
Keywords: traumatic brain injury, brain–gut–microbiome axis, gut microbiota, neuroimmune, immunosuppression, host defense, vagal afferents, bacterial infection, dorsal root ganglia, nociception neural circuitry
Abstract
Traumatic brain injury is a prevalent disorder of the central nervous system. In addition to primary brain parenchymal damage, the enduring biological consequences of traumatic brain injury pose long-term risks for patients with traumatic brain injury; however, the underlying pathogenesis remains unclear, and effective intervention methods are lacking. Intestinal dysfunction is a significant consequence of traumatic brain injury. Being the most densely innervated peripheral tissue in the body, the gut possesses multiple pathways for the establishment of a bidirectional “brain–gut axis” with the central nervous system. The gut harbors a vast microbial community, and alterations of the gut niche contribute to the progression of traumatic brain injury and its unfavorable prognosis through neuronal, hormonal, and immune pathways. A comprehensive understanding of microbiota-mediated peripheral neuroimmunomodulation mechanisms is needed to enhance treatment strategies for traumatic brain injury and its associated complications. We comprehensively reviewed alterations in the gut microecological environment following traumatic brain injury, with a specific focus on the complex biological processes of peripheral nerves, immunity, and microbes triggered by traumatic brain injury, encompassing autonomic dysfunction, neuroendocrine disturbances, peripheral immunosuppression, increased intestinal barrier permeability, compromised responses of sensory nerves to microorganisms, and potential effector nuclei in the central nervous system influenced by gut microbiota. Additionally, we reviewed the mechanisms underlying secondary biological injury and the dynamic pathological responses that occur following injury to enhance our current understanding of how peripheral pathways impact the outcome of patients with traumatic brain injury. This review aimed to propose a conceptual model for future risk assessment of central nervous system-related diseases while elucidating novel insights into the bidirectional effects of the “brain–gut–microbiota axis.”
Introduction
Adverse outcomes in patients with traumatic brain injury (TBI) may persist for a long time and may include complications such as bacterial infections, gastrointestinal (GI) diseases, and further deterioration of neural transmission. These involve a complex range of pathophysiological mechanisms, including excitotoxicity, oxidative stress, inflammation, immune suppression, cell apoptosis, and autophagy (Hanscom et al., 2021). Despite attempts to provide effective defense strategies through animal experiments, the prognosis of patients with severe TBI remains unfavorable. Mounting evidence suggests that the gut microbiota plays a crucial role in maintaining health and managing disease in the host, particularly within the CNS (Benakis et al., 2016; Sundman et al., 2017). The concept of the “microbiota–gut–brain axis,” which governs bidirectional gut–brain communication, has been firmly established, facilitating the maintenance of physiological equilibrium (Sundman et al., 2017).
Peripheral influences play a pivotal role in regulating the physiological processes of the brain in both healthy and diseased states. It serves as the primary mode of signal transmission by integrating incoming and outgoing signals from neural, hormonal, and immune sources to establish a systematic connection between the gut–spinal cord–brain complex (Panther et al., 2022). Dysfunction of this axis occurs as a pathological consequence of disease onset. The role of gut microbiota in neurological and psychiatric disorders has gradually been uncovered, as gut dysbiosis disrupts the development and maturation of the CNS and enteric nervous system (ENS) by unfavorably affecting the host’s immune response (Warner, 2019; Chen et al., 2022). The feedback regulation involving the brain, gut, and microbiota is complex and closely interconnected, and several questions are still worth considering, such as whether CNS injury directly affects changes in gut microbiota and their metabolites, or if it indirectly acts through peripheral neuro-immune crosstalk. How alterations in the gut microbiota and neuronal activity influence the localization of initial injury, the signaling molecules involved, and the sites where potential enduring effects manifest all need to be clarified; therefore, this review on the bidirectional communication between gut microbiota and the CNS aims to clarify these concepts.
This article highlights the significance of the bidirectional “brain–gut–microbiota axis” in influencing the adverse prognosis and functional behavior post-TBI through the involvement of neuro-immune and endocrine factors. The present review utilizes TBI as a case to propose a theoretical framework for comprehending the potential mechanisms underlying risks after CNS injury. Our approach transcends the limited effects of alterations in the gut microbiome to emphasize the interconnections between anatomically independent systems, thereby establishing a foundation for effective clinical prevention and treatment.
Search Strategy
The relevant literature was retrieved by conducting an electronic search of the PubMed database between September 7 and October 22, 2023. During the search strategy and selection criteria, we employed the following keywords: “traumatic brain injury,” “central nervous system injury,” “brain–gut axis,” “sympathetic nervous system,” “vagus nerve,” “intestinal barrier,” “neuroinflammation,” “immunosuppression,” “neuroendocrine,” “enteric nervous system,” “sensory afferent nerve,” “host defense mechanisms post-traumatic pain,” and “post-traumatic depression.” Various combinations of these search terms were comprehensively utilized to access the literature. Additionally, we conducted a thorough review of relevant literature titles and abstracts to further enhance the screening process. Since CNS injury also includes various disease types, such as ischemic stroke, hemorrhagic stroke, and spinal cord injury. In this narrative review, we try to focus only on the study of TBI and its complications, along with the underlying pathogenic mechanisms.
Negative Impacts on Gut Microbiota Post–Traumatic Brain Injury
The occurrence of TBI leads to several secondary effects, and the disruption of the GI environment caused by CNS damage triggers an ecological imbalance, potentially leading to systemic inflammation and compromised neurological outcomes. A better comprehension of alterations in the composition of the gut microbiota following TBI and the underlying mechanisms of brain–gut interaction can enhance the functional prognosis of CNS injury.
Modify the composition and abundance of gut microbiota
CNS damage significantly affects the abundance and diversity of bacteria residing in the gut. Clinical trial data indicate that severe TBI results in a significant reduction in intestinal bacteria, with levels reducing to 1/1000 of those of healthy controls on the day of injury, particularly the levels of anaerobic bacteria and Lactobacillus spp. Moreover, 2 weeks post-TBI, the intestinal flora and concentration of major short-chain fatty acids (SCFAs) do not return to normal levels, while the population of harmful Enterococcus and Pseudomonas gradually increase (Hayakawa et al., 2011). Patients with chronic TBI exhibit enduring alterations in the composition of the gut microbiome, characterized by elevated levels of Actinobacteria, Firmicutes, and Ruminococcaceae and decreased levels of Bacteroidetes and Prevotella (Ma et al., 2017; Urban et al., 2020; Table 1). In mouse models, at 24 hours after controlled cortical impact, the populations of Lactobacillus gastricus, Ruminococcus flavus, and Eubacterium gastroensis deceased significantly, while the populations of Eubacterium sulci and Marwinia forme increased significantly (Treangen et al., 2018). Moreover, previous studies have reported increased abundance of Firmicutes, Proteobacteria, and Ruminococcus, as well as an increase in the population of Bacteroidetes, Lactobacillus, and Clostridium difficile 7 days post TBI in rodents (Treangen et al., 2018; Celorrio et al., 2021; Taraskina et al., 2022; Table 1). Additionally, the population of Bifidobacterium—one of the key microorganisms that promote the production of γ-aminobutyric acid (GABA) in the body to regulate the response of the gut–brain axis—increased significantly 7 days after a severe controlled cortical impact injury (Bao et al., 2023). Therefore, given that the severity of brain damage caused by various methods varies and changes in gut flora at different time points post-TBI exhibit some dissimilarities, it indicates heterogeneity in the cascade of molecular effects directly resulting from TBI.
Table 1.
Effect of traumatic brain injury on the gut microbiome
| Species | Traumatic brain injury method | Study range | Effect of microbiota | Reference |
|---|---|---|---|---|
| Sprague–Dawley male rats | Closed head injury model of engineered rotational acceleration | Both 1 and 9 d post-injury | Firmicutes/Bacteroidetes ratio increased; Perturbations of Firmicutes lineages | Smith et al., 2024 |
| C57BL/6 male and female mice | Controlled cortical impact injury | 3 d or 7 wk post-injury | Lactobacillus is the majority; Dubosiella increased in male mice; Faecalibaculum increased in female mice | Holcomb et al., 2024 |
| C57BL/6 male mice | Controlled cortical impact injury | 3 d post-injury | Alphaproteobacteria, Actinobacteria, Gammaproteobacteria increased; Bacteroidia, Epsilonproteobacteria, 4C0d-2 decreased | Ma et al., 2019 |
| Traumatic brain injury group: 21 males, 1 female; control group: 13 males, 5 females; | Type of traumatic brain injury incurred not reported | 27−502 mon post-injury | Chronic traumatic brain injury increased Actinobacteria, Firmicutes, and Verrucomicrobia and decreased Bacteriodetes; family-level changes: chronic traumatic brain injury increased Unc03qxR and Unc02cwq and decreased Prevotellaceae; species-level changes: chronic traumatic brain injury increased Bacteroides thetaiotaomicron and decreased Prevotella copri, Prevotella spp., and Sutterella spp. | Urban et al., 2020 |
| C57BL/6J mice, male, 6−8 wk | Controlled cortical impact | 7 and 3−90 d post-injury | Depletion of Lactobacillus, Clostridium, and Bacteroides; Atopobium and Ruminococcus increased | Celorrio et al., 2021 |
| Patients with traumatic brain injury in 2015 and provided fecal sample in 2020 | Type of traumatic brain injury incurred not reported | 5 yr post-injury | Traumatic brain injury induced the Corynebacterium genus and Alistipes genus increased; the Corynebacterium and Pseudomonas were detected significantly | Pyles et al., 2024 |
Influence the adhesion and colonization of the gut microbiota
The harmonious symbiosis of intestinal flora in vivo relies on its adhesion and colonization capabilities (Macpherson et al., 2023). The aberrant expansion and migration of intestinal endogenous bacteria during a diseased state constitute a significant risk factor for peripheral infection following TBI. Comparing the cross-colonization of microbiota from different donor sources in the body revealed that the host can select its own microbiota by facilitating microbial adhesion through antimicrobial peptides (AMPs) or mucopolysaccharides (Figure 1). Goblet cells secrete mucins, which are highly glycosylated products that are important for the selection of symbiotic bacteria by providing attachment sites for bacterial adhesins (Arike and Hansson, 2016). A previous study demonstrated that abnormal mucin glycosylation is a consequential outcome of infections and inflammation and serves as a crucial regulatory mechanism for the colonization of intestinal commensal bacteria within the body (Magalhães et al., 2015). Additionally, a specific spectrum of biofilms is formed by microorganisms that colonize the mucus layer of the intestinal epithelium, exhibiting selectivity in subsequent species adhesion (Engevik et al., 2021). Overall, the mechanisms coordinating host-microbiota colonization are vital for resisting infections by opportunistic pathogens.
Figure 1.
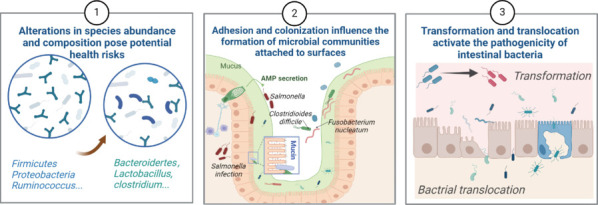
Adverse consequences of TBI on gut microbiota.
TBI induces alterations in the abundance and composition of gut microbiota, which may contribute to the pathogenesis of TBI sequelae by disrupting the symbiotic relationship between microbiota and intestinal epithelial cells. Epithelial cells play a crucial role in maintaining bacterial adhesion through mucin secretion and AMPs, while a unique mutual selection mechanism exists within the bacterial community. Moreover, during disease progression, commensal bacteria undergo degeneration and migration, leading to peripheral infection via intestinal bacterial translocation. Created with BioRender.com. AMPs: Antimicrobial peptides; TBI: traumatic brain injury.
