Abstract
Activation of presynatic histamine H3 receptors (H3R) down-regulates norepinephrine exocytosis from cardiac sympathetic nerve terminals, in both normal and ischemic conditions. Analogous to the effects of α2-adrenoceptors, which also act prejunctionally to inhibit norepinephrine release, H3R-mediated antiexocytotic effects could result from a decreased Ca2+ influx into nerve endings. We tested this hypothesis in sympathetic nerve terminals isolated from guinea pig heart (cardiac synaptosomes) and in a model human neuronal cell line (SH-SY5Y), which we stably transfected with human H3R cDNA (SH-SY5Y-H3). We found that reducing Ca2+ influx in response to membrane depolarization by inhibiting N-type Ca2+ channels with ω-conotoxin (ω-CTX) greatly attenuated the exocytosis of [3H]norepinephrine from both SH-SY5Y and SH-SY5Y-H3 cells, as well as the exocytosis of endogenous norepinephrine from cardiac synaptosomes. Similar to ω-CTX, activation of H3R with the selective H3R-agonist imetit also reduced both the rise in intracellular Ca2+ concentration (Cai) and norepinephrine exocytosis in response to membrane depolarization. The selective H3R antagonist thioperamide prevented this effect of imetit. In the parent SH-SY5Y cells lacking H3R, imetit affected neither the rise in Cai nor [3H]norepinephrine exocytosis, demonstrating that the presence of H3R is a prerequisite for a decrease in Cai in response to imetit and that H3R activation modulates norepinephrine exocytosis by limiting the magnitude of the increase in Cai. Inasmuch as excessive norepinephrine exocytosis is a leading cause of cardiac dysfunction and arrhythmias during acute myocardial ischemia, attenuation of norepinephrine release by H3R agonists may offer a novel therapeutic approach to this condition.
We have found that sympathetic nerve endings in the guinea pig (1) and human heart (2) express the H3 histamine receptor subtype (H3R). Activation of these H3R down-regulates norepinephrine exocytosis in both normal and ischemic conditions (3, 4).
The H3R is a G-protein-coupled receptor linked to the inhibition of adenylyl cyclase (5). The H3R is modestly related in sequence to most biogenic amine receptors, including α2-adrenoceptors (6). Like the H3R, cardiac α2-adrenoceptors act prejunctionally to inhibit norepinephrine exocytosis (3, 7). Moreover, inhibition of Ca2+ influx into sympathetic nerve endings with the selective N-type Ca2+ channel blocker ω-conotoxin (ω-CTX) (8) potentiates both H3R- and α2-adrenoreceptor-mediated attenuation of cardiac adrenergic responses (1). Therefore, it is conceivable that, as for α2-adrenoceptors (9), H3R activation may also inhibit norepinephrine release by diminishing Ca2+ influx into cardiac sympathetic terminals.
The human neuroblastoma cell line SH-SY5Y has been used as a model to study mechanisms of neurotransmitter release (10). To determine whether H3R activation modulates norepinephrine release by impeding Ca2+ influx, we stably transfected SH-SY5Y cells with the cDNA for the H3R (SH-SY5Y-H3). We report that upon K+-induced depolarization, activation of H3R is associated with a marked decrease in intracellular Ca2+ (Cai) and, thus, with an attenuation of norepinephrine exocytosis.
Materials and Methods
Preparation of Cardiac Synaptosomes.
