prepared via pH-driven (93 %) and temperature-driven (76 %) methods achieved substantially higher encapsulation efficiencies compared to the conventional dissolve method (56 %) of curcumin in the oil phase (B. Zheng, Peng, Zhang, & McClements, 2018). Those bibliographic facts indicate that the solubility of hydrophobic bioactive agents in the oil phase plays a vital role in increasing the encapsulation efficiency of nanoemulsions.
The multilayer nanoemulsion system, including oil in O1/W/O2 and W1/O/W2, has been developed to accommodate both hydrophobic and hydrophilic substances. The preparation of multilayer nanoemulsions typically involves a two-step process. (i) Creation of the primary emulsion (W1/O or O1/W) by mixing and emulsifying the oil and aqueous phases; (ii) Formation of W1/O/W2 or O1/W/O2 by using the primary emulsion as either the oil or water phase in a second water (W2) or oil (O2) phase. It is noteworthy that secondary emulsification usually utilizes a milder homogenization process than primary emulsification to avoid the destruction of the primary emulsion. In an illustrative instance, Tang et al. (2021) fabricated encapsulated hydrophilic betanin and hydrophobic curcumin by first dissolving curcumin in ethanol and then adding it into MCT, followed by evaporating ethanol and creating an oil phase. The oil phase was then emulsified to W1/O pre-emulsion with an aqueous phase containing betanin. Finally, the W1/O pre-emulsion was later gradually added into W2 phase by homogenization to obtain W1/O/W2 emulsions with curcumin and betanin encapsulation efficacy of 84.1 % and 65.3 %, respectively (X. Y. Tang et al., 2021). More examples of encapsulation of bioactive ingredients by nanoemulsions are tabulated in Table 2.
Table 2.
Summary of preparation techniques and encapsulation of hydrophilic and hydrophobic bioactives by nanoemulsions.
| Bioactive encapsulant and encapsulant type | Fabrication methods | Nanoemulsions composition | Nanoemulsions type | Encapsulation efficiency (%) | Loading methods of bioactive ingredient | Reference |
|---|---|---|---|---|---|---|
| Curcumin (Hydrophobic) |
High-energy method | W1: gelatin, betanin, water O: medium-chain triglyceride, polyglycerol polyricinoleate, curcumin W2: serum albumin nanoparticles, PBS |
W1/O/W2 | 84 | Curcumin was dissolved in hot ethanol, followed by dropwise the ethanol solution into MCT and evaporating the ethanol. The oil phase was mixed with W1, and a high-pressure process was conducted to produce W1/O emulsion. Afterwards, the W1/O emulsion was added to W2, followed by homogenization to prepare the W1/O/W2 emulsion. | (X. Y. Tang et al., 2021) |
| Proanthocyanidins (hydrophilic) and β-carotene (Hydrophobic) | High-energy method | W1: proanthocyanidins, water O: rice bran wax, corn oil, polyglycerol polyricinoleate W2: whey protein isolate,sodium alginate, water |
W1/O/W2 | 90 | The β-carotene and proanthocyanidins were dissolved in oil and W1 phase, respectively. Primary O/W emulsion was prepared by homogenizing the oil phase and W1 phase. Afterwards, the primary emulsion was mixed with W2 and followed by homogenized to prepare W/O/W emulsion. | (Huang et al., 2021) |
| Honeybee pollen (Hydrophobic) |
High-energy method | W1: water O: lauroglycol 90, span 80, Lipoid P75, honeybee pollen extract W2: water, pluronic F68, chitosan |
W1/O/W2 | 82 | Initiate the process by combining oil phase components and water to form primary W/O emulsions. Next, the internal aqueous phase containing pluronic F68 and chitosan is incorporated. The resulting emulsions are homogenized to obtain nanoemulsion. | (Valdivia-Olivares et al., 2023) |
| Coenzyme Q10, conjugated linoleic acid (Hydrophobic) |
High-energy method | O1: coenzyme Q10, extra virgin olive oil, olive pomace oil, conjugated linoleic acid W: polyoxyethylene sorbitan monopalmitate, polyphenols, deionized water O2: span 80. |
O1/W/O2 | – | The O/W emulsions were prepared through mixing emulsifier, the water phase and oil phase with a high speed homogenizer and a high intensity ultrasonic processor. The O/W/O double emulsion fabricated by adding the above O/W emulsion to O2 phase and followed by homogenization and ultrasonication. | (Katsouli, Giannou, & Tzia, 2020) |
| Pentacyclic triterpenes-rich extract (Hydrophobic) |
High-energy method | Oil phase: soybean, sunflower, and olive oil, pentacyclic triterpenes-rich extract water phase: protein hydrolysate, distilled water |
O/W | 40 | The oil phase containing pentacyclic triterpenes-rich extract was gradrully added to the aqueous phase with agitation to homogenize the both phase. | (Calderon-chiu, Calderon-santoyo, Damasceno-gomes, & Ragazzo-Sanchez, 2021) |
| Curcumin (Hydrophobic) |
Low-energy methods. | Oil phase: soybean oil, tween 80, curcumin water phase: water, glycerol |
O/W | – | Curcumin was dissolved in the soybean oil with Tween 80 to form oil phase. Then, the water phase was added to oil phase by peristaltic pump under mechanically stirred. | (Borrin, Georges, Moraes, & Pinho, 2016) |
| Green tea catechins (Hydrophobic) |
High-energy method | Oil phase: green tea catechins, sunflower oil Water phase: soy protein isolate SPI, water, sodium azide |
O/W | – | Green tea powder was dispersed in sunflower oil to prepare the oil phase, followed by a high-speed homogenizer and high-pressure homogenizer to homogenize the mixture of the oil phase and water phase. | (Bhushani, Karthik, & Anandharamakrishnan, 2016) |
| Quercetin (Hydrophobic) |
Low-energy methods. | Oil phase: soybean oil, Tween 80, Brij 30, quercetin Water phase: water, glycerol |
O/W | – | Mixing quercetin, surfactant and soybean oil to form oil phase with stirring. The water phase was gradually added to the oil phase using a peristaltic pump to form nanoemulsion. | (de Carli, Moraes-Lovison, & Pinho, 2018) |
| Octacosanol (Hydrophobic) |
Low-energy methods. | Oil phase: olive oil, soybean oil, sunflower oil, camellia oil, linoleic acid, glycerol, anhydrous ethanol, propylene glycol Water phase: water |
O/W | – | Combine octacosanol, surfactant, co-surfactant, and oil, then heat the mixture until octacosanol dissolves completely. This process is followed by the gradual addition of deionized water under constant stirring until formation of transparent nanoemulsions. | (Valdivia-Olivares et al., 2023) |
| Tea polyphenols (Hydrophillic) |
High-energy method | Oil phase: corn oil, polysorbate-80 water phase: tea polyphenols, water |
O/W | – | Dissolving tea polyphenols into water to prepare water phase. Coarse emulsions were prepared by mixing corn oil and water phase while adding polysorbate-80 and then homogenized the mixture with a high-speed Tissue-Tearor. Finally, a high-pressure homogenizer was used to prepare nanoemulsion | (Y. R. Peng et al., 2018) |
| Açaí berry anthocyanins (Hydrophilic) |
High-energy method | Water phase: water, açaí berry anthocyanins oil phase: tetraglycerin monolaurate condensed ricinoleic acid esters, medium-chain triglyceride |
W/O | – | Mix the water phase containing açaí berry anthocyanins with the oil phase, followed by homogenization to prepare a coarse emulsion. Afterwards, the coarse emulsions were passed through a high-pressure homogenizer to prepare nanoemulsion. | (Rabelo et al., 2018) |
| Tea polyphenols (Hydrophilic) |
High-energy method | Water phase: tea polyphenols, water, Tween, glycerol oil phase: grape oil, zein, Span |
W/O | 0.053 wt% | Tea polyphones were dissolved in water phase and then dropped it in oil phase at high temperature to form coarse emulsion. Finally, the coarse emulsion was sheared to prepare the W/O emulsion. | (Ye et al., 2020) |
| Anthocyanin extract (Hydrophilic) |
Low-energy methods. | Water phase: water, oil phase: virgin coconut oil, span 80, tween 80 |
W/O | – | Add anthocyanin extract solution to the virgin coconut oil containing surfactant and incubate overnight at room temperature. | (Tamaroh, Sari, Wariyah, Huda, & Candraruna, 2024) |
In summary, the encapsulation of bioactive compounds in nanoemulsions is contingent upon the type of nanoemulsions used. Bioactive substances must be loaded into the dispersed phase of the nanoemulsions to benefit from their protective properties, regulate degradation rates, and enhance absorption. Specifically, O/W, where the hydrophobic cargo is loaded in the internal oil phase core; W/O, which encapsulates hydrophilic compounds in the aqueous core; O/W/O and W/O/W, both capable of encapsulating both hydrophilic and hydrophobic bioactive. Moreover, the solubility of hydrophobic bioactive agents in the oil phase is essential in increasing the encapsulation efficiency of nanoemulsions.
2.3. Solid lipid nanoparticles and nanostructured lipid carriers
2.3.1. Brief overview of SLNs and NLCs
Solid lipid nanoparticles (SLNs) are colloidal dispersions of non-polar lipids, featuring a well-ordered and crystalline lattice core surrounded by a monolayer surfactant shell (Fig. 2c). The distinguishing feature of SLNs is their solid lipid core, which reduces the mobility of encapsulated ingredients within the lipid matrix and enhances stability in challenging environments. However, the highly ordered core structure provides limited space for cargo accommodation, potentially resulting in drug expulsion from the lattice (Bayon-Cordero, Alkorta, & Arana, 2019). To address this limitation, nanostructured lipid carriers (NLCs) were developed by incorporating small quantities of liquid lipids into the solid lipid matrix. This modification reduces the crystalline events and maintains a liquid lipid matrix at room and body temperature, consequently enhancing storage stability and loading capacity (Costa, Moreira, Sousa Lobo, & Silva, 2021).
While both SLNs and NLCs could be characterized as solid lipid-based nanoparticles, the primary difference is the absence (SLNs) and presence (NLCs) of the liquid matrix in the particle core. Generally, solid lipid-based nanoparticles are composed of solid lipids, liquid lipids (used in NLCs), surfactants, and water. The lipid matrix is a fundamental element in both systems, so lipid components could be used to identify SLNs and NLCs. Solid lipids (e.g., stearic acid, glyceryl behenate, tripalmitin, cetyl palmitate, glyceryl monostearate, and tristearin) are predominant components of SLNs and NLCs. In contrast, NLCs contain about 0.1–30 % liquid lipids (medium chain triglycerides, isopropyl palmitate plus capric/caprylic triglyceride, oleic acid, squalene, vegetable and seed oils) (Agrawal et al., 2020). Furthermore, SLNs and NLCs offer the following advantages (Goncalves et al., 2021; C.-H. Tang, Chen, & Dong, 2023):
-
•
Organic solvents could be avoided during preparation
-
•
High stability over the storage period
-
•
Prolonged sustained release against harsh conditions via their solid lipid phase
-
•
Low biotoxicity
-
•
Controlled particle size
-
•
Low-cost and simple fabrication method
2.3.2. Production methods of SLNs and NLCs
Despite variations in lipid composition, both SLNs and NLCs could be prepared using three main methods: (i) high-pressure homogenization (HPH), (ii) microemulsion formation methods, and (iii) Solvent emulsification technique.
Among the various preparation methods, HPH is the predominant technique for SLN and NLC formulation (Fig. 2C), further classified into hot and cold HPH procedures. In the hot HPH method, the solid lipid phase must be heated above the melting temperature into liquid lipid mixture. Then, the heated lipid phase is combined with a hot surfactant solution under continuous agitation. The resulting hot liquid lipid mixture undergoes homogenization and subsequent cooling to room temperature, generating solid SLNs or NLCs. However, this process, involving high temperatures (up to 90 °C) and high-energy mechanical force, is unsuitable for encapsulating thermosensitive and fragile substances. To address this limitation, cold HPH has been proposed to encapsulate heat-sensitive molecules into SLNs and NLCs. In this approach, the completely melted lipid phase is rapidly cooled to solidify lipid particles within a cold solution. The bulk lipid particles are then milled into submicron particles and dispersed in a cold surfactant solution. Finally, the high-pressure homogenizer homogenizes the mixed solution to form nanoparticles at room temperature (Souto et al., 2020).
As for microemulsion formation methods (Fig. 2C), the process entails separately melting lipids and preheating a water phase containing surfactants to the lipid melt point. The hot lipid and preheated water phases are combined under continuous stirring to generate the microemulsion. If necessary, a surfactant solution is gradually added to the mixture until the turbid liquid transforms into a transparent emulsion. The final step entails solidifying the hot microemulsion into nanoparticles by diluting it with cold water (Gonçalves, Vicente, & Pinheiro, 2023).
The solvent emulsification technique utilizes organic solvents to facilitate the dissolution of solid lipids in water and subsequently removes the organic solvents. Initially, both liquid and solid lipids are dissolved in organic solvents. Then, the organic solution is slowly poured into the water solution containing surfactant. The resulting dispersion maintains the high temperature until the organic solvent is completely removed from the system. Finally, the mixture is cooled to form nanoparticles (Duong, Nguyen, & Maeng, 2020).
