Abstract
Precision medicine has the ambition to improve treatment response and clinical outcomes through patient stratification, and holds great potential in mental disorders. However, several important factors are needed to transform current practice into a “precision psychiatry” framework. Most important are (1) the generation of accessible large real-world training and test data including genomic data integrated from multiple sources, (2) the development and validation of advanced analytical tools for stratification and prediction, and (3) the development of clinically useful management platforms for patient monitoring that can be integrated into healthcare systems in real-life settings. This narrative review summarizes strategies for obtaining the key elements – well-powered samples from large biobanks, integrated with electronic health records and health registry data using novel artificial intelligence algorithms – to predict outcomes in severe mental disorders and translate these models into clinical management and treatment approaches. Key elements are massive mental health data and novel artificial intelligence algorithms. For the clinical translation of these strategies, we discuss a precision medicine platform for improved management of mental disorders. We include use cases to illustrate how precision medicine interventions could be brought into psychiatry to improve the clinical outcomes of mental disorders.
Keywords: Precision medicine, Psychiatry, Drug treatment outcomes, Genomics, Real-world data, Prediction algorithms
Background
Mental disorders are among the leading causes of chronic illness, disability, morbidity(1) and mortality(2), representing a major public health concern worldwide(1, 2). People living with severe and enduring mental illness, with onset usually during childhood or adolescence, are reported to have a life expectancy that is reduced by 10–20 years compared to the general population(2, 3). The main cause for the increased mortality rate is comorbidities including additional psychiatric diagnoses(4) and somatic diseases such as type 2 diabetes, hypertension, cardiovascular and respiratory diseases(5–7), but also substance use and suicide(8, 9).
A fundamental challenge in psychiatry is treatment of psychotic and affective symptoms, which are core characteristics of the severe mental disorders schizophrenia (SCZ)(10), bipolar disorder (BIP)(11) and major depressive disorder (MDD)(12). While current medications for psychotic symptoms (antipsychotics) and mood alterations (antidepressants and mood stabilizers) are effective for the majority of patients(13), there is a large variation in efficacy and adverse effects(14). Non-response to these medications is a significant clinical problem, with failure rates around 30% in SCZ(15), and similar rates in BIP(16) and MDD(12). Individuals with symptoms that do not meaningfully improve after ≥2 trials of psychotropic medications (assuming adequate dose and duration) are commonly defined as being treatment resistant(14). However, a significant challenge in the identification of factors related to psychopharmacological treatment response is the high clinical and biological heterogeneity that characterizes psychiatric disorders(17). In addition, adverse effects such as cardiometabolic alterations are common and often cause non-adherence(18, 19). Additional complexity is added by the extensive polypharmacy in psychiatry, increasing the risk for drug-drug interactions and adverse effects(20, 21). Psychopharmacological treatment often involves a trial-and-error approach, balancing between treatment effects and adverse effects(22).
Precision medicine, an approach for treatment and prevention(23, 24), aims to develop and validate clinical prediction models for therapeutic stratification(23–26). For psychopharmacology, the goal of precision medicine is to guide psychopharmacological treatments by considering individual variability in genes, environment, and lifestyle(23). Progress in both psychiatric genetics(27) and pharmacogenomics(28) will create great opportunities for improving treatment outcomes by optimizing the use of existing medications based on the patient’s genetic profile(29, 30). While the application of genomics is crucial for future precision psychiatry, it is anticipated that genomic factors contribute to disease outcomes in concert with environmental factors such as socioeconomic status, education, nutrition, and adverse life events(26, 31). Therefore, there is a need to include environmental exposures as well as non-genetic biomarkers and standard clinical data into prediction models to improve the predictive value of genomic information(31). However, the relevant datasets necessary to develop and validate precision treatment have only recently become available(23). Real-world data (RWD) is defined by the European Medicines Agency (EMA) as any type of data not collected in a randomized clinical trial (RCT)(32). The US Food and Drug Administration (FDA) defines RWD as “the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources”(33). RWD provide a unique opportunity to obtain large datasets with sufficient statistical power to leverage novel analytical methods. This will enable the development of prediction and stratification tools with the precision required for translation into clinically useful decision support tools for precision treatment in psychiatry.
The aim of this narrative review was to summarize important factors needed to bring precision medicine interventions to psychiatry. The cornerstone of these is the use of RWD collected from routine clinical assessments, a yet underexplored source of information that provides a unique opportunity to obtain massive datasets that can power both basic and applied research initiatives(23). As illustrated in Figure 1, the generation of large training and test data by integrating RWD from health care systems and biobanks, the development of advanced artificial intelligence (AI) tools for stratification and prediction, and finally the development of a management platform for clinical monitoring of patients are required to translate precision psychiatry interventions from basic science to clinical practice.
