Abstract
Introduction
Real-world data on bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) fixed-dose combination from resource-constrained settings like Latin America are limited.
Material and methods
We conducted an observational retrospective cohort study of treatment-naive (TN, n= 315) and treatment-experienced (TE, n= 2356) people living with HIV prescribed BIC/FTC/TAF in Argentina from 10/2019 to 12/2021, with 24 and 48-week follow-up data analyzed for virological suppression, persistence, safety, and metabolic parameters. Patient-reported outcomes were assessed via a cross-sectional online survey.
Results
Baseline characteristics: median age 45 years, 72.2% male, 99.6% Hispanic/Latino ethnicity. Treatment persistence at 48 weeks was 99.3% (TN) and 99.5% (TE). Virological suppression rates (<200/<50 copies/mL) at 24 weeks were 97.4/88% (TN) and 99/97% (TE). At 48 weeks were 100/92% (TN) and 99/97% (TE). In the TE group, triglycerides decreased with no other lipid changes. In TN, mild total/LDL/HDL cholesterol increases occurred. eGFR mildly decreased in both groups. The online survey (n=536) showed 91.5% reported no medication concerns. Median quality of life scores were 90 (TN) and 88 (TE). Most reported no self-care, activity, mobility, pain/discomfort, or anxiety/depression issues.
Conclusions
BIC/FTC/TAF demonstrated high persistence, safety, virological efficacy, and favorable metabolic profile over 48 weeks. The cross-sectional survey indicated high treatment satisfaction and good quality of life in this cohort from Argentina.
Keywords: Antiretroviral therapy, HIV, Argentina, Latin America, bictegravir
Abstract
Introducción
Los datos de vida real sobre bictegravir/emtricitabina/tenofovir alafenamida (BIC/FTC/TAF) en entornos con recursos limitados como América Latina son escasos.
Material y métodos
Estudio observacional retrospectivo de cohorte en personas con VIH sin tratamiento previo (ST, n=315) y experimentados en tratamiento (ET, n= 2356) a quienes se les prescribió BIC/FTC/TAF en Argentina desde 10/2019 hasta 12/2021, con datos de seguimiento de 24 y 48 semanas analizados para supresión virológica, persistencia, seguridad y parámetros meta-bólicos. Los resultados reportados por los pacientes se evaluaron mediante una encuesta transversal en línea.
Resultados
Características basales: mediana de edad 45 años, 72,2% hombres, 99,6% etnia hispana/latina. La persistencia del tratamiento a 48 semanas fue del 99,3% (ST) y 99,5% (ET). Las tasas de supresión virológica (<200/<50 copias/mL) a 24 semanas fueron 97,4/88% (ST) y 99/97% (ET). A 48 semanas fueron 100/92% (ST) y 99/97% (ET). En el grupo ET, los triglicéridos disminuyeron sin otros cambios lipídicos. En ST, se produjeron leves aumentos de colesterol total/LDL/HDL. La tasa de filtrado glomerular disminuyó levemente en ambos grupos. La encuesta en línea (n=536) mostró que el 91,5% no reportó preocupaciones sobre la medicación. Las puntuaciones medianas de calidad de vida fueron 90 (ST) y 88 (ET). La mayoría no reportó problemas de autocuidado, actividad, movilidad, dolor/malestar o ansiedad/depresión.
Conclusiones
BIC/FTC/TAF demostró alta persistencia, seguridad, eficacia virológica y un perfil metabólico favorable durante 48 semanas. Los resultados reportados por los pacientes indicaron alta satisfacción con el tratamiento y buena calidad de vida en esta cohorte de Argentina.