2.3.3. Encapsulation strategies for hydrophilic and hydrophobic ingredients in SLNs and NLCs
Due to the excellent compatibility of lipid matrices with most hydrophobic compounds, SLNs, and NLCs have been found to be extensively used in the encapsulation of hydrophobic food bioactives via various preparation methods, including but not limited to rutin, curcumin, carotene, and essential oils (Gunawan & Boonkanokwong, 2024). The loading of hydrophobic substances into SLNs or NLCs is achieved by incorporating hydrophobic substances, with or without organic solvents, into the lipid phase. One prominent method for encapsulating hydrophobic ingredients is the hot-HPH technique. Pezeshki et al. (2019) utilized the hot-HPH method to prepare β-carotene-NLCs to increase the stability of β-carotene. They reported a remarkable encapsulation efficiency of 97.7 % by first melting carotene and mixing it with liquid oil, then introducing this mixture into the melted solid lipid phase supplemented with a surfactant solution. The subsequent homogenization and cooling processes resulted in successfully prepared β-carotene-loaded NLCs (Pezeshki et al., 2019). Another frequently employed method is the solvent emulsification technique, which utilizes organic solvents to enhance the concentration of hydrophobic substances within the aqueous-lipid system. This process involves dissolving both hydrophobic compounds and solid lipids in a minimal amount of organic solvent, followed by the addition of a surfactant solution. The mixture then undergoes homogenization or ultrasonication to form an emulsion, which is subsequently maintained at a mild temperature with stirring to remove the organic solvent and solidify the SLNs (Gonçalves et al., 2021).
While SLNs and NLCs excel in encapsulating hydrophobic compounds, their lipid matrix-based composition presents challenges for encapsulating hydrophilic substances. For encapsulating hydrophilic ingredients into SLNs or NLCs, it is essential to incorporate hydrophilic compound solutions during their preparation (Pimentel-Moral et al., 2019; Ravanfar, Tamaddon, Niakousari, & Moein, 2016). As the solution to this challenge, the W/O or W1/O/W2 emulsion (microemulsion formation methods) has been employed in SLNs and NLCs fabrication to facilitate the encapsulation of hydrophilic compounds. The loading process is similar to the multiple emulsion preparation techniques as presented in Section 2.2.3. Ravanfar et al. (2016) demonstrated the efficacy of this approach in developing anthocyanin-loaded SLNs. Optimizing formation parameters using Plackett-Burman and Box-Behnken experimental designs achieved an impressive encapsulation efficiency of approximately 89 %. Their method involved adding an anthocyanin solution dropwise to a preheated lipid phase containing surfactants to form a W/O pre-emulsion. This pre-emulsion was then dispersed in a cold aqueous surfactant solution and homogenized to produce anthocyanin-loaded NLCs (Ravanfar et al., 2016). Similarly, Pimentel-Moral et al. (2019) prepared Hibiscus sabdariffa extract-loaded NLCs using multiple emulsion technology. Under the appropriate formation parameters, they achieved an encapsulation efficiency of about 84 % and a particle size of approximately 344 nm (Pimentel-Moral et al., 2019). Furthermore, studies reported that the hydrophobic ion pairing technique, which involves linking hydrophilic molecules to surfactants carrying a surface charge opposite to the water-soluble molecules, can facilitate their incorporation into SLNs and NLCs (Abedi, Akhavan, Mohammadi, & Banasaz, 2023). However, this method presents challenges for use in the food industry due to potential toxicity concerns. Table 3 summarizes various studies on encapsulating bioactive ingredients using different preparation methods with SLNs and NLCs, providing a comprehensive overview of the current state of research in this field.
Table 3.
Summary of fabrication methods and encapsulation of hydrophilic and hydrophobic bioactives by SLNs and NLCs.
| Bioactive encapsulant and encapsulant type | Fabrication methods | Nanoemulsions composition | Nanocarrier type | Encapsulation efficiency (%) | Loading methods of bioactive ingredient | Reference |
|---|---|---|---|---|---|---|
| Fisetin (Hydrophobic) |
High-pressure homogenization method | Lipid phase: lexemul AR, Lexemul 561 and Labrasol aqueous phase: pluronic F-127 |
NLCs | 78 | Dissolve fisetin in lipid phase above its melting point; concurrently dissolve surfactant in aqueous medium. Gradually introduce drug-loaded lipid phase into heated aqueous components, followed by homogenization for nanoparticulate dispersion. | (Kumar et al., 2023) |
| Vitamin D3 (Hydrophobic) |
High pressure homogenization method | Lipid phase: MCT, VD3, GM aqueous phase: rhamnolipids, ultra-pure water |
NLCs | 51–72 | Homogenize and heat lipid and aqueous phases at 75 °C, with the lipid phase pre-containing Vitamin D3. Combine both phases under high shear homogenization and followed by sonication. Conclude by cooling in ice to obtain nanoparticles. | (Azevedo, Cerqueira, Goncalves, Amado, & Pastrana, 2023) |
| Citral (Hydrophobic) |
High pressure homogenization method | Lipid phase: glyceryl monostearate, medium chain triglycerides, citral aqueous phase: surfactant, water |
NLCs | 45–73 | Dissolve citral in a melted lipid mixture and combine this lipid phase with an aqueous surfactant solution under high-shear homogenization. The system is then subjected to ultrasonic treatment to yield the NLCs | (Feng, Sheng, Yu, Lin, & Shao, 2023) |
| Vitamin D3 (Hydrophobic) |
High pressure homogenization method | Lipid phase: anhydrous milk fat, vitamin E acetate, polyglycerol ester, vitamin D3 aqueous phase: |
NLCs | >72 | Melt the lipid phase with vitamin D3 at 70 °C. Emulsify sodium caseinate gradually into the lipid phase under homogenization, followed by sonication in a hot bath. Solidify the nanoemulsions at 4 °C to produce the NLCs. | (Barri, Ghanbarzadeh, Mohammadi, & Pezeshki, 2023) |
| β-carotene (Hydrophobic) |
Solvent emulsification method | Lipid phase: corn oil, palmitic acid, β-carotene, vitamin E and butylated hydroxytoluene aqueous phase: Vitamin C, Tween 80, double distilled water |
NLCs | – | Dissolve the β-carotene-containing lipid phase in ethanol at 70 °C. Introduce the solution into the preheated aqueous phase. Cool the resulting dispersion to room temperature for NLCs formation. | (Hejri, Khosravi, Gharanjig, & Davarani, 2019) |
| Formononetin (Hydrophobic) |
melt-emulsification ultrasonic method | Lipid phase: formononetin, glyceryl distearate, perilla seed oil, Tween 80, propylene glycol Water phase: distilled water |
NLCs | 96.3 | Heat and melt the lipid mixture of lipids, surfactants, and formononetin. The mixture is then combined with heated distilled water to form a pre-nanoemulsion. Finally, the system undergoes sonication in an ice bath to produce NLCs. | (L. J. Zheng et al., 2023) |
| Citral (Hydrophobic) |
Microemulsion formation method | Lipid phase: glyceryl monostearate aqueous phase: Tween 80, Span 80 |
SLNs | 57–72 | Heat solid lipid and citral to 65 °C for the lipid phase. Form emulsion by adding surfactant solution under stirring. Cool the emulsion below the melting point to generate SLNs. | (Tian, Lu, Li, & Hu, 2018) |
| β-carotene (Hydrophobic) |
High pressure homogenization method | Lipid phase: octyl octanoate, precirol ATO5 aqueous phase: ploxamer 407, water |
NLCs | 97 | Melt β-carotene, mix with liquid oil, and add to melted solid lipid at 70 °C. A preheated surfactant solution was added to the lipid phase. The NLCs was obtained by homogenizing the lipid mixture and cooling it down to 22 °C for recrystallization. | (Pezeshki et al., 2019) |
| β-carotene (Hydrophobic) |
Solvent emulsification method | Lipid phase: tristearin aqueous phase: sodium caseinate or lactoferrin |
SLNs | – | β-carotene and tristearin were solubilized in ethanol and heated to 80 °C to form the organic phase. Organic phases were gradually added in preheated aqueous phases containing protein under stirring. The dispersions were rapidly cooled to form SLNs | (Oliveira, Furtado, & Cunha, 2019) |
| EGCG (Hydrophillic) |
High pressure homogenization method | Lipid phase: Precirol® ATO 5, Mygliol® 812, Tween® 60 aqueous phase: EGCG, ultrapure water |
NLCs | 85–91 | Pre-heated ultrapure water with EGCG was added to the lipid phase, followed by stirring and ultrasonication. The produced nanoemulsions was cooled at room temperature to obtain NLCs. | (Granja et al., 2017) |
| Anthocyanin (Hydrophillic) |
High pressure homogenization method | Lipid phase: palmitic acid, Span 85, egg lecithin, ethanol or isobutanol aqueous phase: cabbage extract, pluronic F127, distilled water |
SLNs | 27–93 | Heat lipid phase to 60 °C, add ethanol/isobutanol as a co-surfactant. Formulate w/o microemulsion by titrating aqueous phase with active compounds. Disperse microemulsions in Pluronic F127 solution via homogenization to create SLNs. | (Ravanfar et al., 2016) |
| EGCG (Hydrophillic) |
High pressure homogenization method | Lipid phase: cocoa butter, Mono- and diglycerides of fatty acids (MDG), sodium stearoyl-2-lactylate (SSL) aqueous phase: green tea polyphenol, water |
SLNs | 60–70 | Emulsify the green tea polyphenol solution into the organic phase with melted cocoa butter and MDG through homogenization. Re-emulsify the blend into water with SSL. Cool the resulting hot nanoemulsions in a thermostat at 1 °C. | (Shtay, Keppler, Schrader, & Schwarz, 2019) |
| EGCG (Hydrophillic) |
Solvent emulsification method | Lipid phase: glycerol monostearate (GMS), stearic acid (SA), and soya lecithin aqueous phase: EGCG, distilled water, Pluronic F68 |
SLNs | 67–89 | Dissolve GMS, SA, and soya lecithin in dichloromethane. Create a water-in-oil (w/o) primary emulsion by adding EGCG and Pluronic F68 solution to the lipid mixture. Sonicate and transfer the mixture into Pluronic F68 solution for a double emulsion. The emulsion was then centrifuged and washed with water. | (Radhakrishnan et al., 2016) |
| Saffron extract (Hydrophillic) |
High shear homogenization method | Lipid phase: butter, polyglycerol esters, olyglycerol polyricinoleate, thyme and pennyroyal essential oil, aqueous phase: affron extract, Tween 80 |
NLCs | 59–79 | The process integrates an internal aqueous phase containing saffron extract into a heated lipid phase consisting of butter and essential oils with surfactants. Subsequently, this primary emulsion is dispersed into a secondary aqueous phase containing Tween 80 and cool down to form NLCs. | (Binazir et al., 2024) |
It is important to note that despite these advancements, SLNs and NLCs are still primarily employed for loading hydrophobic compounds. The challenges associated with dissolving hydrophilic compounds in the oil phase often result in lower encapsulation efficiency and potential expulsion of the active substance from the solid lipid during storage. To achieve higher encapsulation efficiency and loading capacity for hydrophilic compounds, further research is necessary to explore alternative carriers with improved compatibility.
3. Nature biopolymer-based nanocarrier for encapsulation of hydrophilic and hydrophobic ingredients
3.1. Polysaccharides nanogel
3.1.1. Brief overview of polysaccharides nanogel
Nanogels, also known as nanohydrogels or hydrogel nanoparticles, are innovative nanostructures fabricated through the crosslinking of hydrophilic polymers into a three-dimensional network. Nanogels exhibit both characteristics of hydrogels and nanoparticles, such as non-immunogenicity, flexible chemistry, large surface area, and minimal size. These attributes collectively enhance their ability to overcome adverse environments and penetrate biological barriers, such as intestinal and blood-brain barriers (Z. Zhang et al., 2021).
Over the past decades, there has been a significant emergence of nanogels composed of food-grade polysaccharides. These formulations offer improved biocompatibility and more efficient protection for various food-bioactive molecules and pharmaceutical compounds (Luckanagul et al., 2018). Polysaccharides have garnered considerable attention as desirable materials for nanogel synthesis due to their abundant availability, cost-effectiveness, non-toxic nature, and water solubility. The polysaccharide backbone, rich in reactive functional groups such as hydroxyl and carboxylic acid, facilitates derivatization, thereby enhancing the structural and functional diversity of the nanocarrier (Meng et al., 2024). In the food and biomedical sector, various nanogel have been developed utilizing polysaccharides derived from diverse sources, encompassing animal origins (e.g., dextran, pullulan, xanthan gum, and gellan gum, etc.), plant origins (e.g., pectin, cellulose, starch, etc.), and microbial origins (e.g., agar, alginate, and carrageenan, etc.) to prevent degradation of active substances (Z. Wu, Li, Zhao, Ye, & Zhao, 2022; Z. Zhang et al., 2021). The utilization of these varied polysaccharide sources in nanogel formulation has significantly contributed to the prevention of active substance degradation, thereby enhancing the stability and efficacy of encapsulated bioactive compounds.
The versatility of polysaccharide nanogels is further exemplified by their ability to respond to various stimuli, such as pH, temperature, and enzymatic activity. This responsiveness allows for targeted and controlled release of encapsulated substances, making polysaccharide nanogels particularly attractive for applications in nutraceutical delivery and functional food development. The distinctive advantages of nanogel are as follows (Papagiannopoulos & Sotiropoulos, 2022; Y. Zhang et al., 2022):
-
•
Flexible chemistry of nanogel gives them a better affinity with hydrophilic or hydrophobic compounds.
-
•
Varying fabrication methods could produce stimuli-responsive nanogels for improving drug release behavior in the gastrointestinal tract and protecting bioactive from adverse environments
-
•
Functional groups in polysaccharides offer the potential to produce functional hydrogels
-
•
Many methods could prepare nanogel and easy to scale up for industry
-
•
Plenty of food-based material could be selected to fabricate nanogel and avoid toxicity concerns.