Figure 1:
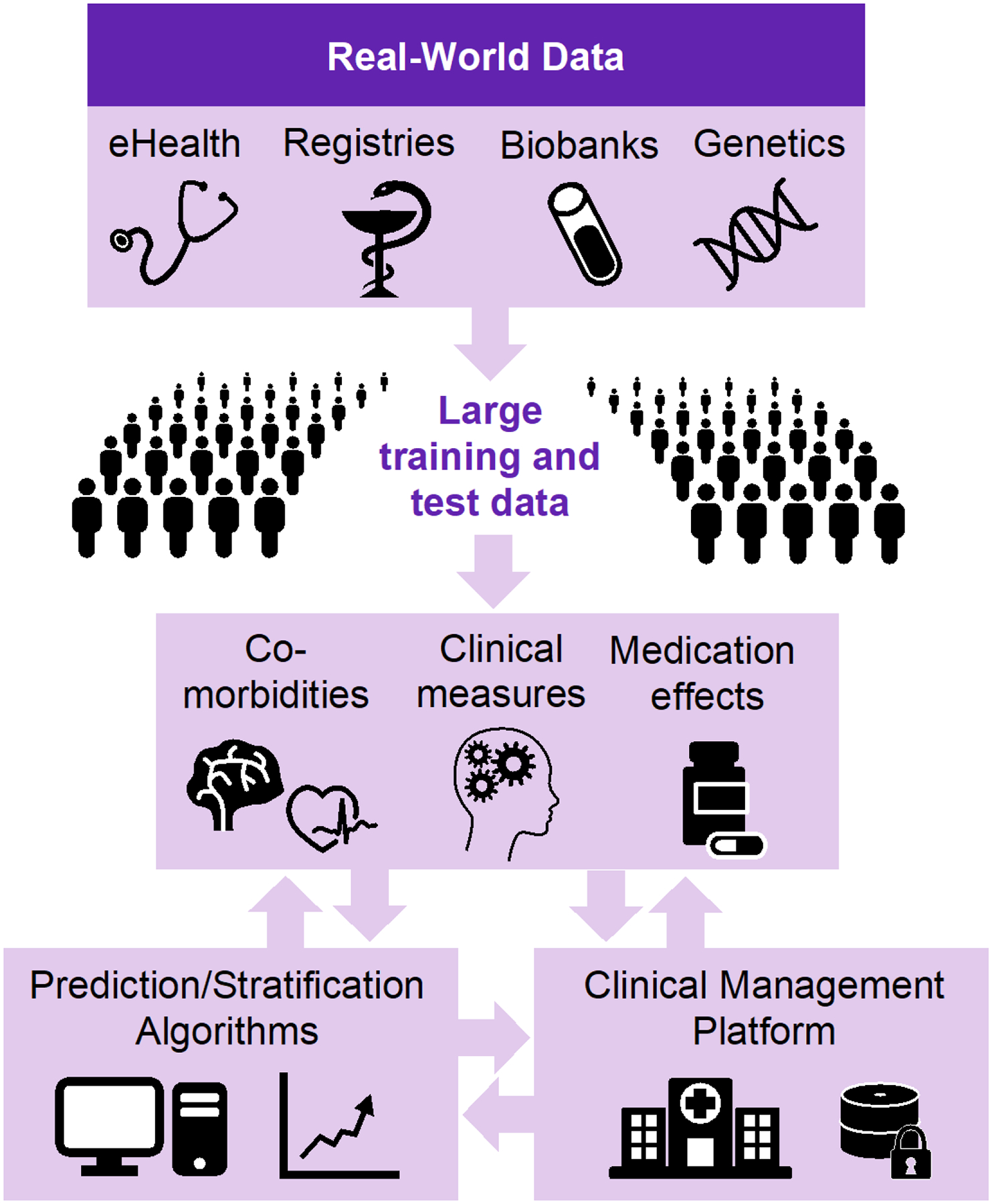
The integration of multiple big real-world data sources and prediction algorithms into a clinical management platform for precision treatment and improved outcomes in psychiatry.
Methods
This is a narrative review focusing on the use of RWD, genetic information, and prediction tools for precision psychiatry. PubMed gathered articles (up to 1st of August 2023) on “precision psychiatry”, “genetics AND precision psychiatry”, “real-world data AND precision psychiatry”, “prediction models AND precision psychiatry”, “electronic health records AND precision psychiatry”, and “treatment stratification AND psychiatry” were collated. We screeded the literature to qualitatively evaluate their relevance for the current objective and selected papers based on expertise in the writing team.
Real-world data sources
Deep phenotyping data of clinical information, including comorbidities and psychopharmacological treatment outcome data, are essential for stratification and prediction of clinical outcomes in mental disorders, but such data are difficult to obtain at a large, homogenous scale. Structured and curated RWD from health registries and hospital records/electronic health records (eHR) linked with genotype data from biobanks, as well as large-scale therapeutic drug monitoring databases or other large clinical samples of individuals with severe mental illness, can provide such data and the sample sizes needed to reach adequate power for discovering genetic factors associated with treatment outcomes in mental disorders. Nationwide prescription records provide insight into individual treatment outcomes that can be deduced from e.g., the duration and changes in type and dosage of medication(34). These proxy phenotypes can be used to estimate treatment response. The Nordic region, i.e., Denmark, Estonia, Finland, Iceland, Norway, and Sweden, offers large population-based genotyped cohorts with longitudinal data valuable for precision medicine(35). Examples of such cohorts include the Danish Neonatal Screening Biobank used by the iPSYCH project (http://www.ipsych.au.dk/), the Estonian Biobank (http://www.biobank.ee/), the FinnGen project (https://www.finngen.fi/), deCODE genetics (http://www.decode.com), and the Norwegian Mother and Child Cohort Study (MoBa) (http://www.fhi.no/MoBa), which all have been linked to drug prescription data and/or self-reported drug use and related treatment response as well as registry data relevant for precision psychiatry.
Combining existing genomics data from biobanks with these collections of RWD overcomes the limitations of data from randomized clinical trials (RCTs), from which patients with multimorbid conditions are excluded because they often require multiple drugs (polypharmacy) and are thus at greater risk of developing adverse effects. Furthermore, treatment adherence is better in RCTs than in the real world(36). Several studies have shown that RWD such as data from eHR can be utilized to identify individuals at risk for treatment resistance in MDD(37, 38) or SCZ(39). Proxies of treatment response or resistance have been defined from prescription registries(37, 38), and natural language processing has been used to refine eHR-derived treatment response definitions(40). In a meta-analysis on antipsychotic treatment discontinuation, it has been demonstrated that results from real-world studies and RCT have good congruency(41). A recent study has shown that treatment-resistant depression can be reliably defined using primary care eHR, and utilized to assess genetic, clinical and demographic characteristics of treatment-resistant depression(37). However, although eHR may facilitate stratification of risk for treatment resistance(38, 42), data from eHR are subject to high variability and confounders, requiring careful curation and validation(42). To combine information from RWD from multiple sources for integrated analysis, the RWD need to fulfil the necessary quality of the measures related to treatment efficacy and adverse effects. Further quality control is required for data harmonization of different types of RWD, collected from registries and biobanks, medical health records, large clinical research data on mental disorder cohorts, and interviews or questionnaires. To apply RWD to precision psychiatry, data quality has to be evaluated and the models have to be further improved using additional measures and external validation to evaluate their performance in real-world clinical settings(38, 42).
Genomic discovery of treatment outcomes
Severe mental disorders are complex chronic conditions with high heritability (40–80%) estimated based on twin studies(43). Recent advances in genotyping technologies have led to the discovery of hundreds of regions in the human genome harbouring risk variants for psychiatric traits, identified from genome-wide association studies (GWAS)(44). Both mental disorders(45–47) and their comorbidities(48–50) are highly polygenic, meaning that they are influenced by many genes with each genetic variant contributing a small effect towards the disorder. In aggregate, however, they explain a substantial portion of the variability of the phenotype(29). Polygenic risk scores (PRS) can be used to study the cumulative effect of disorder-associated SNPs, and may be useful in assessing disease risk. However, the predictive ability of psychiatric PRS is still insufficient for clinical utility(51, 52). With larger GWAS, improved phenotyping, and technological refinement, the predictive performance of PRS is likely to improve in the coming years(51–53), and PRS may become part of clinical psychiatry in the future(51, 54).