Palabras clave: terapia antirretroviral, VIH, Argentina, América Latina, bictegravir
INTRODUCTION
Bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) fixed-dose combination (FDC) is an integrase strand inhibitor (InSTI)-based once-daily, single-tablet regimen for first-line and switching antiretroviral therapy (ART) of people living with HIV (PLWH) in clinical guidelines [1]. Supporting evidence comes from randomized controlled trials (RCT’s) involving treatment-naïve (TN) and treatment-experienced (TE) PLWH. BIC/FTC/TAF quickly suppressed viral load, effectively maintained virologic suppression over time, was as well tolerated as standard comparator regimens, had a high genetic barrier to resistance, and displayed improved metabolic parameters compared to other regimens [2–4]. While clinical trials demonstrate efficacy under controlled conditions, real-world evidence provides additional insights into effectiveness and safety across broader populations in routine clinical settings [5]. Therefore, real-world data on BIC/FTC/TAF is crucial to complement findings from registration trials and inform evidence-based use in clinical practice.
While current data on BIC/FTC/TAF largely stems from cohort studies in high-income regions like Europe [6–11], there needs to be more evidence from Latin American people. Given potential differences in HIV epidemiology, exposure to ART, adherence challenges, and metabolic profiles across Latin American populations, generating real-world data from regional cohorts would provide valuable information supporting evidence-based use of BIC/FTC/TAF in this region.
Argentina has one of the largest HIV epidemics in Latin America, with over 140,000 PLWH. While ART is provided free of charge in public and private health systems, challenges persist including late diagnosis and treatment initiation, suboptimal retention in care, and viral suppression rates [12]. Therefore, further data on optimal ART regimens to improve the HIV care cascade in the Argentine setting are needed. In this context, this study aimed to characterize the baseline traits of PLWH in Argentina initiating or switching to BIC/FTC/TAF, assess its effectiveness and safety, and ascertain the opinion of the prescribed individuals in a real-world clinical setting.
METHODS
Study design and objectives. The BICTARG is a real-world, single-center observational study evaluating the effectiveness, safety, metabolic parameters, and patient-re-ported outcomes of the BIC/FTC/TAF regimen among PLWH in routine clinical practice. The objectives were:
To characterize the demographic and clinical profile of PLWH initiating or switching to BIC/FTC/TAF.
To evaluate effectiveness by assessing virologic suppression, defined as HIV-1 RNA <200 and <50 copies/mL at week 24 and 48 after starting BIC/FTC/TAF.
To characterize the tolerability and safety, including adverse events at week 24 and 48.
To evaluate the impact of this regimen on renal function and lipid profile at week 24 and 48.
To evaluate quality of life and treatment satisfaction using patient-reported outcomes (PRO) surveys among patients on BIC/FTC/TAF.
Study location, data sources and population. The study population comprised HIV-1 infected patients aged ≥18 years who initiated BIC/FTC/TAF through routine care prescription between October 2019 and December 2021 in Helios Salud, Argentina. Helios Salud is a private HIV care center located in Buenos Aires city with a network all over the country. It covers 13,000 health-insured PLWH in its ambulatory program providing medical care, routine laboratory assessments and ART.
Both TN and TE subjects were included. Participants met the following eligibility criteria: confirmed HIV-positive status by serologic testing and detectable viral load, aged 18 years and over, and prescribed BIC/FTC/TAF as part of routine clinical care within the Helios Salud network. Subjects participating in clinical trials involving BIC/FTC/TAF, and those initiating it outside the clinic´s network were excluded.
For the effectiveness, safety and metabolic part of BICTARG, participants were identified through the institution´s electronic medical record system InfHos®. Data on demographics, HIV history, comorbidities, ART exposure, laboratory results and clinical events were retrospectively extracted from medical charts by infectious diseases specialists. For laboratory results, we analyzed viral load, CD4 + T-cell count, fasting blood glucose, total cholesterol (TC), HDL cholesterol (HDL-c), LDL cholesterol (LDL-c), triglycerides, and estimated glomerular filtration rate (eGGR) at baseline, 24 and 48 weeks follow-up. Effectiveness was evaluated with HIV-RNA threshold of <200 and <50 copies/mL, considering PLWH on treatment (discontinuation/missing = excluded).
To evaluate PRO, we conducted a cross-sectional study from November 2022 to February 2023 among patients on BIC/FTC/TAF using electronic surveys sent by WhatsApp®. The following validated Spanish PRO instruments were implemented: 1) The HIV Symptom Distress Module (HIV-SDM) assessed symptom burden; 2) The EuroQol five-dimension questionnaire (EQ-5D-5L) evaluated health-related quality of life; and 3) The HIV/AIDS-targeted quality of life questionnaire (HAT-QoL short form) measured multidimensional impacts of HIV on quality of life. Participants completed the surveys directly without intervention from the study staff.
