Abstract
Background
Hippocampal volume increases throughout early development and is an important indicator of cognitive abilities and mental health. However, hippocampal development is highly vulnerable to exposures during development, as seen by smaller hippocampal volume and differential epigenetic programming in genes implicated in mental health. However, few studies have investigated hippocampal volume in relation to the peripheral epigenome across development, and even less is known about potential genetic moderators. Therefore, in this study, we explored relationships between hippocampal volume and peripheral DNA methylation of mental health–related genes, specifically NR3C1, FKBP5, and SLC6A4, throughout early development and whether these associations were moderated by age or genotype.
Methods
Bilateral hippocampal volume was computed from T2-weighted images through FreeSurfer, and DNA methylation was measured from saliva using the Illumina MethylationEPIC microarray in a pediatric population (N = 248, females = 112, meanage = 5.13 years, SDage = 3.60 years).
Results
Multiple linear regression and bootstrapping analyses revealed that DNA methylation of NR3C1, FKBP5, and SLC6A4 was associated with hippocampal volume and that these relationships were moderated by age and gene-specific variants.
Conclusions
These findings support the validity of peripheral DNA methylation profiles for indirectly assessing hippocampal volume and development and underscore the importance of genotype and age considerations in research. Therefore, peripheral epigenetic profiles may be a promising avenue for investigating the impacts of early-life stress on brain structure and subsequent mental health outcomes.
Keywords: DNA methylation, Epigenetics, Hippocampus, Neuroimaging, Pediatrics
Plain Language Summary
This study explores associations between DNA methylation of mental health–related genes from saliva samples and hippocampal brain volume in children. The findings suggest that DNA methylation across 3 key genes is linked to hippocampal size, with age and genetic variations playing a role in how strong these associations are. Cortisol-related genes, NR3C1 and FKBP5, are stronger predictors in early childhood, while the serotonin transporter, SLC6A4, is stronger in later childhood. These results highlight the importance of studying peripheral epigenetic profiles across development and their relation to neurodevelopment, which may offer insights for personalized mental health treatments.
Plain Language Summary
This study explores associations between DNA methylation of mental health–related genes from saliva samples and hippocampal brain volume in children. The findings suggest that DNA methylation across 3 key genes is linked to hippocampal size, with age and genetic variations playing a role in how strong these associations are. Cortisol-related genes, NR3C1 and FKBP5, are stronger predictors in early childhood, while the serotonin transporter, SLC6A4, is stronger in later childhood. These results highlight the importance of studying peripheral epigenetic profiles across development and their relation to neurodevelopment, which may offer insights for personalized mental health treatments.
The hippocampus is crucial for learning, memory, and the hypothalamic-pituitary-adrenal (HPA) axis stress response. It undergoes significant changes during development, including neurogenesis, dendritic and axonal growth, and synaptic formation and pruning (1). Reduced hippocampal volume is consistently found across adult and pediatric clinical populations, such as those with major depressive disorder (2,3) and posttraumatic stress disorder (4,5). Notably, the hippocampus is highly susceptible to early-life adversity due to its high density of glucocorticoid receptors (NR3C1), FK506-binding protein 51 cochaperones (FKBP5), and serotonin transporters (SLC6A4), all of which are vital for stress regulation (6, 7, 8). Epigenetic processes likely mediate the impact of early experiences on hippocampal development, with subsequent differential gene expression leading to HPA axis dysfunction and reduced hippocampal volume, both of which have been linked to psychiatric disorders (2, 3, 4, 5,9).
Epigenetics involves gene regulation mechanisms independent of DNA sequence, with DNA methylation (DNAm) being the most studied in relation to exposures and mental health in adults (10) and children (11,12). Pioneering rodent studies demonstrated that maternal care variations can modify DNAm and NR3C1 expression in hippocampal tissue (13), a finding extended to human studies using postmortem brain tissue and peripheral samples by our group and others (14,15). Additional animal and human studies support the role of epigenetic action on FKBP5 and SLC6A4 in response to early experiences (15, 16, 17). Notably, human epigenetic studies have often used peripheral samples, raising scientific interest in how well the peripheral epigenome reflects central nervous system processes.
In neuroimaging epigenetics, peripheral samples have shown mixed results regarding correlations between hippocampal volume and DNAm of NR3C1, FKBP5, and SLC6A4. A study found positive associations between blood DNAm of NR3C1 promoter sites and hippocampal subfield volumes in adults and patients with major depressive disorder (18), but this was not replicated in buccal or saliva samples (19). Similarly, FKBP5 intron 7 CpG sites did not correlate significantly with hippocampal volume in blood, buccal, or saliva samples (19,20). Conflicting results were also found for SLC6A4 promoter region CpG sites in relation to hippocampal volume (21,22). A recent epigenome-wide association study (EWAS) in adults linked hippocampal volume to 2 sites (23), neither of which are the current study’s candidate genes. No studies have yet assessed peripheral DNAm and hippocampal volume in pediatric cohorts; only 2 have done so in adolescents, and both showed modest associations from blood (24) and saliva (25) samples. These findings highlight the need to consider development when linking DNAm of NR3C1, FKBP5, and SLC6A4 with hippocampal volume. Past studies were limited by focusing on few CpG sites and lacking genetic moderation considerations. While saliva samples correlate most strongly with brain DNAm (26, 27, 28), variability in peripheral sample types may contribute to inconsistent results.
Genetic context can significantly influence epigenetic patterns and phenotypic expressions. Genetic variations within genes related to neuroplasticity, HPA axis, and dopaminergic and serotonergic systems can moderate the associations between psychiatric symptoms (29, 30, 31, 32) and early-life exposures with DNAm (33,34). Recent neuroimaging multi-omic studies emphasize the need to model both genotype and DNAm when predicting brain development (35), activity (36,37), structure (24,38), and white matter lesions (39). These findings highlight the necessity of considering genetic variation when examining epigenetic associations with brain endophenotypes.
During early development, both the hippocampus and epigenome undergo significant changes and are highly susceptible to environmental influences. NR3C1, FKBP5, and SLC6A4 are among the most studied genes in relation to early-life adversity and psychiatric symptoms, thereby meriting specific consideration. This study addresses the current literature gap by assessing the relationships between DNAm of NR3C1, FKBP5, and SLC6A4 and hippocampal volume across early development. Using data from the ECHO (Environmental influences on Child Health Outcomes) program, we investigated 1) associations between DNAm of these genes at the gene and CpG levels with hippocampal volume across early development, 2) age as a moderator of these relationships, and 3) genetic variants within these genes as moderators of the epigenome-brain relationships.
Methods and Materials
Parent Study
This study used data from the ECHO program, a consortium of 69 pediatric cohort studies that has been collecting data under a common protocol since 2019 (40). Single and cohort-specific institutional review boards oversaw human subject activities, with participants providing written informed consent. Eligibility criteria for the parent study included mothers >18 years old, term gestation (37–41 weeks), healthy singleton pregnancy, no uncontrolled medical conditions, no history of major psychiatric illness, English speaking, and consent to infant brain imaging and a study that is longitudinal in nature. Infants had no significant congenital anomalies, neurological trauma, or disorder. Inclusion criteria for this subset required participants to provide a saliva sample and a magnetic resonance imaging (MRI) scan within 365 days of each other. Written consent was collected from the parent or legal guardian.
Demographics
Demographic information was parent reported for all participants (N = 248) (Table 1). Participants were excluded if the days between saliva sample and MRI scan exceeded 365 (mean = 28.56 days, SD = 70.57). Socioeconomic status was calculated using the Hollingshead Four Factor Index of Social Status (41), with maternal and paternal education scores multiplied by 3 and then averaged, resulting in weighted average socioeconomic status scores.
Table 1.
Sample Descriptive Statistics
| Values | |
|---|---|
| Age | |
| Mean ± SD, Years | 5.13 ± 3.6 |
| Range | 2 months–14 years |
| Sex | |
| Female | 45% |
| Male | 55% |
| Race | |
| American Indian or Alaska Native | 0.4% |
| Asian | 2.0% |
| Asian Indian | 0.8% |
| Black or African American | 7.3% |
| More than one | 13.7% |
| Native Hawaiian or Pacific Islander | 0.4% |
| Other, unknown, declined, or missing | 6.9% |
| White | 68.5% |
| Ethnicity | |
| Non-Hispanic/Latino | 74.6% |
| Hispanic/Latino | 21.0% |
| Declined or missing | 4.4% |
Saliva Collection and DNA Isolation
Saliva was collected using Oragene kits (DNA Genotek) during laboratory visits. DNA was extracted with the supplier’s isolation kit and assessed for purity and yield using a NanoDrop ND-1000 (Thermo Fisher Scientific).