Emerging evidence suggests that treatment response to psychotropic medications may also have a genetic component(55, 56). Pharmacogenomic studies investigate how genetic variation affects drug metabolism (pharmacokinetics), or the molecular, biochemical, and physiologic effects of drugs (pharmacodynamics) and related adverse effects, with the aim of guiding drug prescription to improve treatment response and reduce side effects(57). Several studies have shown that pharmacogenomic testing before starting drug treatment can lead to improved patient outcomes for specific drug-gene combinations(58–61). However, pharmacogenomic information is not widely used in clinical psychiatry(28, 62), primarily due to lack of evidence on therapeutic utility in mental health conditions(63). In addition, most genetic markers identified and validated in psychopharmacogenetic studies are related to variability in pharmacokinetics, in particular drug metabolism mediated by CYP2D6 and CYP2C19(28, 62, 63), while knowledge on how genetic variation affects the pharmacodynamics of psychotropic medications is still weak(63). To provide a pharmacogenetic basis for precision treatment of psychotropic drugs, large-scale studies are therefore needed to discover genetic variants that significantly affect the pharmacotherapeutic outcomes in mental disorders(28, 62).
Knowledge of common and rare variants associated with treatment efficacy and adverse effects may be highly useful for treatment stratification, but the genetics of drug treatment outcomes are poorly understood, making prediction of drug response difficult. In addition, the degree of polygenicity of a phenotype affects the power of the GWAS(64); given that psychotropic drug treatment outcomes are polygenic(55, 56), gene discovery requires large samples. Large RWD samples with both genotypes and longitudinal treatment outcome data could allow for identification of genetic factors associated with response and adverse effects from psychotropic medication. The robust identification of genetic associations in current psychopharmacogenetic studies is limited by insufficient sample sizes as well as variability in defining treatment-related phenotypes(28). For antidepressant response, no robustly replicated associations have been detected to date(65–69). The largest GWAS on antidepressant response (N=5,151), measured using depression symptom scores, did not identify any genome-wide significant loci, likely due to its limited sample size(56). In a GWAS of treatment-resistant SCZ including the worldś largest sample of antipsychotic non-responders (NTRS=10,501 and Nnon-TRS=20,325), no genome-wide significant loci were identified(55). The largest GWAS on lithium response (N=2,563), performed by the International Consortium on Lithium Genetics (ConLiGen), identified one replicable locus(70). While the ConLiGen sample size is even smaller when compared to the GWAS of treatment-resistant SCZ(55) and antidepressant response(56), response to a specific drug, i.e., lithium, can probably be more robustly assessed than other treatment phenotypes.
While current GWAS on psychotropic drug treatment outcomes have not yielded genomic predictors that can be integrated into stratification and prediction of treatment outcomes, data from clozapine clinics in the UK and Norway have been used to conduct analyses linking genomic liability to SCZ with antipsychotic dosing, suggesting that individuals at high genomic risk for SCZ are less likely to respond to clozapine treatment at standard doses(71). A Swedish study demonstrated that lithium dose prediction was improved by using clinical and genomic data(72). Moreover, PRS for SCZ and MDD have been used to predict lithium response(73), with improved prediction when PRS were combined with clinical data using a cross-validated machine-learning regression approach(74). These insights support the strategy of studies that combine genomic information with clinical data to optimize treatment outcome prediction in psychiatry.
Big Data tools development
To transform psychiatric treatment into precision medicine, a main challenge is making multiple data sources and modalities accessible for training of new prediction algorithms.
Identifying and harmonizing phenotypic data is a key initial step towards precision medicine. A solution for distributed data analysis has been developed in the Nordic countries by the Tryggve infrastructure (www.neic.no/tryggve), building on harmonized databases and container solutions(75) for secure and efficient cross-national health research utilizing large sensitive data collections. Container technologies provide platforms to store, share and analyze genomic data in compliance with the General Data Protection Regulation (GDPR), which can be used by users from different countries and across projects to conduct genomic data analyses(75). Big Data analysis tools, such as natural language processing(76) using AI algorithms for extraction of data from eHR, as well as sequence analysis(77) for capturing phenotypic trajectories, can be extended to include nationwide prescription records. Sequence analysis(77) has been used to systematically explore life-course disease trajectories(78).
After harmonized phenotypes and genotypes are linked, the data can be used to identify common and rare risk factors for treatment response, adverse effects, and comorbidities. Differences in phenotype polygenicity and cross-trait genetic overlap motivate the development of tools such as MiXeR(79) that can improve our understanding of the genetic architecture of traits of interest and how they overlap with others. Although standard GWAS approaches can be used to investigate treatment-related phenotypes, the available sample sizes for these traits are often smaller than what is seen for disease phenotypes(27), highlighting a need for more advanced biostatistical tools, such as the following examples. MOSTest(80) exploits multivariate data to improve common variant discovery and replication rates(81–83). The conditional and conjunctional false discovery rate (FDR) approach(84, 85) can be utilized for the identification of polygenic risk factors shared between severe mental disorders and treatment response or comorbid diseases/factors(82, 86), thereby improving prediction and stratification. Applying the conditional FDR approach(85) to boost discovery of genetic variants associated with treatment-resistant SCZ after conditioning on body mass index (BMI), a largely comorbid trait, two novel loci for treatment-resistant SCZ were identified (none were found in the original GWAS of treatment-resistant SCZ)(87). Multi-trait analyses, e.g., using genomic structural equation modelling(88) and multi-trait analysis of GWAS (MTAG)(89), can also be applied for improved discovery of common variants associated with treatment outcomes, by leveraging genetic overlap between related traits.