-
•
High drug encapsulation capacity and diversity of actives that can be encapsulated
3.1.2. Production methods of polysaccharides nanogel
The synthesis of polysaccharide nanogels encompasses a diverse array of methodologies, encompassing coacervation, thermal-denaturation, emulsification, injection or extrusion, shearing, spray chilling, etc. (Silva & Fabi, 2022). These preparation techniques can be broadly categorized based on the crosslinking structure of the resultant nanogel, distinguishing between chemically crosslinked and physically crosslinked nanogels. Chemically crosslinked nanogels utilize the active groups of polymers, such as carbonyl, carboxyl, and amino groups, to form covalent bonds, resulting in a permanent, stable, and rigid three-dimensional structure (Mohammadi, Arabi, & Alibolandi, 2020). In contrast, physically crosslinked nanogel is formed through the entanglements of the polymer chain and physical interactions between several polymers in formulation, such as hydrogen bonds, electrostatic, van der Waals, and hydrophobic interactions (Mauri, Giannitelli, Trombetta, & Rainer, 2021).
Physically crosslinked nanogels are predominantly prepared through amphiphilic self-assembly and electrostatic self-assembly, owing to the mild reaction conditions these methods offer. Amphiphilic polysaccharides, derived from the modification of hydrophilic polysaccharide chains with hydrophobic groups (e.g., fatty acids, steroids, adenylosuccinate, cholesterol, etc.), can spontaneously self-assemble into shell-core structured in water (Z. Wu et al., 2022). Once the amphiphilic polysaccharide is dispersed in water, the hydrophobic moieties have a tendency to assemble via intra- or intermolecular associations and form nanogel a hydrophobic core (Fig. 3A). The hydrophobic core serves to load hydrophobic cargo while the hydrophilic outer shell prolongs circulation time. In the preparation of amphiphilic polysaccharide nanogels, the typical process involves dispersing polysaccharides in water, followed by ultrasonic treatment and dialysis against water. Additionally, the thin film hydration method mentioned above in 2.1.2 has been reported to form nanogels with a liposome structure (Bu et al., 2021).
Fig. 3.
The schematic diagram of biopolymer-based nanocarriers preparation methods and biopolymer-based nanocarriers loading with hydrophilic and hydrophobic bioactive: polysaccharides based nanogel (A, a), protein based nanoparticles (B, b).
The electrostatic self-assembled nanogels are formed by electrostatic interactions between at least two oppositely charged polysaccharides. Electrostatic self-assembly relies on the attraction between oppositely charged molecules, such as cationic and anionic polymers or zwitterionic molecules. These interactions form the backbone of nanogel structures, creating a three-dimensional network. For instance, chitosan's amino groups, which are positively charged in mildly acidic solutions, form electrostatic interactions with anionic polymers or polyanions in aqueous environments (Silva & Fabi, 2022). Yang et al. (2015) prepared the chitosan-hyaluronic acid nanogel via the cationic amino group in chitosan interaction with anionic carboxyl groups of hyaluronic acid, achieved by simply mixing chitosan solution with a hyaluronic acid solution (Yang et al., 2015). Furthermore, the addition of ionic cross-linking agents can facilitate the formation of a nanogel network. For example, anionic sodium tripolyphosphate is commonly used to connect with positively charged amines of chitosan nanogels due to its low toxicity and multivalent properties (Shitrit & Bianco-Peled, 2021). Similarly, divalent cations such as Ca2+, Zn2+, and Mn2+ have been used to fabricate sodium alginate and pectin based nanogels, interacting with hydroxyl and carboxyl groups within polysaccharides (Du, Lu, Zhang, Mata, & Fang, 2021).
Chemical crosslinking differs fundamentally from physical crosslinking as it entails the formation of covalent bonds between polysaccharide chains, thereby imparting stability and rigidity to the nanogel structure. This process is typically facilitated by reactions involving bifunctional or multifunctional crosslinkers that connect the polymer chains. The covalent crosslinking strategies include radical polymerizations, Schiff-base reaction, thiol-disulfide exchange reaction, click chemistries, and photoreactions. The selection of covalent crosslinking strategies depends on the functional group in the polysaccharide chains. A comprehensive review of these methodologies has been conducted by Zhang et al. (X. J. Zhang, Malhotra, Molina, & Haag, 2015). Various crosslinker agents, such as glutaraldehyde, formaldehyde, and glyoxal, have been reported for preparing nanogel (X. Chen et al., 2023; Silva & Fabi, 2022). These crosslinkers induced the reaction in polysaccharides-based nanogel, usually involving hydroxyl or carboxyl groups in polysaccharides. Based on these chemical cross-linking mechanisms, several technologies have been developed to synthesize nanogels, such as precipitation polymerization and nanoprecipitation methods (Paliya et al., 2023). However, the residual monomers or cross-linking agents of chemically crosslinked nanogel have adverse effects on cells or tissues, limiting chemically crosslinked nanogel in the food industry.
3.1.3. Encapsulation strategies for hydrophilic and hydrophobic ingredients in polysaccharides nanogels
The presence of hydrophilic groups, such as -OH, –CONH– and –CONH2– within the polysaccharides chain, enables nanogels to exhibit water absorption properties and the ability to encapsulate hydrophilic ingredients. Furthermore, the insoluble yet hydrophilic polysaccharides forming a three-dimensional network structure through physical or chemical bonds lead to nanogels having superior water absorption capacity and higher hydrophilic drug loading capacity than individual hydrophilic polymers (Hawthorne, Pannala, Sandeman, & Lloyd, 2022).
The majority of studies employ a straightforward approach for encapsulating hydrophilic bioactive ingredients. This typically involves dissolving the hydrophilic compounds in water, followed by the formation of nanogels through either physical or chemical crosslinking within the aqueous solution containing the hydrophilic compounds (Table 4). For instance, Rahaiee, Hashemi, Shojaosadati, Moini, and Razavi (2017) prepared chitosan-alginate nanogels to enhance the bioavailability of crocin by adding the crocin-alginate solution into the chitosan solution (Rahaiee et al., 2017). Similarly, in a study by Zhao et al. (2020), spherical nanogel was fabricated by mixing anthocyanins and cationic chitosan solutions. The obtained chitosan-pectin-anthocyanins nanogel with 66.7 % encapsulation efficiency protects anthocyanins from stomach acid environment damage and delayed release cargo into the intestinal fluid (Zhao et al., 2020).
Table 4.
Summary of fabrication methods and encapsulation of hydrophilic and hydrophobic bioactives by polysaccharide nanogel.
| Bioactive encapsulant and encapsulant type | Encapsulation technique | Nanogel composition | Encapsulation efficiency (%) | Loading methods of bioactive ingredient | Reference |
|---|---|---|---|---|---|
| Curcumin (Hydrophobic) |
Physically crosslinked method | Aminated chitosan, tripolyphosphate, folate acid | 94 | Curcumin was dispersed in an aminated chitosan, folate acid, or tripolyphosphate solution followed by ultrasound treatment. Centrifuged the dispersion and washed the precipitates with acetic acid solution and deionized water to obtain the nanogel. | (X. Sun et al., 2019) |
| Curcumin (Hydrophobic) |
Physically crosslinked method | Sodium alginate, CS, calcium chloride | 10–13 | Incorporating curcumin-ethanol stock solution in calcium chloride solution. The solution was added to the sodium alginate aqueous solution, and the CS solution was dropped into the system. | (R. K. Das, Kasoju, & Bora, 2010) |
| Curcumin (Hydrophobic) |
Physically crosslinked method | Soybean protein isolate-dextran conjugate (SDC) | 89.1 | SDC was dissolved in water through pH adjustment and thermal treatment. Subsequently, incorporate curcumin ethanolic solution into the system through homogenization. The final curcumin-loaded nanogel is obtained through centrifugation and lyophilization processes. | (Y. X. Sun et al., 2024) |
| Resveratrol (Hydrophobic) |
Physically crosslinked method | Trimethyl chitosan | 46–72 | Add RV to TMC aqueous solution. Introduce a TPP, alginate, or a mixed solution of TPP and alginate dropwise into the resultant solution. | (Min et al., 2018) |
| Curcumin (Hydrophobic) |
Chemically crosslinked method | Chitosan hydrochloride, carboxymethyl starch | 89–94 | A curcumin-ethanol solution was added to the CHC solution to prepare the Cur-CHC stock solution. CMC solution of carboxyl groups activated by EDC was added into the stock solution to form amide bond and followed by washing and centrifugation to get nanogel. |
(X. M. Li et al., 2019) |
| β-Carotene (Hydrophobic) |
Physically crosslinked method | Pectin, 2-hydroxypropyl-β-cyclodextrin (HP-CD) | – | β-Carotene-acetone solution was added to the preheated HP-CD solution with stirring. After evaporating acetone, the resultant solution was dropwise to pectin solution to obtain the final nanoparticle. | (Celitan et al., 2022) |
| Riboflavin (Hydrophillic) and quercetin (Hydrophobic) | Physically crosslinked method | Gallic acid modified chitosan (GCS), Ovalbumin (OVA) | 66 (riboflavin) 96 (quercetin) |
Add riboflavin-water or quercetin-ethanol solution to the OVA solution with stirring. Introduce the resultant solution into GCS solution and supplement with calcium chloride solution to obtain nanoparticles. | (L. Li et al., 2022) |
| Green coffee extract (Hydrophillic) |
Chemically crosslinked method | Carrageenan, | – | Dissolve Carrageenan in water, then incorporate green coffee extract at varying concentrations. Subsequently, the mixture is combined with glutaraldehyde to form crosslinked networks and purified through dialysis to yield the final nanogel systems. | (Khalaf, Gouda, Taleb, Heakal, & El-Lateef, 2024) |
| Blueberry anthocyanins (Hydrophillic) |
Physically crosslinked method | Olive pectin (PC), chitosan (CS) | 92 | ANCs and PC solution were added to CS-acetic acid solution and followed by adjusting pH of this system. |
(C. Xie et al., 2023) |
| Oyster peptides (Hydrophillic) |
Physically crosslinked method | Carboxymethyl chitosan, carboxymethyl cellulose | 92.7 | Commence by incorporating oyster peptide solution into carboxymethyl chitosan to form a homogeneous mixture. Subsequently, introduce this mixture into the carboxymethyl cellulose solution. The nanogel was obtained by pH adjustment and thermal treatment | (P. Zhang et al., 2024) |
| Blueberry anthocyanins (Hydrophillic) |
Physically crosslinked method | Pectin, lysozyme | 73 | Mix the pectin, lysozyme, and anthocyanin solution with pH 5 in the ice bath, followed by stirring at room temperature to form a nanogel. | (Rosales, da Silva, Lourenço, Hassimotto, & Fabi, 2021) |
| Anthocyanins (Hydrophoillic) |
Physically crosslinked method | Chitosan, pectin | 67 | Anthocyanin powder was added to the chitosan solution to form CS-ANC complexes, followed by dropwise pectin solution into the mixture to form nanogel. | (Zhao et al., 2020) |
| Cyanidin-3-glucoside (Hydrophillic) |
Physically crosslinked method | Carboxymethyl chitosan (CMC), β-cyclodextrin graft epichlorohydrin (β-CD-EP), CaCl2 | 48 | Combine C3G, β-CD-EP, CMC solution, and CaCl2 solution with carboxymethyl chitosan solution. Stir at 800 rpm for 30 min to form a nanogel. | (J. Sun et al., 2022) |
While the conventional approach involves incorporating the loaded ingredients into the hybrid polysaccharide solution before nanogel formation, studies have shown that bioactive ingredients can also be loaded into the system after nanogel formation. This process is facilitated by passive diffusion and interactions between the bioactive molecules and polysaccharides. Moreover, the unrestricted mobility of small hydrophilic compounds within the expanded nanogel network promotes rapid diffusion of the drug into the surrounding aqueous environment, leading to an undesired burst release phenomenon (J. Li & Mooney, 2016). To address this concern, researchers have developed stimuli-responsive nanogels to address this concern, such as pH-responsive, light-sensitive, and temperature-sensitive nanogels, allowing for the controlled release of encapsulated compounds in specific environmental conditions (Farazi et al., 2020).
Despite the inherent hydrophilic nature of polysaccharide nanogels, several strategies have been developed to encapsulate hydrophobic active substances effectively. One approach involves dissolving hydrophobic actives in water at concentrations below their solubility limit. However, this method often results in low drug loading, limiting the application of such nanomaterials (Min, Kim, Lee, & Lee, 2018; X. Sun et al., 2019). To enhance drug concentration in the nanogel system, researchers employed organic solvents as co-solvents, including ethanol, acetone, and ethyl acetate (S. Das, Singh, Chaudhari, Dwivedy, & Dubey, 2021), to elevate the concentration of hydrophobic substances in nanogel. For example, Wu, Sun, Jiang, Li, and Pang (2021) encapsulated curcumin by dissolving it in ethanol and mixing it with chitosan solution. The carboxymethyl konjac glucomannan was later dropwise added to a chitosan-curcumin solution to form nanogel. This method produces the curcumin-loaded nanogel with 90 % encapsulation efficiency(C. Wu et al., 2021).