Study data were collected and managed using REDCap (Research Electronic Data Capture, Vanderbilt University, Nashville, TN, USA) electronic data capture tools hosted at Fundación Helios Salud [13]. PRO and cohort study databases were independent, data was anonymized, and information could not be matched.
Statistical analysis. Statistical analysis utilized descriptive and analytical approaches. Categorical variables were described using absolute and relative frequencies. Continuous variables were described using medians with interquartile ranges (IQRs) and compared by the Mann–Whitney U test for differences between groups. All tests were two-sided, and a p-value less than 0.05 was considered significant. Variables with missing values were excluded from the analysis. Graphical techniques visualized key results. Analysis used Stata version 14. (STATA/MP 14.0 for Windows).
Ethical statement. The study protocol was reviewed and approved by the Comité de Ética en Investigación Clínica (CEIC) in Buenos Aires, Argentina. For the retrospective component analyzing existing medical record data, a waiver of informed consent was granted as this involved no direct patient contact. For the PRO measures, electronic informed consent was required and obtained as the first question before participants completed the surveys.
RESULTS
Effectiveness, safety and metabolic impact. The study encompassed 2671 PLWH, with 315 being TN and 2356 TE. The median age at baseline was 37 years for TN individuals and 46 years for TE, with the majority being male and 99.6% of Hispanic/Latino ethnicity, as detailed in Table 1. In the TE group, the primary reasons for switching treatment were simplification and toxicity prevention, with a median (IQR) of prior antiretroviral regimens being 2 (1-3). Prior ARTs predominantly involved as anchor drug boosted protease inhibitors (PI’s) (38.2%, mostly ritonavir-boosted darunavir and atazanavir), non-nucleoside reverse transcriptase inhibitors (NNRTIs: 34.3%, mostly efavirenz, and nevirapine) and InSTIs (21.9%, mostly raltegravir).
Table 1.
Baseline characteristics of PLWH initiating or switching to BIC/FTC/TAF fixed-dose combination in the BICTARG cohort, Argentina.
| N (%) or median (IQR) | TN n= 315 |
TE n= 2,356 |
|---|---|---|
| Male sex | 253 (80.3) | 1,675 (71.1) |
| Age, years | 37 (30-45) | 46 (38-53) |
| HIV-1 RNA <50 copies/mL | 5 (2) | 1,799a (89) |
| CD4 count, cells/μL | 329 (171-505) | 621 (436-843)b |
| Comorbiditiesc | 35% | 63.8% |
| Dyslipidemia | 17 (10.2) | 696 (37.3) |
| Hypertension | 12 (7.2) | 320 (17.1) |
| Obesity | 15 (9) | 307 (16.5) |
| Participant reason for initiating or switching to B/F/TAFd | ||
| Simplification | NA | 1,267 (54.8) |
| Prevention of toxicity | NA | 556 (24.1) |
| ART toxicity | NA | 453 (19.6) |
| Virological failure | NA | 46 (2) |
NA: not applicable; IQR: interquartile range; PLWH: people living with HIV; TN: treatment-naïve; TE: treatment-experienced; ART: antiretroviral therapy; an= 2022 with available data; bn = 1998; csample size of 166 for TN and 2265 for TE with available data; dsample size of 1895 TE with available data.
After 24 weeks, 99.9% of TN and 99.6% of TE subjects continued on BIC/FTC/TAF therapy. Similarly, at the 48-week mark, 99.4% of TN and 99.5% of TE individuals remained on this regimen. Adherence levels, immunological status, and virological suppression rates at both time points are outlined in Table 2.
Table 2.