Genotype Array and Data Preprocessing
Genotyping was conducted at the Translational Genomics Research Institute using a Multi-Ethnic Global Array (>1.7 million markers) on an Illumina iScanSystem. Data preprocessing and quality control were done using PLINK version 1.9 (42), applying thresholds for single nucleotide polymorphism (SNP) genotyping rate (≥95%), minor allele frequency (≥5%), Hardy-Weinberg equilibrium (p ≥ 1.0 × 10−5), and sample genotyping rate (≥95%). Sex mismatch analysis and relatedness detection were performed, and principal component analyses (PCAs) were conducted to remove outliers. VCF files were used for imputation via the TOPMed Imputation Server. The first 10 ancestry principal components (PCs) and eigenvalues were calculated using PLINK (--pca) with default minor allele frequency (--maf) and call rate (--geno) filters. Based on the eigenvalue scree plot, the first 3 PCs were used in future analyses and subsequently confirmed as providing the best model fit compared with 4 or more components (Akaike information criterion = 3207.4, adjusted R2 = 0.6394).
DNAm Array and Data Preprocessing
Bisulfite conversion was completed using the EZ-96 DNAm kit (Zymo Research). DNAm levels were measured using an Illumina Infinium MethylationEPIC BeadChip (850K) array. Data preprocessing involved using the minfi package in R, excluding cross-reactive and sex chromosome probes, and performing quality control measures including quantile normalization (43). The proportion of epithelial cells per sample was estimated using the robust partial correlation method from EpiDISH (44).
MRI Scans and Structural Measurement
MRI was conducted by collaborators at Brown University, and detailed methods can be found in Deoni et al. (45). Processing of MRI images was performed using FreeSurfer software, utilizing infant FreeSurfer atlases (46). FreeSurfer then delineated 33 distinct brain regions per hemisphere. Regional brain volumes were reported in voxels (mm3).
Statistical Analyses
PC Analysis
M-values of CpG sites annotated to each candidate gene were used to calculate PCs from PCA, following recommendations (47). M-values, the log2 ratio of beta values, were used to resolve heteroscedasticity in highly methylated or unmethylated probes, stabilizing variance. M-values also enhance detection rates and true positive rates. PCA, performed using SPSS (IBM Corp.), used the first PC (PC1) in analyses. PCs capture data variance more effectively than summed or weighted DNAm scores (48), especially with correlated CpG sites, by transforming them into uncorrelated PCs (49). Using PCs reduces the number of statistical tests needed, thereby minimizing type 1 error. PCA is well established in DNAm studies and commonly used in pediatric cohorts (14,50,51).
Multiple Linear Regressions
The outcome variable was computed by averaging right and left hippocampal volume. The PC1 of each candidate gene was the predictor variable, including age at brain scan, sex, days between genetic collection and brain scan, cell count, batch, and array position as covariates (model 1, N = 248). We also tested subsequent models with the addition of 3 ancestry components (model 2, n = 219) and socioeconomic status (model 3, n = 180). Lastly, we assessed individual CpG sites across all 3 candidate genes as predictors of hippocampal volume in model 3 (false discovery rate p value correction; q < .05). While “predict” is used here as standard regression language, these analyses do not imply causality.
Genotype Interactions
Due to long-recognized difficulties in detecting interactions among continuous variables in moderated multiple regression analysis (52), interactions were tested with model 1 to retain the largest sample size. SNPs assessed can be found in Table S1 (24,53, 54, 55, 56, 57). Multiple linear regressions analyzed interactions between CpG sites and SNPs for each gene, with covariates as before. Due to the smaller sample size available with DNAm, MRI, and genotype data, we used a more lenient false discovery rate threshold common in the literature (q < .1) (58, 59, 60).
Bootstrap Resampling
To validate our sampling distribution, a bootstrap analysis was performed on the correlations between hippocampal volume and the PC1 of each gene using the boot package in R. The simulation was repeated 10,000 times to obtain stable sampling distribution estimates, and confidence intervals were calculated via the percentile method.
Correlations Between Saliva and Brain DNAm
Correlations were performed between DNAm beta values from this study and those of publicly available brain tissue data for matching CpG sites. Brain DNAm values were obtained from the Allen Brain Atlas BrainSpan dataset, a publicly available dataset based on the Infinium HumanMethylation450 BeadChip. The BrainSpan dataset provided DNAm values from 16 brain regions (11 cortex regions, hippocampus, amygdala, thalamus, striatum, and cerebellum) across participants (n = 177 samples from 16 individuals, meanage = 8.35 years, SDage = 10.0 years). To conduct correlation analyses, we calculated the average DNAm value across participants per CpG site annotated to genes of interest. Separate correlation analyses were performed for whole brain (n = 16, meanage = 8.35 years, SDage = 10.0 years), hippocampus only (n = 10, meanage = 5.86 years, SDage = 7.03), and nonhippocampal brain regions (n = 16, meanage = 8.35 years, SDage = 10.0 years).
Results
PCA of DNAm Across NR3C1, FKBP5, and SLC6A4
The descriptions and locations of CpG sites retained in PCA are detailed in Table S2. CpG sites within and outside the promoter regions (e.g., islands, shores, shelves, open seas) strongly contributed to the PC1 of each gene (Table S3), demonstrating high correlation across various CpG types.
NR3C1, FKBP5, and SLC6A4 DNAm Predicting Hippocampal Volume
PC1 DNAm Predicting Hippocampal Volume
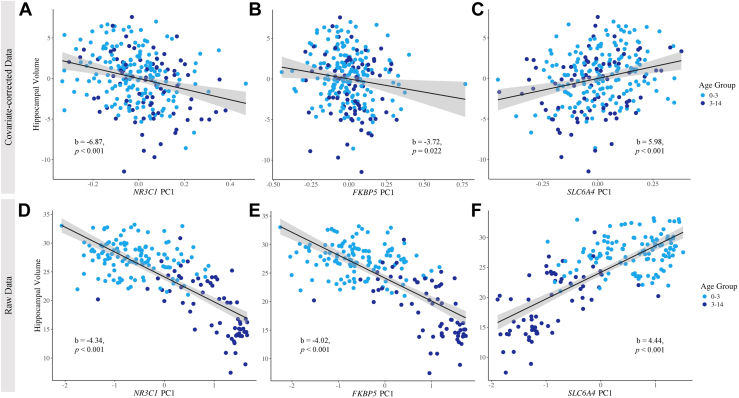
Across all models, all 3 candidate gene PC1s significantly predicted bilateral hippocampal volume (Table 2 and Figure 1). The full model (model 3) produced the highest effect sizes and best model fit across all genes, as evidenced by the highest beta and R2 values (Table 2). Bootstrapping results demonstrated stability and accuracy, with all correlation values falling within the 95% CI estimated distribution (Table 3), supporting the reliability of these findings. To further validate our findings, we used lateral ventricle volume as a control analysis using the full model, revealing that the PC1s of NR3C1 (b = −7.38, p = .44), FKBP5 (b = 5.70, p = .51), and SLC6A4 (b = 7.29, p = .43) were not significant predictors, underscoring the specificity of these associations with hippocampal volume.
Table 2.
Model Fits
| Model | n | Parameters | Beta | p | AIC | Adjusted R2 |
|---|---|---|---|---|---|---|
| NR3C1 | ||||||
| 1 | 248 | 7 | −6.56 | 2.84 × 10−5 | 2624.26 | 0.63 |
| 2 | 219 | 10 | −7.18 | 2.60 × 10−4 | 2623.19 | 0.66 |
| 3 | 180 | 11 | −6.87 | 1.10 × 10−4 | 2612.66 | 0.67 |
| FKBP5 | ||||||
| 1 | 248 | 7 | −3.26 | .025 | 2615.83 | 0.61 |
| 2 | 219 | 10 | −4.27 | .008 | 2612.96 | 0.64 |
| 3 | 180 | 11 | −3.72 | .022 | 2623.48 | 0.65 |
| SLC6A4 | ||||||
| 1 | 248 | 7 | 5.80 | 6.76 × 10−5 | 2618.98 | 0.63 |
| 2 | 219 | 10 | 5.81 | 1.65 × 10−4 | 2615.83 | 0.65 |
| 3 | 180 | 11 | 5.98 | 4.44 × 10−4 | 2615.58 | 0.66 |
Model 1 covariates include age, sex, days between genetic collection and brain scan, cell count, batch location, and batch position. Model 2 covariates include those of model 1 and the first 3 ancestry principal components. Model 3 covariates include those of model 2 and socioeconomic status. Akaike information criterion (AIC) is reported for n = 180.
