Abstract
Background:
Short-chain fatty acids (SCFAs), including propionic acid (PA), are key in immunological research. Supplementing PA has shown benefits for autoimmune diseases. A comprehensive understanding of the PA pharmacokinetics is essential for the optimal design and execution of studies utilizing orally administered PA.
Objective:
We propose two methods of measuring PA in serum, carried out by different laboratories.
Design:
Blood samples from 20 volunteers were collected hourly following PA supplementation.
Methods:
Serum propionate quantification was performed with two independent mass spectrometry-based (MS) analyses, including liquid-chromatography (LC)-MS and direct-infusion (DI)-MS.
Results:
PA levels increased within 1 h of ingestion of 500 mg PA. Serum concentrations ranged from 1.3 to 4.5 µmol/L, rising significantly after 1 h (p < 0.05). Serum levels returned to baseline within 2 h. No significant differences were found regarding sex or diet.
Conclusion:
The shown pharmacokinetics can be used in future PA research.
Keywords: direct infusion mass spectrometry, liquid chromatography mass spectrometry, pharmacokinetics, propionate, propionic-acid, SCFA
Introduction
Short-chain fatty acids (SCFA), such as propionic acid (PA), have been in the focus in immunological research throughout recent years, due to their effects on several systemic functions, including the immune system, glucose and lipid metabolism, and energy levels.1–4 PA, along with acetate and butyrate, is synthesized by the gut microbiome in the colon. This synthesis occurs primarily through the fermentation of dietary fiber. PA serves as a direct energy supplier to the intestinal epithelia and is also absorbed systemically. 5 Neuroprotective properties of PA on the peripheral nervous system have been observed. 6 Beneficial effects have also been observed in in vivo studies and case studies of acute motor and sensory axonal neuropathy (AMSAN), 7 multiple sclerosis,4,8 and other autoimmune diseases 9 through immunomodulatory effects as well as in degenerative processes (such as Parkinson’s disease) 10 and vascular calcification. 11 Although numerous studies have been and are being conducted on PA supplementation, there are no studies on PA absorption and the effect on PA-serum levels after oral administration. This is mainly due to the fact that SCFAs are mainly analyzed in stool samples since they are end products of fermentation of dietary fibers by the anaerobic intestinal microbiota. These SCFAs are transported from the intestinal lumen into the blood of the host and are taken up by organs where they act as substrates or signal molecules. 5
To gain insight into the effects of PA supplementation, in this study, we investigated for the first time, to the best of our knowledge, whether oral PA intake is reflected in serum levels.
Methods
Study design and cohort
The objective of this study was to investigate the pharmacokinetics of orally supplemented PA using two different MS methods. A total of n = 20 healthy volunteers with no history of gastrointestinal, autoimmune, or other serious chronic diseases were included in this study. All subjects were recruited at the Centre for Clinical Research at the St. Josef Hospital, Bochum, Germany, in November 2021 (cohort group B) and in July 2022 (cohort group A).
Healthy volunteers were instructed to ingest 500 mg of sodium propionate (propicum®) in the Centre for Clinical Research at 8:00 am with a prior fasting of 12 h. They were just allowed to drink water and refrained from physical activity until 4 h after propionate intake.
Venous blood samples were collected before propionate supplementation (baseline 0 h) and then at 1-h intervals over a 4-h period. Each serum monovette contained 7.5 mL of blood, which was processed immediately after collection. Blood was centrifuged (2000g, 15 min, 4°C), aliquoted, and immediately stored at −80°C in 500 µL cryotubes for future analysis.
Quantification of propionate in serum
Serum propionate quantification was performed with two independent mass spectrometry-based (MS) analyses, including liquid chromatography (LC)-MS (analysis A) and direct-infusion (DI)-MS (analysis B).
Analysis A: Propionate levels from cohort group A were determined according to Zeng and Cao 12 with slight modifications. Fifty micro liters of serum were mixed with 20 µL of aqueous 13C-PA (10 µM) used as internal standard. Proteins were precipitated by adding of 70 µL of 0.6 M glycine ethyl ester in methanol, incubation at 4°C for 30 min, and centrifugation at 1000 g for 15 min at 4°C. Sixty micro liters of the supernatant were added to 15 µL methanolic 0,3 M N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride. After a reaction time of 2 h, the samples were extracted by dichloromethane, dried, resolved, and analyzed using high performance liquid chromatography with mass spectrometry (HPLC-MS/MS) (API-2000 (Applied Biosystems), Agilent 1100 HPLC with Poroshell HPH-C18, 4 µm, 2,1 × 150 mm2 (Agilent Techologies)).