The majority of existing GWAS approaches assess imputed rather than directly sequenced polymorphisms. For discovery of rare variants that confer risk for development of non-response or adverse effects in mental disorders, the long-range phasing method(90, 91) can be applied. This method imputes variants from sequenced data to large population samples, thereby greatly improving the discovery of rare variants(90, 91). However, discoveries from GWAS may be difficult to interpret. Therefore, various fine-mapping methods aim to identify causal SNPs among the identified variants from GWAS(92). A recently developed variational Bayesian approach for fine mapping of genomic data, Finemap-MiXeR(93), has been shown to outperform most other methods in estimating the genotype-phenotype relationship, because its fine-mapping algorithm detects more causal variants in real applications. Finemap-MiXeR enables the identification of a small number of genetic variants per locus, which are informative for predicting the phenotype in independent samples(93). Gene-set analysis (GSA) has become important to identify biological pathways and relevant tissue- and cell type-specific insights related to GWAS findings(45, 47, 94). GSA methods such as MAGMA(95), Fisher’s exact (hypergeometric) test(96), and stratified linkage disequilibrium (LD) score regression (sLDSC)(97), have become important for understanding the biological implications of GWAS findings(98). A novel GSA tool, GSA-MiXeR(99), estimates fold enrichment and identifies gene-sets with greater biological specificity compared to standard GSA approaches, providing new insights into the pathobiology of complex polygenic disorders, which may help to advance the classification, diagnosis, and treatment of mental disorders(99).
Finally, phenotypic and genetic information obtained using the tools and methods described above can be integrated to improve prediction of treatment outcomes and comorbidities(100, 101). The Polygenic Hazard Score (PHS)(102), a tool for prediction of age of disease onset initially applied to Alzheimer’s disease(102), can be employed for prediction of drug response and adverse effects. PHS(102) applies the Cox proportional hazard model to GWAS data of the disease and information on its age of onset to estimate instantaneous risk of disease development. Thus, PHS provides a fruitful framework to move polygenic information towards clinical utility.
Taken together, to reach the vision of precision treatment, gene discoveries must be leveraged by novel analytical algorithms to enable translation into clinical use. By combining genetic information with clinical and lifestyle data in prediction of treatment outcomes, prediction accuracy can be improved. Novel AI statistical approaches and improved prediction and stratification algorithms both for pharmacological treatment outcomes and multimorbid disease trajectories will open new avenues of treatment of mental disorders and their accompanying comorbidities, to identify an optimal treatment regimen and improve patients’ quality of life.
Validation before clinical use
To test the validity of the genotype-phenotype associations for genetic variants associated with treatment outcomes, replication in independent real-world samples is required. In a recent study(103), an interaction between a previously identified variant in the NFIB gene(104) and CYP1A genes on clozapine serum concentrations in smokers and non-smokers has been identified. Specifically, patients who smoke and carry the studied CYP1A and NFIB variants may need threefold higher doses of clozapine(103). Moreover, the previously mentioned study showing that clozapine dosage is positively correlated with polygenic risk for SCZ, found this association in three independent samples of treatment-resistant SCZ, supporting the clinical impact of pharmacogenetics for precision dosing of clozapine(71). However, large real-world replication cohorts are needed to validate genetic discoveries from GWAS of psychotropic drug treatment outcomes.
RWD offers also opportunities for validation and refining of the prediction models(105), i.e., to determine treatment outcomes in patients for whom accurate prediction is not possible, and to identify additional data to improve the prediction capabilities for other clinical decisions. The ascertainment of individuals with specific genomic variants and subsequent evaluation in recall studies of real-world patients, known as reverse-phenotyping(106), enables validation of a given prediction profile to ensure that the established genetic prediction models are valid. For genotype-phenotype associations of treatment outcomes, reverse-phenotyping of patients who have started psychotropic drug treatment can be done. By splitting those cases into groups of patients with a high predicted likelihood of a positive treatment outcome, patients with a high predicted likelihood of a negative treatment outcome, and those for which the model could not accurately predict outcome status, the developed algorithms can be validated. Likewise, the prediction models can be refined through the collection of additional clinical and outcome information on individuals for which accurate prediction was not possible. Thereby, the outcome of interest can be determined, and additional data can be identified to improve the prediction capabilities of the model in these individuals. This will help to estimate the accuracy of methods and facilitate the collection of additional relevant data, potentially allowing for the development of more accurate prediction and stratification algorithms with clinical utility.
Clinical implementation and utility
Using and combining multi-disciplinary RWD from biobanks, hospitals, registries, self-reports, and medical records, as well as data from clinical research will contribute to advance the knowledge, clinical management, and pharmacological treatment of mental disorders. To implement precision medicine in clinical practice, especially crossing country borders, natural language processing tools(76) can be used for data extraction and harmonization across data sources and countries, and container technologies can be used as a platform for cross-border analysis with tools available for standardizing various data in a unified manner across countries(75). Once large, deep-phenotyped RWD become available for clinical use, the prediction models can be trained and validated for different clinical and ethnic subgroups as well as stratified by age and sex to improve outcome prediction(107, 108). By developing and validating advanced stratification and prediction tools based on measurable biomarkers, namely genotypes in combination with drug treatment outcomes as well as other response predictors (symptoms, disease history, cardiometabolic blood markers, BMI etc.), patients who do not respond to available pharmacological treatments can be identified. Identifying non-responsive patients will enable economic savings while avoiding adverse effects derived from the administration of ineffective and unnecessary treatments. This will enable health and regulatory authorities to improve the standards of care in terms of safety, quality, and effectiveness of medication therapies.
Currently, there are no tools for prediction of treatment outcomes in psychiatry that are used clinically. A clinical decision support tool building on prediction and stratification algorithms integrated with digital tools could potentially improve disease outcomes. Such a clinical management platform should be designed as an integrated software solution that incorporates the baseline information about risk factors and outcome predictors (clinical information, socio-demographics, genetics) with the prediction and stratification algorithms. These algorithms could be integrated with a software system for inclusion of follow-up and outcome data such as specific adverse effects (e.g., obesity, motor disturbance), self-reports (e.g., somnolence, sexual dysfunction), biomarkers (e.g., glucose levels, lipids), and socioeconomic factors collected from registries (e.g., socioeconomic status, education). To make the platform a clinically relevant tool, the monitoring system should build on the integrative clinical decision support analytics, and include specific recommendations for interventions at critical time points during disease progress, such as change of medication type and dose, physical activity, healthier diet, and referral to specialists in other disciplines (cardiology, endocrinology) when needed. The monitoring system should have a user-friendly dashboard, where clinicians can quickly, easily, and securely access their patients’ analytics and reports to inform clinical decision-making for optimal monitoring. Such a platform could contain information that helps clinicians to answer practical, ethical, and user-related questions that must be addressed to implement precision psychiatry. Combining multi-source data and algorithms with new data retrieved from clinical practice while using the platform, the prediction models will be further improved. Through improved prediction, the development of a clinical management platform might ultimately enable earlier diagnosis, including co-morbidities, facilitate planning of individual treatment, and improve clinical strategies to reduce adverse effects as well as preventing complications related to polypharmacy. In sum, a clinical management platform for monitoring of psychiatric patients, integrating prediction tools with clinical information, could have a strong impact on the quality of life of individuals with mental disorders. However, the platform should be used in accordance with the wishes of the patients, ensuring that data can be deleted when a patient revokes consent for data processing.