Figure 1.
DNA methylation across NR3C1, FKBP5, and SLC6A4 predicts bilateral hippocampal volume in a pediatric cohort. The PC1 of (A, D)NR3C1 (b = −6.87, p < .001), (B, E)FKBP5 (b = −3.72, p = .022), and (C, F)SLC6A4 (b = 5.98, p < .001) significantly predict bilateral hippocampal volume. (A–C) depict data points, beta coefficients, and p values as corrected by full model covariates; (D–F) depict raw data points, beta coefficients, and p values with no correction from covariates. PC1, first principal component.
Table 3.
Results of Bootstrap Resampling
| Gene | Original | 95% CI |
|---|---|---|
| NR3C1 | −0.72 | −0.78 to −0.66 |
| FKBP5 | −0.68 | −0.74 to −0.61 |
| SLC6A4 | 0.72 | 0.65 to 0.77 |
CpG Site DNAm Predicting Hippocampal Volume
Based on PC1 best model fit, we tested individual CpG site DNAm as predictors using model 3. Twenty of 113 (17.7%) sites across NR3C1, 10 of 58 (17.2%) sites across FKBP5, and 8 of 35 (22.9%) sites across SLC6A4 significantly predicted bilateral hippocampal volume (qs < .05) (Table 4). Of these 38 CpG sites, 26 (68.4%) were open sea, 7 (18.4%) were shores, and 5 (13.2%) were islands.
Table 4.
CpG Site DNA Methylation Predicting Hippocampal Volume
| Gene | CpG Site | CpG Location | Genomic Location | Beta Direction | abvB | p | q |
|---|---|---|---|---|---|---|---|
| NR3C1 | PC1 | − | 6.87 | 1.10 × 10−4 | |||
| cg21702128 | Island | chr5 142784721 | + | 5.99 | .007 | .040 | |
| cg18718518 | South Shore | chr5 142785455 | − | 5.84 | 3.77 × 10−4 | .004 | |
| cg07197341 | Open Sea | chr5 142916018 | + | 4.64 | 3.80 × 10−8 | 2.15 × 10−6 | |
| cg12969488 | North Shore | chr5 142780984 | − | 4.63 | 4.68 × 10−6 | 8.81 × 10−5 | |
| cg08320082 | Open Sea | chr5 142889494 | + | 4.56 | 2.18 × 10−6 | 5.20 × 10−5 | |
| cg17779063 | Open Sea | chr5 142922486 | + | 3.97 | 1.63 × 10−6 | 5.20 × 10−5 | |
| cg24052866 | Open Sea | chr5 142727470 | + | 3.58 | .002 | .015 | |
| cg13514002 | Open Sea | chr5 142677292 | + | 3.33 | 3.43 × 10−4 | .004 | |
| cg12888360 | Open Sea | chr5 142732502 | − | 3.30 | 1.88 × 10−8 | 2.12 × 10−6 | |
| cg17860381 | Island | chr5 142783569 | + | 3.03 | .002 | .015 | |
| cg23776787 | Open Sea | chr5 142814315 | + | 3.02 | 1.50 × 10−4 | 0.002 | |
| cg15115787 | Open Sea | chr5 142730701 | − | 2.79 | 2.30 × 10−6 | 5.20 × 10−5 | |
| cg20728768 | Open Sea | chr5 142696594 | − | 2.74 | 3.00 × 10−5 | 4.84 × 10−4 | |
| cg15910486 | Island | chr5 142783621 | + | 2.50 | .005 | .032 | |
| cg23430507 | Open Sea | chr5 142798375 | + | 2.44 | .002 | .015 | |
| cg01751279 | Open Sea | chr5 142793924 | + | 2.35 | .002 | .015 | |
| cg05900547 | Open Sea | chr5 142769791 | − | 2.26 | 2.55 × 10−4 | .003 | |
| cg07733851 | North Shore | chr5 142781498 | − | 2.25 | .006 | .038 | |
| cg23484741 | Open Sea | chr5 142936868 | + | 2.09 | .005 | .032 | |
| cg25708981 | Open Sea | chr5 142697868 | − | 1.78 | .001 | .005 | |
| FKBP5 | cg22812853 | Open Sea | chr6 35512865 | + | 6.50 | 1.15 × 10−13 | 6.67 × 10−12 |
| cg03245912 | South Shore | chr6 35657040 | + | 5.56 | 3.56 × 10−4 | .003 | |
| cg01731192 | Open Sea | chr6 35511360 | + | 5.48 | 7.68 × 10−8 | 2.23 × 10−6 | |
| PC1 | − | 3.72 | .022 | ||||
| cg15929276 | Open Sea | chr6 35687457 | + | 3.64 | 5.79 × 10−6 | 6.72 × 10−5 | |
| cg22363520 | Open Sea | chr6 35558488 | + | 3.62 | 1.01 × 10−4 | .001 | |
| cg13344434 | Open Sea | chr6 35570573 | − | 2.95 | 1.03 × 10−5 | 9.96 × 10−5 | |
| cg09318204 | Open Sea | chr6 35511434 | + | 2.88 | 4.39 × 10−7 | 6.37 × 10−6 | |
| cg14339974 | Open Sea | chr6 35687310 | + | 2.79 | 3.86 × 10−7 | 6.37 × 10−6 | |
| cg16912838 | Open Sea | chr6 35551624 | − | 1.97 | .001 | .004 | |
| cg07696519 | Open Sea | chr6 35619165 | − | 1.37 | .004 | .020 | |
| SLC6A4 | PC1 | + | 5.98 | 4.44 × 10−4 | |||
| cg26438554 | Island | chr17 28562733 | − | 5.59 | .002 | .012 | |
| cg20209182 | Open Sea | chr17 28530849 | + | 5.34 | 2.08 × 10−6 | 2.43 × 10−5 | |
| cg06373684 | Island | chr17 28562751 | − | 4.84 | .004 | .015 | |
| cg01991100 | Open Sea | chr17 28555935 | + | 3.94 | 3.35 × 10−4 | 0.002 | |
| cg14352032 | South Shore | chr17 28564834 | + | 3.64 | 2.07 × 10−6 | 2.43 × 10−5 | |
| cg09921370 | Open Sea | chr17 28555315 | + | 3.17 | 2.48 × 10−4 | .002 | |
| cg05951817 | North Shore | chr17 28562142 | + | 3.11 | 4.49 × 10−8 | 1.57 × 10−6 | |
| cg22584138 | North Shore | chr17 28562220 | + | 2.30 | .002 | .012 |
CpG sites that did not pass false discovery rate correction at q < .05 are not reported. Covariates include age, sex, days between genetic collection and brain scan, cell count, batch, position, first 3 ancestry PCs, and socioeconomic status.
abvB, absolute value of beta; chr, chromosome; PC, principal component.
Sex, Age, and Genotype as Moderators of DNAm and Hippocampal Volume
Sex and Age Interactions
Due to sex and age being additional significant predictors of hippocampal volume, we explored models with sex and age interaction terms. To optimize interaction effect detection in multiple regression analyses, model 1 was utilized to retain the largest sample size (52). Sex did not significantly interact with NR3C1, FKBP5, or SLC6A4 PC1 to predict hippocampal volume (Table 5). However, age did significantly interact with NR3C1, FKBP5, and SLC6A4 PC1 in predicting hippocampal volume (Table 5). Next, we compared effect sizes of DNAm PC1 predicting hippocampal volume within 2 age groups based on previous literature (61), highlighting differing epigenetic malleability within these ages (younger: 0–3 years old [n = 81] and older: 3–14 years old [n = 167]). Younger children had larger effect sizes for NR3C1 (b = −1.96) and FKBP5 (b = −3.58) PC1s than older children (NR3C1 b = −0.58, FKBP5 b = −1.05) (Figure 2). In contrast, older children had a larger effect size for SLC6A4 PC1 (b = 1.94) than younger children (b = 1.46) (Figure 2).
Table 5.
Hippocampal Volume and DNA Methylation Interactions With Age and Sex
|
NR3C1 |
FKBP5 |
SLC6A4 |
||||
|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | |
| Age Interaction | ||||||
| PC1 | −5.813 | 2.14 × 10−5a | −4.755 | 2.14 × 10−4a | 4.786 | 2.35 × 10−4a |
| Age (in years) | 0.823 | <2 × 10−16a | 0.852 | <2 × 10−16a | 0.832 | <2 × 10−16a |
| PC1 × age | 0.602 | 5.72 × 10−16a | 0.595 | 3.09 × 10−16a | −0.571 | 1.43 × 10−13a |
| Sex Interaction | ||||||
| PC1 | −6.588 | 2.98 × 10−5a | −3.355 | .026a | 5.811 | 6.84 × 10−5a |
| Sex | 1.809 | 5.02 × 10−5a | 1.859 | 4.87 × 10−5a | 1.714 | 1.30 × 10−4a |
| PC1 × sex | 0.085 | .853 | 0.113 | .806 | 0.210 | .659 |
DNA methylation is represented by first principal components (PC1). Covariates include age at scan, sex, days between genetic collection and brain scan, cell count, batch, and position. Interactions reported with age as a continuous variable.