Another innovative approach involves forming complexes between hydrophobic substances and specific polysaccharides to enhance water solubility. Cyclodextrin (CD) is a cyclic oligosaccharide characterized by its bucket-shaped structure, composed of six to eight glucose subunits linked by α-1,4 glycosidic bonds, and has been extensively utilized for this purpose. Its hydrophobic inner cavity enables the formation of complexes with various hydrophobic food ingredients, while its hydrophilic exterior facilitates integration with hydrogels(Eid et al., 2020). Two methods are commonly used for incorporating CD into nanogels: (i) linking CD with polysaccharides, followed by loading of hydrophobic substances into the CD grafted nanogels (Celitan, Gruskiene, Kavleiskaja, & Sereikaite, 2022); (ii) preparing nanogels without CD and then adding CD loaded with hydrophobic compounds into the nanogel (J. Sun et al., 2022). In a recent example of CD grafting into nanogels, Mohamed et al. grafted β-CD with soy soluble polysaccharide to improve the sustained release of Vitamin E in the gastrointestinal tract, resulting in a prolonged increase in plasma vitamin E levels after administration over 12 h (Eid et al., 2020). In another study performed by Naeem et al. (2023), the inclusion complex of rutin and CD was synthesized to enhance the water solubility of rutin. This involved combining a methanol rutin solution with an aqueous solution of CD. The resultant complex was incorporated into a prepared gel-based hydrogel, facilitating controlled release within the gastrointestinal tract and increasing the bioavailability of rutin (Naeem et al., 2023).
In conclusion, while hydrophilic ingredients are relatively easy to encapsulate in nanogels, increasing the associative interaction between the functional groups of polysaccharides and bioactives is crucial for enhancing encapsulation efficiency and preventing leakage. For hydrophobic compounds, utilizing organic solvents as co-solvents or complexing them with CD to improve their solubility in water is often a prerequisite for achieving high encapsulation efficiency.
3.2. Protein/peptide nanoparticle
3.2.1. Brief overview of protein/peptide nanoparticle
Protein and polypeptides are biopolymers composed of 20 amino acids linked together by peptide bonds. The diverse functional groups within the amino acid chain allow for the encapsulation of actives through different interactions and contribute to forming a three-dimensional structure. For instance, amphiphilic proteins tend to form the micelle structure in an aqueous that provides space to encapsulate the hydrophobic actives (C. H. Tang, 2020). The multitude of hydroxyl, carboxyl, and amino groups in amino acid strings enables the protein nanoparticles to interact with bioactive ingredients and prevent their release from protein nanoparticles (Fig. 3b). Moreover, various functional groups also facile chemical Modifications to produce functional nanoparticles, which not only offer protection for various bioactive molecules but also pave the way for targeted delivery of actives to specific sites of action (T. Zhang et al., 2023).
In the food sector, protein nanoparticles can be fabricated using materials derived from both animal and botanical origins. Commonly employed animal proteins for nanoencapsulation include casein, gelatin, and albumin. Botanical proteins such as zein, soy protein, and gliadin are also extensively utilized (R. Zhang, Han, Xie, Liu, & Chen, 2022). The selection of appropriate protein materials should be based on several factors, including the natural properties of the ingredients to be encapsulated, desired therapeutic goals, and material safety considerations. For instance, botanical proteins generally exhibit superior hydrophobic characteristics compared to animal proteins, making them particularly suitable for encapsulating lipophilic molecules(Shishir, Xie, Sun, Zheng, & Chen, 2018). There are several advantages of protein and polypeptide nanoparticles, which are listed below (T. Zhang et al., 2023):
-
•
Various food proteins can be selected according to the nature of activities
-
•
Plenty of functional groups for the modification and preparation of functional nanoparticles
-
•
Wide range of protein sources and avoid toxicity concerns
-
•
Available in different particle shapes and sizes
3.2.2. Production methods of protein/peptide nanoparticles
Various protein and peptide nanoparticle preparation methods have been proposed, such as desolvation, emulsification, thermal gelation, and self-assembly. These preparation methods are based on the changing protein folding state, controlling molecular interactions with the chosen protein, changing the protein solubility, and separating nanoparticles from the solution. The general process of protein nanoparticle preparation typically comprises three essential steps: (i) dissolving the protein in an aqueous or organic solvent solution. (ii) various methods are adopted to make protein aggregation to nanoparticles, such as changing pH, altering the ratio of water/solvent, imposing temperature cycling, or adding polymers with an opposite charge to the protein. (iii) nanoparticles are solidified and separated from their formation medium.
The desolvation method, a widely employed technique, involves the addition of an agent to the protein solution to induce supersaturation and facilitate protein nano-aggregation formation (G. Liu, An, Li, & Deng, 2023). This umbrella term encompasses several specific approaches, including solvent-nonsolvent, coacervation, and pH-shifting methods. For the solvent-nonsolvent method (Fig. 3B), the addition of the nonsolvent reduces the solubility of the protein and leads to supersaturation. Supersaturation initiates the small protein aggregates to form nuclei that serve as seeds for further growth into nanoparticles (Joye & McClements, 2013). In the process, various molecular interactions, such as hydrophobic forces, hydrogen bonding, and disulfide bonds, are involved in the formation of protein nanoparticles (Dai et al., 2017). This technique involves dissolving the protein in a solvent and subsequently mixing it with a nonsolvent (typically water) to produce the hydrophobic protein based nanoparticles. Conversely, for hydrophilic proteins, ethanol or acetone is often added dropwise to the aqueous protein solution, resulting in protein dehydration and separation from the aqueous phase. This process induces a conformational transition in proteins, shifting their structure from an extended state to a coiled configuration, which facilitates phase separation of hydrophilic proteins from the solution (Elzoghby, Elgohary, & Kamel, 2015). The coacervation preparation method induces phase separation within protein solutions through self-assembly or electrostatic interactions. This method is commonly used to prepare water-soluble protein-based nanoparticles, such as soy protein, gelatin, whey protein, β-lactoglobulin (β-lg), and α-lactalbumin (T. Zhang et al., 2023). This process is initiated by altering temperature, pH, or salt concentration to induce the formation of the small coacervate particles and make the reacting solution become turbidity. If the process continued, the small protein particles coalesce together to form nanoparticles, which lead to the protein nanoparticles settling at the bottom of the container and the formation of a distinctly visible two phase system (Timilsena, Akanbi, Khalid, Adhikari, & Barrow, 2019). The pH-shifting method, also known as pH-cycle or pH-driven, applies to proteins with pH-dependent solubility, such as zein and kafirin (G. Liu et al., 2023). Proteins exhibit different solubility profiles depending on the pH of the solution relative to their isoelectric point (pI). As the pH shifts closer to or away from the pI, proteins undergo conformational rearrangements. For example, at acidic or basic pH levels far from the pI, proteins may unfold due to electrostatic repulsion or protonation/deprotonation of amino acid side chains. This unfolding exposes hydrophobic regions and reactive groups, facilitating intermolecular interactions such as hydrophobic interactions and hydrogen bonding (S. F. Peng et al., 2020; Wei et al., 2021). At the pI, proteins carry no net charge, leading to reduced electrostatic repulsion and increased propensity for aggregation (Mao et al., 2023). The method ususally involves dissolving the protein in an extremely acidic or alkaline solution, followed by the addition of a base or acid to reduce protein solubility and induce nanoparticle formation.
Desolvation is popular in preparing protein nanoparticles due to its simplicity and efficiency. However, the desolvation process is always accompanied by organic solvent or cross-link regent. Physical preparation methods such as spray drying and gelation are introduced in protein nanoparticle preparation to avoid potential toxicity concerns. Spray drying is a method for converting protein or peptide solution into solid particles under a continuous process, which could treat heat-sensitive food compounds, preventing their degradation (Fig. 3B). The bioactive molecules and proteins are prepared into a suspension, which is then fed into drying chamber through the atomizer. In the drying chamber, the protein suspension is formed into micron-sized droplets, and the temperature is set high enough to evaporate the solvent, leading to protein nanoparticle formation (Marante, Viegas, Duarte, Macedo, & Fonte, 2020). Spray drying provides the advantage of drying and particle formation in a single-step, continuous, and scalable process, which makes the technique widely used in various food and pharmaceutical companies.
The gelation method is well-suited for preparing heat-sensitive protein-based nanoparticles, such as those derived from whey protein, β-lactoglobulin, and lactoferrin(Q. Liu et al., 2020). This method utilizes the denaturation and unfolding of heat-sensitive proteins upon heating processes (Fig. 3B). During the heating process, the native tertiary and secondary structures of proteins are disrupted, exposing hydrophobic regions and reactive groups (e.g., sulfhydryl groups). Then, the nanoparticles are formed via the interact among the exposed groups, such as disulfide bonds, hydrogen bonds, hydrophobic interactions, and van der Waals forces (Martinez-Lopez et al., 2020). Moreover, polysaccharides are usually added to protein solutions to strengthen the structural stability of protein nanoparticles.
3.2.3. Encapsulation strategies for hydrophilic and hydrophobic ingredients in protein/peptide nanoparticles
The encapsulation of bioactive compounds within protein nanoparticles is a critical aspect of their application in food science. Three primary strategies have been developed to facilitate the incorporation of hydrophilic or hydrophobic cargos into protein nanoparticles: (i) direct dissolution, (ii) solvent-assisted dissolution, (iii) pH-driven dissolution.
Direct dissolution is the simplest method for encapsulating hydrophobic ingredients in protein nanoparticles. This strategy requires both protein and food compounds to be soluble in the same solvent. The interaction between cargos and proteins is driven by electrostatic attraction, hydrophobic interactions, and hydrogen bonding, which facilitate protein-cargo complex formation. Afterward, the drug-loaded nanoparticles can be separated from the original system by evaporation, centrifugation, desolvation, etc. Alcohol-soluble proteins, such as zein and gliadin, are particularly suitable for the preparation of hydrophobic cargo-loaded nanoparticles using the direct dissolution strategy. For instance, Shi et al. (2024) achieved a high encapsulation efficacy (94.7 %) of curcumin by dropping the mixed zein and curcumin aqueous ethanol solution in water to form nanoparticles (Shi et al., 2024). Likewise, Zhong et al. (2022) dissolved resveratrol and gliadin in a 75 % aqueous ethanol solution and then dropped the gliadin/ resveratrol mixture into water, achieving an encapsulation rate of 78.8 % after ethanol evaporation(Zhong et al., 2022).
In the case of loading hydrophilic components by direct dissolution methods, physicochemical methods such as heat treatment, pH cycling, and chemical modification can be employed to increase the affinity between hydrophilic molecules and proteins. For example, heat treatment of β-lactoglobulin results in a 3.5-fold higher association constant with EGCG than the original protein state. After preheating β-lactoglobulin, EGCG-β-lactoglobulin nanoparticles have a small diameter and better protection of EGCG (Shpigelman, Israeli, & Livney, 2010). Otherwise, the divalent calcium ions could enhance the interaction of deprotonated polyphenol with the carboxylic or phosphate group of casein, potentially promoting more cargo entry into casein nanoparticles(Carnovale, Huppertz, Britten, & Bazinet, 2020). Furthermore, it is widely recognized that altering the pH of a protein solution can induce a shift in its surface charge, rendering it negatively charged at pH levels above its isoelectric point (pI) and positively charged at pH levels below its pI. Thus, it becomes feasible to create a solution where proteins bear an opposite charge to that of the active substance, facilitating ingredients entry into nanoparticles through electrostatic interactions. Tavares et al. discovered that up to 10 anions of folic acid, at pH 5.5, can bind to positively charged lactoferrin (pI of 8.6–8.9) to form 15 nm aggregates (Tavares et al., 2015). In another study, authors altered the solution pH to 3.3 to promote the combination of whey protein, with pI of 5.1–5.2, and folic acid (Corfield et al., 2020).
The solvent-assisted dissolution strategy is commonly used to load hydrophobic substances into hydrophilic protein nanoparticles and vice versa. In order to load hydrophobic substances on hydrophilic protein matrix nanoparticles, organic solvents such as ethanol are often used to assist in the dissolution of the hydrophobic substances and then added dropwise to the protein solution. The protein nanoparticles with encapsulated hydrophobic compounds are obtained after stirring, incubation, and consolidation (Table 5). Liu et al. (2022) mixed an ethyl acetate solution of coenzyme Q10 with an aqueous whey protein solution and formed coenzyme-loaded whey protein nanoparticles after homogenization and rotating evaporation. The results showed that hydrogen bonding and hydrophobic interactions contribute to the binding of coenzymes Q10 to whey proteins with a maximum encapsulation of over 98 % (Q. G. Liu et al., 2022). Conversely, for incorporating hydrophilic substances into hydrophobic proteins, the proteins are dissolved in aqueous solutions containing various ratios of organic solvents to provide a dissolution medium for the cargo, after which the nanoparticles are prepared by different preparation approaches. This approach has been successfully applied to encapsulate water-soluble bioactive compounds such as EGCG-zein (Jin et al., 2022), EGCG-hordein (He, Guan, Song, Li, & Huang, 2020), and anthocyanins-zein (S. J. Li et al., 2023).
Table 5.