Ethical considerations
The use of RWD and prediction models for precision psychiatry carries ethical challenges(23, 109), in particular privacy protection for individuals contributing to RWD. Ethical concerns have particularly raised about using genomic information, including informed consent, sample collection, storage, identifiability of the samples, re-identification, sharing samples throughout the world, and privacy and confidentiality(110, 111). Informed consent for genetic material should contain information about sample storage, anonymity, and an option for withdrawing the samples(112, 113). Data protection issues must be addressed by data protection legislation(114) and the implementation of secure data systems to ensure that the RWD are impossible to identify and the data are securely handled. In Europe, secure data handling environments must align with requirements from the GDPR and upcoming European Health Data Space (EHDS), especially when databases are cross-linked. Software container technologies with tools for data capture, harmonization and standard analysis can fulfil these requirements and be used across borders to conduct large-scale genomic and phenotypic data analyses(75).
For the clinical use of prediction and stratification tools, the requirements of regulations such as the EU Medical Device Regulations must be fulfilled. In addition, the safety, performance, and benefit-risk ratios of the software tools need to be established prior to their clinical use. By applying secure cloud-based solutions in accordance with GDPR and clinical security systems, it is possible to build a versatile infrastructure that can support management platforms across health care systems.
Conclusions
To bring precision medicine interventions to psychiatry, RWD from health care systems combined with biobanks and research data can solve the need for large-scale data necessary for training and testing of prediction models related to treatment outcomes in mental disorders. The implementation of a RWD infrastructure, novel tools to exploit these large datasets, and a clinical management platform with prediction algorithms for medication response and adverse effects offers large opportunities for precision psychiatry to improve treatment outcomes and quality of life of individuals with mental disorders.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 964874. We also acknowledge support from the Research Council of Norway (296030, 223273), grants from South-Eastern Norway Regional Health Authority (2020060), and the Estonian Research Council (PRG184).
Disclosures
Dr. Andreassen reported grants from Stiftelsen Kristian Gerhard Jebsen, South-East Regional Health Authority, Research Council of Norway, and European Union’s Horizon 2020 during the conduct of the study; personal fees from cortechs.ai (stock options), Lundbeck (speaker’s honorarium), and Sunovion (speaker’s honorarium) and Janssen (speaker’s honorarium) outside the submitted work. Drs. Walters and O’Donovan have received grant funding from Takeda for work unrelated to this paper and from the Medical research Council (UK), from European Union’s Horizon 2020, and Akrivia Health to develop linked genomic and electronic health resources. Dr. Sullivan is a shareholder and SAB member for Neumora Therapeutics. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.G. B. D. Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM. Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. British Journal of Psychiatry. 2018;199(6):453–8. [DOI] [PubMed] [Google Scholar]
- 4.Plana-Ripoll O, Pedersen CB, Holtz Y, Benros ME, Dalsgaard S, de Jonge P, et al. Exploring Comorbidity Within Mental Disorders Among a Danish National Population. JAMA Psychiatry. 2019;76(3):259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plana-Ripoll O, Pedersen CB, Agerbo E, Holtz Y, Erlangsen A, Canudas-Romo V, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. The Lancet. 2019;394(10211):1827–35. [DOI] [PubMed] [Google Scholar]
- 6.Corell CU, Solomi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–81. [DOI] [PubMed] [Google Scholar]
- 8.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlangsen A, Andersen PK, Toender A, Laursen TM, Nordentoft M, Canudas-Romo V. Cause-specific life-years lost in people with mental disorders: a nationwide, register-based cohort study. The Lancet Psychiatry. 2017;4(12):937–45. [DOI] [PubMed] [Google Scholar]
- 10.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. The Lancet. 2022;399(10323):473–86. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar disorders. The Lancet. 2020;396(10265):1841–56. [DOI] [PubMed] [Google Scholar]
- 12.Malhi GS, Mann JJ. Depression. The Lancet. 2018;392(10161):2299–312. [DOI] [PubMed] [Google Scholar]
- 13.Huhn M, Tardy M, Spineli LM, Kissling W, Förstl H, Pitschel-Walz G, et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry. 2014;71(6):706–15. [DOI] [PubMed] [Google Scholar]
- 14.Howes OD, Thase ME, Pillinger T. Treatment resistance in psychiatry: state of the art and new directions. Mol Psychiatry. 2022;27(1):58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane JM, Correll CU. The Role of Clozapine in Treatment-Resistant Schizophrenia. JAMA Psychiatry. 2016;73(3):187–8. [DOI] [PubMed] [Google Scholar]
- 16.Gitlin M Treatment-resistant bipolar disorder. Mol Psychiatry. 2006;11(3):227–40. [DOI] [PubMed] [Google Scholar]
- 17.Kinon BJ. The Group of Treatment Resistant Schizophrenias. Heterogeneity in Treatment Resistant Schizophrenia (TRS). Front Psychiatry. 2018;9:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–60. [DOI] [PubMed] [Google Scholar]
- 19.Novick D, Haro JM, Suarez D, Perez V, Dittmann RW, Haddad PM. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2–3):109–13. [DOI] [PubMed] [Google Scholar]
- 20.Möller HJ, Seemüller F, Schennach-Wolff R, Stübner S, Rüther E, Grohmann R. History, background, concepts and current use of comedication and polypharmacy in psychiatry. The International Journal of Neuropsychopharmacology. 2013;17(07):983–96. [DOI] [PubMed] [Google Scholar]
- 21.Wolff J, Hefner G, Normann C, Kaier K, Binder H, Hiemke C, et al. Polypharmacy and the risk of drug–drug interactions and potentially inappropriate medications in hospital psychiatry. Pharmacoepidemiology and Drug Safety. 2021;30(9):1258–68. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida K, Takeuchi H. Dose-dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia. Behav Brain Res. 2021;402:113098. [DOI] [PubMed] [Google Scholar]