Indicates significant p value (< .05).
Figure 2.
Age moderates the relationship between DNAm and bilateral hippocampal volume. DNAm across (A)NR3C1 and (B)FKBP5 is a stronger predictor of bilateral hippocampal volume at ages 0 to 3 years old (NR3C1 b = −1.96, p = .41; FKBP5 b = −3.58, p = .20) than at ages 3 to 14 years (NR3C1 b = −0.58, p = .72; FKBP5 b = −1.05, p = .41), whereas (C) DNAm across SLC6A4 is a stronger predictor of bilateral hippocampal volume in ages 3 to 14 years old (b = 1.94, p = .19) than ages 0 to 3 years old (b = 1.46, p = .50). Beta and p values included reflect results of model interaction term. DNAm, DNA methylation; PC1, first principal component.
SNP Interactions
For major allele homozygotes versus minor allele carrier comparisons, those with the homozygous major allele (TT) of SLC6A4 SNP rs6354 had significantly larger hippocampal volumes than minor allele carriers (GT/GG). There were no other significant main effects of SNP group on hippocampal volume. We found a significant interaction between NR3C1 PC1 and rs6189/90 (b = −1.86, p = .027) (Figure 3A), such that carriers of the minor allele (TC/TT) had a stronger negative association between DNAm and hippocampal volume than homozygous major allele carriers (CC). There were no significant interactions between FKBP5 or SLC6A4 PC1s and SNPs in predicting hippocampal volume. At an uncorrected p value threshold, 19 NR3C1 CpG sites, 32 FKBP5 sites, and 3 SLC6A4 sites were significantly moderated by genotype, 2 of which passed false discovery rate 0.1 correction (Table 6; an example is visualized in Figure 3B).
Figure 3.
Genotype moderates the relationship between DNAm and hippocampal volume. (A) The association between the NR3C1 DNAm PC1 and bilateral hippocampal volume depends on rs6189/90 (b = −1.86, p = .027), such that carriers of the minor allele (TC/TT) have a stronger negative association than homozygous carriers of major allele (CC). (B) The association between FKBP5 cg21789597 DNAm and hippocampal volume depends on rs9394309 (b = −3.11, q = 0.04), such that carriers of the minor allele (GA/AA) have a negative association, and homozygous carriers of the major allele (AA) have a positive association. Beta and p values included reflect results of the model interaction term. DNAm, DNA methylation; PC1, first principal component.
Table 6.
SNP × CpG Site Interactions Predicting Hippocampal Volume
| Gene | SNP | CpG | Location | CpG Location | Distance | Direction | Strand | q |
|---|---|---|---|---|---|---|---|---|
| FKBP5 | rs9394309 | cg21789597 | Open Sea | chr6 35633557 | 11,676 | Upstream | cis | .03588 |
| rs4713916 | cg21789597 | Open Sea | chr6 35633557 | 36,325 | Downstream | cis | .04786 |
Covariates include age at scan, sex, days between genetic collection and brain scan, cell count, batch, and position. Table includes only interactions that passed false discovery rate multiple corrections at q < .1.
SNP, single nucleotide polymorphism.
Correlations Between Buccal DNAm and Brain DNAm
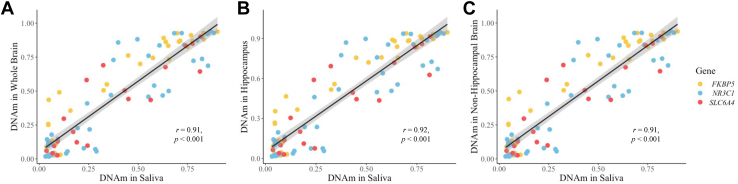
DNAm measured from saliva samples in this study were highly correlated with DNAm measured in brain tissue samples in the Allen Brain Atlas BrainSpan dataset across 16 brain regions for NR3C1 (r = 0.91, p < .001), FKBP5 (r = 0.94, p < .001), SLC6A4 (r = 0.89, p < .001), and for all genes combined (r = 0.91, p < .001) (Table 7 and Figure 4). We also correlated DNAm from saliva with only hippocampal tissue or only nonhippocampal tissue, which did not change the results (Table 7 and Figure 4).
Table 7.
DNA Methylation Correlation in Brain vs. Saliva
| Gene | No. of CpGs | Whole Brain |
Hippocampus Only |
Non-Hippocampus |
|||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| NR3C1 | 46 | 0.91 | <2.2 × 10−16a | 0.91 | <2.2 × 10−16a | 0.91 | <2.2 × 10−16a |
| FKBP5 | 33 | 0.94 | <2.2 × 10−16a | 0.95 | <2.2 × 10−16a | 0.94 | 2.37 × 10−16a |
| SLC6A4 | 19 | 0.89 | 4.17 × 10−7a | 0.88 | 6.48 × 10−7a | 0.89 | 4.07 × 10−7a |
| Overall | 98 | 0.91 | <2.2 × 10−16a | 0.92 | <2.2 × 10−16a | 0.91 | <2.2 × 10−16a |
Indicates significant p value (< .05).
Figure 4.
DNAm in saliva samples is correlated with DNAm in brain tissue. DNAm of saliva samples in the current study was highly correlated with DNAm in brain tissue samples obtained from the Allen Brain Atlas BrainSpan dataset across 16 brain regions. High correlations were present for all 3 candidate genes with DNAm measured in (A) whole brain (r = 0.91, p < .001), (B) hippocampus only (r = 0.92, p < .001), and (C) nonhippocampal regions only (r = 0.91, p < .001). Data points represent average DNAm values for individual CpG sites. DNAm, DNA methylation.
Discussion
This study contributes to neuroimaging epigenetics by showing that DNAm across NR3C1, FKBP5, and SLC6A4 from saliva is linked to hippocampal volume in a healthy pediatric population. Age moderated these relationships, with NR3C1 and FKBP5 DNAm being stronger predictors in early childhood, while SLC6A4 DNAm was stronger in later childhood. Genetic variants also moderated these relationships. Most research on peripheral epigenome and brain structure/function relationships has been conducted with adults (62), but exploring early development is key to understanding how peripheral epigenetic profiles are related to brain and behavior in pediatric populations (63).
Our PCA-based gene-wide DNAm score approach diverges from the literature. We found that NR3C1 and SLC6A4 DNAm PC1s were stronger predictors of hippocampal volume than individual CpG sites. For FKBP5, an open sea CpG site was the strongest predictor. This highlights the importance of using gene-wide DNAm and examining nonpromoter regions. Previous studies in adults on NR3C1 or SLC6A4 focused mainly on CpG sites near promoters (18,19,21,22), or in FKBP5 intron 7 (19,20), with modest results. Similarly, adolescent studies are limited in the number of CpG sites measured across SLC6A4 and FKBP5 and have yielded only modest results (25). About half of these studies also examined the average across a few sites (19,21,24). Alternatively, our approach of using PCA to assess many CpG sites reduces type 1 error and captures gene DNAm variance while also minimizing information loss. This method is robust for predicting brain structure, and we previously reported strong associations between gene PC1 and behavior, physiology, and early-life experiences (14,50,51). However, PCA limits biological interpretation of results.
To date, only one study has employed an EWAS to investigate the relationship between hippocampal volume and DNAm. This study used blood samples from adults and found no significant associations with CpG sites in the NR3C1, FKBP5, or SLC6A4 genes (23). While the EWAS approach is valuable for identifying novel, hypothesis-independent, differentially methylated regions, candidate gene approaches provide targeted analysis of specific genes of interest, which may help detect associations that could be overlooked due to the stringent p value corrections required in EWAS. In this context, a comprehensive epigenome approach may lack specificity because it evaluates associations that involve CpG sites located near genes that may not be expressed in the hippocampus. Moreover, similar to the correlation that has been observed in genotype SNP and RNA expression data (64,65), DNAm at adjacent CpG sites tends to be highly correlated (66), which presents challenges in applying unbiased p value correction methods effectively.