Rates of adherence (pharmacy withdrawals), virologic suppression and immunological status at 24 and 48 weeks in PLWH initiating or switching to BIC/FTC/TAF fixed-dose combination in the BICTARG cohort, Argentina.
| 24-week follow-up | TN | TE |
|---|---|---|
| N= 190 | N=1,610 | |
| Adherence | 99.9% | 99.6% |
| Viral load <200 c/mL | 97.4% | 99% |
| Viral load <50 c/mL | 88% | 97% |
| CD+ T-cell/uL (median, IQR) | 468 (288-702) | 628 (461-838) |
| 48-week follow-up | N= 131 | N= 1,464 |
| Adherence | 99.4% | 99.5% |
| Viral load <200 c/mL | 100% | 99% |
| Viral load <50 c/mL | 92% | 97% |
| CD+ T-cell/uL (median, IQR) | 550 (374-797) | 642 (470-857) |
PLWH: people living with HIV; TN: treatment-naïve; TE: treatment-experienced
Discontinuations at 24 weeks (absolute numbers) were 1 for TN and 8 for TE, with reasons including intolerance (n = 1), renal toxicity (n = 1), weight gain (n = 1), patient preference (n = 1), and other causes (n = 4). At the 48-week assessment, overall discontinuation rates were 10 (1 for TN and 9 for TE), primarily due to weight gain (n = 2), hepatic toxicity (n = 1), patient preference (n = 1), other causes (n = 3), and unknown reasons (n = 3). The overall prevalence of adverse events at the end of the study was low at 1%. No virological failures were detected throughout the study period.
Baseline metabolic parameters were available for 1542 TE PLWH. No statistically significant differences were observed at 24 and 48 weeks in the median values of blood glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c). A decrease in triglyceride levels was observed (136 mg/dL vs. 123 mg/dL, p <0.001), as illustrated in Figure 1. In contrast, among the TN (n = 87 with available data), slight increases were observed in the median TC, HDL-c, and LDL-c levels at 24 weeks (158 mg/dL vs. 175 mg/dL; 38 mg/dL vs. 42 mg/dL; 95 mg/dL vs. 106 mg/dL, respectively; p <0.05) and in TC and HDL-c at 48 weeks (158 mg/dL vs. 169.5 mg/dL; 38 mg/dL vs. 41 mg/dL, respectively, p <0.05), while fasting blood glucose and triglyceride levels remained unchanged (Figure 2). Both groups documented a slight decrease in the estimated glomerular filtration rate at 24 and 48 weeks (p<0.001).
Figure 1.

Evolution of total cholesterol, HDL cholesterol, LDL cholesterol, fasting blood glucose, triglycerides, and estimated glomerular filtration rate (GRF) at baseline vs. 24 and 48 weeks of follow-up in antiretroviral therapy-experienced people living with HIV who switched to BIC/FTC/TAF (n = 1,542).
Figure 2.
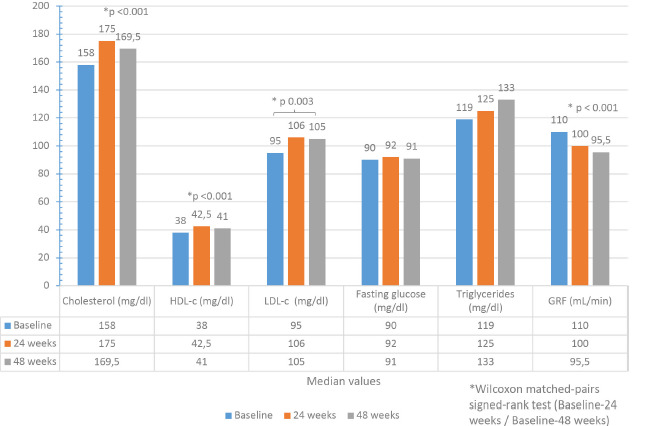
Evolution of total cholesterol, HDL cholesterol, LDL cholesterol, fasting blood glucose, triglycerides, and estimated glomerular filtration rate (GRF) at baseline vs. 24 and 48 weeks of follow-up in treatment-naïve individuals initiating BIC/FTC/TAF (n = 87).