Unleash the potential pathogenicity of the gut microbiota
A previous study shows that the gut microbiota may be capable of restoring CNS damage in neurological disorders (Howard et al., 2017). However, in most cases, a shift in the host’s commensal microbiota towards opportunistic pathogens can significantly impair the prognosis of TBI (Figure 1). After severe TBI, there is a significant increase in the abundance of Lachnoclostridium, Acinetobacter, Bacteroides, and Streptococcus in the gut of mice 3 hours post-injury. After 7 days, the relative abundance of Acinetobacter in the lungs increases to 16.2%, and an analysis of lung tissue microbiota revealed that 49.69% of microorganisms originated from the gut, ultimately leading to lung infection (Yang et al., 2022b). The disruption of the intestinal mucosal epithelial barrier serves as the primary mechanism for opportunistic pathogens to invade, encompassing various multicellular pathways such as downregulation of paracellular tight junction complexes, impaired secretion of AMPs by Paneth cells, and dysregulation of homeostasis in microfold cells within intestinal lymph nodes (Iacob et al., 2018; Drolia and Bhunia, 2019; Fekete and Buret, 2023). Hence, tracing the origins, functions, and changes of microorganisms in the brain, lungs, and urinary tract is an effective approach for preventing poor prognosis after TBI.
Top-Down Modulation of the Brain–Gut–Microbiota Axis: Dysregulation of the Microbiota Following Central Nervous System Injury
Gut–brain communication maintains the homeostasis of both the CNS and GI, and this influences the composition and function of the gut microbiota, as well as the integrity of the intestinal barrier. It also plays a role in immune monitoring within both visceral organs and the circulatory system through neuro-immune–endocrine efferent signals. These processes collectively shape a healthy ecological environment within the GI (Li et al., 2020; Neufeld et al., 2024; Zhang et al., 2024). An acute TBI implies a dynamic process wherein significant therapeutic opportunities arise from identifying and addressing potential delays. However, the precise interplay between the brain-gut-microbiota triad and its impact on TBI recovery remains elusive.
Autonomic nervous system perspectives for traumatic brain injury
Sympathetic nervous system disorders
Paroxysmal sympathetic hyperactivity is a primary etiology of secondary nerve injury in patients with TBI. Diffuse or focal brain injury disrupts the connectivity between one or more brain centers and cortical inhibitory centers, such as the hippocampus, amygdala, insular cortex, cingulate cortex, and other brainstem nuclei. This disruption leads to the interruption of descending inhibitory pathways, loss of inhibition of spinal sympathetic reflexes, increased excitatory interneurons activity, and increased sympathetic activity (Jafari et al., 2022). The SNS releases more neurotransmitters after TBI, including catecholamines, epinephrine, norepinephrine, and dopamine (Straub et al., 2006). These neurotransmitters trigger opposing pro-inflammatory and anti-inflammatory responses in the gut, depending on the receptors they bind to (Straub et al., 2000, 2002). The induction of sympathetic denervation by 6-hydroxydopamine results in an increase in activated CD68+CD86+ macrophages and monocytes within the intestinal mucosa. This leads to an up-regulation of proinflammatory, such as cytokines interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and interferon gamma (IFN-γ), while down-regulating the expression of arginase-1 and CD163. Consequently, there is a decrease in intestinal epithelial barrier function and an enhancement of antimicrobial defense (Mallesh et al., 2022). The SNS also plays a regulatory role in the modulation of T helper 1 (Th1), Th17, and regulatory T cells (Tregs) within the intestinal environment (Kasper and Shoemaker, 2010; Figure 2). Demyelination occurs when proinflammatory Th1/Th17 cells migrate to the CNS (Lee et al., 2020; Lin et al., 2021). SNS-induced dendritic cells can partially counteract the effects of Th1/Th17 cells and restore homeostasis within the gut-brain axis under pathological conditions. Additionally, ablation of β-adrenergic receptors inhibits systemic immune responses (resulting in a decrease in the number of circulating CD4+ T cells) and also induces elevated levels of beneficial SCFAs within the colon.
Figure 2.

The impact of traumatic brain injury on the brain–gut–microbiome axis from a top-down perspective.
(1) After TBI, the SNS regulates the presence of CD68+CD86+ macrophages and monocytes in the gut, thereby playing a crucial role in maintaining intestinal barrier integrity and enhancing antimicrobial defense ability. (2) The activation of the VN leads to the stimulation of the descending cholinergic anti-inflammatory pathway. The release of ACh by VN binds to receptors on immune cells and inhibits inflammatory responses. In addition, ACh induced the activation of the WNT pathway contributes to AMP secretion and initiates host defense. The activation of VN suppresses the inflammatory response of myometrial macrophages via the JAK2/STAT3 signaling pathway. (3) TBI results in damage to the intestinal mucosal barrier, characterized by epithelial cell apoptosis and an increased population of microfold cells. (4) TBI induces activation of the HPA axis, resulting in the elevated release of ACTH and corticosterone levels, which subsequently leads to increased paracellular permeability of intestinal epithelial cells. (5) Moreover, TBI results in the hyperactivation of adrenergic neurons within the ENS and triggers the conversion of monocytes into an anti-inflammatory phenotype. (6) TBI results in peripheral immunosuppression, leading to a shift from Th1 cells towards Th2 cells and inducing spleen atrophy. Created with BioRender.com. ACh: Acetylcholine; ACTH: adrenocorticotropic hormone; AMP: antimicrobial peptide; ENS: enteric nervous system; HPA axis: hypothalamic–pituitary–epinephrine axis; JAK2: Janus kinase 2; SNS: sympathetic nervous system; STAT3: signal transducer and activator of transcription 3; VN: vagus nerve; WNT: canonical Wnt/β-catenin pathway.
The innervation effect of the SNS on the colon of mice gradually increases in a proximal-to-distal manner (Muller et al., 2020). A previous study has demonstrated an upregulation of proto-oncogene protein c-fos expression in both the celiac ganglion and superior mesenteric ganglion of germ-free (GF) mice, indicating evident neuronal activation (Muller et al., 2020). However, following fecal microbiota transplantation (FMT), there is a decrease in c-fos expression, suggesting that exogenous sympathetic nerves possess direct sensing capabilities and provide feedback responses to intestinal microorganisms. Following CNS injury, increased SNS activity in the gut results in increased permeability of the intestinal mucosal barrier and peripheral immunosuppression (Stanley et al., 2016). This is evidenced by the upregulation of triggering receptors expressed on myeloid cells 1 in inflammatory macrophages, leading to intestinal bacterial translocation and adverse outcomes associated with peripheral infection (Liu et al., 2019; Figure 2). Therefore, the SNS pathway is responsible for the disruption of the gut microbiota post-TBI.
Vagus nerve dysfunction
TBI leads to dysfunction of the autonomic nervous system. The vagus nerve (VN), which originates from the paraventricular nucleus (PVN) of the hypothalamus, serves as a primary component of the parasympathetic nervous system. Its descending nerve fibers project towards the pituitary gland and ventral tegmental area, where they influence both the hypothalamic–pituitary–adrenal (HPA) axis and dopamine system. Various studies have demonstrated that post-TBI, VN stimulation (VNS) can effectively restore forelimb movement and coordination capabilities in rats, thereby leading to improved neurological function scores (Pruitt et al., 2016; Du et al., 2024). The application of VNS can attenuate the upregulation of aquaporin protein-4, restore the integrity of the blood–brain barrier (BBB), induce angiogenesis, enhance metabolic activity in key regions, such as the forebrain, thalamus, and reticular structure, elicit a broad spectrum of cortical activation, facilitate brain arousal, and ultimately promote restoration of the injured brain area (Neren et al., 2016; Collins et al., 2021; Divani et al., 2023). Following neurological trauma, a cascade of secondary damage occurs, including excessive generation of free radicals, which surpasses the capacity of antioxidant mechanisms and leads to irreversible oxidative injury. VNS can safeguard against nerve injury in rats after TBI by suppressing oxidative stress, inflammation, and apoptosis, which is mediated through the nuclear factor kappa-B/nucleotide-binding domain-like receptor protein 3 signaling pathway (Tang et al., 2020). Furthermore, it has been discovered that the PVN of the VN nucleus synthesizes oxytocin, which exerts an inhibitory effect on microglial activation by downregulating extracellularly regulated protein kinases and P38 phosphorylation (Yuan et al., 2016). The findings imply that the activation of the VN plays a crucial role in ameliorating neurodevelopmental abnormalities and neurological impairments.
VN input is the main anti-inflammatory pathway in the gut, as it releases acetylcholine, which binds to muscarinic receptors, promotes proper differentiation, maintains peripherally derived Tregs (Nakata et al., 2022), and enhances the secretion of AMPs and lysozyme (Shi et al., 2014; Labed et al., 2018), while simultaneously inhibiting the release of inflammatory factors by macrophages (Stakenborg et al., 2019; Yang et al., 2021; Figure 2). This intricate mechanism serves as a crucial regulator of the immune ecological niche in the gut. VNS treatment can ameliorate the reduction of CD103+ cells in intestinal lymph nodes, increase the Treg/Th17 ratio, and promote intestinal tolerance to inflammation in rats with hemorrhagic shock after a traumatic injury (Morishita et al., 2015). The expression of GABAA receptors in the dorsal nucleus of the neurons of the VN can be suppressed by electroacupuncture therapy. Furthermore, simultaneously stimulating the efferent pathway of the VN and activating the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway mediated by the α7 nicotinic acetylcholine receptor on intestinal macrophages inhibits intestinal inflammation (Yang et al., 2021; Figure 2). Additionally, the prophylactic administration of a 5-hydroxytryptamine receptor 4 inhibitor results in a significant reduction in intestinal inflammation. However, this anti-inflammatory effect is not observed in α7 nicotinic acetylcholine receptor knockout mice, indicating an interaction between 5-HT and the cholinergic anti-inflammatory pathway (Stakenborg et al., 2019).
Although the gut microbiota can communicate with the CNS through endocrine and immune pathways, vagal signaling is one of the fastest and most direct routes (Yuan et al., 2021). The VN remains intact following TBI, and sensory afferents drive motor output to establish a neural reflex circuitry. Clinical studies have demonstrated that in patients with heightened intestinal barrier permeability, brief stimulation of the VN prevents an increase in paracellular permeability and impedes the dissemination of pathogenic microorganisms (Reese, 1987; Krzyzaniak et al., 2012; Bonaz, 2022). The findings of infection-related studies suggest that VNS treatment effectively enhances the release of acetylcholine, which enables the host mucosa to effectively combat intestinal pathogens (Ramirez et al., 2019; Chu et al., 2021). On one hand, the egl-30–egl-8 axis, which encodes Gaq and phospholipase Cb in muscarinic receptors, has been found to confer resistance against Staphylococcus aureus (Labed et al., 2018). On the other hand, intestinal infection triggers the secretion of acetylcholine by ENS neurons, which in turn activates the downstream WNT/β-catenin pathway and initiates the barrier defense mechanisms by intestinal epithelial cells (Labed et al., 2018; Figure 2). Additionally, T cell-derived Ach can promote the transcription of Ifny and other genes, which effectively reduces the bacterial load of Citrobacter rodentium in the gut and establishes a stable host-symbiotic environment (Ramirez et al., 2019). Therefore, cholinergic signaling is a key mediator in maintaining the brain–gut–microbial ecological balance.