Historically, CpG island sites within promoter regions have been the primary focus of exposure- and behavioral-epigenetic studies, likely due to their well-characterized effects on gene transcription (67). For example, a recent meta-analysis on the effects of early-life stress and NR3C1 DNAm in humans reported that the number of assayed CpGs ranged from <10 (5 records; 15%) to more than 50 (2 records; 6%), with the majority of research reporting on a pool of 10 to 50 CpG sites of the NR3C1 gene (9 studies; 26%) (68). However, CpG sites outside of islands, such as shore, shelf, and open sea sites, are gaining attention for their regulatory roles (69,70). For example, shore CpG sites may play gene regulation roles similar to those played by island sites (70). In the context of development, CpG sites with increased and decreased age-differential DNAm are found not only in islands but also in open seas, shelfs, and shores (69). A more recent study found that age-differential DNAm was not random; CpGs with decreasing DNAm were enriched in gene bodies and enhancers and were annotated to genes enriched in immune-developmental functions. In contrast, CpGs with increasing DNAm were enriched in promoter regions and annotated to genes enriched in neurodevelopmental functions (71). Therefore, it is likely that DNAm at CpGs outside of islands is important for regulating gene transcription and should be investigated with the exposure or phenotype of interest. Results from the current study demonstrate that the majority of predictive CpG sites were open sea sites. Such findings highlight the need for further characterization of the functional consequences of DNAm at all CpG site location types.
Evidence from both rodent (72) and human (73) literature points to a hyporesponsive period in the HPA axis during early life, resulting in lower cortisol. In contrast, peak serotonin levels occur during the first 2 years of life in humans (74). Furthermore, the HPA axis is hypersensitive to exposures during the first 1000 days of life (75), whereas there is increasing evidence of heightened serotonergic sensitivity during adolescence (76,77). Interestingly, results from this study mirror these developmental stages, such that saliva-derived DNAm of NR3C1 and FKBP5 was a stronger predictor of hippocampal volume in early life, during the hypocortisol/hypersensitive stage, while SLC6A4 DNAm was a stronger predictor in later development, during the hyposerotonin/potentially hypersensitive period. While the reason for these developmental interactions cannot be ascertained by this study, it is interesting to note that correlations between the peripheral epigenome and brain metrics may be spatially and temporally dependent, with stronger relationships occurring when the relative system is hypoactive and more sensitive to exposures. Recognizing age-dependent relationships between the peripheral epigenome and brain traits is crucial for prospective studies of epigenetics and mental health in children and adolescents, which still lag behind adult studies (78). Throughout development, differing brain networks that subserve social, emotional, and cognitive development undergo increased plasticity that is sensitive to social exposures (79). Thus, mapping which gene families may best serve as peripheral biomarkers for brain development at specific periods will be an important pursuit. Replication of these results in an independent cohort and further exploration of the epigenome and brain regions involved in mental health could lead to improved understanding of which systems may be more vulnerable across specific developmental windows.
Interindividual DNAm variation is influenced by genetic variation (80, 81, 82, 83, 84), and DNAm plays a role in genetic regulatory mechanisms (81,85, 86, 87, 88, 89, 90). Thus, incorporating genetic variants in neuroimaging epigenetic studies is crucial for understanding the peripheral epigenome. For example, variations across NR3C1, FKBP5, and SLC6A4 appear to moderate the effects of early-life stress on gene expression, DNAm, brain structure and function, and behavior, such that variation in genotype can convey increased vulnerability or resiliency (91, 92, 93, 94, 95). The results of this study are consistent with this view, suggesting that HPA gene variants moderate the relationship between DNAm and hippocampal volume. One open sea site, cg21789597, significantly interacted with 2 FKBP5 SNPs, again highlighting the important role of nonisland CpG sites. NR3C1 SNP rs6189/90 had the only significant interaction with PC1 but had the least diverse genotype sample. Results also replicated similar adult findings, such that SLC6A4 SNPs did not moderate DNAm predictors of hippocampal volume (22). Taken together, this body of evidence highlights the complexity and importance of underlying genetic variance when elucidating pathways between exposures, DNAm, brain metrics, and mental health.
Structural MRI alone does not reveal transcripts, protein levels, or cellular morphology, limiting insight into the pathways that link DNAm of candidate genes with hippocampal volume. MRI-detected volumetric changes can stem from glial or endothelial cell proliferation, neurogenesis, alterations in cellular size, dendritic spine size/density, axonal remodeling, or changes in extracellular space (96,97). Few animal studies have assessed these mechanisms and have focused instead on spine density and markers for neurons and astrocytes in the mouse hippocampus (97, 98, 99). However, gray matter volume changes are more complex, involving nuclear volume, cell number, and spatial clustering characteristics (100). A recent study across 2 large adult cohorts showed that genetic variation (NR3C1 rs56149945), NR3C1 expression, and blood cortisol levels interacted to predict hippocampal volume, with higher NR3C1 expression being linked to smaller hippocampal volume (101). Additionally, FKBP5 expression in female mice is associated with reduced hippocampal neuron density (7). No significant associations were found between peripheral DNAm and serotonergic markers in postmortem brain samples (102). Overall, hippocampal development is shaped by experiences, likely through epigenetic mechanisms in the peripheral and central nervous systems at both the single-cell and population levels (103). Further study of the peripheral epigenome’s relationship with brain traits is needed to understand experience-driven hippocampal circuits that underlie cognitive and mental health.
Despite these important findings, several limitations should be noted. Saliva, buccal, and blood samples vary in correlation with brain DNAm (26, 27, 28), so using different peripheral sample types, such as blood or buccal cells, could yield different results. Adding a second peripheral sample type may improve interpretation. Our sample, primarily from a northeastern state with 83.1% White and 17.1% Hispanic/Latino individuals (104), is relatively diverse at 68.5% White and 21.0% Hispanic/Latino. However, broader racial diversity is limited, which may restrict generalizability. Prenatal nicotine exposure, known to impact DNAm and hippocampal volumes (105,106), was not controlled, potentially affecting associations and warranting future study. While no SLC6A4 SNP moderators of DNAm and hippocampal volume were detected, our small sample size limits these exploratory results. Larger cohorts are needed to replicate these findings and explore relationships across additional brain regions relevant to mental health. As an observational study, causality between brain structure and peripheral epigenetics should be inferred cautiously; here, predict refers only to associations, not causation. Lastly, it is known that serotonin influences spine density in the hippocampus (107,108), but such changes may not alter overall hippocampal volume (109) and thus would go undetected in this analysis.
Conclusions
Epigenetic processes like DNAm offer a promising molecular system for understanding the gene-environment-neurodevelopment interactions that influence mental health. This study is a valuable contribution, addressing gaps in psychiatric and neuroimaging epigenetics research (78), especially in pediatric or adolescent cohorts (62). Evidence increasingly shows that epigenetic mechanisms are crucial in shaping neurodevelopment, which influences behavior across the life span. Three key themes have emerged from animal and human studies: first, early environments interact with genetics to influence peripheral and central nervous system epigenetic markers; second, variations in these profiles shape typical and atypical neurodevelopment and mental health; and third, peripheral epigenetic markers may reflect brain changes in a spatially and temporally dependent way. Thus, further exploration of peripheral brain–epigenome relationships could help identify biological dysregulation and inform targeted, personalized treatments for maladaptive behavior.
Acknowledgments and Disclosures
This work was supported by the ECHO Program, Office of the Director, National Institutes of Health (Grant Nos. U2COD023375 [Coordinating Center], U24OD023382 [Data Analysis Center], and U24OD023319) with cofunding from the Office of Behavioral and Social Sciences Research (PRO Core) (Grant No. UH3OD023313 [to DK-M]), ECHO Opportunities and Infrastructure Fund (Grant No. 5U2COD023375-06 [to CRL]), and National Institute of Child Health and Human Development (Grant No. R00HD099307 [to CRL]).
We thank our ECHO Colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO Program collaborators: Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: P.B. Smith, L.K. Newby Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: L.P. Jacobson; Research Triangle Institute, Durham, North Carolina: D.J. Catellier; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: R. Gershon, D. Cella.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2024.100421.