Patient reported outcomes. The survey was administered to 1500 PLWH on therapy with BIC/FTC/TAF. The response rate was 35.7% (n=536). Eighty-one respondents (15.1%) received BIC/FTC/TAF as initial treatment, while 455 (84.9%) switched to BIC/FTC/TAF. The majority, 72%, were male, and 53.8% identified as non-heterosexual. The median age was 48 (40-56) years. HIV-1 RNA was suppressed <50 copies/mL in 91.9% of respondents, and 69.5% had a CD4+ T-cell count >350 cells/mm3. Sixty-five percent had been taking BIC/FTC/TAF for at least one year.
Regarding treatment satisfaction, 91.5% reported no concerns with their medication. The median quality of life (QoL) score was 90 (IQR: 77-100) for those initiating and 88 (IQR: 75-98) for those who switched to BIC/FTC/TAF.
The majority reported no difficulties with self-care, activities of daily living, and mobility as shown in Figure 3. Seventy-eight percent expressed overall life satisfaction. Absence of pain or discomfort was reported by 74%, while 56.4% reported no anxiety or depression. Concerning body alterations, 88% described no weight or muscular mass loss concerns, whereas 62% reported no issues with weight gain or fat accumulation. Overall medication worries were low (Figure 4).
Figure 3.

Responses to EuroQol Five-Dimension questionnaire in people living with HIV under BIC/FTC/TAF therapy in Argentina (n = 536).
Figure 4.
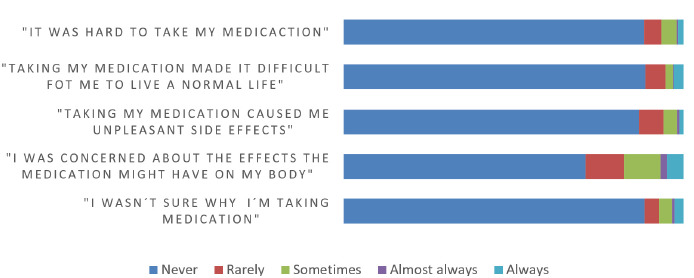
HIV/AIDS medication worries in people living with HIV under BIC/FTC/TAF therapy in Argentina (n = 536).
DISCUSSION
This study presents a substantial real-world cohort of PLWH from Argentina who receive BIC/FTC/TAF therapy, providing valuable insights into the tolerability, effectiveness, metabolic impact and patient´s perspective. The demographic characteristics of the PLWH here align with the epidemiology of the HIV epidemic in Argentina and Latin America more broadly, with a high proportion of men and young adults represented [14,15].
The majority of PLWH in this study had previous exposure to ART. Similar findings have been reported across different cohorts examining BIC/FTC/TAF as a switch option for TE persons. The frequency of TE in our cohort is comparable to rates between 80-90% noted in other studies [6,9,11,16]. The specific prior regimens taken by PLWH vary across different countries and regional contexts. In our cohort, regimens containing PIs and NNRTIs were most common, whereas others reported higher rates of InSTIs between 60-80%. This is consistent with the trends in ART prescription in Latin America [17].
Documented reasons for switching antiretroviral regimens include simplification, improved tolerance, prevention of adverse events or toxicity, and drug-drug interactions, among others. As prior regimens in BICTARG contained primarily PIs and NNRTIs, known concerns like high pill burden and interactions with co-medications likely motivated many of the switches. These motivations align with those reported across European and other regional cohorts [9,11].
Notably, a substantial proportion of PLWH in our cohort had comorbid conditions at the time of BIC/FTC/TAF initiation, including about 60% of the TE subgroup. The most frequent comorbidities were dyslipidemia, hypertension, and obesity. Similar patterns have been reported in other cohorts, with neuropsychiatric conditions, dyslipidemia, and hypertension being most common [6,9,11]. TE PLWH tend to have a higher frequency of comorbidities compared to treatment-naive PLWH. Given BIC/FTC/TAF favorable safety profile and limited drug interactions, it remains an appropriate choice for PLWH with one or more comorbid conditions requiring polypharmacy [18].