Neuroendocrine perspectives for traumatic brain injury
A significant risk factor for TBI is the presence of post-traumatic stress disorder, and clinical evidence suggests that approximately 25% of patients with TBI exhibit adrenal insufficiency resulting from the inhibition of HPA axis activation (Powner and Boccalandro, 2008). The occurrence of TBI results in hypopituitarism, which suppresses the stress response of the HPA axis and diminishes adrenocorticotropic hormone production, thereby impairing adrenal gland stimulation and reducing corticosterone synthesis (Figure 2). The apoptosis rate and Caspase-3 expression of adrenal cells are elevated during the acute stage of mild TBI, while the apoptotic protein expression in pituitary cells is increased during the chronic stage of mild TBI, indicating that apoptosis plays a role in the occurrence and progression of endocrine dysfunction in the HPA axis following TBI (Taheri et al., 2022).
Intestinal barrier damage is a pathological characteristic of intestinal complications resulting from post-traumatic stress. However, studies have demonstrated that probiotics actively participate in inhibiting the excessive reaction of the HPA axis, thereby reducing plasma adrenocorticotropic hormone and corticosterone concentrations. This ultimately preserves the paracellular permeability of colonic epithelial cells and plays a protective role in maintaining intestinal integrity (Ait-Belgnaoui et al., 2012; Figure 2). Gut microbiota can modulate hormone release by influencing the activity of key components of the limbic system, such as the amygdala, hippocampus, and thalamus (Bastiaanssen et al., 2019). Similarly, the HPA axis can impact changes in gut microbiota through endocrine factors (Sudo, 2014; Figure 2). Excessive activation of the β-adrenergic system can lead to cortisol depletion, which further perpetuates the pathological mechanism of chronic inflammation (Bugajski et al., 1995; Abdullahi et al., 2020; Zhao et al., 2022). It has been demonstrated that the disruption of the sympathetic–adrenal medullary system mediates immune suppression and increases susceptibility to infection in mice with high-level spinal cord injury (Prüss et al., 2017). Prophylactic adrenalectomy effectively mitigates the occurrence of glucocorticoid overdose and lymphocyte depletion induced by spinal cord injury; however, it is not protective against pneumonia.
Intestinal microenvironment perspectives for traumatic brain injury
Breach of intestinal barrier integrity
The bidirectional communication between the CNS and ENS is crucial for the regulation of various GI functions, including motility, secretion, blood flow, intestinal permeability, mucosal immune activity, and visceral sensation (Rhee et al., 2009; Liu et al., 2024). The functional and structural dysfunction of the GI tract after TBI has been demonstrated in both human subjects and experimental models (Bansal et al., 2010; Urban et al., 2020). Adrenergic hyperactivity mediates small intestinal mucosal injury and increased permeability in experimental rats 72 hours post-TBI (Yang et al., 2022b). This disruption results in the translocation of intestinal commensal bacteria, along with their by-products, such as lipopolysaccharide (LPS), leading to peripheral infection and potentially sepsis.
Apoptosis of intestinal epithelial cells and loss of tight junction proteins between epithelial cells result in increased gut barrier permeability after TBI, relying on the release of metalloproteinases and reactive oxygen species by neutrophils (Pan et al., 2019). Mitochondrial autophagy effectively attenuates oxidative stress-induced epithelial cell apoptosis through the inhibition of the extracellular regulated protein kinases/nuclear factor erythroid 2-related factor 2/heme oxygenase-1 signaling pathway (Liu et al., 2017), for which CCAAT/enhancer binding protein deta may be an upstream target (Banerjee et al., 2019). Moreover, gut microbial disorders are associated with mucus barrier breakdown, when the mucus layer thins, which exposes more antigens and bacteria to the epithelium (Paone and Cani, 2020). Ablation of recombinant forkhead box protein O1, which maintains goblet cell function, significantly increases susceptibility to GI inflammation (Chen et al., 2021). The polysaccharide structure of mucus facilitates the function of the intestinal immune barrier by binding to lectin-like proteins present in immune cells, such as the mucin 2 receptor complex, thereby inhibiting the proinflammatory effects of dendritic cells (Shan et al., 2013).
Once pathogens break through the physical and mucus barriers, innate immunity is first activated, followed by adaptive immunity. The host has evolved a large amount of mucosa-associated lymphoid tissue (enrichment of T and B lymphocytes) for host defense, which can stimulate the secretion of regenerating islet–derived protein 3 gamma by intestinal epithelial cells during Gram-negative bacteria activity (Abreu, 2010). Moreover, GF mice exhibit diminished sizes of Peyer’s patches, decreased production of immunoglobulin A, and lowered expression of Toll-like receptors (Shirakashi et al., 2022). The mucosa-associated lymphoid tissues in the GI encompass a unique subset of microfold cells that function as a conduit for specific microorganisms to enter and exit (Kobayashi et al., 2019; Figure 2). When the differentiation and maturation of microfold cells are impaired, it results in reduced B cell follicle size and diminished secretion of immunoglobulin A. Consequently, the initiation of adaptive immunity in the gut is hindered, thereby facilitating the escape of pathogenic microorganisms from the gut after central impairment (Nagashima et al., 2017).
Abnormalities of the enteric nervous system
Multiple genes implicated in CNS dysfunction, such as Chd8, Tcf4, Slc6a4, Shank3, and Nlgn3, also contribute to ENS dysfunction and ultimately result in functional GI disorders associated with CNS damage (Niesler and Rappold, 2021). The excessive activation of the adrenergic system has been demonstrated to result in impaired intestinal physiological function in TBI experiments. Noradrenergic neurons in the ENS exert their effects on intestinal monocytes via β2 adrenergic receptors, as they promote the differentiation of myometrial macrophages into an M2 phenotype (Gabanyi et al., 2016), suppress the production of Th1 cytokines by dendritic cells, and impede the differentiation of monocytes into Th1 cells in vivo (Panina-Bordignon et al., 1997; Ramer-Quinn et al., 1997; Figure 2). The ENS neurons induce goblet cell secretion of AMP and mucus by producing IL-18, thereby impeding the colonization of Salmonella bacteria to facilitate host defenses (Jarret et al., 2020). In addition, the absence of neuromodulatory mechanisms in enteric glial cells affects the immunoregulatory function of IL-22 release from ILC3 cells, which impairs the defense function of intestinal epithelial cells, leads to dysbiosis of the microflora, and increases susceptibility to intestinal infections (Sonnenberg et al., 2011; Ibiza et al., 2016).
Peripheral immunosuppression perceptions for traumatic brain injury
Stage I: burst of central inflammation
The immune system must appropriately respond to injury, and TBI is accompanied by a range of secondary effects that continuously pose challenges to the peripheral immune system. Damage to the CNS and BBB after TBI leads to leakage of brain-derived antigens and inflammatory mediators, leading to a systemic inflammatory reaction syndrome while triggering increased activity of resident innate immune cells within the brain. Additionally, circulating leukocytes, complement proteins, and inflammatory cytokines are activated. Following the activation of the myeloid cell system, neutrophils initially infiltrate the injured brain tissue, followed by varying degrees of natural killer cell and T cell aggregation on the ipsilateral side of the injury. These immune cells are recruited through multiple pathways, including lymphoid tissue, blood vessels, arachnoid membranes or the choroid plexus (Celorrio et al., 2024; Figure 2). The initiation of adaptive immune responses and the establishment of unique immune memory are facilitated by antigen presenting cells, which present antigenic peptides to immune cells (Jha et al., 2019). The SNS and the HPA axis act as mediators that coordinate peripheral immune responses with central inflammatory reactions (Wong et al., 2011; McCulloch et al., 2017).
Stage II: peripheral immunodeficiency
TBI stimulation leads to an elevation in the levels of inflammatory factors, such as TNF-α, IL-1β, as well as chemokines chemokine (C–X–C motif), ligand 1 protein (CXCL1), CXCL2, and CXCL3, among others. The expression of triggering receptors that are expressed on myeloid cells 1 on peripheral myeloid cells amplifies the pro-inflammatory response of both brain-derived and gut-derived immune components (Liu et al., 2019). When the proinflammatory response becomes excessive, the body will release anti-inflammatory factors as a compensatory mechanism; however, this may result in an increased susceptibility to peripheral infections (Celorrio et al., 2024). The primary mechanism underlying CNS injury-induced immunosuppression involves a transition from a Th1 to a Th2 lymphocyte phenotype, accompanied by reduced lymphocyte and NK cell counts in both the bloodstream and spleen, as well as compromised neutrophil and monocyte defense mechanisms (Prass et al., 2003; Faura et al., 2021; Figure 2). The occurrence of pulmonary edema and pneumonia with systemic immunosuppression has been observed 24 hours after experimental TBI (Vermeij et al., 2013). The immune dysfunction persists for several weeks following TBI, and studies conducted on mice with closed head injuries have confirmed a reduction in the number of peripheral blood monocytes within 24 hours post-injury, which continues for up to 1 month (Schwulst et al., 2013). Additionally, there is a shift towards an M2 phenotype characterized by anti-inflammatory abilities. Administration of Streptococcus pneumoniae infected TBI mice resulted in elevated levels of high mobility group box 1 in the peripheral circulation and elevated IL-1β and TNF-α in the cerebral cortex, whereas TNF-α in the lung was relatively reduced compared to that in the lungs of mice that were given Streptococcus pneumoniae infection with sham surgery (Doran et al., 2020). Furthemore, the expression of M2-like macrophage markers was increased, leading to the manifestation of more severe bacterial infections in the lungs, thereby confirming that TBI compromises monocyte immune function.
Role of the hypothalamic–pituitary–adrenal axis in peripheral immunity
Activation of the HPA axis following brain injury has been demonstrated to stimulate the secretion of glucocorticoids (Jehn et al., 2010), which subsequently modulates B cell differentiation and suppresses B cell lymphopoiesis (Igarashi et al., 2005). The administration of glucocorticoids in mice infected with Trypanosoma cruzi and Mycobacterium avium induces immune organ atrophy, which can be reversed to restore peripheral immune function to a state of normal physiological function by inhibiting the HPA axis (Majlessi et al., 2006; Pérez et al., 2007). In addition, mice exhibit evident signs of systemic immunosuppression, and atrophy of the spleen and thymus accompanied by lymphopenia have been observed during brain injury caused by Angiostrongylus cantonensis infection; however, the reduction in B and T cells was not attributed to apoptosis but rather resulted from impaired development following thymocyte activation and compromised B cell genesis (Chen et al., 2016; Qiao et al., 2023). Further preclinical and randomized controlled trials are required to elucidate the profound impact of alternative therapies on the activation of the HPA axis–induced immunosuppression, specifically focusing on their effects on the gut microbiota in patients with TBI.
Role of brain-spleen crosstalk on peripheral immunity
Acute brain injury results in spleen atrophy, highlighting the crucial role of the spleen in maintaining immune homeostasis both centrally and peripherally (Yu et al., 2021). Studies have shown that mice treated with LPS exhibit depressive-like phenotypes, resulting in abnormal expression of ionized calcium binding adapter molecule 1 and postsynaptic density protein 95 in the hippocampus, while denervation of the spleen effectively restores elevated plasma IL-6 and leads to significant changes in microbiota composition (Erny et al., 2015; Ma et al., 2022a). The SNS and HPA axis serve as mechanisms for brain–spleen communication, wherein norepinephrine binds to β2 adrenergic receptors and activates glucocorticoid receptors to directly suppress lymphocyte activity or cellular immunity (Figure 2). Furthermore, they enhance the secretion of anti-inflammatory cytokines or cortisol and even promote apoptosis of immune cells by interacting with VN, leading to increased synthesis of Th2 cytokines (Sharma et al., 2019; Celorrio et al., 2024). The far-reaching effects of neuro-immune regulatory mechanisms between the brain, spleen, and gut after TBI necessitate further elucidation.