Supplementary Material
Tables S1 and S2
References
- 1.Leuner B., Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmaal L., Veltman D.J., Van Erp T.G.M., Sämann P.G., Frodl T., Jahanshad N., et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barch D.M., Tillman R., Kelly D., Whalen D., Gilbert K., Luby J.L. Hippocampal volume and depression among young children. Psychiatry Res Neuroimaging. 2019;288:21–28. doi: 10.1016/j.pscychresns.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logue M.W., van Rooij S.J.H., Dennis E.L., Davis S.L., Hayes J.P., Stevens J.S., et al. Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83:244–253. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L., Pan N., Zhang L., Lui S., Huang X., Xu X., et al. Hippocampal subfield alterations in pediatric patients with post-traumatic stress disorder. Soc Cogn Affect Neurosci. 2021;16:334–344. doi: 10.1093/scan/nsaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berumen L.C., Rodríguez A., Miledi R., García-Alcocer G. Serotonin receptors in hippocampus. ScientificWorldJournal. 2012;2012 doi: 10.1100/2012/823493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criado-Marrero M., Smith T.M., Gould L.A., Kim S., Penny H.J., Sun Z., et al. FKBP5 and early life stress affect the hippocampus by an age-dependent mechanism. Brain Behav Immun Health. 2020;9 doi: 10.1016/j.bbih.2020.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjorth O.R., Frick A., Gingnell M., Hoppe J.M., Faria V., Hultberg S., et al. Expression and co-expression of serotonin and dopamine transporters in social anxiety disorder: A multitracer positron emission tomography study. Mol Psychiatry. 2021;26:3970–3979. doi: 10.1038/s41380-019-0618-7. [DOI] [PubMed] [Google Scholar]
- 9.Dahmen B., Puetz V.B., Scharke W., von Polier G.G., Herpertz-Dahlmann B., Konrad K. Effects of early-life adversity on hippocampal structures and associated HPA axis functions. Dev Neurosci. 2018;40:13–22. doi: 10.1159/000484238. [DOI] [PubMed] [Google Scholar]
- 10.Katrinli S., Maihofer A.X., Wani A.H., Pfeiffer J.R., Ketema E., Ratanatharathorn A., et al. Epigenome-wide meta-analysis of PTSD symptom severity in three military cohorts implicates DNA methylation changes in genes involved in immune system and oxidative stress. Mol Psychiatry. 2022;27:1720–1728. doi: 10.1038/s41380-021-01398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boström A.E., Ciuculete D.M., Attwood M., Krattinger R., Nikontovic L., Titova O.E., et al. A MIR4646 associated methylation locus is hypomethylated in adolescent depression. J Affect Disord. 2017;220:117–128. doi: 10.1016/j.jad.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Cicchetti D., Handley E.D. Methylation of the glucocorticoid receptor gene, nuclear receptor subfamily 3, group C, member 1 (NR3C1), in maltreated and nonmaltreated children: Associations with behavioral undercontrol, emotional lability/negativity, and externalizing and internalizing symptoms. Dev Psychopathol. 2017;29:1795–1806. doi: 10.1017/S0954579417001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver I.C.G., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 14.Lewis C.R., Breitenstein R.S., Henderson A., Sowards H.A., Piras I.S., Huentelman M.J., et al. Harsh parenting predicts novel HPA receptor gene methylation and NR3C1 methylation predicts cortisol daily slope in middle childhood. Cell Mol Neurobiol. 2021;41:783–793. doi: 10.1007/s10571-020-00885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parade S.H., Huffhines L., Daniels T.E., Stroud L.R., Nugent N.R., Tyrka A.R. A systematic review of childhood maltreatment and DNA methylation: Candidate gene and epigenome-wide approaches. Transl Psychiatry. 2021;11:134. doi: 10.1038/s41398-021-01207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soga T., Teo C.H., Parhar I. Genetic and epigenetic consequence of early-life social stress on depression: Role of serotonin-associated genes. Front Genet. 2020;11 doi: 10.3389/fgene.2020.601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M., Fu X., Xie W., Guo W., Li B., Cui R., Yang W. Effect of early life stress on the epigenetic profiles in depression. Front Cell Dev Biol. 2020;8:867. doi: 10.3389/fcell.2020.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na K.S., Chang H.S., Won E., Han K.M., Choi S., Tae W.S., et al. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Sante J., Ismaylova E., Nemoda Z., Gouin J.-P., Yu W.-J., Caldwell W., et al. Peripheral DNA methylation of HPA axis-related genes in humans: Cross-tissue convergence, two-year stability and behavioural and neural correlates. Psychoneuroendocrinology. 2018;97:196–205. doi: 10.1016/j.psyneuen.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Tozzi L., Farrell C., Booij L., Doolin K., Nemoda Z., Szyf M., et al. Epigenetic Changes of FKBP5 as a Link Connecting Genetic and Environmental Risk Factors with Structural and Functional Brain Changes in Major Depression. Neuropsychopharmacology. 2018;43:1138–1145. doi: 10.1038/npp.2017.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booij L., Szyf M., Carballedo A., Frey E.M., Morris D., Dymov S., et al. DNA methylation of the serotonin transporter gene in peripheral cells and stress-related changes in hippocampal volume: A study in depressed patients and healthy controls. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dannlowski U., Kugel H., Redlich R., Halik A., Schneider I., Opel N., et al. Serotonin transporter gene methylation is associated with hippocampal gray matter volume. Hum Brain Mapp. 2014;35:5356–5367. doi: 10.1002/hbm.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia T., Chu C., Liu Y., van Dongen J., Papastergios E., Armstrong N.J., et al. Epigenome-wide meta-analysis of blood DNA methylation and its association with subcortical volumes: Findings from the ENIGMA Epigenetics Working Group. Mol Psychiatry. 2021;26:3884–3895. doi: 10.1038/s41380-019-0605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Womersley J.S., Roeh S., Martin L., Ahmed-Leitao F., Sauer S., Rex-Haffner M., et al. FKBP5 intron 7 methylation is associated with higher anxiety proneness and smaller right thalamus volume in adolescents. Brain Struct Funct. 2022;227:2809–2820. doi: 10.1007/s00429-022-02577-9. [DOI] [PubMed] [Google Scholar]
- 25.Chiarella J., Schumann L., Pomares F.B., Frodl T., Tozzi L., Nemoda Z., et al. DNA methylation differences in stress-related genes, functional connectivity and gray matter volume in depressed and healthy adolescents. J Affect Disord. 2020;271:160–168. doi: 10.1016/j.jad.2020.03.062. [DOI] [PubMed] [Google Scholar]
- 26.Smith A.K., Kilaru V., Klengel T., Mercer K.B., Bradley B., Conneely K.N., et al. DNA extracted from saliva for methylation studies of psychiatric traits: Evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:36–44. doi: 10.1002/ajmg.b.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe R., Gemma C., Beyan H., Hawa M.I., Bazeos A., Leslie R.D., et al. Buccals are likely to be a more informative surrogate tissue than blood for epigenome-wide association studies. Epigenetics. 2013;8:445–454. doi: 10.4161/epi.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun P.R., Han S., Hing B., Nagahama Y., Gaul L.N., Heinzman J.T., et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry. 2019;9:47. doi: 10.1038/s41398-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Januar V., Ancelin M.L., Ritchie K., Saffery R., Ryan J. BDNF promoter methylation and genetic variation in late-life depression. Transl Psychiatry. 2015;5 doi: 10.1038/tp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chagnon Y.C., Potvin O., Hudon C., Préville M. DNA methylation and single nucleotide variants in the brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) genes are associated with anxiety/depression in older women. Front Genet. 2015;6:230. doi: 10.3389/fgene.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L., Pan H., Tuan T.A., Teh A.L., MacIsaac J.L., Mah S.M., et al. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism influences the association of the methylome with maternal anxiety and neonatal brain volumes. Dev Psychopathol. 2015;27:137–150. doi: 10.1017/S0954579414001357. [DOI] [PubMed] [Google Scholar]
- 32.Ursini G., Cavalleri T., Fazio L., Angrisano T., Iacovelli L., Porcelli A., et al. BDNF rs6265 methylation and genotype interact on risk for schizophrenia. Epigenetics. 2016;11:11–23. doi: 10.1080/15592294.2015.1117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M., et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijayendran M., Beach S.R.H., Plume J.M., Brody G.H., Philibert R.A. Effects of genotype and child abuse on DNA methylation and gene expression at the serotonin transporter. Front Psychiatry. 2012;3:55. doi: 10.3389/fpsyt.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong M.-L., Tuan T.A., Poh J., Teh A.L., Chen L., Pan H., et al. Neonatal amygdalae and hippocampi are influenced by genotype and prenatal environment, and reflected in the neonatal DNA methylome. Genes Brain Behav. 2019;18 doi: 10.1111/gbb.12576. [DOI] [PubMed] [Google Scholar]