This study found a high rate of viral suppression at both 6 and 12 months in TN individuals. This rate was even higher among those with previous ART exposure. These real-world effectiveness results align with observations from other cohort studies, which have also shown higher rates of viral suppression in TE patients, with approximately 92-94% suppressed at 6 months and around 96% at 12 months. For TN individuals, the viral suppression rates tend to range from 80-85% at 6 months, and then reach up to >90% by the 12-month time point [4,6,9,11,19].
Our cohort demonstrates a remarkably low rate of treatment discontinuation at 6 and 12 months, highlighting the tolerability of BIC/FTC/TAF. Clinical trials have consistently reported a low prevalence of discontinuation due to adverse events, typically 2% or less. The most commonly observed adverse events were related to gastrointestinal and neuropsychiatric disorders [2,4]. On the other hand, real-world data show a discontinuation prevalence between less than 2% and 3.5%, predominantly due to gastrointestinal and neuropsychiatric side effects, weight gain, and renal complications. These findings underscore the overall favorable tolerability profile of BIC/FTC/TAF-based regimens, albeit with varying discontinuation rates across different cohorts, likely influenced by factors such as study design, population characteristics, and regional variations in clinical practice [7,10,11,16].
There are ongoing questions about the metabolic effects, particularly on serum lipids, of the BIC/FTC/TAF. This study found differential lipid changes between the TN and TE patient groups. In the TN patients, we observed increases in cholesterol levels at both 6 and 12 months of treatment. This aligns with the clinical trial findings of lipid changes seen in those initiating BIC/FTC/TAF. Real-world evidence also links the initiation of this regimen to rises in total and LDL cholesterol. The lipid changes observed, while statistically significant, may not be of sufficient magnitude to warrant clinical concern or intervention. In contrast, the TE patients showed reductions in triglyceride levels. This is consistent with clinical trials and observational studies, which have resulted in declines in total cholesterol, triglycerides, or both, especially when switching from protease inhibitor-based therapy [7,16,20,21].
As treatment guidelines emphasize achieving the “fourth 90” goal of good health-related quality of life, considering PROs along with standard efficacy endpoints is crucial for selecting optimal HIV treatment strategies [22]. Our study shows that after initiating or switching to BIC/FTC/TAF most real-world PLWH reported high treatment satisfaction and an absence of physical or psychological symptoms or alterations in activities of daily living. Despite lack of a comparator group, the results align with a secondary analysis by Wohl et al. of two phase 3 trials that also demonstrated a lower prevalence symptoms that negatively affect quality of life with BIC/FTC/TAF compared to ABC/DTG/3TC over 48 weeks in both treatment-naive and viro-logically suppressed PLWH [23]. Our study also supports findings in asiatic population: Chen et al. (2023) examined PROs of vi-rally suppressed PLWH in Taiwan who switched to BIC/FTC/TAF. The investigators found that after 48 weeks of switching to BIC/FTC/TAF, the overall bothersome symptom burden decreased significantly in both prevalence and severity, suggesting potential benefits of switching to BIC/FTC/TAF in virally-suppressed PLWH. The improvement was more pronounced in participants who switched from elvitegravir-based regimens compared to dolutegravir-based regimens [24].
This study has several limitations that must be acknowledged. Firstly, the research was conducted in a private clinic setting, which may limit the generalizability of the findings to PLWH from other healthcare settings or centers. However, it is important to note that in Argentina, the BIC/FTC/TAF regimen is not available in the public health system. Secondly, the retrospective design of the study raises concerns about the completeness and variability of the available data: analyses were done considering participants with available data and on treatment. Thirdly, the lack of a comparator group further limits the interpretability of the results in the context of other therapeutic options. Additionally, the PROs were not linked to the clinical and metabolic data and also lacked a comparator group or a baseline survey in the experienced PLWH, which could have provided valuable insights. Despite these limitations, this study has several strengths. It is one of the largest real-world cohorts of its kind, comprising a homogeneous population of Hispanic-Latino ethnicity. This unique population offers important observations into the baseline characteristics, metabolic profiles, and participant’s perspective of this ethnic group, which may differ from other cohorts with diverse backgrounds and heritage. The availability of metabolic data and the analysis of PROs, an aspect often overlooked in other observational studies, further enhances the study’s significance.