Male Hartley guinea pigs (Charles River Breeding Laboratories) weighing 250–300 g were killed by cervical dislocation under light anesthesia with CO2 vapor. The rib cage was rapidly opened and the heart was dissected away. A cannula was inserted into the aorta, and the heart was perfused for 5 min at constant pressure (40 cm H2O) in a Langendorff apparatus (11) with Ringer's solution (154 mM NaCl/5.61 mM KCl/2.16 mM CaCl2/5.95 mM NaHCO3/5.55 mM glucose) equilibrated with 100% O2 at 37°C. This procedure ensured that no blood traces remained in the coronary circulation. Hearts were then freed from fat and connective tissue and minced in ice-cold 0.32 M sucrose containing 1 mM EGTA (pH 7.4). Synaptosomes were isolated as described (2), with the following modifications. Minced tissue was digested with 40–75 mg collagenase (type II; Worthington)/10 ml Hepes-buffered saline solution [50 mM Hepes, pH 7.4/144 mM NaCl/5 mM KCl/1.2 mM CaCl2/1.2 mM MgCl2/10 mM glucose/1 mM pargyline (pargyline hydrochloride, Sigma–Aldrich), to prevent enzymatic destruction of synaptosomal norepinephrine]/g wet heart weight for 1 h at 37°C. After low-speed centrifugation (10 min at 120 × g at 4°C), the resulting pellet was suspended in 10 vol of 0.32 M sucrose and homogenized with a Teflon/glass homogenizer. The homogenate was spun at 650 × g for 10 min at 4°C, and the pellet was rehomogenized and respun. The pellet, which contained cellular debris, was discarded, and the supernatants from the last two spins were combined and equally subdivided into 10–12 tubes. Each tube was centrifuged for 20 min at 20,000 × g at 4°C. Each pellet, which contained cardiac synaptosomes, was resuspended in Hepes-buffered saline solution to a final volume of 500 μl and incubated with KCl (3–30 mM) in the presence or absence of pharmacological agents in a water bath at 37°C. Each suspension functioned as an independent sample and was used only once. In every experiment, one sample was untreated (control, basal release), and the others were treated with high K+ alone, high K+ and drugs, or with drugs alone. When high K+ was used, osmolarity was kept constant by adjusting the NaCl concentration. Treated samples were incubated with a given agent for 20 min and then with high K+ for 5 min. When antagonists were used, samples were incubated with the antagonist for 10 min before incubation with the agonist. Controls were incubated for an equivalent length of time without drugs. At the end of the incubation period each sample was centrifuged again for 20 min (20,000 × g at 4°C). The supernatant was assayed for norepinephrine content by HPLC with electrochemical detection (11). The pellet was assayed for protein content, by a modified Lowry procedure (12).
SH-SY5Y Parent Cells.
Materials.
The human neuroblastoma SH-SY5Y cell line, Eagle's MEM, Ham's F-12 nutrient mixture, and FBS were obtained from the American Type Culture Collection. Trypsin, PBS, geneticin, glutamine, penicillin, and streptomycin were purchased from GIBCO. Desipramine hydrochloride, EGTA, imetit dihydrobromide, thioperamide maleate, Triton X-100, and ω-conotoxin GVIA were obtained from Sigma–Aldrich. [3H]Norepinephrine (28.0 Ci/mmol) was purchased from Amersham Pharmacia. Ionomycin was obtained from Calbiochem. Fura-2 AM was obtained from Molecular Probes.
Tissue culture.
SH-SY5Y cells were cultured with the use of a 1:1 ratio of Eagles' MEM and Ham's F-12 nutrient mixture, supplemented with 10% FBS, 2 mM glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin (complete medium), maintained at 37°C in 5% CO2. Cells were plated 4 days before the [3H]norepinephrine release experiments onto 6-well plates; for Cai measurements, cells were grown on 22-mm2 standard glass coverslips (No. 1).
[3H]norepinephrine release assay.
The [3H]norepinephrine release method was adapted from that described by Murphy et al. (13). Culture medium was removed and cells were washed once with Hepes buffer (2.6 mM CaCl2/25 mM Hepes/1.2 mM MgSO4/5.6 mM glucose/1.2 mM KH2PO4/125 mM NaCl/4.8 mM KCl/0.2 mM pargyline, pH 7.4) and then incubated in 1 ml per well of Hepes buffer containing 50 nM [3H]norepinephrine for 60 min at 37°C. This procedure was followed by three washes per well with 1 ml Hepes buffer (containing 1 μM desipramine). One milliliter of release buffer (Hepes buffer with 100 mM K+, adjusted to maintain osmolarity) was then added to each well for 3 min at room temperature. A 300-μl aliquot of the supernatant was taken from each well for counting, and the remaining solution was discarded. One milliliter (0.3%) of Triton X-100 was added to the cells for 30 min, and 300 μl of lysate was taken for counting. Samples taken for counting were each added to 4 ml of Bio-Safe II scintillation mixture and counted on a Beckman LS6000 scintillation counter. For drug experiments, after the three washes cells were incubated in 1 ml of Hepes buffer containing the given drug for 10 min at 37°C, followed by release as described above. [3H]norepinephrine release was expressed as a percentage of the total [3H]norepinephrine content.