Summary of fabrication methods and encapsulation of hydrophilic and hydrophobic bioactives by protein nanaoparticles.
| Bioactive encapsulant and encapsulant type | Fabrication methods | Protein nanoparticles composition | Encapsulation efficiency (%) | Loading methods of bioactive ingredient | Reference |
|---|---|---|---|---|---|
| Curcumin (Hydrophobic) |
Desolvation | Bovine serum albumin | 85 | Bovine serum albumin solution was mixed with ethanolic solution of curcumin. Supplemental ethanol and glutaraldehyde solution were added to the protein solution to induce cross-linking. Evaporation and water wash to remove ethanol and glutaraldehyde to obtain nanoparticles. | (S. S. Zhang et al., 2019) |
| Resveratrol (Hydrophobic) |
Desolvation | Zein, pectin | 78 | Add resveratrol to zein-containing aqueous ethanol solution. Rapidly introduce the resveratrol-zein solution to acid water with stirring. After ethanol evaporation, combine the resultant solution with pectin solution to coat pectin on the protein nanoparticles. | (Y. Liu et al., 2020) |
| Curcumin (Hydrophobic) |
pH-driven | Whey protein isolate (WPI), zein | 45–84 | WPI was dissolved in water and adjusted its pH to 12, followed by adding zein to the WPI solution. Curcumin was added to the WPI-Zein solution and adjusted to pH 7.0 to form Curcumin-loaded nanoparticles. | (Zhan et al., 2020) |
| Quercetin (Que) and avenanthramide 2c (AV 2c) (Hydrophobic) |
pH-driven | Caseinate | Que 94 AV 2c 80 |
Que was dissolved in caseinate solution with pH 12 and adjusted its pH to 7. AV 2c was added to the neutral mixture and followed by adjusting its pH to 6 to form Que. and AV 2c loaded nanoparticle. | (C. Chen, Chen, & Zhong, 2022) |
| Coenzyme Q10 (Hydrophobic) |
Gelation | WPI | 98 | Heat the WPI solution to 85 °C, then cool to room temperature with an ice bath. Mix the WPI solution with Coenzyme Q10-ethyl acetate solution and homogenize the resulting mixture. Evaporate ethyl acetate and supplement water to obtain CoQ10-loaded nanoparticles | (Q. G. Liu et al., 2022) |
| Rutin (Hydrophobic) |
Spray dry | Bovine serum albumin | 32 | Rutin and Bovine serum albumin were dissolved in a 30 % ethanol-water solution. The solution was filtered and dried by spraying to form dried nanoparticles. | (Pedrozo, Antônio, Khalil, & Mainardes, 2020) |
| Anthocyanins (Hydrophillic) |
Desolvation | Zein | – | Dissolve anthocyanin in a zein solution with 70 % ethanol and adjust its pH. Supplement with water and evaporate ethanol to obtain anthocyanin-loaded nanoparticles. | (S. J. Li et al., 2023) |
| EGCG (Hydrophillic) |
Desolvation | Hordein | 74–84 | EGCG powder was mixed with a hordein solution with 55 % isopropanol, and the resulting EGCG-hordein solution was added to water to form the nanoparticle. | (He et al., 2020) |
| Folic acid (Hydrophillic) |
Desolvation | β-lactoglobulin, whey protein isolate | – | Combine the neutral aqueous solution of β-lg, WPI, and FA. The mixture pH was adjusted to 3.0 to form the FA-protein precipitation. The precipitate was frozen and freeze-dried to form nanoparticles. | (Corfield et al., 2020) |
| EGCG (Hydrophillic) | Desolvation | Zein, lecithin | 68 | EGCG was added to the ethanol-water solution containing zein and lecithin. The pH of the formed Zein-Lecithin-EGCG nanocomplex was adjusted to 4 to form dispersion. | (H. J. Xie et al., 2021) |
| Olive leaf extract (Hydrophillic) |
Spray dry | Whey protein | 40–80 | Add an aqueous solution with olive leaf extract to the WPC solution. Introduce the blend solution to an electrospraying system to obtain electrospray WPC particles. | (Soleimanifar, Jafari, & Assadpour, 2020) |
The pH-driven strategy involves manipulating the pH of a system, transitioning it from a neutral state to either highly acidic or alkaline conditions, and subsequently readjusting the pH back to neutrality to facilitate nanocomplex formation (Table 5). This method is effective in increasing solute solubility, inducing structural changes in the molecules, and promoting their interaction with each other. It should be the notion that this method is not only suitable for enhancing protein solubility by folding and unfolding its structure (e.g., zein, casein, bovine serum albumin (X. Chen et al., 2023) but also for improving the water solubility of several hydrophobic molecules (e.g., curcumin, quercetin, diosmetin). A typical example is that curcumin exhibits poor solubility in neutral and acidic aqueous solutions but can dissolve in an alkaline environment due to the deprotonation of its -OH group. The study by Zhan, Dai, Zhang, and Gao (2020) formed spherical curcumin-loaded nanoparticles by dissolving hydrophobic zein and curcumin in an alkaline aqueous solution at pH 12 and then adjusting the pH to neutral (Zhan et al., 2020). The encapsulated curcumin exhibited desirable storage, physical stability, and re-dispersibility. In addition, extreme pH changes drive the proteins to carry different charges, providing the feasibility of preparing stable multiple nanocomplexes by adding biopolymers with opposite charges.
In conclusion, achieving high drug loading in protein nanoparticles depends on the physicochemical properties, such as solubility, charge, and pI, of the proteins and the active compounds. The most convenient approach is typically to prepare nano-carriers loaded with bioactive compounds by dissolving hydrophilic substances in water-soluble protein solutions or dissolving hydrophobic proteins and active substances together in organic solvents. When these direct methods are not feasible, leveraging the interaction between proteins and active molecules through the use of organic solvents or pH manipulation can effectively immobilize the actives within the nanoparticles. The choice of encapsulation strategy should be tailored to the specific properties of the protein and bioactive compound to optimize loading efficiency and stability.
4. Conclusion and future perspective
Whether the composition of the nanocarriers is hydrophilic or lipophilic, all types of nanocarriers have been reported to be loaded with hydrophilic and hydrophobic substances. However, the loading capacity and form vary among the different nanocarriers. Liposomes facilitate the simultaneous encapsulation of hydrophilic and hydrophobic compounds due to their unique structure, whereas SLN and NLC are more effective for hydrophobic ingredients because of their lipid matrix. The encapsulation capacity of nanoemulsion depends on the emulsion type, and the bioactive ingredients should be dissolved in the dispersed phase. Nanogels exhibit a strong ability to absorb water for loading hydrophilic substances while also accommodating hydrophobic compounds through intermolecular forces. For protein nanoparticles, selecting proteins with a higher affinity for the active compounds enhances loading efficiency.
Despite present research demonstrating the loading capabilities of both lipid-based and biopolymer-based nanocarriers for hydrophilic and hydrophobic ingredients, challenges remain in optimizing encapsulation efficacy for food ingredients. For hydrophilic compounds, enhancing interactions between bioactive ingredients and the components of the nanocarrier is essential; insufficient interaction can result in the leakage of encapsulated ingredients from the nanoparticles. Consequently, using biopolymer-based nanocarriers with abundant functional groups is a viable strategy. The most usual encapsulation method for hydrophobic ingredients is to incorporate them into oils or organic solvents during the formation of lipid-based nanocarriers. Moreover, increasing their water solubility—through strategies such as the use of organic solvents as co-solvents or the complexation of bioactives substances with polysaccharides and proteins— can expand the range of compatible nanocarriers.
CRediT authorship contribution statement
Ruihan Huang: Writing – original draft, Investigation. Hongdong Song: Writing – review & editing. Sen Li: Visualization. Xiao Guan: Supervision, Funding acquisition.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 32172247).
Data availability
No data was used for the research described in the article.
References
- Abedi E., Akhavan H.-R., Mohammadi H., Banasaz S. Structure-based modifications of nano lipid carriers: Comparative review on release properties and anti-microbial activities of bioactive compounds. Food Control. 2023 [Google Scholar]
- Agrawal M., Saraf S., Saraf S., Dubey S.K., Puri A., Patel R.J., Alexander A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. Journal of Controlled Release. 2020;321:372–415. doi: 10.1016/j.jconrel.2020.02.020. [DOI] [PubMed] [Google Scholar]
- Ajeeshkumar K.K., Aneesh P.A., Raju N., Suseela M., Ravishankar C.N., Benjakul S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Comprehensive Reviews in Food Science and Food Safety. 2021;20(2):1280–1306. doi: 10.1111/1541-4337.12725. [DOI] [PubMed] [Google Scholar]
- Andra V., Pammi S.V.N., Bhatraju L., Ruddaraju L.K. A comprehensive review on novel liposomal methodologies, commercial formulations, Clinical Trials and Patents. Bionanoscience. 2022;12(1):274–291. doi: 10.1007/s12668-022-00941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo M.A., Cerqueira M.A., Goncalves C., Amado I.R., Pastrana L. Encapsulation of vitamin D3 using rhamnolipids-based nanostructured lipid carriers. Food Chemistry. 2023;427 doi: 10.1016/j.foodchem.2023.136654. [DOI] [PubMed] [Google Scholar]
- Barri A., Ghanbarzadeh B., Mohammadi M., Pezeshki A. Application of sodium caseinate as protein based coating/stabilizing agent in development and optimization of vitamin D3-loaded nanostructured lipid carriers (NLCs) Food Hydrocolloids. 2023;144 doi: 10.1016/j.foodhyd.2023.108747. [DOI] [Google Scholar]
- Bayon-Cordero L., Alkorta I., Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials (Basel) 2019;9(3) doi: 10.3390/nano9030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushani J.A., Karthik P., Anandharamakrishnan C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocolloids. 2016;56:372–382. doi: 10.1016/j.foodhyd.2015.12.035. [DOI] [Google Scholar]
- Binazir E., Ghanbarzadeh B., Hanifian S., Gharekhani M., Kafil H.S., Falcone P.M. Development and optimization of thyme-pennyroyal essential oils based active nanostructured lipid carrier (NLC) containing saffron extract with antioxidant and antimicrobial properties. Food Bioscience. 2024;61 doi: ARTN 104992 10.1016/j.fbio.2024.104992. [Google Scholar]
- Borrin T.R., Georges E.L., Moraes I.C., Pinho S.C. Curcumin-loaded nanoemulsions produced by the emulsion inversion point (EIP) method: An evaluation of process parameters and physico-chemical stability. Journal of Food Engineering. 2016;169:1–9. [Google Scholar]
- Bu X.T., Ji N., Dai L., Dong X.Y., Chen M., Xiong L., Sun Q.J. Self-assembled micelles based on amphiphilic biopolymers for delivery of functional ingredients. Trends in Food Science & Technology. 2021;114:386–398. doi: 10.1016/j.tifs.2021.06.001. [DOI] [Google Scholar]
- Calderon-chiu C., Calderon-santoyo M., Damasceno-gomes S., Ragazzo-Sanchez J.A. Use of jackfruit leaf (Artocarpus heterophyllus L.) protein hydrolysates as a stabilizer of the nanoemulsions loaded with extract-rich in pentacyclic triterpenes obtained from Coccoloba uvifera L. leaf. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carli C., Moraes-Lovison M., Pinho S.C. Production, physicochemical stability of quercetin-loaded nanoemulsions and evaluation of antioxidant activity in spreadable chicken pates. LWT- Food Science and Technology. 2018;98:154–161. doi: 10.1016/j.lwt.2018.08.037. [DOI] [Google Scholar]
- Carnovale V., Huppertz T., Britten M., Bazinet L. Impact of calcium on the interactions between epigallocatechin-3-gallate and α-casein. International Dairy Journal. 2020;102 doi: ARTN 10460810.1016/j.idairyj.2019.104608. [Google Scholar]
- Celitan E., Gruskiene R., Kavleiskaja T., Sereikaite J. β-Carotene-2-hydroxypropyl-β-cyclodextrin complexes coated with pectin. Food Hydrocolloids. 2022;133 doi: ARTN 107990 10.1016/j.foodhyd.2022.107990. [Google Scholar]
- Chai C., Park J. Food liposomes: Structures, components, preparations, and applications. Food Chemistry. 2024;432 doi: 10.1016/j.foodchem.2023.137228. [DOI] [PubMed] [Google Scholar]
- Chen C., Chen Z.X., Zhong Q.X. Caseinate nanoparticles co-loaded with quercetin and avenanthramide 2c using a novel two-step pH-driven method: Formation, characterization, and bioavailability. Food Hydrocolloids. 2022;129 doi: 10.1016/j.foodhyd.2022.107669. [DOI] [Google Scholar]