Down-Top Alterations in the Brain–Gut–Microbiota Axis: Disruptions in Gut Microbiota Exacerbate Central Nervous System Symptoms
Post-traumatic pain is a common clinical manifestation after TBI. The inflammatory response and microbial disorders are the main causes of this pain. A study showed that inhibiting the release of substance P from transient receptor potential vanilloid 1 (TRPV1) mechanoreceptors can reduce damage-related Tau hyperphosphorylation and improve cognitive and behavioral disorders after TBI (Corrigan et al., 2021). Moreover, this hinders the up-regulation of TRPV1 in cerebral vascular endothelium cells induced by TBI, and assumes an anti-apoptotic role to protect the integrity of the BBB (Yang et al., 2019). It is evident that sensory afferents play a crucial role in the pathophysiology and pathology of TBI. However, recent studies have demonstrated that the depletion of the gut microbiota can effectively prevent or significantly suppress neuropathic pain induced by chronic nerve injury (Ma et al., 2022b; Chen et al., 2023). The transplantation of fresh fecal bacteria has been shown to restore the manifestation of neuropathic pain behavior (Ma et al., 2022b). This suggests that targeting the gut microbiota could be a promising therapeutic approach for treating neuropathic pain. The host’s response and coordination with gut bacterial pathogens is a highly intricate phenomenon that integrates multiple signaling pathways (Góralczyk-Bińkowska et al., 2022; Rastogi et al., 2022). Herein, we elucidate the underlying mechanisms of “down-top” signaling triggered by alterations in the microbiota after TBI and its unfavorable prognosis.
Sensory perception and defense of the gut microbiome
The role of microbes in the regulation of the CNS in neurological disorders has been well established. However, it is important to acknowledge that sensory afferents from the spinal cord and the vagal ganglion represent the two primary pathways through which information from the GI tract is transmitted to the CNS (Breit et al., 2018; Perna et al., 2021; Ma et al., 2022b). The cell bodies of these neurons are located in the dorsal root ganglia (DRG) and tuberous/jugular ganglia (Figure 3). Spinal afferents terminate at the dorsal horn of the spinal cord, while vagal afferents project to the nucleus of the solitary tract in the brainstem (Adidharma et al., 2022). The activation of nerve terminal nociceptors is commonly believed to be initiated by inflammatory mediators produced by immune cells (Chavan et al., 2017). This means that afferent signals are transmitted from the periphery to the spinal cord, while efferent impulses propagate from nerve axons back to nerve terminals, resulting in the local release of vasoactive mediators in the surrounding tissues. This phenomenon is known as “neurogenic inflammation” (Matsuda et al., 2019). Recent studies have demonstrated that sensory neurons directly respond to various bacterial products, including bacterial toxins and metabolites (Lai et al., 2017; Baral et al., 2018; Saelinger et al., 2019). Pain is elicited upon bacterial invasion of the body, and nociceptive neurons transmit this aversive sensation upwards through a variety of mechanical and chemical molecular sensors. Peripheral involvement in the prevention and control of bacterial infection has emerged as a prominent research focus in recent years. Therefore, we examined the potential mechanisms underlying host sensing and defense against microbial alterations, translocation, invasion, and infection following TBI.
Figure 3.

The potential mechanisms by which the brain senses and responds to microbial changes.
The ascending transmission involves vagal afferents terminating in the DMV and dorsal root afferents of the spinal cord, whose terminals are equipped with molecular sensors to detect inflammatory mediators and microbiota alterations. The secretions and metabolites produced by bacteria can also activate nociceptors and induce an increase in intracellular calcium flux, which in turn, stimulates the nociceptors to release neurotransmitters (such as CGRP) for signal transmission to the host brain. Additionally, EECs possess intrinsic sensory conduction properties, enabling the formation of neural circuits with vagal afferents to facilitate the transmission of alterations in the GI milieu upwards. Created with BioRender.com. CGRP: Calcitonin-gene–related peptide; DMV: dorsal nucleus of vagus nerve; EECs: enteroendocrine cells.
Vagal afferent perspectives
The chemoreceptors of vagal afferents play a crucial role in the transmission of glucose, fat, and appetite-related signals (Williams et al., 2016; Han et al., 2018; Berthoud et al., 2021). A previous study involving experimental bacterial infection models revealed that the microbial sensing ability of vagal afferents and oral administration of Helicobacter pylori or Salmonella typhimurium significantly activated the expression of c-fos in the vagal ganglia and nucleus of the solitary tract (Riley et al., 2013). Therefore, the vagal afferent pathway may serve as a potential monitoring mechanism in the progression of TBI-induced augmented intestinal barrier permeability and subsequent peripheral infection resulting from gut bacterial translocation.
The activation of vagal afferents contributes to the regulation of neuro-immune interactions through the expression of immune factor-related receptors, thereby maintaining body homeostasis. When degeneration of neurons in the dorsal nucleus of the VN impairs complete signal transmission within the VN circuit, there is an increase in the number of inflammatory monocytes and mature macrophages, resulting in a significant release of pro-inflammatory factors (such as TNF-α and CXCL1), thereby exacerbating intestinal inflammation (Wirtz et al., 2007; Qi et al., 2012). TRPV1+ nociceptors have been shown to regulate innate immune responses in fatal pneumonia caused by drug-resistant Staphylococcus aureus. Targeted depletion of these neurons by diphtheria toxin improves neutrophil and γδT cell responses, thereby promoting efficient bacterial clearance and improving host survival (Baral et al., 2018). After Staphylococcus aureus infection, Myd88–/– mice exhibited heightened pain-like hypersensitivity at 72 hours post-infection. The aforementioned studies provide compelling evidence implicating bacterial activity in sensory signaling via immune activation (Hanke et al., 2012; Figure 3). Additionally, bacteria directly trigger calcium influx and action potentials in nociceptors through N-formylated peptides and pore-forming toxin α-hemolysin (Chiu, 2018; Figure 3), implying an unsuspected role in the host-pathogen interaction of GI dysfunction following CNS injury.
Neuropodocytes, a specific subset of enteroendocrine cells (EECs), actively participate in ascending signal transmission through the establishment of synaptic connections with the VN. Neuropodocytes exhibit electrical excitability and employ glutamate as the neurotransmitter for synaptic transmission with the vagal nerve, thereby establishing a neuroepithelial circuit that connects the GI lumen to the brainstem (Figure 3). This helps the brain to quickly perceive intestinal stimuli (Bellono et al., 2017; Kaelberer et al., 2018). The activation of the Gaolf/adenylate cyclase signaling cascade in EECs by isovaleric acid, a type of SCFA, promotes intracellular Ca2+ influx mediated by the sensory receptors Trpa1 and Olfr558 (Psichas et al., 2015; Bellono et al., 2017; Figure 3). The process activates intracellular signaling factors in EECs, including prominent molecular sensors, such as G protein-coupled receptors, Toll-like receptors, and microbial associated molecular pattern receptors (Lai et al., 2017; Kaelberer et al., 2020). When the EEC senses LPS, G protein-coupled receptors are activated, leading to the release of glucagon-like peptide-1, which compromises the integrity of the intestinal mucosa (Lebrun et al., 2017; Figure 3). In the context of TBI, the role of intestinal epithelial and immune cells in GI surveillance and the initiation of protective responses following gut barrier breakdown is well established, while the contribution of vagal afferents remains underestimated.
Spinal afferent nerve perspectives
Spinal afferent nerves undoubtedly play a crucial role in rapid signal transmission between the GI and CNS. Anterograde tracing experiments in mouse DRGs have revealed the presence of exogenous efferent terminals in the GI tract. These external neurons originate in the DRG of the spinal cord, project throughout the GI tract and relevant immune sites (lymph nodes, mucosa-associated lymphoid tissues), and are responsive to noxious stimuli, such as bacterial pathogens and toxins (Lai et al., 2017; Figure 3). The majority of spinal afferents projecting to the gut consist of slowly conducting unmyelinated C fibers, which are commonly characterized by the expression of transient receptor potential channels. These fibers often exhibit terminals that contain various peptides, including calcitonin-gene-related peptide (CGRP) or substance P. The activation of TRP channels triggers the release of peptides from efferent terminals through axonal reflexes, subsequently exerting their effects by binding to neuropeptide receptors on immune cells (Chiu et al., 2013; Meseguer et al., 2014; Figure 3).
Numerous studies have been conducted to explore the role of sensory nerves in the neuro-immune interactions of the peripheral tissue barrier (Talbot et al., 2015; Gabanyi et al., 2016; Liu et al., 2016). Skin nociceptors interact with IL-33 through the expression of growth stimulation-expressed gene 2 to induce immune activation (Liu et al., 2016). Activation of Nav1.8+ neurons in the DRG induces cytokine production by CD4 and ILC2 cells, including IL-5, via the vasoactive intestinal peptide/vasoactive intestinal peptide receptor 2 axis (Talbot et al., 2015). A study showed that colonization of the colonic epithelium of TRPV1–/– mice by Citrobacter rodentium resulted in increased species abundance in fecal samples, which was inversely correlated with decreased expression of neutrophil chemokines and adhesion factors (Chiu et al., 2013). These findings provide strong evidence for the role of peripheral neural-immune crosstalk in enhancing barrier defense capabilities.
Nociceptors are capable of expressing Toll-like receptors and other pattern recognition receptors to detect microbe-associated molecular patterns present on the surfaces of bacteria (Peters and Emrick, 2023). With the discovery that intestinal Salmonella infection triggers nociceptive circuit activation to initiate the host’s defenses (Lai et al., 2020), an increasing number of studies have focused on the role of nociception and pain perception in the microbial domain. TRPV1+ nociceptive neurons transmit signals from enteral stimuli to the spinal cord in response to exogenous bacterial invasion, leading to the release of CGRP from peripheral terminals. The secretion of CGRP plays a crucial role in regulating the population of microfold cells within Peyer’s patches in the small intestine, thereby restricting the access points of pathogenic microorganisms entering or exiting the intestinal tract (Figure 3). Moreover, the sensory neurons that innervate the gut have a regulatory role in the tissue programming of macrophages at the distal level during Salmonella infection (Gabanyi et al., 2016). However, in experimental models of colitis or irritable bowel syndrome, TRP receptors exhibit exaggerated responses, which contribute to peripheral inflammation and nociception through the expression of related receptors on immune cells or induce local inflammation by releasing vasoactive substances (Kihara et al., 2003; Szitter et al., 2010; Guo et al., 2021; Perna et al., 2021). Hence, the combination of immunotherapy and nociceptor activation can synergistically enhance the host’s defenses and concurrently coordinate the immune response, thereby yielding improved defensive effects.
A recent study demonstrated that Nav1.8+ nociceptors transmit signals via the CGRP/receptor activity-modifying protein 1 signaling axis to intestinal goblet cells, thereby regulating mucus production and facilitating barrier protection (Yang et al., 2022a). Depletion of these nociceptors in mice results in a decrease in the mucus layer and dysbiosis of the gut microbiota. Although the capacity of DRG neurons to perceive and react to bacteria and their metabolites has been elucidated in the context of infection and inflammation-related disorders, the role of DRG neurons in TBI and its unfavorable prognosis remains unknown, thus representing a future avenue for exploring alternative therapies for secondary peripheral infection following TBI.