Transfection of SH-SY5Y Cells with the Human H3 Receptor.
Cells were grown to 70–80% confluence and removed from the plate with trypsin and pelleted in a clinical centrifuge. The pellet was then resuspended in 400 μl of complete medium and transferred to an electroporation cuvette with a 0.4-cm gap between the electrodes (Bio-Rad no. 165-2088). One microgram of supercoiled H3R cDNA was added to the cells and mixed. The voltage for the electroporation was set at 0.25 kV, and the capacitance was set at 960 μF. After electroporation the cells were diluted into 10 ml of complete medium and were plated onto four 10-cm dishes at the following ratios: 1:20, 1:10, 1:5, and 1:2. The cells were incubated for 24 h before 100 μg/ml geneticin was added. Colonies that survived selection were isolated and expanded for testing. Because these cells grow poorly in geneticin, 4 days after isolation geneticin was completely removed from the medium. The individual clones were then tested for binding to [3H]Nα-methyl-histamine as described by Lovenberg et al. (14).
Cai Measurements.
Cells grown on coverslips were loaded with the membrane-permeant form of the Cai indicator Fura-2 ester (5 μM) for 20 min at room temperature. After loading with the dye, the cells were rinsed with Hepes-buffered saline Ringer's solution (140 mM NaCl/5 mM KCl/10 mM Hepes/2.6 mM CaCl2/1.0 mM MgCl2, pH 7.4). The coverslip was attached to the bottom of a flow-through superfusion chamber and mounted on the stage of an inverted epifluorescence microscope (Nikon Diaphot). The cells in the chamber were superfused and maintained at 37°C as described (15). Cells were first visualized under transmitted light with a Nikon CF Fluor (×40/1, 3 NA oil immersion objective) before fluorescence measurements were started. Cells were depolarized with a high-K+ solution (based on the Na Ringer's composition described above, with 100 mM KCl replacing 100 mM NaCl). Calibration of the emitted Fura-2 signal from each cell in the field was carried out in the presence of the Ca2+ ionophore, ionomycin (10 μM), in the presence of Hepes buffer containing either 2.6 mM Ca2+ or 10 mM EGTA titrated to pH 7.4. Cai levels were calculated as described by Grynkiewicz et al. (16). Cells in the experimental field of view were analyzed singularly and independently from their neighbors.
Reagents.
Ionomycin was prepared in DMSO to give a final concentration of 10 μM. Individual vials (50 μg) of the acetoxymethyl derivative of Fura-2 were stored dry at 0°C and reconstituted in DMSO, at a concentration of 5 mM, for each experiment.
Equipment.
The basic components of the experimental apparatus have been described (15, 17). The imaging workstation was controlled with the metafluor software package (Universal Imaging, Westchester, PA). Quantitative image pairs at 340-nm and 380-nm excitation with emission at 510 nm were obtained either every 15 s or every 0.1 s immediately preceding and during depolarization. The fluorescence excitation was shuttered off except during the brief periods required to record an image. To check for interference from intrinsic autofluorescence and background, images were obtained on cells with the same exposure time and filter combination used for the experiments and found to be minor compared with the fluorescence signal.
Statistics.
Results are expressed as means ± SEM, where n refers to the number of cells. Cells were analyzed individually unless otherwise noted. Significant differences were determined by one-way ANOVA. Significance was asserted if P < 0.05.
Results
Exocytosis of Endogenous Norepinephrine from Cardiac Sympathetic Nerve Terminals.
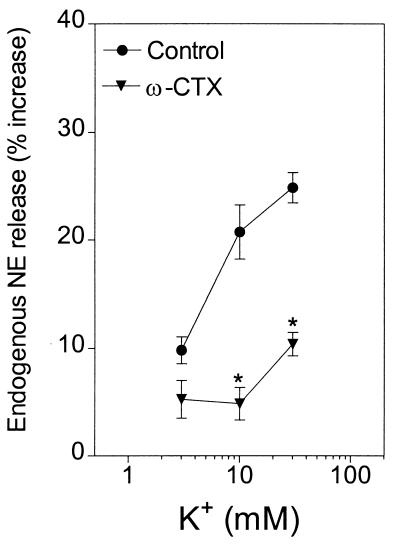
Depolarization of cardiac synaptosomes with K+ (3–30 mM) elicited a 10–30% increase in the release of endogenous norepinephrine (Figs. 1 and 2). In the presence of the selective N-type Ca2+ channel blocker ω-CTX (100 nM), the concentration–response curve for the K+-induced norepinephrine exocytosis was shifted markedly downward (Fig. 1).