- Chen W., Zou M., Ma X., Lv R., Ding T., Liu D. Co-encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. Journal of Food Science. 2019;84(1):111–120. doi: 10.1111/1750-3841.14405. [DOI] [PubMed] [Google Scholar]
- Chen X., Yu C., Zhang Y., Wu Y.C., Ma Y., Li H.J. Co-encapsulation of curcumin and resveratrol in zein-bovine serum albumin nanoparticles using a pH-driven method. Food & Function. 2023;14(7):3169–3178. doi: 10.1039/d2fo03929j. [DOI] [PubMed] [Google Scholar]
- Chung C.H., Jung W., Keum H., Kim T.W., Jon S. Nanoparticles derived from the natural antioxidant Rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano. 2020;14(6):6887–6896. doi: 10.1021/acsnano.0c01018. [DOI] [PubMed] [Google Scholar]
- Cong L.X., Wang J., Lu H., Tian M.W., Ying R.F., Huang M.G. Influence of different anionic polysaccharide coating on the properties and delivery performance of nanoliposomes for quercetin. Food Chemistry. 2023;409 doi: 10.1016/j.foodchem.2022.135270. [DOI] [PubMed] [Google Scholar]
- Corfield R., Martínez K.D., Allievi M.C., Santagapita P., Mazzobre F., Schebor C., Pérez O.E. Whey proteins-folic acid complexes: Formation, isolation and bioavailability in a model. Food Structure-Netherlands. 2020;26 doi: ARTN 100162 10.1016/j.foostr.2020.100162. [Google Scholar]
- Costa C.P., Moreira J.N., Sousa Lobo J.M., Silva A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharmaceutica Sinica B. 2021;11(4):925–940. doi: 10.1016/j.apsb.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Sun C.X., Li R.R., Mao L.K., Liu F.G., Gao Y.X. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chemistry. 2017;237:1163–1171. doi: 10.1016/j.foodchem.2017.05.134. [DOI] [PubMed] [Google Scholar]
- Dammak I., Sobral P., Aquino A., Neves M.A.D., Conte-Junior C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones-a review. Comprehensive Reviews in Food Science and Food Safety. 2020;19(5):2721–2746. doi: 10.1111/1541-4337.12606. [DOI] [PubMed] [Google Scholar]
- Das R.K., Kasoju N., Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine: Nanotechnology, Biology, and Medicine. 2010;6(1):153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Das S., Singh V.K., Chaudhari A.K., Dwivedy A.K., Dubey N.K. Fabrication, physico-chemical characterization, and bioactivity evaluation of chitosan-linalool composite nano-matrix as innovative controlled release delivery system for food preservation. International Journal of Biological Macromolecules. 2021;188:751–763. doi: 10.1016/j.ijbiomac.2021.08.045. [DOI] [PubMed] [Google Scholar]
- Das S.S., Verma P.R.P., Singh S.K. Screening and preparation of quercetin doped nanoemulsion: Characterizations, antioxidant and anti-bacterial activities. LWT- Food Science and Technology. 2020;124 doi: ARTN 109141 10.1016/j.lwt.2020.109141. [Google Scholar]
- Dong W.Y., Tang C.Q., Xia M.Q., Sheng L., Cai Z.X. Preparation and characterization of egg yolk immunoglobulin loaded chitosan-liposome assisted by supercritical carbon dioxide. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130934. [DOI] [PubMed] [Google Scholar]
- Du M., Lu W., Zhang Y., Mata A., Fang Y. Natural polymer-sourced interpenetrating network hydrogels: Fabrication, properties, mechanism and food applications. Trends in Food Science & Technology. 2021;116:342–356. doi: 10.1016/j.tifs.2021.07.031. [DOI] [Google Scholar]
- Duong V.A., Nguyen T.T., Maeng H.J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25(20):4781. doi: 10.3390/molecules25204781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid M., Sobhy R., Zhou P.Y., Wei X.L., Wu D., Li B. β-Cyclodextrin- soy soluble polysaccharide based core-shell bionanocomposites hydrogel for vitamin E swelling controlled delivery. Food Hydrocolloids. 2020;104 doi: ARTN 105751 10.1016/j.foodhyd.2020.105751. [Google Scholar]
- Elzoghby A.O., Elgohary M.M., Kamel N.M. In: Implications of protein- and peptide-based nanoparticles as potential vehicles for anticancer drugs. Donev R., editor. Protein and peptide nanoparticles for drug delivery; 2015. pp. 169–221. [DOI] [PubMed] [Google Scholar]
- Fan C.L., Feng T.T., Wang X.W., Xia S.Q., Swing C.J. Liposomes for encapsulation of liposoluble vitamins (a, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Research International. 2023;163 doi: 10.1016/j.foodres.2022.112264. [DOI] [PubMed] [Google Scholar]
- Farazi S., Chen F., Foster H., Boquiren R., McAlpine S.R., Chapman R. Real time monitoring of peptide delivery using high payload pH responsive nanogels. Polymer Chemistry. 2020;11(2):425–432. doi: 10.1039/c9py01120j. [DOI] [Google Scholar]
- Favarin F.R., Forrati E.M., Bassoto V.A., da Silva Gundel S., Velho M.C., Ledur C.M., Ourique A.F. Ascorbic acid and ascorbyl palmitate-loaded liposomes: Development, characterization, stability evaluation, in vitro security profile, antimicrobial and antioxidant activities. Food Chemistry. 2024;460(Pt 2) doi: 10.1016/j.foodchem.2024.140569. [DOI] [PubMed] [Google Scholar]
- Feng S.M., Sheng J.L., Yu J.H., Lin Y., Shao P. Encapsulation and release of citral using nanostructured lipid carriers: A study on the impact of different preparation methods. Food Bioscience. 2023;56 doi: ARTN 103185 10.1016/j.fbio.2023.103185. [Google Scholar]
- Gonçalves R.F., Martins J.T., Abrunhosa L., Baixinho J., Matias A.A., Vicente A.A., Pinheiro A.C. Lipid-based nanostructures as a strategy to enhance curcumin bioaccessibility: Behavior under digestion and cytotoxicity assessment. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110278. [DOI] [PubMed] [Google Scholar]
- Gonçalves R.F., Vicente A.A., Pinheiro A.C. Incorporation of curcumin-loaded lipid-based nano delivery systems into food: Release behavior in food simulants and a case study of application in a beverage. Food Chemistry. 2023;405 doi: 10.1016/j.foodchem.2022.134740. [DOI] [PubMed] [Google Scholar]
- Goncalves R.F.S., Martins J.T., Abrunhosa L., Baixinho J., Matias A.A., Vicente A.A., Pinheiro A.C. Lipid-based nanostructures as a strategy to enhance curcumin bioaccessibility: Behavior under digestion and cytotoxicity assessment. Food Research International. 2021;143 doi: 10.1016/j.foodres.2021.110278. [DOI] [PubMed] [Google Scholar]
- Gouda A., Sakr O.S., Nasr M., Sammour O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. Journal of Drug Delivery Science and Technology. 2021;61 doi: ARTN 102174 10.1016/j.jddst.2020.102174. [Google Scholar]
- Granja A., Vieira A.C., Chaves L.L., Nunes C., Neves A.R., Pinheiro M., Reis S. Folate-targeted nanostructured lipid carriers for enhanced oral delivery of epigallocatechin-3-gallate. Food Chemistry. 2017;237:803–810. doi: 10.1016/j.foodchem.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Guldiken B., Gibis M., Boyacioglu D., Capanoglu E., Weiss J. Ascorbic acid-induced degradation of liposome-encapsulated acylated and non-acylated anthocyanins of black carrot extract. Journal of the Science of Food and Agriculture. 2021;101(13):5707–5714. doi: 10.1002/jsfa.11225. [DOI] [PubMed] [Google Scholar]
- Gunawan M., Boonkanokwong V. Current applications of solid lipid nanoparticles and nanostructured lipid carriers as vehicles in oral delivery systems for antioxidant nutraceuticals: A review. Colloids and Surfaces. B, Biointerfaces. 2024;233 doi: 10.1016/j.colsurfb.2023.113608. doi: ARTN 113608. [DOI] [PubMed] [Google Scholar]
- Gupta A., Eral H.B., Hatton T.A., Doyle P.S. Nanoemulsions: Formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi: 10.1039/c5sm02958a. [DOI] [PubMed] [Google Scholar]
- Hamadou A.H., Zhang J.Y., Chao C., Xu B. Stability of rutin using pectin-chitosan dual coating nanoliposomes. LWT- Food Science and Technology. 2022;170 doi: 10.1016/j.lwt.2022.114084. [DOI] [Google Scholar]
- Hanley L., Ghazani S.M., Marangoni A.G. Giant multilamellar and large unilamellar lecithin vesicles for the encapsulation and oral delivery of cannabinoids. Food Chemistry. 2024;433 doi: 10.1016/j.foodchem.2023.137291. [DOI] [PubMed] [Google Scholar]
- Has C., Sunthar P. A comprehensive review on recent preparation techniques of liposomes. Journal of Liposome Research. 2020;30(4):336–365. doi: 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- Hawthorne D., Pannala A., Sandeman S., Lloyd A. Sustained and targeted delivery of hydrophilic drug compounds: A review of existing and novel technologies from bench to bedside. Journal of Drug Delivery Science and Technology. 2022;78 doi: 10.1016/j.jddst.2022.103936. [DOI] [Google Scholar]
- He A.J., Guan X., Song H.D., Li S., Huang K. Encapsulation of (−)-epigallocatechin-gallate (EGCG) in hordein nanoparticles. Food Bioscience. 2020;37 doi: ARTN 100727 10.1016/j.fbio.2020.100727. [Google Scholar]
- Hejri A., Khosravi A., Gharanjig K., Davarani M.M. Effect of edible antioxidants on chemical stability of beta-carotene loaded nanostructured lipid carriers. LWT- Food Science and Technology. 2019;113 doi: 10.1016/j.lwt.2019.108272. [DOI] [Google Scholar]
- Huang Z.H., Guo B.Z., Deng C., Tang C., Liu C.M., Hu X.T. Fabrication and characterization of the W/O/W multiple emulsion through oleogelation of oil. Food Chemistry. 2021;358 doi: 10.1016/j.foodchem.2021.129856. doi: ARTN 129856 10.1016/j.foodchem.2021.129856. [DOI] [PubMed] [Google Scholar]
- Inapurapu S.P., Ibrahim A., Kona S.R., Pawar S.C., Bodiga S., Bodiga V.L. Development and characterization of ω-3 fatty acid nanoemulsions with improved physicochemical stability and bioaccessibility. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2020;606 doi: ARTN 125515 10.1016/j.colsurfa.2020.125515. [Google Scholar]
- Javadi B., Farahmand A., Soltani-Gorde-Faramarzi S., Hesarinejad M.A. Chitosan-coated nanoliposome: An approach for simultaneous encapsulation of caffeine and roselle-anthocyanin in beverages. International Journal of Biological Macromolecules. 2024;275(Pt 1) doi: 10.1016/j.ijbiomac.2024.133469. [DOI] [PubMed] [Google Scholar]
- Jin J.C., Liu C.Z., Tong H.F., Sun Y.L., Huang M., Ren G.R., Xie H.J. Encapsulation of EGCG by Zein-gum Arabic complex nanoparticles and in vitro simulated digestion of complex nanoparticles. Foods. 2022;11(14) doi: 10.3390/foods11142131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joye I.J., McClements D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends in Food Science & Technology. 2013;34(2):109–123. doi: 10.1016/j.tifs.2013.10.002. [DOI] [Google Scholar]
- Karimi M., Gheybi F., Zamani P., Mashreghi M., Golmohammadzadeh S., Darban S.A., Jaafari M.R. Preparation and characterization of stable nanoliposomal formulations of curcumin with high loading efficacy: In vitro and in vivo anti-tumor study. International Journal of Pharmaceutics. 2020;580 doi: 10.1016/j.ijpharm.2020.119211. [DOI] [PubMed] [Google Scholar]
- Katsouli M., Giannou V., Tzia C. Enhancement of physicochemical and encapsulation stability of O-1/W/O(2)multiple nanoemulsions loaded with coenzyme Q(10)or conjugated linoleic acid by incorporating polyphenolic extract. Food & Function. 2020;11(10):8878–8892. doi: 10.1039/d0fo01707h. [DOI] [PubMed] [Google Scholar]
- Khalaf M.M., Gouda M., Taleb M.F.A., Heakal F.E., El-Lateef H.M.A. Fabrication of smart nanogel based on carrageenan and green coffee extract as a long-term antifouling agent to improve biofilm prevention in food production. Food Chemistry. 2024;461 doi: 10.1016/j.foodchem.2024.140719. doi: ARTN 140719 10.1016/j.foodchem.2024.140719. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Na K., Bae Y.H. Nanoparticle oral absorption and its clinical translational potential. Journal of Controlled Release. 2023;360:149–162. doi: 10.1016/j.jconrel.2023.06.024. [DOI] [PubMed] [Google Scholar]
- Komaiko J.S., McClements D.J. Formation of food-grade Nanoemulsions using low-energy preparation methods: A review of available methods. Comprehensive Reviews in Food Science and Food Safety. 2016;15(2):331–352. doi: 10.1111/1541-4337.12189. [DOI] [PubMed] [Google Scholar]
- Koroleva M., Nagovitsina T., Yurtov E. Nanoemulsions stabilized by non-ionic surfactants: Stability and degradation mechanisms. Physical Chemistry Chemical Physics. 2018;20(15):10369–10377. doi: 10.1039/c7cp07626f. [DOI] [PubMed] [Google Scholar]
- Kumar H., Chand P., Pachal S., Mallick S., Jain R., Madhunapantula S.V., Jain V. Fisetin-loaded nanostructured lipid carriers: Formulation and evaluations against advanced and metastatic melanoma. Molecular Pharmaceutics. 2023;20(12):6035–6055. doi: 10.1021/acs.molpharmaceut.3c00309. [DOI] [PubMed] [Google Scholar]