Microbiota and brain
The comprehension of the initiation events and outcome endpoints of microbial stimulation impacting neural circuits is a crucial objective in mitigating adverse outcomes following TBI. The neural pathways involved in the bidirectional brain-gut-microbe connection after TBI can either descend locally from the brain or ascend from nerve terminals to reach specific nuclei in the brain. Utilizing optogenetics and chemical genetics has facilitated our understanding of the relative contribution of these neural circuits to behavioral changes dependent on microbes.
The hypothalamus (which receives sensory and visceral information), PVN (involved in physiological homeostasis and stress regulation), central amygdala (associated with fear processing and memory formation), anterior cingulate cortex, supplementary somatosensory cortex, and insular cortex effectively respond to peripheral neuronal inputs and perform their respective functions. Activation of neurons in the gigantocellular paralateral nucleus/anterior ventrolateral medulla region of the brainstem, which innervate sympathetic preganglionic neurons projecting to the GI, is triggered by alterations in microbial composition, resulting in a deceleration of GI transport (Muller et al., 2020). The agouti-related peptide and pro-opiomelanocortin neurons in the arcuate nucleus of the hypothalamus project to the sympathetic preganglionic brain–derived neurotrophic factor neurons in the PVN and establish a neural circuit that regulates the plasticity of the sympathetic postganglionic nerve, thereby exerting direct control over energy homeostasis within the body (Wang et al., 2020). The insular cortex plays a crucial role in the integration of external immune responses, and its association with sympathetic innervation and visceral information processing highlights its significance in mice with colitis (Han et al., 2018; Koren et al., 2021). After LPS infection, the nucleus of the solitary tract-area postrema region of mice responded rapidly, with the activation of adenylate cyclase activating polypeptide 1 (ADCYAP1+) neurons playing a pivotal role in the brain’s perception mechanism (Ilanges et al., 2022). By suppressing ADCYAP1+ neurons, the manifestation of LPS-induced phenomena, such as anorexia, obesity, and acinesia can be significantly attenuated, thereby establishing a crucial connection between central processes and infection. Furthermore, vasopressin cells in the PVN can further diminish sucrose preference in both male and female mice during LPS infection, while specifically inducing social behavioral deficits in male mice (Sgritta et al., 2019; Whylings et al., 2021). These findings imply that sex hormones exert specific nuclear regulation on brain regions. Due to the cascading nature of multiple biological processes in TBI complications, drug interventions targeting a single nervous system have been shown to be ineffective in achieving therapeutic outcomes. The aforementioned active regions, which exhibit microbial effects on sensing, offer compelling evidence to elucidate the interplay between the central and peripheral nervous systems triggered by TBI. The intricate bidirectional regulation can be disentangled through the identification of distinct brain nuclei that fulfill separate functions in priming and outcome.
Consequences of Dysregulation in the Brain–Gut–Microbiota Axis
Exacerbation of neural inflammation
There is increasing evidence suggesting that the CNS and the immune system are closely interconnected. This functional interaction explains the occurrence of immune responses following CNS injury. Resident microglia activation and peripheral blood neutrophil recruitment in early TBI are followed by the infiltration of macrophages derived from lymphocytes and monocytes. Proinflammatory and anti-inflammatory cytokines promote each other to terminate the neuroinflammatory response after trauma, while chemokine signaling leads to the activation and recruitment of immune cells (Simon et al., 2017). Some risk factors establish a bias toward the immune response in the injured brain, such as altered microbiota, impaired immune surveillance, abnormal signal transmission, and the release of brain-derived antigens, which are freshly recognized by the immune system and then turn on the autoimmune response. Furthermore, antibiotic-induced modifications of the gut microbiota result in a reduction in ischemic brain injury. Specifically, dysbiosis hinders the migration of effector T cells from the GI tract to the leptomeninges following stroke. Within the brain, these cells contribute to heightened neuroinflammation. Gut dysbiosis also impacts the integrity and permeability of the BBB. When combined with the physical disruption caused by TBI, it facilitates easier passage of intestinal contents and the associated upregulation of proinflammatory immune responses into the CNS. This leads to heightened microglia activity, neuroinflammation, and neuropathology (Erny et al., 2015; Sun et al., 2016). TBI-induced gut dysbiosis may contribute to heightened activation of microglia following CNS injury (Witcher et al., 2021). The observed mitochondrial dysfunction in TBI may also be influenced by gut dysbiosis, which exerts an impact on mitochondrial function (Pandya et al., 2014).
Although the findings discussed so far detail the potential mechanisms by which altered microbial composition aggravates neurological damage, most of these are experimental studies; therefore, further clinical studies are needed to elucidate these interactions and confirm these scientific findings. Additionally, small molecules secreted by bacteria also support CNS function. Notably, microglial developmental defects in GF-conditioned FFAR2–/– (SCFA receptor) mice lead to impaired brain connectivity and social behavioral alterations (Erny et al., 2015). Supplementation with SCFAs restores the expression of c-fos both peripherally and centrally, thereby ameliorating neuroinflammation. The attenuation of CNS inflammation is accompanied by a reduction in white matter demyelination and axonal loss, as propionic acid induces a population of gut-derived Tregs, promoting neuroprotection (Pandya et al., 2014). Overall, transplantation of SCFA is similar to the effects of gut probiotics, as it exerts a protective function on the injured brain through the gut-brain axis.
Abnormal neurotransmission
The development of depression following CNS injury is typically a slow process triggered by negative emotional responses that hinder the recovery of neurological function, including anxiety, depression, and social behavioral disorders (Margolis et al., 2021; Chen et al., 2022). Summarizing the underlying mechanisms of bidirectional communication in the “brain–gut–microbe” axis, makes it easy to understand that the interwoven networks of the nervous system and neurosecretory monoamines play an essential role in modulating emotions. The primary injury of TBI leads to the loss of hippocampal neurogenesis, transient activation and long-term inhibition of pain sensing in the sensory cortex, reduction of brain-derived neurotrophic factor release at the injury site, and disruption of the balance between excitatory and inhibitory neurotransmitters, which are risk factors for the interlinkage of multiple regions in the brain. For instance, cortical–striatal–pallidal–thalamic–cortical projection dysfunction mediates the development of post-stroke depression (Loubinoux et al., 2012). Moreover, the gut microbiota modulates the function of central GABAergic nerves (Jiang et al., 2015) and the metabolism of D-glutamate (Chang et al., 2020), which play a crucial role in emotional-behavioral functions, such as anxiety, cognition, and learning post-CNS injury. Furthermore, the gut microbiota play significant roles in the regulation of neurogenesis and neuroplasticity; however, their roles in depression following TBI remain uncertain. A study reported that hippocampal neurogenesis in adult GF mice exhibited significantly higher proliferation compared with that in conventional mice (Ogbonnaya et al., 2015). Furthermore, bacterial cell wall components have been reported to have the ability to traverse the maternal–fetal barrier, resulting in increased cortical nerve proliferation and cognitive impairment in the offspring (Humann et al., 2016). Antibiotic treatment decreases spatial and object recognition abilities in mice but could be restored by the administration of the probiotic VSL3 (Möhle et al., 2016). Using magnetic resonance imaging to monitor brain network connectivity in diseased states can explore potential therapeutic targets for functional changes or weakened connectivity.
Studies have demonstrated that pre-TBI restoration of the gut microbial community structure through fecal microbiota transfer can mitigate neurocognitive deficits in mice following TBI (Du et al., 2021; Davis et al., 2022; Medel-Matus et al., 2022). Within 90 days post-injury, FMT-treated TBI mice exhibited reduced cortical volume loss, preserved white matter connectivity, and increased microglial activation in the brain (Davis et al., 2022). These activated microglia facilitate the maintenance of functional connectivity among brain regions, including the amygdala, hippocampus, and prefrontal cortex. However, clinical TBI encompasses a spectrum of injury progression, ranging from mild to severe, diffuse to focal, and single to multiple occurrences. Therefore, the generalizability of FMT to the entire spectrum of TBI processes requires further investigation. Additionally, controlling for differences in age, sex hormones, and physical conditions poses significant challenges in FMT treatment.
Secondary peripheral infection
Experimental and clinical studies have shown that TBI can lead to severe peripheral organ and systemic functional impairments. The most common complications include respiratory and urinary tract infections caused by Gram-negative bacteria. While risk factors, such as hospitalization, drowsiness, swallowing difficulties, aspiration, and bladder dysfunction make patients susceptible to infection (Cook et al., 2020; Shepetovsky et al., 2021; Zhang et al., 2022), there is evidence supporting two independent risk factors (Sharma et al., 2019): gut bacterial translocation (Yang et al., 2022b) and immune suppression (Stephens et al., 2023). Although antibiotics are clinically administered for the treatment of infections following TBI, some patients treated with moxifloxacin experience persistent infections and exhibit elevated levels of plasma IL-10 (Chamorro et al., 2007). After conducting a comprehensive analysis of the aforementioned potential mechanisms, it has been postulated that catecholamines exhibit impaired regulatory effects on immune factors, consequently leading to the loss of inhibitory capacity of IL-10 towards IFN-γ. This subsequently leads to a shift from Th1 to Th2 responses, resulting in an inadequate resolution of bacterial invasions and infections. The neuroimmune regulatory systems constitute a dynamic and intricate network of interconnected responses and pathways that facilitate specific mechanisms for immune suppression and infection. The above chapters provide a cutting-edge review on the regulatory mechanisms of the “brain–gut” axis on microbiota and the effects of microbiota in the “gut–brain” axis. There is a pathological similarity in CNS injury–related diseases, such as stroke, spinal cord injury, and TBI; however, there is heterogeneity in the mechanisms of bidirectional communication between the brain and gut, and infections are not completely associated with neurodegeneration.
Conclusion
The microbial–gut–brain axis is a widely recognized concept in neuro-immunology. It encompasses ascending neural pathways that transmit information through the spinal cord and brain stem for host defense, and the descending ANS that regulates a range of GI functions by binding receptors to affect immunity and metabolism. Changes in microbial colonization or deficiency could affect CNS efficacy, orchestrate local and systemic immune homeostasis, and facilitate crosstalk between the ENS and the CNS (Table 1). These interactions highlight the value of bidirectional brain–gut communication after CNS injury. Although invisible lines connect these three parts, they still belong to different regional modules in terms of organizational structure. By investigating the interconnection between these nodes through biogenetic means, we aim to explore the unique properties of neuro-immunity while largely preserving anatomical structure and functionality. However, further research is needed to specifically elucidate the dynamic processes through which gut microbiota signals and their products traverse disease-affected states, such as the gut barrier, BBB, and leptomeninges.
It should be noted that this review does not primarily focus on examining the role played by mechanical barriers in systemic spread. Additionally, GI complications of CNS injury involve multiple branches, including the metabolic, endocrine, and immune systems, and each has distinct anatomical characteristics. Understanding the commonalities and specificities of potential signaling molecules in the peripheral system is a challenging task; however, it will ultimately contribute to defining the most applicable interventions in clinical practice.
Traditionally, the utilization of antibiotics, dietary supplementation, and FMT are beneficial in terms of facilitating the colonization of commensal bacteria, enhancing resistance against pathogenic microorganisms, preserving mucosal barrier integrity, and collectively shaping a healthy intestinal niche. Moreover, these approaches serve as effective strategies to maintain a harmonious host-microbe symbiotic balance (Figure 4). Almost all neurological diseases or injuries have a common feature: prolonged neuroinflammation. Studies comparing rodent microbiomes before and after exposure have found this inflammation to be a result of reciprocal causality. Given the intricate nature of the GI environment, it is imperative to further investigate the temporal alterations in pathways that regulate and perceive microbial changes through signal transmission.