Figure 1.
Release of endogenous norepinephrine (NE) from guinea pig heart synaptosomes by depolarization with 3–30 mM K+ in the absence and presence of the selective N-type Ca2+ channel inhibitor ω-conotoxin (100 nM). Points represent mean increases in norepinephrine release above the basal level (± SEM; n = 8). The basal norepinephrine level was 1.63 ± 0.02 pmol/mg of protein. *, P < 0.05 from the corresponding control norepinephrine level by unpaired t test.
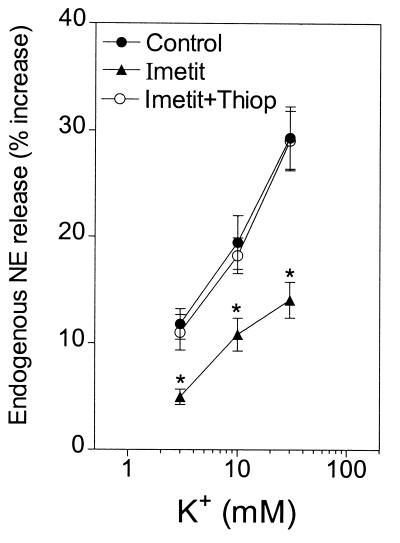
Figure 2.
Release of endogenous norepinephrine (NE) from guinea pig heart synaptosomes by depolarization with 3–30 mM K+ in the absence and presence of the selective H3R agonist imetit (100 nM), either alone or in combination with the selective H3R antagonist thioperamide (300 nM). Points represent mean increases in norepinephrine release above the basal level (± SEM; n = 8). The basal norepinephrine level was 1.14 ± 0.01 pmol/mg of protein. *, P < 0.05 from the corresponding control norepinephrine level by unpaired t test.
In the presence of the selective H3R agonist imetit (100 nM), the concentration–response curve for the K+-induced exocytosis of endogenous norepinephrine was also significantly shifted downward. This effect was prevented by the selective H3R antagonist thioperamide (300 nM) (Fig. 2).
Exocytosis of Tritiated Norepinephrine from Cultured Neuroblastoma Cells.
Depolarization of neuroblastoma cells SH-SY5Y and SH-SY5Y-H3 with K+ (10–100 mM) elicited a concentration-dependent release of [3H]norepinephrine (10–40% of stored [3H]norepinephrine; Figs. 3 A and B and 4 A and B). In the presence of ω-CTX (100 nM), the concentration–response curve for the K+-induced exocytosis of [3H]norepinephrine was shifted markedly downward in both SH-SY5Y and SH-SY5Y-H3 cells (Fig. 3 A and B).
Figure 3.
Selective inhibition of N-type Ca2+ channels with ω-CTX (100 nM) attenuates both Ca2+ influx and the resulting exocytosis of tritiated norepinephrine (NE) in K+-depolarized, cultured neuroblastoma cells. (Upper) Release of [3H]norepinephrine from parent SH-SY5Y (A) and H3R-transfected SH-SY5Y (SH-SY5Y-H3) (B) cells, by depolarization with 10–100 mM K+, in the absence and presence of ω-CTX (100 nM). Points represent mean [3H]norepinephrine release expressed as a percentage of total [3H]norepinephrine content (± SEM; n = 3–5). *, P < 0.05 from the corresponding control [3H]norepinephrine level by unpaired t test. (Lower) Peak Cai concentration in SH-SY5Y (C) and SH-SY5Y-H3 (D) cells depolarized with 100 mM K+ in the absence and presence of ω-CTX (100 nM). Bars represent means (± SEM) of 157 control and 87 ω-CTX-treated SH-SY5Y cells (C), and 232 control and 203 ω-CTX-treated SH-SY5Y-H3 cells (D). *, P < 0.05 from the corresponding control peak Cai level by unpaired t test.
Figure 4.