Analysis B: For cohort group B, a single-phase extraction protocol utilizing 2-propanol was employed for the extraction process. Fifty micro liters of serum were spiked with 100 μL of D5-propionate (10 μg/mL). Isopropanol was added to the samples to a final concentration of 70%. After incubation for 60 min at 4°C, the samples were centrifuged at 3500g for 10 min at 4°C, and the supernatant containing metabolites was transferred to a new glass vial. Samples were derivatized with 3-nitrophenylhydrazine hydrochloride and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (Sigma-Aldrich, Saint Louis, MO, USA) as previously described. 13 Ten micro liters of the derivate were transferred to a 384-well plate. Samples were analyzed by direct infusion mass spectrometry, using a Triversa Nanomate (Advion BioSciences Inc.) source coupled to a Q Exactive-HF Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany). MS-data were aquired using parallel reaction monitoring and subsequently analyzed using FreeStyle software version 1.8.65.0 (Thermo Fisher Scientific, Rockford, IL, USA). Endogenous PA concentrations were determined using the internal standard (D5-propionate).
Statistical analysis was performed using Prism 8 (GraphPad Software, La Jolla, USA). Diet- and gender-related group differences were analyzed using area under the curve calculations, Welch’s t-test, and Mann–Whitney test. p < 0.05 was considered statistically significant.
Results
Oral PA supplementation increases serum PA levels 1 h after ingestion
To elucidate whether oral PA supplementation is reflected in serum levels, the serum concentration of PA after oral PA intake was determined for the first time in this study. For this purpose, serum from two cohorts of healthy volunteers (groups A and B) was analyzed before and 1, 2, 3, and 4 h after oral ingestion of 500 mg PA using two independent MS approaches, including LC-MS (analysis A) and DI-MS (analysis B). Cohort group A, studied with analysis A, consisted of five men and five women, six of whom followed an omnivorous diet and four a predominantly plant-based diet. Cohort B, studied with analysis B, consisted of four men and six women, four of whom were omnivores and six of whom were on a predominantly plant-based diet. One sample from cohort B was excluded from the study due to technical issues encountered during the measurement of the sample.
For the respective cohorts at the different time points, both analyses showed comparable PA serum concentrations in the range of 1.3 and 4.5 µmol/L, consistent with reported physiological values (Figure 1).8,14,15 Moreover, a significant increase in the PA serum level (Baseline to T1) was observed with both approaches 1 h after oral intake (A: p = 0.0208; B: p = 0.0001), returning to fasting baseline levels 2 h after supplementation. No differences were found in the evaluations of the PA baseline levels in relation to gender and diet (data not shown).
Figure 1.
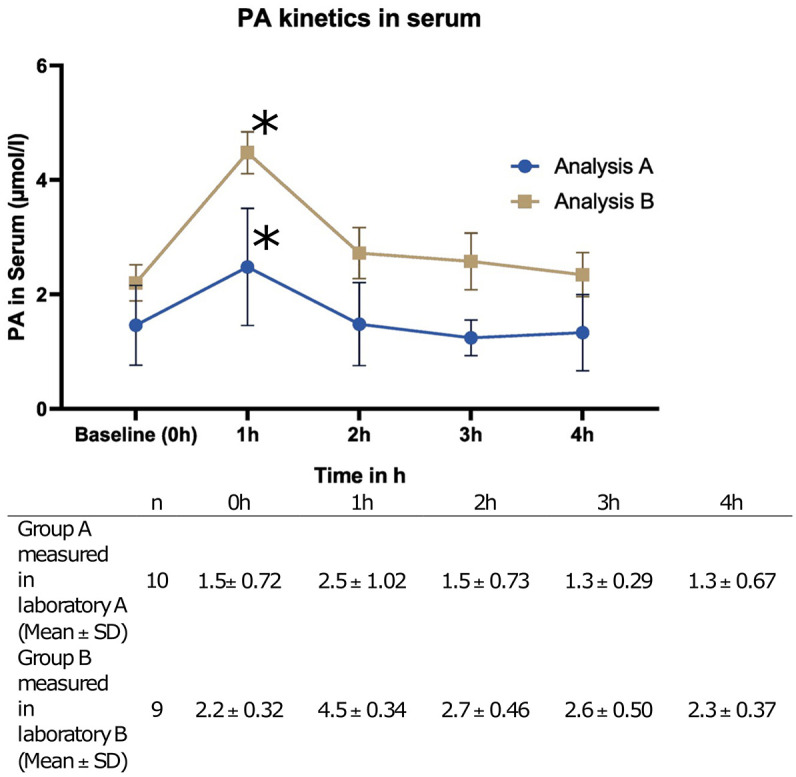
PA levels in serum after supplementation of 500 mg PA(mean ± SD).