Figure 4.

Timeline showing the role of brain–gut–microbiota axis in TBI in the literature.
GI: Gastrointestinal; TBI: traumatic brain injury.
Additional file: Open peer review report 1 (81.5KB, pdf) .
Funding Statement
Funding: This work was supported by the National Natural Science Foundation of China, No. 82174112 (to PZ); Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine, No. 22HHZYSS00015 (to PZ); and State-Sponsored Postdoctoral Researcher Program, No. GZC20231925 (to LN).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
P-Reviewers: Celorrio M, Lucke-Wold B; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
Data availability statement:
Not applicable.
References
- Abdullahi PR, Raeis-Abdollahi E, Sameni H, Vafaei AA, Ghanbari A, Rashidy-Pour A. Protective effects of morphine in a rat model of post-traumatic stress disorder: role of hypothalamic-pituitary-adrenal axis and beta- adrenergic system. Behav Brain Res. 2020;395:112867. doi: 10.1016/j.bbr.2020.112867. [DOI] [PubMed] [Google Scholar]
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Adidharma W, Khouri AN, Lee JC, Vanderboll K, Kung TA, Cederna PS, Kemp SWP. Sensory nerve regeneration and reinnervation in muscle following peripheral nerve injury. Muscle Nerve. 2022;66:384–396. doi: 10.1002/mus.27661. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Arike L, Hansson GC. The densely O-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. J Mol Biol. 2016;428:3221–3229. doi: 10.1016/j.jmb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Fu Q, Shah SK, Melnyk SB, Sterneck E, Hauer-Jensen M, Pawar SA. C/EBPδ protects from radiation-induced intestinal injury and sepsis by suppression of inflammatory and nitrosative stress. Sci Rep. 2019;9:13953. doi: 10.1038/s41598-019-49437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V, Costantini T, Ryu SY, Peterson C, Loomis W, Putnam J, Elicieri B, Baird A, Coimbra R. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68:1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Sun Y, Lin Y, Yang X, Chen Z. An integrated analysis of gut microbiota and the brain transcriptome reveals host-gut microbiota interactions following traumatic brain injury. Brain Res. 2023;1799:148149. doi: 10.1016/j.brainres.2022.148149. [DOI] [PubMed] [Google Scholar]
- Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat Med. 2018;24:417–426. doi: 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making sense of … the microbiome in psychiatry. Int J Neuropsychopharmacol. 2019;22:37–52. doi: 10.1093/ijnp/pyy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Albaugh VL, Neuhuber WL. Gut-brain communication and obesity: understanding functions of the vagus nerve. J Clin Invest. 2021;131:e143770. doi: 10.1172/JCI143770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B. Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction. Neurogastroenterol Motil. 2022;34:e14456. doi: 10.1111/nmo.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajski J, Gadek-Michalska A, Ołowska A, Borycz J, Głód R, Bugajski AJ. Adrenergic regulation of the hypothalamic-pituitary-adrenal axis under basal and social stress conditions. J Physiol Pharmacol. 1995;46:297–312. [PubMed] [Google Scholar]
- Celorrio M, Shumilov K, Friess SH. Gut microbial regulation of innate and adaptive immunity after traumatic brain injury. Neural Regen Res. 2024;19:272–276. doi: 10.4103/1673-5374.379014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celorrio M, Abellanas MA, Rhodes J, Goodwin V, Moritz J, Vadivelu S, Wang L, Rodgers R, Xiao S, Anabayan I, Payne C, Perry AM, Baldridge MT, Aymerich MS, Steed A, Friess SH. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol Commun. 2021;9:40. doi: 10.1186/s40478-021-01137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gómez-Choco M, Torres F, Planas AM. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Chang CH, Lin CH, Lane HY. d-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci. 2020;21:2676. doi: 10.3390/ijms21082676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SS, Pavlov VA, Tracey KJ. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity. 2017;46:927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AL, Sun X, Wang W, Liu JF, Zeng X, Qiu JF, Liu XJ, Wang Y. Activation of the hypothalamic-pituitary-adrenal (HPA) axis contributes to the immunosuppression of mice infected with Angiostrongylus cantonensis. J Neuroinflammation. 2016;13:266. doi: 10.1186/s12974-016-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, McCormick B, Sampson TR, Alam A, Ye K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71:2233–2252. doi: 10.1136/gutjnl-2021-326269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Bharadwaj V, Irvine KA, Clark JD. Mechanisms and treatments of chronic pain after traumatic brain injury. Neurochem Int. 2023;171:105630. doi: 10.1016/j.neuint.2023.105630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Luo J, Li J, Kim G, Chen ES, Xiao S, Snapper SB, Bao B, An D, Blumberg RS, Lin CH, Wang S, Zhong J, Liu K, Li Q, Wu C, Kuchroo VK. Foxo1 controls gut homeostasis and commensalism by regulating mucus secretion. J Exp Med. 2021;218:e20210324. doi: 10.1084/jem.20210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM. Infection, pain, and itch. Neurosci Bull. 2018;34:109–119. doi: 10.1007/s12264-017-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Parkhurst CN, Zhang W, Zhou L, Yano H, Arifuzzaman M, Artis D. The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity. Sci Immunol. 2021;6:eabe3218. doi: 10.1126/sciimmunol.abe3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Boddington L, Steffan PJ, McCormick D. Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr Biol. 2021;31:2088–2098.e3. doi: 10.1016/j.cub.2021.02.049. [DOI] [PubMed] [Google Scholar]
- Cook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, Samuel S, Tokumaru S, Venkatasubramanian C, Zacko C, Zimmermann LL, Hirsch K, Shutter L. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care. 2020;32:647–666. doi: 10.1007/s12028-020-00959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan F, Cernak I, McAteer K, Hellewell SC, Rosenfeld JV, Turner RJ, Vink R. NK1 antagonists attenuate tau phosphorylation after blast and repeated concussive injury. Sci Rep. 2021;11:8861. doi: 10.1038/s41598-021-88237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BTt, Chen Z, Islam M, Timken ME, Procissi D, Schwulst SJ. Fecal microbiota transfer attenuates gut dysbiosis and functional deficits after traumatic brain injury. Shock. 2022;57:251–259. doi: 10.1097/SHK.0000000000001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divani AA, Salazar P, Ikram HA, Taylor E, Wilson CM, Yang Y, Mahmoudi J, Seletska A, SantaCruz KS, Torbey MT, Liebler EJ, Bragina OA, Morton RA, Bragin DE. Non-invasive vagus nerve stimulation improves brain lesion volume and neurobehavioral outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2023;40:1481–1494. doi: 10.1089/neu.2022.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran SJ, Henry RJ, Shirey KA, Barrett JP, Ritzel RM, Lai W, Blanco JC, Faden AI, Vogel SN, Loane DJ. Early or late bacterial lung infection increases mortality after traumatic brain injury in male mice and chronically impairs monocyte innate immune function. Crit Care Med. 2020;48:e418–428. doi: 10.1097/CCM.0000000000004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolia R, Bhunia AK. Crossing the intestinal barrier via listeria adhesion protein and internalin A. Trends Microbiol. 2019;27:408–425. doi: 10.1016/j.tim.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Du D, Tang W, Zhou C, Sun X, Wei Z, Zhong J, Huang Z. Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neurological deficits after traumatic brain injury. Oxid Med Cell Longev. 2021;2021:5816837. doi: 10.1155/2021/5816837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, He X, Xiong X, Zhang X, Jian Z, Yang Z. Vagus nerve stimulation in cerebral stroke: biological mechanisms, therapeutic modalities, clinical applications, and future directions. Neural Regen Res. 2024;19:1707–1717. doi: 10.4103/1673-5374.389365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Danhof HA, Auchtung J, Endres BT, Ruan W, Bassères E, Engevik AC, Wu Q, Nicholson M, Luna RA, Garey KW, Crawford SE, Estes MK, Lux R, Yacyshyn MB, Yacyshyn B, Savidge T, Britton RA, Versalovic J. Fusobacteriumnucleatum adheres to clostridioides difficile via the RadD adhesin to enhance biofilm formation in intestinal mucus. Gastroenterology. 2021;160:1301–1314.e8. doi: 10.1053/j.gastro.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. 2021;18:127. doi: 10.1186/s12974-021-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete E, Buret AG. The role of mucin O-glycans in microbiota dysbiosis, intestinal homeostasis, and host-pathogen interactions. Am J Physiol Gastrointest Liver Physiol. 2023;324:G452–G465. doi: 10.1152/ajpgi.00261.2022. [DOI] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. 2022;23:11245. doi: 10.3390/ijms231911245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Patel S, Shah D, Chi L, Emadi S, Pierce DM, Han M, Brumovsky PR, Feng B. Optical clearing reveals TNBS-induced morphological changes of VGLUT2-positive nerve fibers in mouse colorectum. Am J Physiol Gastrointest Liver Physiol. 2021;320:G644–G657. doi: 10.1152/ajpgi.00363.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohórquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE. A neural circuit for gut-induced reward. Cell. 2018;175:665–678.e23. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Angle A, Kielian T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS One. 2012;7:e42476. doi: 10.1371/journal.pone.0042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanscom M, Loane DJ, Shea-Donohue T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest. 2021;131:e143777. doi: 10.1172/JCI143777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, Minami Y, Sugano M, Kubota N, Uegaki S, Kamoshida H, Sawamura A, Nomoto K, Gando S. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56:2361–2365. doi: 10.1007/s10620-011-1649-3. [DOI] [PubMed] [Google Scholar]
- Holcomb M, Marshall A, Flinn H, Lozano M, Soriano S, Gomez-Pinilla F, Treangen TJ, Villapol S. Probiotic treatment causes sex-specific neuroprotection after traumatic brain injury in mice. Research Square. 2024 doi: 10.21203/rs.3.rs-4196801/v1. [Google Scholar]
- Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, Callcut RA, Calfee CS, Lamere BJ, Fadrosh DW, Lynch S, Cohen MJ. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2017;2:e000108. doi: 10.1136/tsaco-2017-000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, Farr A, Hu Y, Durick-Eder K, Fillon SA, Smeyne RJ, Tuomanen EI. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe. 2016;19:388–399. doi: 10.1016/j.chom.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob S, Iacob DG, Luminos LM. Intestinal microbiota as a host defense mechanism to infectious threats. Front Microbiol. 2018;9:3328. doi: 10.3389/fmicb.2018.03328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, Eberl G, Grice EA, Veiga-Fernandes H. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi H, Medina KL, Yokota T, Rossi MI, Sakaguchi N, Comp PC, Kincade PW. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int Immunol. 2005;17:501–511. doi: 10.1093/intimm/dxh230. [DOI] [PubMed] [Google Scholar]