- Lee C., Na K. Anthocyanin-loaded liposomes prepared by the pH-gradient loading method to enhance the anthocyanin stability, Antioxidation effect and skin permeability. Macromolecular Research. 2019;28(3):289–297. doi: 10.1007/s13233-020-8039-7. [DOI] [Google Scholar]
- Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nature Reviews Materials. 2016;1(12):1–17. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lv H., Chen Y., Song H., Zhang Y., Wang S., Guan X. N-trimethyl chitosan coated targeting nanoparticles improve the oral bioavailability and antioxidant activity of vitexin. Carbohydrate Polymers. 2022;286 doi: 10.1016/j.carbpol.2022.119273. [DOI] [PubMed] [Google Scholar]
- Li S.J., Wang X., Zhang X.Q., Zhang H., Li S.Y., Zhou J.Z., Fan L.L. Interactions between zein and anthocyanins at different pH: Structural characterization, binding mechanism and stability. Food Research International. 2023;166 doi: 10.1016/j.foodres.2023.112552. [DOI] [PubMed] [Google Scholar]
- Li X.M., Wu Z.Z., Zhang B., Pan Y., Meng R., Chen H.Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chemistry. 2019;293:197–203. doi: 10.1016/j.foodchem.2019.04.096. [DOI] [PubMed] [Google Scholar]
- Liu G., An D., Li J., Deng S. Zein-based nanoparticles: Preparation, characterization, and pharmaceutical application. Frontiers in Pharmacology. 2023;14 doi: 10.3389/fphar.2023.1120251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Cui H.P., Muhoza B., Duhoranimana E., Xia S.Q., Hayat K., Zhang X.M. Fabrication of low environment-sensitive nanoparticles for cinnamaldehyde encapsulation by heat-induced gelation method. Food Hydrocolloids. 2020;105 doi: ARTN 105789 10.1016/j.foodhyd.2020.105789. [Google Scholar]
- Liu Q.G., Sun Y.X., Cui Q., Cheng J.J., Killpartrik A., Kemp A.H., Guo M.R. Characterization, antioxidant capacity, and bioaccessibility of coenzyme Q10 loaded whey protein nanoparticles. LWT- Food Science and Technology. 2022;160 doi: ARTN 113258 10.1016/j.lwt.2022.113258. [Google Scholar]
- Liu W.L., Hou Y.Y., Jin Y.Y., Wang Y.P., Xu X.K., Han J.Z. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends in Food Science & Technology. 2020;104:177–189. doi: 10.1016/j.tifs.2020.08.012. [DOI] [Google Scholar]
- Liu X.Z., Lin X., Wei Y.J., Fei T., Hu X.P., Wang L. Enhancing the stability and bio-accessibility of carotenoids extracted from canistel (Lucuma nervosa) via liposomes encapsulation. LWT- Food Science and Technology. 2024;204 doi: ARTN 116455 10.1016/j.lwt.2024.116455. [Google Scholar]
- Liu X.Z., Lin X., Wei Y.J., Fei T., Hu X.P., Wang L. Enhancing the stability and bio-accessibility of carotenoids extracted from canistel (Lucuma nervosa) via liposomes encapsulation. LWT- Food Science and Technology. 2024;204 doi: ARTN 116455 10.1016/j.lwt.2024.116455. [Google Scholar]
- Liu Y., Liang X., Zou Y., Peng Y.Q., McClements D.J., Hu K. Resveratrol-loaded biopolymer core-shell nanoparticles: Bioavailability and anti-inflammatory effects. Food & Function. 2020;11(5):4014–4025. doi: 10.1039/d0fo00195c. [DOI] [PubMed] [Google Scholar]
- Lombardo D., Kiselev M.A. Methods of liposomes preparation: Formation and control factors of versatile Nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3):543. doi: 10.3390/pharmaceutics14030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckanagul J.A., Pitakchatwong C., Ratnatilaka Na Bhuket P., Muangnoi C., Rojsitthisak P., Chirachanchai S., Rojsitthisak P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydrate Polymers. 2018;181:1119–1127. doi: 10.1016/j.carbpol.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Ma J.Q., Guan R.F., Chen X.Q., Wang Y.B., Hao Y.B., Ye X.Q., Liu M.Q. Response surface methodology for the optimization of beta-lactoglobulin nano-liposomes. Food & Function. 2014;5(4):748–754. doi: 10.1039/c3fo60476d. [DOI] [PubMed] [Google Scholar]
- Machado T.O., Grabow J., Sayer C., de Araujo P.H.H., Ehrenhard M.L., Wurm F.R. Biopolymer-based nanocarriers for sustained release of agrochemicals: A review on materials and social science perspectives for a sustainable future of Agri- and horticulture. Advances in Colloid and Interface Science. 2022;303 doi: 10.1016/j.cis.2022.102645. [DOI] [PubMed] [Google Scholar]
- Mao Y.H., Huang W.T., Jia R.J., Bian Y.Y., Pan M.H., Ye X.Y. Correlation between protein features and the properties of pH-driven-assembled nanoparticles: Control of particle size. Journal of Agricultural and Food Chemistry. 2023;71(14):5686–5699. doi: 10.1021/acs.jafc.3c00147. [DOI] [PubMed] [Google Scholar]
- Marante T., Viegas C., Duarte I., Macedo A.S., Fonte P. An overview on spray-drying of protein-loaded polymeric nanoparticles for dry powder inhalation. Pharmaceutics. 2020;12(11) doi: 10.3390/pharmaceutics12111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhamati M., Ranjbar G., Rezaie M. Effects of emulsifiers on the physicochemical stability of oil-in-water Nanoemulsions: A critical review. Journal of Molecular Liquids. 2021;340 doi: ARTN 117218 10.1016/j.molliq.2021.117218. [Google Scholar]
- Martinez-Lopez A.L., Pangua C., Reboredo C., Campion R., Morales-Gracia J., Irache J.M. Protein-based nanoparticles for drug delivery purposes. International Journal of Pharmaceutics. 2020;581 doi: 10.1016/j.ijpharm.2020.119289. [DOI] [PubMed] [Google Scholar]
- Mauri E., Giannitelli S.M., Trombetta M., Rainer A. Synthesis of Nanogels: Current trends and future outlook. Gels. 2021;7(2):36. doi: 10.3390/gels7020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Qiu C., Li X., McClements D.J., Sang S., Jiao A., Jin Z. Polysaccharide-based nano-delivery systems for encapsulation, delivery, and pH-responsive release of bioactive ingredients. Critical Reviews in Food Science and Nutrition. 2024;64(1):187–201. doi: 10.1080/10408398.2022.2105800. [DOI] [PubMed] [Google Scholar]
- Michelon M., Huang Y.T., de la Torre L.G., Weitz D.A., Cunha R.L. Single-step microfluidic production of W/O/W double emulsions as templates for β-carotene-loaded giant liposomes formation. Chemical Engineering Journal. 2019;366:27–32. doi: 10.1016/j.cej.2019.02.021. [DOI] [Google Scholar]
- Min J.B., Kim E.S., Lee J.S., Lee H.G. Preparation, characterization, and cellular uptake of resveratrol-loaded trimethyl chitosan nanoparticles. Food Science and Biotechnology. 2018;27(2):441–450. doi: 10.1007/s10068-017-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani Y., Patravale V.B., S, B. Solid lipid nanoparticles for hydrophilic drugs. Journal of Controlled Release. 2021;335:457–464. doi: 10.1016/j.jconrel.2021.05.032. [DOI] [PubMed] [Google Scholar]
- Modi S., Xiang T.X., Anderson B.D. Enhanced active liposomal loading of a poorly soluble ionizable drug using supersaturated drug solutions. Journal of Controlled Release. 2012;162(2):330–339. doi: 10.1016/j.jconrel.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Arabi L., Alibolandi M. Doxorubicin-loaded composite nanogels for cancer treatment. Journal of Controlled Release. 2020;328:171–191. doi: 10.1016/j.jconrel.2020.08.033. [DOI] [PubMed] [Google Scholar]
- Mushtaq A., Wani S.M., Malik A.R., Gull A., Ramniwas S., Nayik G.A., Bari A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chemistry: X. 2023;18 doi: 10.1016/j.fochx.2023.100684. doi: ARTN 100684 10.1016/j.fochx.2023.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem A., Yu C., Zang Z., Zhu W., Deng X., Guan Y. Synthesis and evaluation of Rutin-Hydroxypropyl beta-Cyclodextrin inclusion complexes embedded in xanthan gum-based (HPMC-g-AMPS) hydrogels for Oral controlled drug delivery. Antioxidants (Basel) 2023;12(3):552. doi: 10.3390/antiox12030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z., Acevedo-Fani A., McDowell A., Barnett A., Loveday S.M., Singh H. Nanoemulsion structure and food matrix determine the gastrointestinal fate and in vivo bioavailability of coenzyme Q10. Journal of Controlled Release. 2020;327:444–455. doi: 10.1016/j.jconrel.2020.08.025. [DOI] [PubMed] [Google Scholar]
- Oliveira D.R.B., Furtado G.D., Cunha R.L. Solid lipid nanoparticles stabilized by sodium caseinate and lactoferrin. Food Hydrocolloids. 2019;90:321–329. doi: 10.1016/j.foodhyd.2018.12.025. [DOI] [Google Scholar]
- Ozogul Y., Karsli G.T., Durmus M., Yazgan H., Oztop H.M., McClements D.J., Ozogul F. Recent developments in industrial applications of nanoemulsions. Advances in Colloid and Interface Science. 2022;304 doi: 10.1016/j.cis.2022.102685. [DOI] [PubMed] [Google Scholar]
- Paliya B.S., Sharma V.K., Sharma M., Diwan D., Nguyen Q.D., Aminabhavi T.M., Gupta V.K. Protein-polysaccharide nanoconjugates: Potential tools for delivery of plant-derived nutraceuticals. Food Chemistry. 2023;428 doi: 10.1016/j.foodchem.2023.136709. [DOI] [PubMed] [Google Scholar]
- Papagiannopoulos A., Sotiropoulos K. Current advances of polysaccharide-based Nanogels and microgels in food and biomedical sciences. Polymers (Basel) 2022;14(4) doi: 10.3390/polym14040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic N., Mijalkovic J., Dordevic V., Pecarski D., Bugarski B., Knezevic-Jugovic Z. Ultrasonication for production of nanoliposomes with encapsulated soy protein concentrate hydrolysate: Process optimization, vesicle characteristics and in vitro digestion. Food Chemistry: X. 2022;15 doi: 10.1016/j.fochx.2022.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrozo R.C., Antônio E., Khalil N.M., Mainardes R.M. Bovine serum albumin-based nanoparticles containing the flavonoid rutin produced by nano spray drying. Brazilian Journal of Pharmaceutical Sciences. 2020;56 doi: ARTN e17692 10.1590/s2175-97902019000317692. [Google Scholar]
- Peng S.F., Zhou L., Cai Q.Z., Zou L.Q., Liu C.M., Liu W., McClements D.J. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocolloids. 2020;107 doi: 10.1016/j.foodhyd.2020.105963. [DOI] [Google Scholar]
- Peng Y.R., Meng Q.L., Zhou J., Chen B., Xi J.J., Long P.P., Hou R.Y. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chemistry. 2018;242:527–532. doi: 10.1016/j.foodchem.2017.09.094. [DOI] [PubMed] [Google Scholar]
- Pezeshki A., Hamishehkar H., Ghanbarzadeh B., Fathollahy I., Nahr F.K., Heshmati M.K., Mohammadi M. Nanostructured lipid carriers as a favorable delivery system for β-carotene. Food Bioscience. 2019;27:11–17. doi: 10.1016/j.fbio.2018.11.004. [DOI] [Google Scholar]
- Pimentel-Moral S., Teixeira C., Fernandes R., Borras-Linares I., Arráez-Román D., Martínez-Férez A., Souto E.B. Polyphenols-enriched extract-loaded nanostructured lipid carriers (NLC): Optimization by multi-response surface methodology. Journal of Drug Delivery Science and Technology. 2019;49:660–667. doi: 10.1016/j.jddst.2018.12.023. [DOI] [Google Scholar]
- Rabelo C.A.S., Taarji N., Khalid N., Kobayashi I., Nakajima M., Neves M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with acai berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Research International. 2018;106:542–548. doi: 10.1016/j.foodres.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R., Kulhari H., Pooja D., Gudem S., Bhargava S., Shukla R., Sistla R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chemistry and Physics of Lipids. 2016;198:51–60. doi: 10.1016/j.chemphyslip.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Rahaiee S., Hashemi M., Shojaosadati S.A., Moini S., Razavi S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. International Journal of Biological Macromolecules. 2017;99:401–408. doi: 10.1016/j.ijbiomac.2017.02.095. [DOI] [PubMed] [Google Scholar]
- Ravanfar R., Tamaddon A.M., Niakousari M., Moein M.R. Preservation of anthocyanins in solid lipid nanoparticles: Optimization of a microemulsion dilution method using the placket-Burman and box-Behnken designs. Food Chemistry. 2016;199:573–580. doi: 10.1016/j.foodchem.2015.12.061. [DOI] [PubMed] [Google Scholar]
- Rodríguez J., Martín M.J., Ruiz M.A., Clares B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Research International. 2016;83:41–59. [Google Scholar]
- Rosales T.K.O., da Silva M.P., Lourenço F.R., Hassimotto N.M.A., Fabi J.P. Nanoencapsulation of anthocyanins from blackberry (spp.) through pectin and lysozyme self-assembling. Food Hydrocolloids. 2021;114 doi: ARTN 106563 10.1016/j.foodhyd.2020.106563. [Google Scholar]
- Safaya M., Rotliwala Y.C. Vol. 27. 1st international conference on recent advances in materials and manufacturing (ICRAMM); Belagavi, INDIA: 2019. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique; pp. 454–459. [Google Scholar]
- Saifullah M., Shishir M.R.I., Ferdowsi R., Rahman M.R.T., Vuong Q.V. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends in Food Science & Technology. 2019;86:230–251. doi: 10.1016/j.tifs.2019.02.030. [DOI] [Google Scholar]