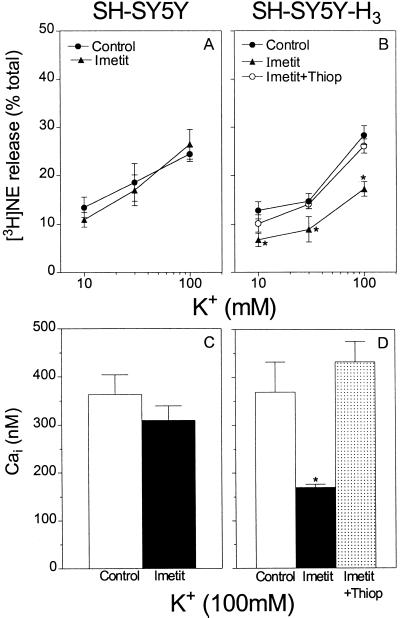
Activation of H3R with the selective agonist imetit (100 nM) attenuates both Ca2+ influx and resulting exocytosis of tritiated norepinephrine (NE) in K+-depolarized, cultured neuroblastoma cells transfected with the H3R (SH-SY5Y-H3; B and D). Parent SH-SY5Y cells fail to respond to imetit (A and C). (Upper) Release of [3H]norepinephrine from SH-SY5Y cells (A) and SH-SY5Y-H3 cells (B), by depolarization with 10–100 mM K+, in the absence and presence of imetit (100 nM), either alone or in combination with the selective H3R antagonist thioperamide (300 nM; B). Points represent mean [3H]norepinephrine release expressed as a percentage of total [3H]norepinephrine content (± SEM; n = 3–7). *, P < 0.05 from the corresponding control [3H]norepinephrine level by unpaired t test. (Lower) Peak Cai concentration in SH-SY5Y cells (C) and SH-SY5Y-H3 cells (D) depolarized with 100 mM K+ in the absence and presence of imetit ± thioperamide (D). Bars represent means (± SEM) of 157 control and 174 imetit-treated SH-SY5Y cells (C), and 232 control, 197 imetit-treated, and 231 imetit + thioperamide-treated SH-SY5Y-H3 cells (D). *, P < 0.05 from the corresponding control peak Cai level by unpaired t test.
In contrast, in the presence of the selective H3R agonist imetit (100 nM), the concentration–response curve for the K+-induced exocytosis of [3H]norepinephrine was significantly shifted downward only in neuroblastoma cells transfected with the H3R, but not in the nontransfected parent cells (Fig. 4, compare A and B). When present, the effect of imetit was prevented by the selective H3R antagonist thioperamide (300 nM) (Fig. 4B).
Ca2+ Influx in Cultured Neuroblastoma Cells.
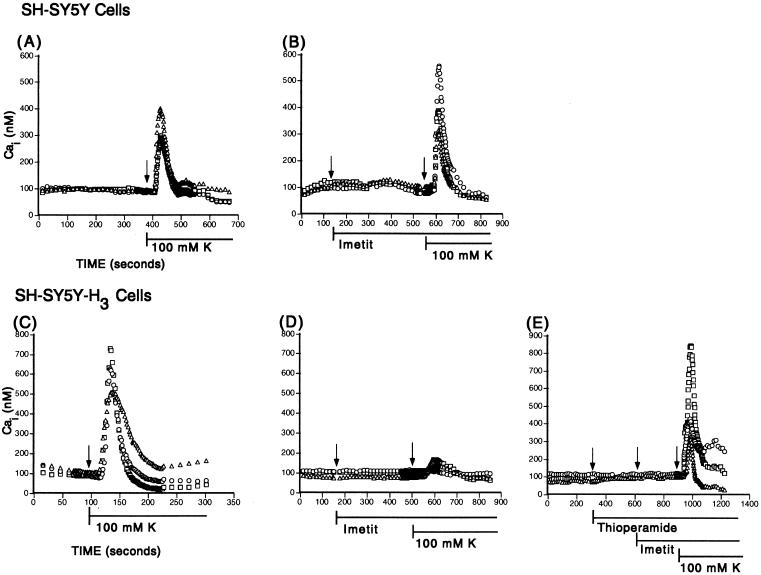
To test directly the hypothesis that H3R activation diminishes Ca2+ influx, we measured Cai in response to K+-induced membrane depolarization in parent SH-SY5Y cells and SH-SY5Y-H3 cells loaded with the Cai indicator Fura-2. Fig. 5 shows representative Cai responses at the individual cell level in the parent (A and B) and H3R-transfected neuroblastoma cells (C–E). As shown for both SH-SY5Y and SH-SY5Y-H3 cells, exposure to high extracellular K+ (100 mM) led to a rapid increase in Cai followed by a rapid return to initial levels (Fig. 5 A and C).