A significant increase in serum level is indicated with*
h, hour; PA, Propionate; SD, Standard Deviation.
A statistically significant difference was observed between the absolute serum PA level values of both laboratory methodologies across all time points (Baseline: p = 0.0157, T1: p = 0.0001, T2: p = 0.0006, T3: p = 0.0001, T4: p = 0.0011). Furthermore, the differences in serum levels between the baseline and the distinct time points (delta) were also consistently found to be significantly different between the two laboratory methods (Delta: Baseline-T1: p = 0.0001, Delta Baseline-T2: p = 0.0001, Delta Baseline-T3: p = 0.0016, Delta Baseline-T4: p = 0.0001).
Discussion
This study is the first to describe the pharmacokinetics of orally administered PA in serum, with a peak in serum levels 1 h after oral administration and return to the fasting serum levels within 2 h.
The rapid absorption within 1 h indicates that the orally supplemented PA is absorbed in the small intestine, in contrast to the physiological PA which is produced by the microbiome in the colon. 15 Also, it is striking that serum levels return to baseline levels after 2 h. This raises the question of how and where exactly supplemented PA is metabolized. Physiological colon-derived PA is largely consumed as an energy source in colonocytes and hepatocytes and in gluconeogenesis, and only about 9% of colon-derived PA enters the blood system. 15 It is possible that also the orally administered PA is used as an energy source and that more stable serum levels and a better immunoregulatory effect could be achieved with a higher dosing frequency or a different formulation. Repeated administration of PA may be more effective than single high doses in achieving sustained serum PA levels.
As plant- and fiber-rich diets may lead to endogenous PA production by the gut microbiome, we recommend further investigation of the missing differences in uptake, distribution, and initial serum PA levels between omnivorous and plant-based diets in larger cohorts. Whether gender affects the absorption and distribution also remains to be determined in future larger studies.
LC-MS separates compounds through liquid chromatography before mass spectrometry, allowing for better resolution and identification, while DI-MS introduces samples directly into the mass spectrometer, making it faster but with less separation. Overall, LC-MS is suitable for complex samples with multiple analytes and offers higher sensitivity, whereas DI-MS is used for simpler analyses and rapid screenings. We found significant differences in PA levels between the two methods. However, both methods did not measure the exact same samples, but different cohorts, and this report was not primarily designed to compare the two methods, as this would have required more samples and multiple measurements. Therefore, this data do not permit the conclusion that one methodology is superior to the other.
Given the exploratory nature of this study, a priori determination of the number of cases was not feasible. The number of subjects was therefore set at 10 per examined method.
A further limitation of our work is, that the implementation of both methods in laboratories was not feasible simultaneously, leading to a one-year temporal discrepancy between the measurements of cohorts A and B. The absence of further reference samples from cohort A at the time of measurement of cohort B made testing with both quantification techniques in both cohorts untenable.
Conclusion
In conclusion, we report for the first time the pharmacokinetics of orally administered PA in serum, with a peak in serum levels 1 h after oral administration and return to the fasting serum levels within 2 h. No differences in serum PA levels were observed with respect to gender or diet.
Acknowledgments
This research was supported by the GBS CIDP Foundation International – Discovery Grant 2021. We thank our colleagues from the Institute of Agricultural and Nutritional Sciences, Martin Luther University Halle-Wittenberg, Halle (Saale) and the Center for protein diagnostics (PRODI), Ruhr-University Bochum, Germany who provided insight and expertise that greatly assisted the research.
Appendix
Abbreviations
SCFA short-chain fatty acid
PA propionic acid
MS mass-spectrometry
LC liquid chromatography
DI direct-infusion
PNS peripheral nervous system
AMSAN acute motor and sensory axonal neuropathy
Footnotes
ORCID iDs: Maximilian Schröder  https://orcid.org/0009-0009-0928-3178
https://orcid.org/0009-0009-0928-3178
Anna Lena Fisse  https://orcid.org/0000-0003-0493-8656
https://orcid.org/0000-0003-0493-8656
Contributor Information
Maximilian Schröder, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Arijan Pasic, Medizinisches Proteom-Center, Medical Faculty, Ruhr-University Bochum, Germany; Medical Proteome Analysis, Center for protein diagnostics (PRODI), Ruhr-University Bochum, Germany.
Frank Hirche, Institute of Agricultural and Nutritional Sciences, Martin Luther University Halle-Wittenberg, Halle (Saale), Germany.