- 36.Almli L.M., Stevens J.S., Smith A.K., Kilaru V., Meng Q., Flory J., et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:327–336. doi: 10.1002/ajmg.b.32315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhrif A., Romanos M., Peters K., Furtmann A.-K., Caspers J., Lesch K.-P., et al. Serotonergic modulation of normal and abnormal brain dynamics: The genetic influence of the TPH2 G-703T genotype and DNA methylation on wavelet variance in children and adolescents with and without ADHD. PLoS One. 2023;18 doi: 10.1371/journal.pone.0282813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han K.-M., Won E., Sim Y., Kang J., Han C., Kim Y.-K., et al. Influence of FKBP5 polymorphism and DNA methylation on structural changes of the brain in major depressive disorder. Sci Rep. 2017;7 doi: 10.1038/srep42621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raina A., Zhao X., Grove M.L., Bressler J., Gottesman R.F., Guan W., et al. Cerebral white matter hyperintensities on MRI and acceleration of epigenetic aging: The atherosclerosis risk in communities study. Clin Epigenetics. 2017;9:21. doi: 10.1186/s13148-016-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillman M.W., Blaisdell C.J. Environmental influences on Child Health Outcomes, a Research Program of the National Institutes of Health. Curr Opin Pediatr. 2018;30:260–262. doi: 10.1097/MOP.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollingshead A. Yale University Press Department of Psychology; New Haven, CT: 1975. Four Factor Index of Social Status. [Google Scholar]
- 42.Chang C.C., Chow C.C., Tellier L.C., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng S.C., Breeze C.E., Beck S., Dong D., Zhu T., Ma L., et al. EpiDISH web server: Epigenetic Dissection of Intra-Sample-Heterogeneity with online GUI. Bioinformatics. 2019;36:1950–1951. doi: 10.1093/bioinformatics/btz833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deoni S.C.L., Bruchhage M.M.K., Beauchemin J., Volpe A., D'Sa V., Huentelman M., Williams S.C.R. Accessible pediatric neuroimaging using a low field strength MRI scanner. Neuroimage. 2021;238 doi: 10.1016/j.neuroimage.2021.118273. [DOI] [PubMed] [Google Scholar]
- 46.De Macedo Rodrigues K., Ben-Avi E., Sliva D.D., Choe M.S., Drottar M., Wang R., et al. A FreeSurfer-compliant consistent manual segmentation of infant brains spanning the 0-2 year age range. Front Hum Neurosci. 2015;9:21. doi: 10.3389/fnhum.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du P., Zhang X., Huang C.C., Jafari N., Kibbe W.A., Hou L., Lin S.M. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jolliffe I.T. 2nd ed. Springer; New York, NY: 2002. Principal Component Analysis. [Google Scholar]
- 49.Wilhelm-Benartzi C.S., Koestler D.C., Karagas M.R., Flanagan J.M., Christensen B.C., Kelsey K.T., et al. Review of processing and analysis methods for DNA methylation array data. Br J Cancer. 2013;109:1394–1402. doi: 10.1038/bjc.2013.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis C.R., Henderson-Smith A., Breitenstein R.S., Sowards H.A., Piras I.S., Huentelman M.J., et al. Dopaminergic gene methylation is associated with cognitive performance in a childhood monozygotic twin study. Epigenetics. 2019;14:310–323. doi: 10.1080/15592294.2019.1583032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis C.R., Sowards H.A., Huentelman M.J., Doane L.D., Lemery-Chalfant K. Epigenetic differences in inflammation genes of monozygotic twins are related to parent-child emotional availability and health. Brain Behav Immun Health. 2020;5 doi: 10.1016/j.bbih.2020.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shieh G. Detecting interaction effects in moderated multiple regression with continuous variables power and sample size considerations. Organ Res Methods. 2009;12:510–528. doi: 10.3758/BRM.42.3.824. [DOI] [PubMed] [Google Scholar]
- 53.Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Djordjevic V., Despotovic M., Stankovic I., Jevtovic Stoimenov T. Association of ER22/23EK glucocorticoid receptor gene polymorphism with glucocorticoids dosage in COPD. Eur Respir J. 2018;52 [Google Scholar]
- 55.Li W., Yang Y., Lin J., Wang S., Zhao J., Yang G., et al. Association of serotonin transporter gene (SLC6A4) polymorphisms with schizophrenia susceptibility and symptoms in a Chinese-Han population. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:290–295. doi: 10.1016/j.pnpbp.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Su S., Zhao J., Bremner J.D., Miller A.H., Tang W., Bouzyk M., et al. Serotonin transporter gene, depressive symptoms, and interleukin-6. Circ Cardiovasc Genet. 2009;2:614–620. doi: 10.1161/CIRCGENETICS.109.870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szczepankiewicz A., Leszczyńska-Rodziewicz A., Pawlak J., Narozna B., Rajewska-Rager A., Wilkosc M., et al. FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J Affect Disord. 2014;164:33–37. doi: 10.1016/j.jad.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Tong T., Zhao H. Practical guidelines for assessing power and false discovery rate for a fixed sample size in microarray experiments. Stat Med. 2008;27:1960–1972. doi: 10.1002/sim.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edillor C.R., Parks B.W., Mehrabian M., Lusis A.J., Pellegrini M. DNA methylation changes more slowly than physiological states in response to weight loss in genetically diverse mouse strains. Front Endocrinol. 2019;10:882. doi: 10.3389/fendo.2019.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shu J., Jelinek J., Chen H., Zhang Y., Qin T., Li M., et al. Genome-wide screening and functional validation of methylation barriers near promoters. Nucleic Acids Res. 2024;52:4857–4871. doi: 10.1093/nar/gkae302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linnér A., Almgren M. Epigenetic programming-The important first 1000 days. Acta Paediatr. 2020;109:443–452. doi: 10.1111/apa.15050. [DOI] [PubMed] [Google Scholar]
- 62.Walton E., Baltramonaityte V., Calhoun V., Heijmans B.T., Thompson P.M., Cecil C.A.M. A systematic review of neuroimaging epigenetic research: calling for an increased focus on development. Mol Psychiatry. 2023;28:2839–2847. doi: 10.1038/s41380-023-02067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alisch R.S., Barwick B.G., Chopra P., Myrick L.K., Satten G.A., Conneely K.N., Warren S.T. Age-associated DNA methylation in pediatric populations. Genome Res. 2012;22:623–632. doi: 10.1101/gr.125187.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson R.C., Nelson G.W., Troyer J.L., Lautenberger J.A., Kessing B.D., Winkler C.A., O’Brien S.J. Accounting for multiple comparisons in a genome-wide association study (GWAS) BMC Genomics. 2010;11:724. doi: 10.1186/1471-2164-11-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fulcher B.D., Arnatkeviciute A., Fornito A. Overcoming false-positive gene-category enrichment in the analysis of spatially resolved transcriptomic brain atlas data. Nat Commun. 2021;12:2669. doi: 10.1038/s41467-021-22862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Li X., Aryee M.J., Ekström T.J., Padyukov L., Klareskog L., et al. GeMes, clusters of DNA methylation under genetic control, can inform genetic and epigenetic analysis of disease. Am J Hum Genet. 2014;94:485–495. doi: 10.1016/j.ajhg.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Leung F.C.C. An evaluation of new criteria for CpG islands in the human genome as gene markers. Bioinformatics. 2004;20:1170–1177. doi: 10.1093/bioinformatics/bth059. [DOI] [PubMed] [Google Scholar]
- 68.Berretta E., Guida E., Forni D., Provenzi L. Glucocorticoid receptor gene (NR3C1) methylation during the first thousand days: Environmental exposures and developmental outcomes. Neurosci Biobehav Rev. 2021;125:493–502. doi: 10.1016/j.neubiorev.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Xu C.-J., Bonder M.J., Söderhäll C., Bustamante M., Baïz N., Gehring U., et al. The emerging landscape of dynamic DNA methylation in early childhood. BMC Genomics. 2017;18:25. doi: 10.1186/s12864-016-3452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Irizarry R.A., Ladd-Acosta C., Wen B., Wu Z., Montano C., Onyango P., et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulder R.H., Neumann A., Cecil C.A.M., Walton E., Houtepen L.C., Simpkin A.J., et al. Epigenome-wide change and variation in DNA methylation in childhood: Trajectories from birth to late adolescence. Hum Mol Genet. 2021;30:119–134. doi: 10.1093/hmg/ddaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sapolsky R.M., Meaney M.J. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 73.Gunnar M., Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 74.Sodhi M.S.K., Sanders-Bush E. Int Rev Neurobiol. Vol. 59. Elsevier; Amsterdam, Netherlands: 2004. Serotonin and brain development; pp. 111–174. [DOI] [PubMed] [Google Scholar]