Figure 5.
Cai transients in K+-depolarized SH-SY5Y and SH-SY5Y-H3 cultured neuroblatoma cells. Ordinates represent the Cai concentration determined by intracellular calibration of the Fura-2 signal emitted from three individual representative cells in each panel. All cells were initially superfused with Hepes-buffered saline Ringer's solution and subsequently exposed to 100 mM K+ in the absence or presence of the pharmacological agents indicated. Note that the selective H3R agonist imetit fails to affect Cai transients in SH-SY5Y (B) but markedly suppresses Cai transients in SH-SY5Y-H3 cells (D), an effect that is prevented by the selective H3R antagonist thioperamide (E).
In the presence of the selective H3R agonist imetit (100 nM), K+-elicited Ca2+ transients were markedly depressed in the SH-SY5Y-H3, but not in the SH-SY5Y cells (Fig. 5, compare B and D). The response to imetit was prevented by the selective H3R antagonist thioperamide (300 nM; Fig. 5E). A quantitative evaluation of the effects of imetit is shown in Fig. 4 C and D. Whereas imetit caused a 55% decrease in the peak Cai concentration elicited by 100 mM K+ in the H3R-transfected cells (Fig. 4D), it failed to affect peak Cai concentration in the nontransfected cells (Fig. 4C). The imetit-induced depression of the rise in Cai concentration was completely prevented by the selective H3R antagonist thioperamide (Fig. 4D).
Relationship Between Cai and Norepinephrine Exocytosis.
The relationship between Cai concentration and norepinephrine release is shown in Fig. 3. When neuroblastoma cells were depolarized with K+, the increase in peak Cai concentration was markedly attenuated in the presence of ω-CTX (100 nM) in both parent and H3R-transfected cells (Fig. 3 C and D). In both cells, this decrease in Cai was associated with a marked decrease in [3H]norepinephrine release (Fig. 3 A and B). A decrease in endogenous norepinephrine release was also observed in sympathetic nerve endings in the presence of ω-CTX (100 nM; see Fig. 1).
The same association between Cai and norepinephrine release was observed also in cells depolarized with K+ in the absence and presence of H3R ligands. When imetit was used in parent SH-SY5Y cells, the increases in Cai and norepinephrine release in response to K+ were unaffected (Fig. 4 A and C). In contrast, when imetit was used in SH-SY5Y-H3 cells, the marked decrease in peak Cai concentration was associated with a marked attenuation in norepinephrine release. Moreover, in the presence of thioperamide, the abolition of the effect of imetit on Cai was associated with a restoration of norepinephrine release (Fig. 4 B and D).
Discussion
The exocytotic release of norepinephrine from postganglionic sympathetic neurons requires entry of Ca2+ through voltage-dependent Ca2+ channels (10, 18, 19). Having first established that H3R activation down-regulates norepinephrine exocytosis from cardiac sympathetic terminals (1, 2, 7), we subsequently found that the selective N-type Ca2+ channel blocker ω-CTX (8) potentiates the effects of H3R activation (1). Interestingly, ω-CTX also potentiated the cardiac responses to α2-adrenoreceptor activation (1). This finding implied that, similar to α2-adrenoreceptor activation, which inhibits norepinephrine release and reduces Ca2+ influx into cardiac sympathetic terminals (9), H3R activation could also inhibit norepinephrine release by diminishing Cai. We have now investigated this implication in sympathetic nerve terminals (i.e., cardiac synaptosomes) expressing native H3R (7) and in a human neuroblastoma cell line [SH-SY5Y (10)], stably transfected with the H3R cDNA (SH-SY5Y-H3). The results demonstrate that the attenuation of norepinephrine exocytosis caused by activation of the H3R is associated with a marked decrease in Cai concentration, probably secondary to a decreased Ca2+ influx into sympathetic nerve terminals.