Svitlana Rozanova, Medizinisches Proteom-Center, Medical Faculty, Ruhr-University Bochum, Germany; Medical Proteome Analysis, Center for protein diagnostics (PRODI), Ruhr-University Bochum, Germany.
Melissa Sgodzai, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Barbara Gisevius, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Lea Horstkemper, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Ralf Gold, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Katrin Marcus, Medizinisches Proteom-Center, Medical Faculty, Ruhr-University Bochum, Germany; Medical Proteome Analysis, Center for protein diagnostics (PRODI), Ruhr-University Bochum, Germany.
Kalliopi Pitarokoili, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Jeremias Motte, Department of Neurology, St. Josef-Hospital, Ruhr-University Bochum, Germany.
Katalin Barkovits, Medizinisches Proteom-Center, Medical Faculty, Ruhr-University Bochum, Germany; Medical Proteome Analysis, Center for protein diagnostics (PRODI), Ruhr-University Bochum, Germany.
Gabriele Stangl, Institute of Agricultural and Nutritional Sciences, Martin Luther University Halle-Wittenberg, Halle (Saale), Germany.
Anna Lena Fisse, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Gudrunstr. 56, Bochum 44791, Germany.
Declarations
Ethics approval and consent to participate: This study was approved by the ethics committee of the Ruhr-University Bochum (registration number 20-6915). Written informed consent was obtained from all participants. We used the STROBE cross-sectional checklist when writing our report. 16
Consent of publication: Consent of publication was obtained from all participants.
Author contributions: Maximilian Schröder: Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Visualization; Writing – original draft.
Arijan Pasic: Formal analysis; Investigation; Methodology; Writing – review & editing.
Frank Hirche: Formal analysis; Investigation; Methodology; Writing – review & editing.
Svitlana Rozanova: Formal analysis; Investigation; Methodology; Writing – review & editing.
Melissa Sgodzai: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Barbara Gisevius: Conceptualization; Writing – review & editing.
Lea Horstkemper: Conceptualization; Investigation; Writing – review & editing.
Ralf Gold: Conceptualization; Funding acquisition; Resources; Supervision; Writing – review & editing.
Katrin Marcus: Conceptualization; Resources; Supervision; Writing – review & editing.
Kalliopi Pitarokoili: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Jeremias Motte: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Katalin Barkovits: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Gabriele Stangl: Conceptualization; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Anna Lena Fisse: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the GBS CIDP Foundation International – Discovery Grant 2021.
Competing interests: R.G. is the Editor-in-Chief of Therapeutic Advances in Neurological Disorders. Therefore, the peer review was handled by alternative members of the Board, and the submitting Editor was not involved in the decision-making process.
All other authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author, AL.F., upon reasonable request.
References
- 1. Adler GK, Hornik ES, Murray G, et al. Acute effects of the food preservative propionic acid on glucose metabolism in humans. BMJ Open Diabetes Res Care 2021; 9: e002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers ES, Byrne CS, Aspey K, et al. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes Metab 2018; 20: 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haghikia A, Zimmermann F, Schumann P, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J 2022; 43: 518–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tobin D, Vige R, Calder PC. Review: the nutritional management of multiple sclerosis with propionate. Front Immunol 2021; 12: 676016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013; 54: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grüter T, Mohamad N, Rilke N, et al. Propionate exerts neuroprotective and neuroregenerative effects in the peripheral nervous system. Proc Natl Acad Sci U S A 2023; 120: e2216941120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon M-S, Pitarokoili K, Sturm D, et al. Treatment of an acute motor and sensory axonal neuropathy with propionate in a 33-year-old male. Ther Adv Neurol Disord 2018; 11: 1756286418809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020; 180: 1067–1080.e16. [DOI] [PubMed] [Google Scholar]
- 9. Golpour F, Abbasi-Alaei M, Babaei F, et al. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed Pharmacother Biomedecine Pharmacother 2023; 163: 114763. [DOI] [PubMed] [Google Scholar]
- 10. Chen S-J, Chen C-C, Liao H-Y, et al. Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology 2022; 98: e848–e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan J, Pan Y, Shao W, et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome 2022; 10: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng M, Cao H. Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction. J Chromatogr B 2018; 1083: 137–145. [DOI] [PubMed] [Google Scholar]
- 13. Han J, Lin K, Sequeira C, et al. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 2015; 854: 86–94. [DOI] [PubMed] [Google Scholar]
- 14. Pagonas N, Seibert FS, Liebisch G, et al. Association of plasma propionate concentration with coronary artery disease in a large cross-sectional study. Front Cardiovasc Med 2023; 10: 1063296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dalile B, Van Oudenhove L, Vervliet B, et al. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol 2019; 16: 461–478. [DOI] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]