- 75.Van Bodegom M., Homberg J.R., Henckens M.J.A.G. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 2017;11:87. doi: 10.3389/fncel.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spinelli S., Müller T., Friedel M., Sigrist H., Lesch K.-P., Henkelman M., et al. Effects of repeated adolescent stress and serotonin transporter gene partial knockout in mice on behaviors and brain structures relevant to major depression. Front Behav Neurosci. 2013;7:215. doi: 10.3389/fnbeh.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheth C., McGlade E., Yurgelun-Todd D. Chronic stress in adolescents and its neurobiological and psychopathological consequences: An RDoC perspective. Chronic Stress (Thousand Oaks) 2017;1 doi: 10.1177/2470547017715645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cecil C.A.M., Neumann A., Walton E. Epigenetics applied to child and adolescent mental health: Progress, challenges and opportunities. JCPP Adv. 2023;3 doi: 10.1002/jcv2.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bossong M.G., Niesink R.J.M. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 80.Kaminsky Z.A., Tang T., Wang S.-C., Ptak C., Oh G.H.T., Wong A.H.C., et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41:240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- 81.Chen L., Ge B., Casale F.P., Vasquez L., Kwan T., Garrido-Martín D., et al. Genetic drivers of epigenetic and transcriptional variation in human immune cells. Cell. 2016;167:1398–1414.e24. doi: 10.1016/j.cell.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Dongen J., Nivard M.G., Willemsen G., Hottenga J.-J., Helmer Q., Dolan C.V., et al. Genetic and environmental influences interact with age and sex in shaping the human methylome. Nat Commun. 2016;7 doi: 10.1038/ncomms11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung W.A., Shao X., Morin A., Siroux V., Kwan T., Ge B., et al. Functional variation in allelic methylomes underscores a strong genetic contribution and reveals novel epigenetic alterations in the human epigenome. Genome Biol. 2017;18:50. doi: 10.1186/s13059-017-1173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Volkov P., Olsson A.H., Gillberg L., Jørgensen S.W., Brøns C., Eriksson K.-F., et al. A genome-wide mQTL analysis in human adipose tissue identifies genetic variants associated with DNA methylation, gene expression and metabolic traits. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hannon E., Spiers H., Viana J., Pidsley R., Burrage J., Murphy T.M., et al. Methylation QTLs in the developing brain and their enrichment in schizophrenia risk loci. Nat Neurosci. 2016;19:48–54. doi: 10.1038/nn.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morrow J.D., Glass K., Cho M.H., Hersh C.P., Pinto-Plata V., Celli B., et al. Human lung DNA methylation quantitative trait loci colocalize with chronic obstructive pulmonary disease genome-wide association loci. Am J Respir Crit Care Med. 2018;197:1275–1284. doi: 10.1164/rccm.201707-1434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taylor D.L., Jackson A.U., Narisu N., Hemani G., Erdos M.R., Chines P.S., et al. Integrative analysis of gene expression, DNA methylation, physiological traits, and genetic variation in human skeletal muscle. Proc Natl Acad Sci U S A. 2019;116:10883–10888. doi: 10.1073/pnas.1814263116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huan T., Joehanes R., Song C., Peng F., Guo Y., Mendelson M., et al. Genome-wide identification of DNA methylation QTLs in whole blood highlights pathways for cardiovascular disease. Nat Commun. 2019;10:4267. doi: 10.1038/s41467-019-12228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andrews S.V., Ellis S.E., Bakulski K.M., Sheppard B., Croen L.A., Hertz-Picciotto I., et al. Cross-tissue integration of genetic and epigenetic data offers insight into autism spectrum disorder. Nat Commun. 2017;8:1011. doi: 10.1038/s41467-017-00868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng B., White C.C., Klein H.-U., Sieberts S.K., McCabe C., Patrick E., et al. An xQTL map integrates the genetic architecture of the human brain’s transcriptome and epigenome. Nat Neurosci. 2017;20:1418–1426. doi: 10.1038/nn.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeo S., Enoch M.A., Gorodetsky E., Akhtar L., Schuebel K., Roy A., Goldman D. The influence of FKBP5 genotype on expression of FKBP5 and other glucocorticoid-regulated genes, dependent on trauma exposure. Genes Brain Behav. 2017;16:223–232. doi: 10.1111/gbb.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hartling C., Fan Y., Weigand A., Trilla I., Gärtner M., Bajbouj M., et al. Interaction of HPA axis genetics and early life stress shapes emotion recognition in healthy adults. Psychoneuroendocrinology. 2019;99:28–37. doi: 10.1016/j.psyneuen.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 93.Di Iorio C.R., Carey C.E., Michalski L.J., Corral-Frias N.S., Conley E.D., Hariri A.R., Bogdan R. Hypothalamic-pituitary-adrenal axis genetic variation and early stress moderates amygdala function. Psychoneuroendocrinology. 2017;80:170–178. doi: 10.1016/j.psyneuen.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu C., Xu L., Li J., Zhou F., Yang X., Zheng X., et al. Serotonin and early life stress interact to shape brain architecture and anxious avoidant behavior – A TPH2 imaging genetics approach. Psychol Med. 2021;51:2476–2484. doi: 10.1017/S0033291720002809. [DOI] [PubMed] [Google Scholar]
- 95.Palma-Gudiel H., Fañanás L. An integrative review of methylation at the serotonin transporter gene and its dialogue with environmental risk factors, psychopathology and 5-HTTLPR. Neurosci Biobehav Rev. 2017;72:190–209. doi: 10.1016/j.neubiorev.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 96.Streitbürger D.-P., Möller H.E., Tittgemeyer M., Hund-Georgiadis M., Schroeter M.L., Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keifer O.P., Hurt R.C., Gutman D.A., Keilholz S.D., Gourley S.L., Ressler K.J. Voxel-based morphometry predicts shifts in dendritic spine density and morphology with auditory fear conditioning. Nat Commun. 2015;6:7582. doi: 10.1038/ncomms8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lerch J.P., Yiu A.P., Martinez-Canabal A., Pekar T., Bohbot V.D., Frankland P.W., et al. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 99.Biedermann S.V., Fuss J., Steinle J., Auer M.K., Dormann C., Falfán-Melgoza C., et al. The hippocampus and exercise: Histological correlates of MR-detected volume changes. Brain Struct Funct. 2016;221:1353–1363. doi: 10.1007/s00429-014-0976-5. [DOI] [PubMed] [Google Scholar]
- 100.Asan L., Falfán-Melgoza C., Beretta C.A., Sack M., Zheng L., Weber-Fahr W., et al. Cellular correlates of gray matter volume changes in magnetic resonance morphometry identified by two-photon microscopy. Sci Rep. 2021;11:4234. doi: 10.1038/s41598-021-83491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van der Auwera S., Klinger-König J., Wittfeld K., Terock J., Hannemann A., Bülow R., et al. The interplay between genetic variation and gene expression of the glucocorticoid receptor gene NR3C1 and blood cortisol levels on verbal memory and hippocampal volumes. Eur Arch Psychiatry Clin Neurosci. 2022;272:1505–1516. doi: 10.1007/s00406-022-01420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bruzzone S.E.P., Ozenne B., Fisher P.M., Ortega G., Jensen P.S., Dam V.H., et al. No association between peripheral serotonin-gene-related DNA methylation and brain serotonin neurotransmission in the healthy and depressed state. Clin Epigenetics. 2024;16:71. doi: 10.1186/s13148-024-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cossart R., Khazipov R. How development sculpts hippocampal circuits and function. Physiol Rev. 2022;102:343–378. doi: 10.1152/physrev.00044.2020. [DOI] [PubMed] [Google Scholar]
- 104.United States Census Bureau QuickFacts: Rhode Island. https://www.census.gov/quickfacts/RI Available at:
- 105.Zeid D., Kutlu M.G., Gould T.J. Differential effects of nicotine exposure on the hippocampus across lifespan. Curr Neuropharmacol. 2018;16:388–402. doi: 10.2174/1570159X15666170714092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cosin-Tomas M., Cilleros-Portet A., Aguilar-Lacasaña S., Fernandez-Jimenez N., Bustamante M. Prenatal maternal smoke, DNA methylation, and multi-omics of tissues and child health. Curr Environ Health Rep. 2022;9:502–512. doi: 10.1007/s40572-022-00361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feria-Velasco A., Del Angel A.R., Gonzalez-Burgos I. Vol. 136. Elsevier; Amsterdam, Netherlands: 2002. Chapter 11: Modification of dendritic development; pp. 135–143. (Prog Brain Res). [DOI] [PubMed] [Google Scholar]
- 108.Hajszan T., MacLusky N.J., Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 109.Gogtay N., Nugent T.F., Herman D.H., Ordonez A., Greenstein D., Hayashi K.M., et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2