Our claim is based on the findings that (i) ω-CTX, which is known to impede Ca2+ influx by means of N-type channels (8), markedly reduced the increase in Cai in response to membrane depolarization with 100 mM K+ in both parent and H3R-transfected SH-SY5Y neuroblastoma cells; (ii) in association with the decrease in Cai, ω-CTX also greatly attenuated the exocytosis of tritiated norepinephrine from both SH-SY5Y and SH-SY5Y-H3 cells, and of endogenous norepinephrine from cardiac synaptosomes; (iii) similar to ω-CTX, H3R activation with the selective H3R agonist imetit (20) markedly reduced the increase in Cai in response to high K+, a response that was prevented by the selective H3R antagonist thioperamide (21); (iv) in association with the decrease in Cai, imetit also greatly attenuated the exocytosis of tritiated norepinephrine from SH-SY5Y-H3 cells and of endogenous norepinephrine from cardiac synaptosomes; (v) in sharp contrast, imetit affected neither the Cai response nor the exocytosis of norepinephrine in parent SH-SY5Y cells, not only demonstrating that H3R expression is a prerequisite for a decrease in Cai in response to imetit, but also linking the magnitude of the increase in Cai to the extent of norepinephrine exocytosis.
We chose to experiment with SH-SY5Y, because this neuroblastoma cell line derived from the sympathetic nervous system expresses several properties of mature sympathetic neurons (10). In addition, our findings in neuroblastoma cells were strengthened by their similarity to the findings obtained in native cardiac sympathetic terminals. Indeed, we found all responses to be equivalent in both neuroblastoma cells and cardiac sympathetic terminals.
Some speculations can be attempted as to the possible mechanisms of the association between activation of H3R, decreased Cai concentration, and attenuation of norepinephrine exocytosis. Because both ω-CTX and imetit decrease Cai and norepinephrine exocytosis, and ω-CTX decreases Cai by inhibiting Ca2+ influx through N-type Ca2+ channels (8), it is likely that imetit also decreases Cai by inhibiting Ca2+ influx into sympathetic nerve terminals. Indeed, recent work on primary cultures of histaminergic neurons from the rat hypothalamus suggests an H3R-mediated inhibition of N-type Ca2+ channel current (22). Presynaptic receptors that inhibit depolarization-evoked release of norepinephrine most commonly rely on a G protein-mediated blockade of voltage-gated Ca2+ channels (18). In fact, H3R are likely to be coupled to Gi/Go proteins, inasmuch as we found that pertussis toxin attenuates the H3R-mediated inhibition of adrenergic responses in the heart (1). Moreover, H3R activation inhibits forskolin-stimulated adenylyl cyclase activity in SKNMC neuroblastoma cells stably transfected with the human H3R cDNA (5). Inasmuch as phosphorylation of the N-type Ca2+ channel increases its activity (23), a decreased phosphorylation resulting from inhibition of the cAMP/protein kinase A pathway could be involved in H3R-mediated attenuation of N-type Ca2+ channel activity and, thus, norepinephrine exocytosis.
Our discovery that H3R activation results in a decrease in axoplasmic Ca2+ concentration, seemingly because of an impaired entrance of Ca2+ through voltage-gated ion channels, pertains not only to cardiac physiology, but also to pathological conditions, such as acute myocardial ischemia, characterized by exaggerated norepinephrine exocytosis (4). Previous work from our laboratories had demonstrated that in protracted myocardial ischemia H3R activation inhibits the Na+/H+ exchanger in sympathetic neurons (4, 17) and thus prevents carrier-mediated norepinephrine release, because of a Ca2+-independent intraneuronal Na+ accumulation resulting from activation of the Na+/H+ exchanger. Collectively, our findings emphasize the multiplicity of protective roles played by H3R activation in the setting of myocardial ischemia. Because excess norepinephrine release can trigger severe arrhythmias and sudden cardiac death, negative modulation of norepinephrine release by H3R agonists may offer a novel therapeutic approach to myocardial ischemia.
Acknowledgments
This work was supported by Grants HL 34215, HL46403, and DK45828 from the National Institutes of Health and by the Underhill and Wild Wings Foundation.