- 23.Denny JC, Collins FS. Precision medicine in 2030-seven ways to transform healthcare. Cell. 2021;184(6):1415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17(9):507–22. [DOI] [PubMed] [Google Scholar]
- 25.Adams ST, Leveson SH. Clinical prediction rules. BMJ. 2012;344:d8312. [DOI] [PubMed] [Google Scholar]
- 26.Franks PW, Melen E, Friedman M, Sundstrom J, Kockum I, Klareskog L, et al. Technological readiness and implementation of genomic-driven precision medicine for complex diseases. J Intern Med. 2021;290(3):602–20. [DOI] [PubMed] [Google Scholar]
- 27.Andreassen OA, Hindley G, Frei O, Smeland OB. New insights from the last decade of research in psychiatric genetics- discoveries, challenges and clinical implications. World Psychiatry. 2023;22:4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardinas AF, Owen MJ, Walters JTR. Pharmacogenomics: A road ahead for precision medicine in psychiatry. Neuron. 2021;109(24):3914–29. [DOI] [PubMed] [Google Scholar]
- 29.Breen G, Li Q, Roth BL, O’Donnell P, Didriksen M, Dolmetsch R, et al. Translating genome-wide association findings into new therapeutics for psychiatry. Nat Neurosci. 2016;19(11):1392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jukic MM, Haslemo T, Molden E, Ingelman-Sundberg M. Impact of CYP2C19 Genotype on Escitalopram Exposure and Therapeutic Failure: A Retrospective Study Based on 2,087 Patients. Am J Psychiatry. 2018;175(5):463–70. [DOI] [PubMed] [Google Scholar]
- 31.Johansson A, Andreassen OA, Brunak S, Franks PW, Hedman H, Loos RJF, et al. Precision medicine in complex diseases-Molecular subgrouping for improved prediction and treatment stratification. J Intern Med. 2023;294(4):378–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arlett P, Kjaer J, Broich K, Cooke E. Real-World Evidence in EU Medicines Regulation: Enabling Use and Establishing Value. Clin Pharmacol Ther. 2022;111(1):21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Food US and Administration Drug. Real-World Evidence. 2023. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Accessed 27 Sep 2023. [Google Scholar]
- 34.Wettermark B, Zoega H, Furu K, Korhonen M, Hallas J, Norgaard M, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research--a literature review. Pharmacoepidemiol Drug Saf. 2013;22(7):691–9. [DOI] [PubMed] [Google Scholar]
- 35.Njolstad PR, Andreassen OA, Brunak S, Borglum AD, Dillner J, Esko T, et al. Roadmap for a precision-medicine initiative in the Nordic region. Nat Genet. 2019;51(6):924–30. [DOI] [PubMed] [Google Scholar]
- 36.Allemann SS, Nieuwlaat R, Navarro T, Haynes B, Hersberger KE, Arnet I. Congruence between patient characteristics and interventions may partly explain medication adherence intervention effectiveness: an analysis of 190 randomized controlled trials from a Cochrane systematic review. J Clin Epidemiol. 2017;91:70–9. [DOI] [PubMed] [Google Scholar]
- 37.Fabbri C, Hagenaars SP, John C, Williams AT, Shrine N, Moles L, et al. Genetic and clinical characteristics of treatment-resistant depression using primary care records in two UK cohorts. Mol Psychiatry. 2021;26(7):3363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lage I, McCoy TH, Jr., Perlis RH, Doshi-Velez F. Efficiently identifying individuals at high risk for treatment resistance in major depressive disorder using electronic health records. J Affect Disord. 2022;306:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadra-Scalzo G, Fonseca de Freitas D, Agbedjro D, Francis E, Ridler I, Pritchard M, et al. A predictor model of treatment resistance in schizophrenia using data from electronic health records. PLoS One. 2022;17(9):e0274864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlis RH, Iosifescu DV, Castro VM, Murphy SN, Gainer VS, Minnier J, et al. Using electronic medical records to enable large-scale studies in psychiatry: treatment resistant depression as a model. Psychol Med. 2012;42(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katona L, Bitter I, Czobor P. A meta-analysis of effectiveness of real-world studies of antipsychotics in schizophrenia: Are the results consistent with the findings of randomized controlled trials? Transl Psychiatry. 2021;11(1):510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grzenda A, Widge AS. Electronic health records and stratified psychiatry: bridge to precision treatment? Neuropsychopharmacology. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47(7):702–9. [DOI] [PubMed] [Google Scholar]
- 44.Wendt FR, Pathak GA, Tylee DS, Goswami A, Polimanti R. Heterogeneity and Polygenicity in Psychiatric Disorders: A Genome-Wide Perspective. Chronic Stress (Thousand Oaks). 2020;4:2470547020924844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trubetskoy V, Pardinas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604(7906):502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nature Genetics. 2021;53(6):817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience. 2019;22(3):343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingelsson E, McCarthy MI. Human Genetics of Obesity and Type 2 Diabetes Mellitus. Circulation: Genomic and Precision Medicine. 2018;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schunkert H, von Scheid M, Kessler T, Stiller B, Zeng L, Vilne B. Genetics of coronary artery disease in the light of genome-wide association studies. Clin Res Cardiol. 2018;107:2–9. [DOI] [PubMed] [Google Scholar]
- 51.Smeland OB, Andreassen OA. Polygenic risk scores in psychiatry - Large potential but still limited clinical utility. Eur Neuropsychopharmacol. 2021;51:68–70. [DOI] [PubMed] [Google Scholar]
- 52.Lewis ACF, Green RC, Vassy JL. Polygenic risk scores in the clinic: Translating risk into action. HGG Adv. 2021;2(4):100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai N, Revez JA, Adams MJ, Andlauer TFM, Breen G, Byrne EM, et al. Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet. 2020;52(4):437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray GK, Lin T, Austin J, McGrath JJ, Hickie IB, Wray NR. Could Polygenic Risk Scores Be Useful in Psychiatry?: A Review. JAMA Psychiatry. 2021;78(2):210–9. [DOI] [PubMed] [Google Scholar]