- Ilanges A, Shiao R, Shaked J, Luo JD, Yu X, Friedman JM. Brainstem ADCYAP1(+) neurons control multiple aspects of sickness behaviour. Nature. 2022;609:761–771. doi: 10.1038/s41586-022-05161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari AA, Shah M, Mirmoeeni S, Hassani MS, Nazari S, Fielder T, Godoy DA, Seifi A. Paroxysmal sympathetic hyperactivity during traumatic brain injury. Clin Neurol Neurosurg. 2022;212:107081. doi: 10.1016/j.clineuro.2021.107081. [DOI] [PubMed] [Google Scholar]
- Jarret A, et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell. 2020;180:50–63.e12. doi: 10.1016/j.cell.2019.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn CF, Kühnhardt D, Bartholomae A, Pfeiffer S, Schmid P, Possinger K, Flath BC, Lüftner D. Association of IL-6, hypothalamus-pituitary-adrenal axis function, and depression in patients with cancer. Integr Cancer Ther. 2010;9:270–275. doi: 10.1177/1534735410370036. [DOI] [PubMed] [Google Scholar]
- Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2019;145:230–246. doi: 10.1016/j.neuropharm.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Rupprecht LE, Liu WW, Weng P, Bohórquez DV. Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu Rev Neurosci. 2020;43:337–353. doi: 10.1146/annurev-neuro-091619-022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Shoemaker J. Multiple sclerosis immunology: The healthy immune system vs the MS immune system. Neurology 74 Suppl. 2010;1:S2–8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Takahashi D, Takano S, Kimura S, Hase K. The roles of Peyer’s patches and microfold cells in the gut immune system: relevance to autoimmune diseases. Front Immunol. 2019;10:2345. doi: 10.3389/fimmu.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, Azulay-Debby H, Zalayat I, Avishai E, Hajjo H, Schiller M, Haykin H, Korin B, Farfara D, Hakim F, Kobiler O, Rosenblum K, Rolls A. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184:5902–5915.e17. doi: 10.1016/j.cell.2021.10.013. [DOI] [PubMed] [Google Scholar]
- Krzyzaniak M, Ortiz-Pomales Y, Lopez N, Reys LG, Cheadle G, Wolf P, Eliceiri B, Bansal V, Baird A, Coimbra R. CPSI-121 pharmacologically prevents intestinal barrier dysfunction after cutaneous burn through a vagus nerve-dependent mechanism. J Trauma Acute Care Surg. 2012;72:355–361. doi: 10.1097/TA.0b013e31824484fe. discussion 361-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labed SA, Wani KA, Jagadeesan S, Hakkim A, Najibi M, Irazoqui JE. Intestinal epithelial wnt signaling mediates acetylcholine-triggered host defense against infection. Immunity. 2018;48:963–978.e3. doi: 10.1016/j.immuni.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NY, Mills K, Chiu IM. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J Intern Med. 2017;282:5–23. doi: 10.1111/joim.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NY, et al. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell. 2020;180:33–49.e22. doi: 10.1016/j.cell.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros JP, Le Guern N, Plesnik J, Thomas C, Bourgeois T, Dejong CHC, Kox M, Hundscheid IHR, Khan NA, Mandard S, Deckert V, Pickkers P, Drucker DJ, Lagrost L, Grober J. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 2017;21:1160–1168. doi: 10.1016/j.celrep.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hall JA, Kroehling L, Wu L, Najar T, Nguyen HH, Lin WY, Yeung ST, Silva HM, Li D, Hine A, Loke P, Hudesman D, Martin JC, Kenigsberg E, Merad M, Khanna KM, Littman DR. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell. 2020;183:2036–2039. doi: 10.1016/j.cell.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu C, Xiao W, Song T, Wang S. Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit Care. 2020;32:272–285. doi: 10.1007/s12028-019-00773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Liu Y, Ma L, Ma X, Shen L, Ma X, Chen Z, Chen H, Li D, Su Z, Chen X. Constipation induced gut microbiota dysbiosis exacerbates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Transl Med. 2021;19:317. doi: 10.1186/s12967-021-02995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, Jordt SE. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A. 2016;113:E7572–7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Johnson EM, Lam RK, Wang Q, Bo Ye H, Wilson EN, Minhas PS, Liu L, Swarovski MS, Tran S, Wang J, Mehta SS, Yang X, Rabinowitz JD, Yang SS, Shamloo M, Mueller C, James ML, Andreasson KI. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol. 2019;20:1023–1034. doi: 10.1038/s41590-019-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu Y, Liu J, Zhang H, Shan C, Guo Y, Gong X, Cui M, Li X, Tang M. Correlation between the gut microbiome and neurodegenerative diseases: a review of metagenomics evidence. Neural Regen Res. 2024;19:833–845. doi: 10.4103/1673-5374.382223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bao Z, Xu X, Chao H, Lin C, Li Z, Liu Y, Wang X, You Y, Liu N, Ji J. Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J Neurotrauma. 2017;34:2119–2131. doi: 10.1089/neu.2016.4764. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, Kaczmarek L, Popa-Wagner A. Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med. 2012;16:1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EL, Smith AD, Desai N, Cheung L, Hanscom M, Stoica BA, Loane DJ, Shea-Donohue T, Faden AI. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun. 2017;66:56–69. doi: 10.1016/j.bbi.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang J, Fujita Y, Shinno-Hashimoto H, Shan J, Wan X, Qu Y, Chang L, Wang X, Hashimoto K. Effects of spleen nerve denervation on depression-like phenotype, systemic inflammation, and abnormal composition of gut microbiota in mice after administration of lipopolysaccharide: A role of brain-spleen axis. J Affect Disord. 2022;317:156–165. doi: 10.1016/j.jad.2022.08.087. [DOI] [PubMed] [Google Scholar]
- Ma P, Mo R, Liao H, Qiu C, Wu G, Yang C, Zhang Y, Zhao Y, Song XJ. Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice. J Neuroinflammation. 2022;19:169. doi: 10.1186/s12974-022-02523-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Liu T, Fu J, Fu S, Hu C, Sun B, Fan X, Zhu J. Lactobacillus acidophilus exerts neuroprotective effects in mice with traumatic brain injury. J Nutr. 2019;149:1543–1552. doi: 10.1093/jn/nxz105. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Pachnis V, Prinz M. Boundaries and integration between microbiota, the nervous system, and immunity. Immunity. 2023;56:1712–1726. doi: 10.1016/j.immuni.2023.07.011. [DOI] [PubMed] [Google Scholar]
- Magalhães A, Marcos-Pinto R, Nairn AV, Dela Rosa M, Ferreira RM, Junqueira-Neto S, Freitas D, Gomes J, Oliveira P, Santos MR, Marcos NT, Xiaogang W, Figueiredo C, Oliveira C, Dinis-Ribeiro M, Carneiro F, Moremen KW, David L, Reis CA. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim Biophys Acta. 2015;1852:1928–1939. doi: 10.1016/j.bbadis.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, Nouzé C, Ladant D, Cole ST, Sebo P, Leclerc C. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74:2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallesh S, Ten Hove AS, Schneider R, Schneiker B, Efferz P, Kalff JC, de Jonge WJ, Wehner S. Sympathetic innervation modulates mucosal immune homeostasis and epithelial host defense. Cells. 2022;11:2606. doi: 10.3390/cells11162606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131–139. doi: 10.1007/s00540-018-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch L, Smith CJ, McColl BW. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat Commun. 2017;8:15051. doi: 10.1038/ncomms15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medel-Matus JS, Simpson CA, Ahdoot AI, Shin D, Sankar R, Jacobs JP, Mazarati AM. Modification of post-traumatic epilepsy by fecal microbiota transfer. Epilepsy Behav. 2022;134:108860. doi: 10.1016/j.yebeh.2022.108860. [DOI] [PubMed] [Google Scholar]
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. Ly6C(hi) Monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- Morishita K, Coimbra R, Langness S, Eliceiri BP, Costantini TW. Neuroenteric axis modulates the balance of regulatory T cells and T-helper 17 cells in the mesenteric lymph node following trauma/hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2015;309:G202–208. doi: 10.1152/ajpgi.00097.2015. [DOI] [PubMed] [Google Scholar]
- Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Mármol J, Castro TBR, Furuichi M, Perkins M, Han W, Rao A, Pickard AJ, Cross JR, Honda K, de Araujo I, Mucida D. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Sawa S, Nitta T, Tsutsumi M, Okamura T, Penninger JM, Nakashima T, Takayanagi H. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol. 2017;18:675–682. doi: 10.1038/ni.3732. [DOI] [PubMed] [Google Scholar]
- Nakata Y, Miura K, Yamasaki N, Ogata S, Miura S, Hosomi N, Kaminuma O. Expression and function of nicotinic acetylcholine receptors in induced regulatory T cells. Int J Mol Sci. 2022;23:1779. doi: 10.3390/ijms23031779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neren D, Johnson MD, Legon W, Bachour SP, Ling G, Divani AA. Vagus nerve stimulation and other neuromodulation methods for treatment of traumatic brain injury. Neurocrit Care. 2016;24:308–319. doi: 10.1007/s12028-015-0203-0. [DOI] [PubMed] [Google Scholar]
- Neufeld PM, Nettersheim RA, Matschke V, Vorgerd M, Stahlke S, Theiss C. Unraveling the gut-brain axis: the impact of steroid hormones and nutrition on Parkinson’s disease. Neural Regen Res. 2024;19:2219–2228. doi: 10.4103/1673-5374.391304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Rappold GA. Emerging evidence for gene mutations driving both brain and gut dysfunction in autism spectrum disorder. Mol Psychiatry. 2021;26:1442–1444. doi: 10.1038/s41380-020-0778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78:e7–9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Pan P, Song Y, Du X, Bai L, Hua X, Xiao Y, Yu X. Intestinal barrier dysfunction following traumatic brain injury. Neurol Sci. 2019;40:1105–1110. doi: 10.1007/s10072-019-03739-0. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, Rabchevsky AG, Sullivan PG. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp Neurol. 2014;257:106–113. doi: 10.1016/j.expneurol.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panther EJ, Dodd W, Clark A, Lucke-Wold B. Gastrointestinal microbiome and neurologic injury. Biomedicines. 2022;10:500. doi: 10.3390/biomedicines10020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232–2243. doi: 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez AR, Roggero E, Nicora A, Palazzi J, Besedovsky HO, Del Rey A, Bottasso OA. Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun. 2007;21:890–900. doi: 10.1016/j.bbi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Perna E, Aguilera-Lizarraga J, Florens MV, Jain P, Theofanous SA, Hanning N, De Man JG, Berg M, De Winter B, Alpizar YA, Talavera K, Vanden Berghe P, Wouters M, Boeckxstaens G. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut. 2021;70:1275–1286. doi: 10.1136/gutjnl-2020-321530. [DOI] [PubMed] [Google Scholar]
- Peters SB, Emrick JJ. Nociceptors are needed to guide tooth development, function, repair, and regeneration. Neural Regen Res. 2023;18:1503–1504. doi: 10.4103/1673-5374.360280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powner DJ, Boccalandro C. Adrenal insufficiency following traumatic brain injury in adults. Curr Opin Crit Care. 2008;14:163–166. doi: 10.1097/MCC.0b013e3282f57528. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma. 2016;33:871–879. doi: 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H, Tedeschi A, Thiriot A, Lynch L, Loughhead SM, Stutte S, Mazo IB, Kopp MA, Brommer B, Blex C, Geurtz LC, Liebscher T, Niedeggen A, Dirnagl U, Bradke F, Volz MS, DeVivo MJ, Chen Y, von Andrian UH, Schwab JM. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat Neurosci. 2017;20:1549–1559. doi: 10.1038/nn.4643. [DOI] [PubMed] [Google Scholar]
- Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles RB, Miller AL, Urban RJ, Sheffield-Moore M, Wright TJ, Maxwell CA, Randolph KM, Danesi CP, McGovern KA, Vargas J, Armstrong P, Kreber L, Cumpa G, Randall K, Morrison M, Masel BE. The altered TBI fecal microbiome is stable and functionally distinct. Front Mol Neurosci. 2024;17:1341808. doi: 10.3389/fnmol.2024.1341808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Cui X, Dong W, Barrera R, Nicastro J, Coppa GF, Wang P, Wu R. Ghrelin attenuates brain injury after traumatic brain injury and uncontrolled hemorrhagic shock in rats. Mol Med. 2012;18:186–193. doi: 10.2119/molmed.2011.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Xu Q, Xu Y, Zhao Y, He N, Tang J, Zhao J, Liu Y. Molecular chaperones in stroke-induced immunosuppression. Neural Regen Res. 2023;18:2638–2644. doi: 10.4103/1673-5374.373678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer-Quinn DS, Baker RA, Sanders VM. Activated T helper 1 and T helper 2 cells differentially express the beta-2-adrenergic receptor: a mechanism for selective modulation of T helper 1 cell cytokine production. J Immunol. 1997;159:4857–4867. [PubMed] [Google Scholar]
- Ramirez VT, Godinez DR, Brust-Mascher I, Nonnecke EB, Castillo PA, Gardner MB, Tu D, Sladek JA, Miller EN, Lebrilla CB, Bevins CL, Gareau MG, Reardon C. T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog. 2019;15:e1007719. doi: 10.1371/journal.ppat.1007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Mohanty S, Sharma S, Tripathi P. Possible role of gut microbes and host’s immune response in gut-lung homeostasis. Front Immunol. 2022;13:954339. doi: 10.3389/fimmu.2022.954339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE. The distribution of axons according to diameter in the optic nerve and optic tract of the rat. Neuroscience. 1987;22:1015–1024. doi: 10.1016/0306-4522(87)92977-0. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley TP, Neal-McKinney JM, Buelow DR, Konkel ME, Simasko SM. Capsaicin-sensitive vagal afferent neurons contribute to the detection of pathogenic bacterial colonization in the gut. J Neuroimmunol. 2013;257:36–45. doi: 10.1016/j.jneuroim.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger CM, McNabb MC, McNair R, Bierbower S, Cooper RL. Effects of bacterial endotoxin on regulation of the heart, a sensory-CNS-motor nerve circuit and neuromuscular junctions: crustacean model. Comp Biochem Physiol A Mol Integr Physiol. 2019;237:110557. doi: 10.1016/j.cbpa.2019.110557. [DOI] [PubMed] [Google Scholar]
- Schwulst SJ, Trahanas DM, Saber R, Perlman H. Traumatic brain injury-induced alterations in peripheral immunity. J Trauma Acute Care Surg. 2013;75:780–788. doi: 10.1097/TA.0b013e318299616a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101:246–259.e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, Comerma L, Huang B, Blander JM, Xiong H, Mayer L, Berin C, Augenlicht LH, Velcich A, Cerutti A. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Shultz SR, Robinson MJ, Belli A, Hibbs ML, O’Brien TJ, Semple BD. Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain Behav Immun. 2019;79:63–74. doi: 10.1016/j.bbi.2019.04.034. [DOI] [PubMed] [Google Scholar]
- Shepetovsky D, Mezzini G, Magrassi L. Complications of cranioplasty in relationship to traumatic brain injury: a systematic review and meta-analysis. Neurosurg Rev. 2021;44:3125–3142. doi: 10.1007/s10143-021-01511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Wang L, Zhou Z, Liu R, Li Y, Song L. Acetylcholine modulates the immune response in Zhikong scallop Chlamys farreri. Fish Shellfish Immunol. 2014;38:204–210. doi: 10.1016/j.fsi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Shirakashi M, Maruya M, Hirota K, Tsuruyama T, Matsuo T, Watanabe R, Murata K, Tanaka M, Ito H, Yoshifuji H, Ohmura K, Elewaut D, Sakaguchi S, Fagarasan S, Mimori T, Hashimoto M. Effect of impaired T cell receptor signaling on the gut microbiota in a mouse model of systemic autoimmunity. Arthritis Rheumatol. 2022;74:641–653. doi: 10.1002/art.42016. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Challagundla L, McGee IG, Warfield ZJ, Santos C, Garrett MR, Grayson BE. Temporal shifts to the gut microbiome associated with cognitive dysfunction following high-fat diet consumption in a juvenile model of traumatic brain injury. Physiol Genomics. 2024;56:301–316. doi: 10.1152/physiolgenomics.00113.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakenborg N, et al. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2019;68:1406–1416. doi: 10.1136/gutjnl-2018-317263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, Wong CH. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- Stephens R, Grainger JR, Smith CJ, Allan SM. Systemic innate myeloid responses to acute ischaemic and haemorrhagic stroke. Semin Immunopathol. 2023;45:281–294. doi: 10.1007/s00281-022-00968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Wiest R, Strauch UG, Härle P, Schölmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut. 2006;55:1640–1649. doi: 10.1136/gut.2006.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Günzler C, Miller LE, Cutolo M, Schölmerich J, Schill S. Anti-inflammatory cooperativity of corticosteroids and norepinephrine in rheumatoid arthritis synovial tissue in vivo and in vitro. FASEB J. 2002;16:993–1000. doi: 10.1096/fj.02-0085com. [DOI] [PubMed] [Google Scholar]
- Straub RH, Schaller T, Miller LE, von Hörsten S, Jessop DS, Falk W, Schölmerich J. Neuropeptide Y cotransmission with norepinephrine in the sympathetic nerve-macrophage interplay. J Neurochem. 2000;75:2464–2471. doi: 10.1046/j.1471-4159.2000.0752464.x. [DOI] [PubMed] [Google Scholar]
- Sudo N. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv Exp Med Biol. 2014;817:177–194. doi: 10.1007/978-1-4939-0897-4_8. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 2017;66:31–44. doi: 10.1016/j.bbi.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Szitter I, Pozsgai G, Sandor K, Elekes K, Kemeny A, Perkecz A, Szolcsanyi J, Helyes Z, Pinter E. The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J Mol Neurosci. 2010;42:80–88. doi: 10.1007/s12031-010-9366-5. [DOI] [PubMed] [Google Scholar]
- Taheri S, Karaca Z, Mehmetbeyoglu E, Hamurcu Z, Yilmaz Z, Dal F, Çınar V, Ulutabanca H, Tanriverdi F, Unluhizarci K, Rassoulzadegan M, Kelestimur F. The role of apoptosis and autophagy in the hypothalamic-pituitary-adrenal (HPA) axis after traumatic brain injury (TBI) Int J Mol Sci. 2022;23:15699. doi: 10.3390/ijms232415699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot S, et al. Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron. 2015;87:341–354. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Dong X, Chen G, Ye W, Kang J, Tang Y, Feng Z. Vagus nerve stimulation attenuates early traumatic brain injury by regulating the NF-κB/NLRP3 signaling pathway. Neurorehabil Neural Repair. 2020;34:831–843. doi: 10.1177/1545968320948065. [DOI] [PubMed] [Google Scholar]
- Taraskina A, et al. Effects of traumatic brain injury on the gut microbiota composition and serum amino acid profile in rats. Cells. 2022;11:1409. doi: 10.3390/cells11091409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen TJ, Wagner J, Burns MP, Villapol S. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. 2018;9:2757. doi: 10.3389/fimmu.2018.02757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban RJ, Pyles RB, Stewart CJ, Ajami N, Randolph KM, Durham WJ, Danesi CP, Dillon EL, Summons JR, Singh CK, Morrison M, Kreber LA, Masel B, Miller AL, Wright TJ, Sheffield-Moore M. Altered fecal microbiome years after traumatic brain injury. J Neurotrauma. 2020;37:1037–1051. doi: 10.1089/neu.2019.6688. [DOI] [PubMed] [Google Scholar]
- Vermeij JD, Aslami H, Fluiter K, Roelofs JJ, van den Bergh WM, Juffermans NP, Schultz MJ, Van der Sluijs K, van de Beek D, van Westerloo DJ. Traumatic brain injury in rats induces lung injury and systemic immune suppression. J Neurotrauma. 2013;30:2073–2079. doi: 10.1089/neu.2013.3060. [DOI] [PubMed] [Google Scholar]
- Wang P, Loh KH, Wu M, Morgan DA, Schneeberger M, Yu X, Chi J, Kosse C, Kim D, Rahmouni K, Cohen P, Friedman J. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583:839–844. doi: 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- Warner BB. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res. 2019;85:216–224. doi: 10.1038/s41390-018-0191-9. [DOI] [PubMed] [Google Scholar]
- Whylings J, Rigney N, de Vries GJ, Petrulis A. Removal of vasopressin cells from the paraventricular nucleus of the hypothalamus enhances lipopolysaccharide-induced sickness behaviour in mice. J Neuroendocrinol. 2021;33:e12915. doi: 10.1111/jne.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Witcher KG, Bray CE, Chunchai T, Zhao F, O’Neil SM, Gordillo AJ, Campbell WA, McKim DB, Liu X, Dziabis JE, Quan N, Eiferman DS, Fischer AJ, Kokiko-Cochran ON, Askwith C, Godbout JP. Traumatic brain injury causes chronic cortical inflammation and neuronal dysfunction mediated by microglia. J Neurosci. 2021;41:1597–1616. doi: 10.1523/JNEUROSCI.2469-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Jenne CN, Lee WY, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell. 2022;185:4190–4205.e25. doi: 10.1016/j.cell.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DX, Jing Y, Liu YL, Xu ZM, Yuan F, Wang ML, Geng Z, Tian HL. Inhibition of transient receptor potential vanilloid 1 attenuates blood-brain barrier disruption after traumatic brain injury in mice. J Neurotrauma. 2019;36:1279–1290. doi: 10.1089/neu.2018.5942. [DOI] [PubMed] [Google Scholar]
- Yang NN, Yang JW, Ye Y, Huang J, Wang L, Wang Y, Su XT, Lin Y, Yu FT, Ma SM, Qi LY, Lin LL, Wang LQ, Shi GX, Li HP, Liu CZ. Electroacupuncture ameliorates intestinal inflammation by activating α7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics. 2021;11:4078–4089. doi: 10.7150/thno.52574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yuan Q, Li Z, Du Z, Wu G, Yu J, Hu J. Translocation and dissemination of gut bacteria after severe traumatic brain injury. Microorganisms. 2022;10:2082. doi: 10.3390/microorganisms10102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Cai Y, Zhong A, Zhang Y, Zhang J, Xu S. The “dialogue” between central and peripheral immunity after ischemic stroke: focus on spleen. Front Immunol. 2021;12:792522. doi: 10.3389/fimmu.2021.792522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Lu XJ, Wu Q. Gut microbiota and acute central nervous system injury: a new target for therapeutic intervention. Front Immunol. 2021;12:800796. doi: 10.3389/fimmu.2021.800796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflammation. 2016;13:77. doi: 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang Y, Zhu M, Liu K, Zhang HL. Gut flora in multiple sclerosis: implications for pathogenesis and treatment. Neural Regen Res. 2024;19:1480–1488. doi: 10.4103/1673-5374.387974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou H, Shen H, Wang M. Pulmonary infection in traumatic brain injury patients undergoing tracheostomy: predicators and nursing care. BMC Pulm Med. 2022;22:130. doi: 10.1186/s12890-022-01928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Shi W, Lu Y, Gao X, Wang A, Zhang S, Du Y, Wang Y, Li L. Alterations of monoamine neurotransmitters, HPA-axis hormones, and inflammation cytokines in reserpine-induced hyperalgesia and depression comorbidity rat model. BMC Psychiatry. 2022;22:419. doi: 10.1186/s12888-022-04065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.