In conclusion, the findings from this real-world cohort study conducted in Argentina demonstrate that the BIC/FTC/TAF regimen exhibited favorable safety and efficacy profiles with high persistence and overall treatment satisfaction, both in treatment-naive and experienced PLWH.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the statistical analysis contributions of Sofia Diana Menendez and Gustavo Castro, as well as the statistical advice provided by Macarena Roel. The authors thank the administrative support teams at Helios Salud for their assistance.
Acknowledgments
Parts of this study have been presented at the 12th IAS Conference on HIV Science. Brisbane (IAS), Australia, 23-26 July 2023 (EPB0210) and 19th European AIDS Conference, Warsaw, Poland. 18-21 October 2023 (abstracts 428/461).
FUNDING
This study was sponsored by Gilead.
CONFLICT OF INTEREST
DC has received an educational grant and speaker fees from Gilead Sciences. IC has received an educational grant, speaker fees, and an investigational grant from Gilead Sciences. The other authors declare no conflicts of interest
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Accessed 4th May 2024. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed May 4th 2024
- 2.Sax PE, Arribas JR, Orkin C, Lazzarin A, Pozniak A, De Jesus E, et al. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. E Clinical Medicine. 2023;59:101991. Available from: 10.1016/j.eclinm.2023.101991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daar ES, De Jesus E, Ruane P, Crofoot G, Oguchi G, Creticos C, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e347–56. Available from: 10.1016/S2352-3018(18)30091-2 [DOI] [PubMed] [Google Scholar]
- 4.Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, López-Cortés L, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, jactive-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e357–65. Available from: 10.1016/S2352-3018(18)30092-4 [DOI] [PubMed] [Google Scholar]
- 5.Dang A. Real-World Evidence: A Primer. Pharmaceut Med. 2023;37:25–36. Available from: 10.1007/s40290-022-00456-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torralba M, Rodríguez G, González Gasca FJ, Cuadra F, Barberá J, Geijo P, et al. Bictegravir/Emtricitabine/Tenofovir Alafenamide in a Multicentre Cohort: Real-Life Experience From Spain. Ann Pharmacother. 2024;58:140–7. Available from: 10.1177/10600280231168852 [DOI] [PubMed] [Google Scholar]
- 7.Squillace N, Ricci E, Maggi P, Taramasso L, Menzaghi B, De Socio GV, et al. Real-life safety of Emtricitabine/Tenofovir Alafenamide/Bictegravir. PLoS One. 2023;18:e0289132. Available from: 10.1371/journal.pone.0289132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocabert A, Borjabad B, Berrocal L, Blanch J, Inciarte A, Chivite I, et al. Tolerability of bictegravir/tenofovir alafenamide/emtricitabine versus dolutegravir/lamivudine as maintenance therapy in a real-life setting. J Antimicrob Chemother. 2023;78:2961–7. Available from: https://academic.oup.com/jac/article-pdf/78/12/2961/53850551/dkad338.pdf [DOI] [PubMed] [Google Scholar]
- 9.Ambrosioni J, Rojas Liévano J, Berrocal L, Inciarte A, de la Mora L, González-Cordón A, et al. Real-life experience with bictegravir/emtricitabine/tenofovir alafenamide in a large reference clinical centre. J Antimicrob Chemother. 2022;77:1133–9. Available from: https://academic.oup.com/jac/article-pdf/77/4/1133/43192415/dkab481.pdf [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine R, Florence E, Yombi JC, Henrard S, Darcis G, Van Praet J, et al. Efficacy, durability, and tolerability of bictegravir/emtricitabine/tenofovir alafenamide for the treatment of HIV in a real-world setting in Belgium. HIV Med. 2023;24:914–24. Available from: 10.1111/hiv.13493 [DOI] [PubMed] [Google Scholar]
- 11.Esser S, Brunetta J, Inciarte A, Levy I, D’Arminio Monforte A, Lambert JS, et al. Twelve-month effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide in people with HIV: Real-world insights from BICSTaR cohorts. HIV Med. 2024;25:440–53. Available from: 10.1111/hiv.13593 [DOI] [PubMed] [Google Scholar]