- 55.Pardinas AF, Smart SE, Willcocks IR, Holmans PA, Dennison CA, Lynham AJ, et al. Interaction Testing and Polygenic Risk Scoring to Estimate the Association of Common Genetic Variants With Treatment Resistance in Schizophrenia. JAMA Psychiatry. 2022;79(3):260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pain O, Hodgson K, Trubetskoy V, Ripke S, Marshe VS, Adams MJ, et al. Identifying the Common Genetic Basis of Antidepressant Response. Biol Psychiatry Glob Open Sci. 2022;2(2):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF, et al. Pharmacogenomics. Lancet. 2019;394(10197):521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coenen MJ, de Jong DJ, van Marrewijk CJ, Derijks LJ, Vermeulen SH, Wong DR, et al. Identification of Patients With Variants in TPMT and Dose Reduction Reduces Hematologic Events During Thiopurine Treatment of Inflammatory Bowel Disease. Gastroenterology. 2015;149(4):907–17 e7. [DOI] [PubMed] [Google Scholar]
- 59.Henricks LM, Lunenburg C, de Man FM, Meulendijks D, Frederix GWJ, Kienhuis E, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 2018;19(11):1459–67. [DOI] [PubMed] [Google Scholar]
- 60.Mallal S, Phillips E, Carosi G, Molina J-M, Workman C, Tomažič T, et al. HLA-B*5701 Screening for Hypersensitivity to Abacavir. N Engl J Med. 2008;358:568–79. [DOI] [PubMed] [Google Scholar]
- 61.Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, et al. A Genotype-Guided Strategy for Oral P2Y(12) Inhibitors in Primary PCI. N Engl J Med. 2019;381(17):1621–31. [DOI] [PubMed] [Google Scholar]
- 62.van Schaik RHN, Muller DJ, Serretti A, Ingelman-Sundberg M. Pharmacogenetics in Psychiatry: An Update on Clinical Usability. Front Pharmacol. 2020;11:575540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bousman CA, Bengesser SA, Aitchison KJ, Amare AT, Aschauer H, Baune BT, et al. Review and Consensus on Pharmacogenomic Testing in Psychiatry. Pharmacopsychiatry. 2021;54(1):5–17. [DOI] [PubMed] [Google Scholar]
- 64.Holland D, Wang Y, Thompson WK, Schork A, Chen CH, Lo MT, et al. Estimating Effect Sizes and Expected Replication Probabilities from GWAS Summary Statistics. Front Genet. 2016;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.GENDEP, MARS, STAR*D. Common Genetic Variation and Antidepressant Efficacy in Major Depressive Disorder: A Meta-Analysis of Three Genome-Wide Pharmacogenetic Studies. Am J Psychiatry. 2013;170(2):207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biernacka JM, Sangkuhl K, Jenkins G, Whaley RM, Barman P, Batzler A, et al. The International SSRI Pharmacogenomics Consortium (ISPC): a genome-wide association study of antidepressant treatment response. Transl Psychiatry. 2015;5:e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabbri C, Kasper S, Kautzky A, Bartova L, Dold M, Zohar J, et al. Genome-wide association study of treatment-resistance in depression and meta-analysis of three independent samples. Br J Psychiatry. 2019;214(1):36–41. [DOI] [PubMed] [Google Scholar]
- 68.Tansey KE, Guipponi M, Perroud N, Bondolfi G, Domenici E, Evans D, et al. Genetic predictors of response to serotonergic and noradrenergic antidepressants in major depressive disorder: a genome-wide analysis of individual-level data and a meta-analysis. PLoS Med. 2012;9(10):e1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabbri C, Tansey KE, Perlis RH, Hauser J, Henigsberg N, Maier W, et al. New insights into the pharmacogenomics of antidepressant response from the GENDEP and STAR*D studies: rare variant analysis and high-density imputation. Pharmacogenomics J. 2018;18(3):413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet. 2016;387(10023):1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kappel DB, Legge SE, Hubbard L, Willcocks IR, O’Connell KS, Smith RL, et al. Genomic stratification of clozapine prescription patterns using schizophrenia polygenic scores. Biological Psychiatry. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Millischer V, Matheson GJ, Bergen SE, Coombes BJ, Ponzer K, Wikstrom F, et al. Improving lithium dose prediction using population pharmacokinetics and pharmacogenomics: a cohort genome-wide association study in Sweden. Lancet Psychiatry. 2022;9(6):447–57. [DOI] [PubMed] [Google Scholar]
- 73.Schubert KO, Thalamuthu A, Amare AT, Frank J, Streit F, Adl M, et al. Combining schizophrenia and depression polygenic risk scores improves the genetic prediction of lithium response in bipolar disorder patients. Transl Psychiatry. 2021;11(1):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cearns M, Amare AT, Schubert KO, Thalamuthu A, Frank J, Streit F, et al. Using polygenic scores and clinical data for bipolar disorder patient stratification and lithium response prediction: machine learning approach. Br J Psychiatry. 2022:1–10. [DOI] [PubMed] [Google Scholar]
- 75.Akdeniz B, Frei O, Hagen E, Filiz T, Karthikeyan S, Pasman J, et al. COGEDAP: A COmprehensive GEnomic Data Analysis Platform. arXiv. 2022. [Google Scholar]
- 76.Kreimeyer K, Foster M, Pandey A, Arya N, Halford G, Jones SF, et al. Natural language processing systems for capturing and standardizing unstructured clinical information: A systematic review. J Biomed Inform. 2017;73:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguena Nguefack HL, Page MG, Katz J, Choiniere M, Vanasse A, Dorais M, et al. Trajectory Modelling Techniques Useful to Epidemiological Research: A Comparative Narrative Review of Approaches. Clin Epidemiol. 2020;12:1205–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Momen NC, Plana-Ripoll O, Agerbo E, Benros ME, Borglum AD, Christensen MK, et al. Association between Mental Disorders and Subsequent Medical Conditions. N Engl J Med. 2020;382(18):1721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frei O, Holland D, Smeland OB, Shadrin AA, Fan CC, Maeland S, et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat Commun. 2019;10(1):2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Meer D, Frei O, Kaufmann T, Shadrin AA, Devor A, Smeland OB, et al. Understanding the genetic determinants of the brain with MOSTest. Nat Commun. 2020;11(1):3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schork AJ, Thompson WK, Pham P, Torkamani A, Roddey JC, Sullivan PF, et al. All SNPs are not created equal: genome-wide association studies reveal a consistent pattern of enrichment among functionally annotated SNPs. PLoS Genet. 2013;9(4):e1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andreassen OA, Thompson WK, Schork AJ, Ripke S, Mattingsdal M, Kelsoe JR, et al. Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9(4):e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smeland OB, Frei O, Dale AM, Andreassen OA. The polygenic architecture of schizophrenia - rethinking pathogenesis and nosology. Nat Rev Neurol. 2020;16(7):366–79. [DOI] [PubMed] [Google Scholar]