Abbreviations
- Cai
intracellular Ca2+ concentration
- H3R
histamine H3 receptors
- SH-SY5Y-H3 cells
human SH-SY5Y neuroblastoma cells stably transfected with H3R cDNA
- ω-CTX
ω-conotoxin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Endou M, Poli E, Levi R. J Pharmacol Exp Ther. 1994;269:221–229. [PubMed] [Google Scholar]
- 2.Imamura M, Seyedi N, Lander H M, Levi R. Circ Res. 1995;77:206–210. doi: 10.1161/01.res.77.1.206. [DOI] [PubMed] [Google Scholar]
- 3.Imamura M, Poli E, Omoniyi A T, Levi R. J Pharmacol Exp Ther. 1994;271:1259–1266. [PubMed] [Google Scholar]
- 4.Levi R, Smith N C E. J Pharmacol Exp Ther. 2000;292:825–830. [PubMed] [Google Scholar]
- 5.Lovenberg T W, Roland B L, Wilson S J, Jiang X, Pyati J, Huvar A, Jackson M R, Erlander M G. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- 6.Leurs R, Hoffmann M, Wieland K, Timmerman H. Trends Pharmacol Sci. 2000;21:11–12. doi: 10.1016/s0165-6147(99)01411-x. [DOI] [PubMed] [Google Scholar]
- 7.Seyedi N, Win T, Lander H M, Levi R. Circ Res. 1997;81:774–784. doi: 10.1161/01.res.81.5.774. [DOI] [PubMed] [Google Scholar]
- 8.Sher E, Biancardi E, Passafaro M, Clementi F. FASEB J. 1991;5:2677–2683. doi: 10.1096/fasebj.5.12.1655547. [DOI] [PubMed] [Google Scholar]
- 9.Lipscombe D, Kongsamut S, Tsien R W. Nature (London) 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan P F, Peers C, Walker J H. Gen Pharmacol. 1995;26:1191–1201. doi: 10.1016/0306-3623(94)00312-b. [DOI] [PubMed] [Google Scholar]
- 11.Imamura M, Lander H M, Levi R. Circ Res. 1996;78:475–481. doi: 10.1161/01.res.78.3.475. [DOI] [PubMed] [Google Scholar]
- 12.Markwell M A, Haas S M, Bieber L L, Tolbert N E. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 13.Murphy N P, Ball S G, Vaughan P F. J Neurochem. 1991;56:1810–1815. doi: 10.1111/j.1471-4159.1991.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 14.Lovenberg T W, Pyati J, Chang H, Wilson S J, Erlander M G. J Pharmacol Exp Ther. 2000;293:771–778. [PubMed] [Google Scholar]
- 15.Cardone M H, Smith B L, Mennitt P A, Mochly-Rosen D, Silver R B, Mostov K E. J Cell Biol. 1996;133:997–1005. doi: 10.1083/jcb.133.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 17.Silver R B, Mackins C J, Smith N C E, Koritchneva I L, Lefkowitz K, Lovenberg T W, Levi R. Proc Natl Acad Sci USA. 2001;98:2855–2859. doi: 10.1073/pnas.051599198. . (First Published February 20, 2001; 10.1073/pnas.051599198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boehm S, Huck S. Prog Neurobiol. 1997;51:225–242. doi: 10.1016/s0301-0082(96)00056-1. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y A, Scales S J, Duvvuri V, Murthy M, Patel S M, Schulman H, Scheller R H. J Biol Chem. 2001;276:26680–26687. doi: 10.1074/jbc.M103522200. [DOI] [PubMed] [Google Scholar]
- 20.Garbarg M, Arrang J M, Rouleau A, Ligneau X, Tuong M D, Schwartz J C, Ganellin C R. J Pharmacol Exp Ther. 1992;263:304–310. [PubMed] [Google Scholar]
- 21.Arrang J M, Garbarg M, Lancelot J C, Lecomte J M, Pollard H, Robba M, Schunack W, Schwartz J C. Nature (London) 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita Y, Watanabe T, Sakata T, Munakata M, Ishibashi H, Akaike N. Neuroscience. 1998;87:797–805. doi: 10.1016/s0306-4522(98)00152-3. [DOI] [PubMed] [Google Scholar]
- 23.Swartz K J. Neuron. 1993;11:305–320. doi: 10.1016/0896-6273(93)90186-u. [DOI] [PubMed] [Google Scholar]