- Seyedabadi M.M., Rostami H., Jafari S.M., Fathi M. Development and characterization of chitosan-coated nanoliposomes for encapsulation of caffeine. Food Bioscience. 2021;40 doi: ARTN 100857 10.1016/j.fbio.2020.100857. [Google Scholar]
- Sharma S., Cheng S.F., Bhattacharya B., Chakkaravarthi S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends in Food Science & Technology. 2019;91:305–318. doi: 10.1016/j.tifs.2019.07.030. [DOI] [Google Scholar]
- Shi Y.F., Rong S., Guo T.X., Zhang R.Y., Xu D.X., Han Y.H., Chen S. Fabrication of compact zein-chondroitin sulfate nanocomplex by anti-solvent co-precipitation: Prevent degradation and regulate release of curcumin. Food Chemistry. 2024;430 doi: 10.1016/j.foodchem.2023.137110. [DOI] [PubMed] [Google Scholar]
- Shishir M.R.I., Karim N., Gowd V., Zheng X.D., Chen W. Liposomal delivery of natural product: A promising approach in health research. Trends in Food Science & Technology. 2019;85:177–200. doi: 10.1016/j.tifs.2019.01.013. [DOI] [Google Scholar]
- Shishir M.R.I., Xie L.H., Sun C.D., Zheng X.D., Chen W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends in Food Science & Technology. 2018;78:34–60. doi: 10.1016/j.tifs.2018.05.018. [DOI] [Google Scholar]
- Shitrit Y., Bianco-Peled H. Insights into the formation mechanisms and properties of pectin hydrogel physically cross-linked with chitosan nanogels. Carbohydrate Polymers. 2021;269 doi: 10.1016/j.carbpol.2021.118274. doi: ARTN 118274 10.1016/j.carbpol.2021.118274. [DOI] [PubMed] [Google Scholar]
- Shpigelman A., Israeli G., Livney Y.D. Thermally-induced protein-polyphenol co-assemblies: beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocolloids. 2010;24(8):735–743. doi: 10.1016/j.foodhyd.2010.03.015. [DOI] [Google Scholar]
- Shtay R., Keppler J.K., Schrader K., Schwarz K. Encapsulation of (−)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. Journal of Food Engineering. 2019;244:91–100. doi: 10.1016/j.jfoodeng.2018.09.008. [DOI] [Google Scholar]
- Silva M.P., Fabi J.P. Food biopolymers-derived nanogels for encapsulation and delivery of biologically active compounds: A perspective review. Food Hydrocolloids for Health. 2022;2 doi: 10.1016/j.fhfh.2022.100079. [DOI] [Google Scholar]
- Sneha K., Kumar A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innovative Food Science & Emerging Technologies. 2022;76 doi: 10.1016/j.ifset.2021.102914. [DOI] [Google Scholar]
- Soleimanifar M., Jafari S.M., Assadpour E. Encapsulation of olive leaf phenolics within electrosprayed whey protein nanoparticles; production and characterization. Food Hydrocolloids. 2020;101 doi: 10.1016/j.foodhyd.2019.105572. [DOI] [Google Scholar]
- Souri J., Almasi H., Hamishehkar H., Amjadi S. Sodium caseinate-coated and beta-cyclodextrin/vitamin E inclusion complex-loaded nanoliposomes: A novel stabilized nanocarrier. LWT- Food Science and Technology. 2021;151 doi: 10.1016/j.lwt.2021.112174. [DOI] [Google Scholar]
- Souto E.B., Baldim I., Oliveira W.P., Rao R., Yadav N., Gama F.M., Mahant S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opinion on Drug Delivery. 2020;17(3):357–377. doi: 10.1080/17425247.2020.1727883. [DOI] [PubMed] [Google Scholar]
- Sun J., Chen J., Bi Y., Xiao Y., Ding L., Bai W. Fabrication and characterization of beta-cyclodextrin-epichlorohydrin grafted carboxymethyl chitosan for improving the stability of Cyanidin-3-glucoside. Food Chemistry. 2022;370 doi: 10.1016/j.foodchem.2021.130933. [DOI] [PubMed] [Google Scholar]
- Sun X., Yu D., Ying Z., Pan C., Wang N., Huang F., Ouyang X.K. Fabrication of ion-crosslinking Aminochitosan nanoparticles for encapsulation and slow release of curcumin. Pharmaceutics. 2019;11(11):584. doi: 10.3390/pharmaceutics11110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.X., Yue W.T., Xiang X.R., Chen Z.H., Chen J.P., Li S.S., Zhang Q. Curcumin-loaded soybean-dextran conjugate nanogels: Construction, characterization, and incorporation into orange juice beverage. Food Bioscience. 2024;59 doi: ARTN 104140 10.1016/j.fbio.2024.104140. [Google Scholar]
- Tamaroh S., Sari Y.P., Wariyah C., Huda N., Candraruna D.B. Formulation and characterization of nanoemulsion containing anthocyanin extract from purple yam (L.) Food Science and Biotechnology. 2024:1–7. doi: 10.1007/s10068-024-01713-x. [DOI] [Google Scholar]
- Tan C., Wang J., Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnology Advances. 2021;48 doi: 10.1016/j.biotechadv.2021.107727. [DOI] [PubMed] [Google Scholar]
- Tang C.H. Nanocomplexation of proteins with curcumin: From interaction to nanoencapsulation (a review) Food Hydrocolloids. 2020;109 doi: ARTN 106106 10.1016/j.foodhyd.2020.106106. [Google Scholar]
- Tang C.-H., Chen H.-L., Dong J.-R. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as food-grade Nanovehicles for hydrophobic nutraceuticals or bioactives. Applied Sciences. 2023;13(3) doi: 10.3390/app13031726. [DOI] [Google Scholar]
- Tang X.Y., Wang Z.M., Meng H.C., Lin J.W., Guo X.M., Zhang T., Yu S.J. Robust W/O/W emulsion stabilized by Genipin-cross-linked sugar beet pectin-bovine serum albumin nanoparticles: Co-encapsulation of Betanin and curcumin. Journal of Agricultural and Food Chemistry. 2021;69(4):1318–1328. doi: 10.1021/acs.jafc.0c05212. [DOI] [PubMed] [Google Scholar]
- Tavares G.M., Croguennec T., Le S., Lerideau O., Hamon P., Carvalho A.F., Bouhallab S. Binding of folic acid induces specific self-aggregation of Lactoferrin: Thermodynamic characterization. Langmuir. 2015;31(45):12481–12488. doi: 10.1021/acs.langmuir.5b02299. [DOI] [PubMed] [Google Scholar]
- Tian H., Lu Z., Li D., Hu J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chemistry. 2018;248:78–85. doi: 10.1016/j.foodchem.2017.11.091. [DOI] [PubMed] [Google Scholar]
- Timilsena Y.P., Akanbi T.O., Khalid N., Adhikari B., Barrow C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. International Journal of Biological Macromolecules. 2019;121:1276–1286. doi: 10.1016/j.ijbiomac.2018.10.144. [DOI] [PubMed] [Google Scholar]
- Tripathy S., Srivastav P.P. Encapsulation of Centella asiatica leaf extract in liposome: Study on structural stability, degradation kinetics and fate of bioactive compounds during storage. Food Chemistry Advances. 2023;2 [Google Scholar]
- Valdivia-Olivares R.Y., Martinez-González E.A., Montenegro G., Bridi R., Alvarez-Figueroa M.J., González-Aramundiz J.V. Innovative multiple nanoemulsion (W/O/W) based on Chilean honeybee pollen improves their permeability, antioxidant and antibacterial activity. Food Research International. 2023;168 doi: 10.1016/j.foodres.2023.112767. doi: ARTN 112767 10.1016/j.foodres.2023.112767. [DOI] [PubMed] [Google Scholar]
- Wei Y., Zhan X.Y., Dai L., Zhang L., Mao L.K., Yuan F., Gao Y.X. Formation mechanism and environmental stability of whey protein isolate-zein core-shell complex nanoparticles using the pH-shifting method. LWT- Food Science and Technology. 2021;139 doi: 10.1016/j.lwt.2020.110605. [DOI] [Google Scholar]
- William B., Noémie P., Brigitte E., Géraldine P. Supercritical fluid methods: An alternative to conventional methods to prepare liposomes. Chemical Engineering Journal. 2020;383 doi: ARTN 123106 10.1016/j.cej.2019.123106. [Google Scholar]
- Wu C., Sun J., Jiang H., Li Y., Pang J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chemistry. 2021;362 doi: 10.1016/j.foodchem.2021.130242. https://www.sciencedirect.com/science/article/pii/S0308814621012486?via%3Dihub [DOI] [PubMed] [Google Scholar]
- Wu Z., Li H., Zhao X.W., Ye F.Y., Zhao G.H. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications. Carbohydrate Polymers. 2022;284 doi: 10.1016/j.carbpol.2022.119182. doi: ARTN 119182 10.1016/j.carbpol.2022.119182. [DOI] [PubMed] [Google Scholar]
- Xie C., Huang M., Ying R., Wu X., Hayat K., Shaughnessy L.K., Tan C. Olive pectin-chitosan nanocomplexes for improving stability and bioavailability of blueberry anthocyanins. Food Chemistry. 2023;417 doi: 10.1016/j.foodchem.2023.135798. [DOI] [PubMed] [Google Scholar]
- Xie H.J., Liu C.Z., Gao J., Shi J.Y., Ni F.F., Luo X., Luo Z.S. Fabrication of Zein-lecithin-EGCG complex nanoparticles: Characterization, controlled release in simulated gastrointestinal digestion. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.130542. [DOI] [PubMed] [Google Scholar]
- Yang L., Gao S., Asghar S., Liu G., Song J., Wang X., Xiao Y. Hyaluronic acid/chitosan nanoparticles for delivery of curcuminoid and its in vitro evaluation in glioma cells. International Journal of Biological Macromolecules. 2015;72:1391–1401. doi: 10.1016/j.ijbiomac.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Ye, Q. Z., Li, T., Li, J. H., Liu, L., Dou, X. H., & Zhang, X. R. (2020). Development and evaluation of tea polyphenols loaded water in oil emulsion with zein as stabilizer. Journal of Drug Delivery Science and Technology, 56, 101528. doi: ARTN 101528 10.1016/j.jddst.2020.101528.
- Yi X.Z., Gao X., Zhang X., Xia G.H., Shen X.R. Preparation of liposomes by glycolipids/phospholipids as wall materials: Studies on stability and digestibility. Food Chemistry. 2023;402 doi: 10.1016/j.foodchem.2022.134328. [DOI] [PubMed] [Google Scholar]
- Zhan X.Y., Dai L., Zhang L., Gao Y.X. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocolloids. 2020;106 doi: 10.1016/j.foodhyd.2020.105839. [DOI] [Google Scholar]
- Zhang R., Han Y., Xie W., Liu F., Chen S. Advances in protein-based Nanocarriers of bioactive compounds: From microscopic molecular principles to Macroscopical structural and functional attributes. Journal of Agricultural and Food Chemistry. 2022;70(21):6354–6367. doi: 10.1021/acs.jafc.2c01936. [DOI] [PubMed] [Google Scholar]
- Zhang S.S., Asghar S., Yu F., Chen Z.P., Hu Z.Y., Ping Q.N., Xiao Y.Y. BSA nanoparticles modified with N-acetylcysteine for improving the stability and Mucoadhesion of curcumin in the gastrointestinal tract. Journal of Agricultural and Food Chemistry. 2019;67(33):9371–9381. doi: 10.1021/acs.jafc.9b02272. [DOI] [PubMed] [Google Scholar]
- Zhang T., Li L., Chunta S., Wu W., Chen Z., Lu Y. Enhanced oral bioavailability from food protein nanoparticles: A mini review. Journal of Controlled Release. 2023;354:146–154. doi: 10.1016/j.jconrel.2022.12.043. [DOI] [PubMed] [Google Scholar]
- Zhang X.J., Malhotra S., Molina M., Haag R. Micro- and nanogels with labile crosslinks - from synthesis to biomedical applications. Chemical Society Reviews. 2015;44(7):1948–1973. doi: 10.1039/c4cs00341a. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Dong L., Liu L., Wu Z., Pan D., Liu L. Recent advances of stimuli-responsive polysaccharide hydrogels in delivery systems: A review. Journal of Agricultural and Food Chemistry. 2022;70(21):6300–6316. doi: 10.1021/acs.jafc.2c01080. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Ding R., Zhang K., Guo H., Lin Y. Self-assembled Nanocarrier delivery Systems for Bioactive Compounds. Small. 2024;20(26) doi: 10.1002/smll.202310838. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Hao G., Liu C., Fu J., Hu D., Rong J., Yang X. Recent progress in the preparation, chemical interactions and applications of biocompatible polysaccharide-protein nanogel carriers. Food Research International. 2021;147 doi: 10.1016/j.foodres.2021.110564. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhang X.D., Tie S.S., Hou S., Wang H.T., Song Y.K., Tan M.Q. Facile synthesis of nano-nanocarriers from chitosan and pectin with improved stability and biocompatibility for anthocyanins delivery: An and study. Food Hydrocolloids. 2020;109 doi: ARTN 106114 10.1016/j.foodhyd.2020.106114. [Google Scholar]
- Zheng B., Peng S., Zhang X., McClements D.J. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded Nanoemulsions with commercial curcumin supplements. Journal of Agricultural and Food Chemistry. 2018;66(41):10816–10826. doi: 10.1021/acs.jafc.8b03174. [DOI] [PubMed] [Google Scholar]
- Zheng L.J., Xu H.J., Zhang H.X., Shi C.H., Zhou W., Zhang X.R. Nanostructured lipid carrier as a strategy for encapsulation of formononetin and perilla seed oil: In vitro characterization and stability studies. Food Bioscience. 2023;53 doi: 10.1016/j.fbio.2023.102707. [DOI] [Google Scholar]
- Zhong W.Q., Zhi Z.J., Zhao J.B., Li D.J., Duan M.X., Xu J.T., Wu C.H. Oxidized chitin nanocrystals greatly strengthen the stability of resveratrol-loaded gliadin nanoparticles. Journal of Agricultural and Food Chemistry. 2022 doi: 10.1021/acs.jafc.2c04174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.