- 84.Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophrenia bulletin. 2014;40(1):13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smeland OB, Frei O, Shadrin A, O’Connell K, Fan CC, Bahrami S, et al. Discovery of shared genomic loci using the conditional false discovery rate approach. Hum Genet. 2020;139(1):85–94. [DOI] [PubMed] [Google Scholar]
- 86.Andreassen OA, Djurovic S, Thompson WK, Schork AJ, Kendler KS, O’Donovan MC, et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. American journal of human genetics. 2013;92(2):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Connell KS, Koch E, Lenk HC, Akkouh IA, Hindley G, Jaholkowski P, et al. Polygenic overlap with body-mass index improves prediction of treatment-resistant schizophrenia. Psychiatry Res. 2023;325:115217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav. 2019;3(5):513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sedlazeck FJ, Lee H, Darby CA, Schatz MC. Piercing the dark matter: bioinformatics of long-range sequencing and mapping. Nat Rev Genet. 2018;19(6):329–46. [DOI] [PubMed] [Google Scholar]
- 91.Kong A, Masson G, Frigge ML, Gylfason A, Zusmanovich P, Thorleifsson G, et al. Detection of sharing by descent, long-range phasing and haplotype imputation. Nat Genet. 2008;40(9):1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schaid DJ, Chen W, Larson NB. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat Rev Genet. 2018;19(8):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akdeniz BC, Frei O, Shadrin A, Vetrov D, Kropotov D, Hovig E, et al. Finemap-MiXeR: A variational Bayesian approach for genetic finemapping. bioRxiv. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hill WD, Marioni RE, Maghzian O, Ritchie SJ, Hagenaars SP, McIntosh AM, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 2019;24(2):169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11(4):e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simillion C, Liechti R, Lischer HE, Ioannidis V, Bruggmann R. Avoiding the pitfalls of gene set enrichment analysis with SetRank. BMC Bioinformatics. 2017;18(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47(11):1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Leeuw CA, Neale BM, Heskes T, Posthuma D. The statistical properties of gene-set analysis. Nat Rev Genet. 2016;17(6):353–64. [DOI] [PubMed] [Google Scholar]
- 99.Frei O, Hindley G, Shadrin AA, van der Meer D, Akdeniz BC, Cheng W, et al. Improved functional mapping with GSA-MiXeR implicates biologically specific gene-sets and estimates enrichment magnitude. medRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beam AL, Kohane IS. Big Data and Machine Learning in Health Care. JAMA. 2018;319(13):1317–8. [DOI] [PubMed] [Google Scholar]
- 101.Fogel AL, Kvedar JC. Artificial intelligence powers digital medicine. NPJ Digit Med. 2018;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, et al. Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score. PLoS Med. 2017;14(3):e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lenk HC, Lovsletten Smith R, O’Connell KS, Jukic MM, Kringen MK, Andreassen OA, et al. Impact of NFIB and CYP1A variants on clozapine serum concentration-A retrospective naturalistic cohort study on 526 patients with known smoking habits. Clin Transl Sci. 2023;16(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith RL, O’Connell K, Athanasiu L, Djurovic S, Kringen MK, Andreassen OA, et al. Identification of a novel polymorphism associated with reduced clozapine concentration in schizophrenia patients-a genome-wide association study adjusting for smoking habits. Transl Psychiatry. 2020;10(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riley RD, Ensor J, Snell KI, Debray TP, Altman DG, Moons KG, et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: opportunities and challenges. BMJ. 2016;353:i3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilczewski CM, Obasohan J, Paschall JE, Zhang S, Singh S, Maxwell GL, et al. Genotype first: Clinical genomics research through a reverse phenotyping approach. American journal of human genetics. 2023;110(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walsh CG, Chaudhry B, Dua P, Goodman KW, Kaplan B, Kavuluru R, et al. Stigma, biomarkers, and algorithmic bias: recommendations for precision behavioral health with artificial intelligence. JAMIA Open. 2020;3(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koutsouleris N, Dwyer DB, Degenhardt F, Maj C, Urquijo-Castro MF, Sanfelici R, et al. Multimodal Machine Learning Workflows for Prediction of Psychosis in Patients With Clinical High-Risk Syndromes and Recent-Onset Depression. JAMA Psychiatry. 2021;78(2):195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fusar-Poli P, Manchia M, Koutsouleris N, Leslie D, Woopen C, Calkins ME, et al. Ethical considerations for precision psychiatry: A roadmap for research and clinical practice. Eur Neuropsychopharmacol. 2022;63:17–34. [DOI] [PubMed] [Google Scholar]
- 110.Haga SB, Beskow LM. Ethical, legal, and social implications of biobanks for genetics research. Adv Genet. 2008;60:505–44. [DOI] [PubMed] [Google Scholar]
- 111.Breckenridge A, Lindpaintner K, Lipton P, McLeod H, Rothstein M, Wallace H. Pharmacogenetics: ethical problems and solutions. Nat Rev Genet. 2004;5(9):676–80. [DOI] [PubMed] [Google Scholar]
- 112.Hansson MG, Dillner J, Bartram CR, Carlson JA, Helgesson G. Should donors be allowed to give broad consent to future biobank research? Lancet Oncol. 2006;7(3):266–9. [DOI] [PubMed] [Google Scholar]
- 113.Rotimi CN, Marshall PA. Tailoring the process of informed consent in genetic and genomic research. Genome Med. 2010;2(3):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hallinan D A Normative Framework for the Reconciliation of EU Data Protection Law and Medical Research Ethics. Med Law Rev. 2021;29(3):446–67. [DOI] [PubMed] [Google Scholar]