- 12.Dirección de respuesta al VIH, ITS, hepatitis virales y tuberculosis . Ministerio de Salud de la Nación. Boletín n° 40. Respuesta al VIH y las ITS en la Argentina. Año XXVI. Diciembre 2023. Accessed May 4th 2024. Available from: https://bancos.salud.gob.ar/recurso/boletin-ndeg-40-respuesta-al-vih-y-las-its-en-la-argentina [in spanish]
- 13.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. Available from: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caro-Vega Y, Rebeiro PF, Shepherd BE, Belaunzarán-Zamudio PF, Crabtree-Ramirez B, Cesar C, et al. Clinical effects of durability of immunosuppression in virologically suppressed ART-initiating persons with HIV in Latin America. A retrospective cohort study. The Lancet Regional Health–Americas. 2022;8:100175 Available from: http://www.thelancet.com/article/S2667193X2100171X/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff MJ, Giganti MJ, Cortes CP, Cahn P, Grinsztejn B, Pape JW, et al. A decade of HAART in Latin America: Long term outcomes among the first wave of HIV patients to receive combination therapy. PLoS One. 2017;12:e0179769. Available from: https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0179769&-type=printable [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gidari A, Benedetti S, Tordi S, Zoffoli A, Altobelli D, Schiaroli E, et al. Bictegravir/Tenofovir Alafenamide/Emtricitabine: A Real-Life Experience in People Living with HIV (PLWH). Infect Dis Rep. 2023;15:766–77. Available from: 10.3390/idr1506006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zitko P, Hojman M, Sabato S, Parenti P, Cuini R, Calanni L, et al. Antiretroviral therapy use in selected countries in Latin America during 2013-2017: results from the Latin American Workshop in HIV Study Group. Int J Infect Dis. 2021;113:288–96. Available from: 10.1016/j.ijid.2021.09.047 [DOI] [PubMed] [Google Scholar]
- 18.Deeks ED. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs. 2018;78:1817–28. Available from: 10.1007/s40265-018-1010-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troya J, Pousada G, Micán R, Galera C, Sanz J, de los Santos I, et al. Real-life data of immune recovery using bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed people living with HIV. Results at 48–96 weeks of RETROBIC Study. J Antimicrob Chemother. 2024;79:595–607. Available from: https://academic.oup.com/jac/article-pdf/79/3/595/56796849/dkae011.pdf [DOI] [PubMed] [Google Scholar]
- 20.Orkin C, DeJesus E, Sax PE, Arribas JR, Gupta SK, Martorell C, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomized, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV. 2020;7:e389–400. Available from: 10.1016/S2352-3018(20)30099-0 [DOI] [PubMed] [Google Scholar]
- 21.Stellbrink HJ, Arribas JR, Stephens JL, Albrecht H, Sax PE, Maggiolo F, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6:e364–72. Available from: 10.1016/S2352-3018(19)30080-3 [DOI] [PubMed] [Google Scholar]
- 22.Lazarus JV, Safreed-Harmon K, Barton SE, Costagliola D, Dedes N, del Amo Valero J, et al. Beyond viral suppression of HIV–the new quality of life frontier. BMC Med . 2016;14:1–5. Available from: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0640-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohl D, Clarke A, Maggiolo F, Garner W, Laouri M, Martin H, et al. Patient-Reported Symptoms Over 48 Weeks Among Participants in Randomized, Double-Blind, Phase III Non-inferiority Trials of Adults with HIV on Co-formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Co-formulated Abacavir, Dolutegravir, and Lamivudine. The Patient-Patient-Centered Outcomes Research. 2018;11:561–73. Available from: https://link.springer.com/article/10.1007/s40271-018-0322-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LY, Sun HY, Chuang YC, Huang YS, Liu WD, Lin KY, et al. Patient-reported outcomes among virally suppressed people living with HIV after switching to Co-formulated bictegravir, emtricitabine and tenofovir alafenamide. J Microbiol Immunol Infect. 2023;3:575–85. Available from: 10.1016/j.jmii.2023.01.015 [DOI] [PubMed] [Google Scholar]


