Abstract
Acid–base abnormalities are common in the critically ill. The traditional classification of acid–base abnormalities and a modern physico-chemical method of categorizing them will be explored. Specific disorders relating to mortality prediction in the intensive care unit are examined in detail. Lactic acidosis, base excess, and a strong ion gap are highlighted as markers for increased risk of death.
Keywords: acid–base disorders, base excess, lactic acidosis, metabolic acidosis, strong ion gap
Introduction
Deranged acid–base physiology drives admission to a critical care arena for vast numbers of patients. Management of diverse disorders ranging from diabetic ketoacidosis to hypoperfusion with lactic acidosis from hemorrhagic or septic shock shares a variety of common therapies for disordered acid–base balance. It is encumbent upon the intensivist to decode the deranged physiology and to categorize the disorder in a meaningful fashion to direct effective repair strategies [1].
Besides the traditional classification of respiratory versus metabolic, acidosis versus alkalosis, and gap versus nongap (normal gap), the intensivist benefits from classifying acid–base disorders into three discrete groups: iatrogenically induced (i.e. hyperchloremic metabolic acidosis), a fixed feature of a pre-existing disease process (i.e. chronic renal failure, hyperlactatemia), or a labile feature of an evolving disease process (i.e. lactic acidosis from hemorrhage, shock of any cause). The therapy for, and the outcome from, each of these three categories may be distinctly different. A review of the genesis of acid–base abnormalities is appropriate but will be limited to metabolic derangements, as respiratory acid–base abnormalities are usually reparable with adjustments in sedative or ventilator prescription.
Acid–base abnormality genesis
Traditional paradigms of acid–base abnormalities hinge on generation of protons from the liberation of metabolic acids such as lactate or carbonic acid from increased CO2. Most traditional views rely on the Henderson–Hasselbach equation to determine the pH and proton concentration. Other attempts at classification rely upon nomograms with imprecise 'grey zones' to account for the imprecision in the Henderson–Hasselbach equation solutions. The key fault with these determinations is reliance upon bicarbonate as a determinant of the pH. In 1983, Peter Stewart clarified the physical chemistry principles that describe the independent determinants of proton concentration and pH, allowing the clinician to precisely and accurately determine the pH and to understand the genesis of each acid–base disturbance encountered [2].
The Stewartian methodology relies upon the relationships between ions that completely dissociate at physiologic pH – so-called 'strong ions'. There exist strong cations (Na+, K+, Ca2+ and Mg2+) as well as strong anions (Cl-, lactate, and sulphates [most notable in renal failure]). These strong ions establish a readily apparent strong ion difference (SID) that is net strong ion-positive (normal approximately +40). Since human acid–base physiology derives its homeostasis from charge balance, according to the physical chemistry principles articulated by Stewart the SID must be counterbalanced by an equal and opposing charge termed the effective strong ion difference (SIDe) (normal approximately -40). The SIDe negative charge principally stems from the dissociated moieties of plasma proteins (~78% albumin) and phosphate (~20%). The sum of these weak acids is known as ATOT since they exist in a dissociated form (A-) as well as an associated form (AH). When the SID and SIDe are equal, the plasma pH is exactly 7.4 at a pCO2 of 40 torr. These relationships are demonstrated in Fig. 1.
Figure 1.
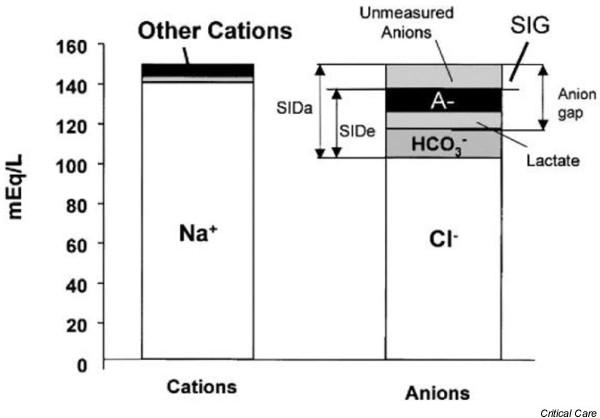
Charge balance in human plasma. SIDa, apparent strong ion difference; SIDe, effective strong ion difference; SIG, strong ion gap. Reproduced with permission from [1].
Note that when the SID and SIDe are unequal, the difference between the two is termed the strong ion gap (SIG) (SID – SIDe, normal = 0). This value is not discoverable by interrogation of any other acid–base variables or scheme, and is buried within the anion gap along with A- and lactate. It is important to note that the generation or consumption of protons is driven by the law of mass action upon the relationships identified in Fig. 2.
Figure 2.
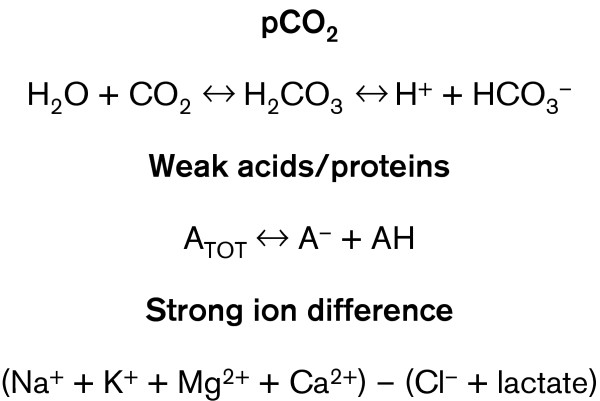
Charge interaction in human plasma. The equations demonstrate the charge interactions in human plasma that serve as independent control mechanisms for pH determination (pCO2, sum of weak acids and proteins in human plasma [ATOT], and strong ion difference).
Saline is comprised of equal parts of sodium and chloride, and as such appears electrically neutral. When equal amounts of sodium and chloride are added to plasma, however, the effects are different from those expected. The plasma chloride level is less than that of sodium. The net impact of adding equal amounts of sodium and chloride will therefore raise the chloride to a greater degree than the sodium. This results in a narrowed SID and a reduced plasma positive net strong ion charge. When plasma positive charge is reduced, as commonly occurs with significant chloride loading (reduced SID), an immediate and compensatory response is proton generation to aid in restoring charge equilibrium. The clinician identifies this physiologic process as a decreased pH. The genesis of hyperchloremic metabolic acidosis is thus readily understandable based on the Stewart principles [3]. It is important to recognize that the changes in plasma electrolyte concentration are millimolar in scale while the corresponding changes in proton concentration are nanomolar. There is therefore an unfavorable electrochemical gradient for simple plasma electrolyte and proton 'exchange'; the mechanism that underpins these changes is well explained by Stewart [2].
Relatedly, an individual with chloride loss (vomiting, large volume nasogastric losses without proton pump blockade) would have a net increase in plasma positive charge. Exactly the opposite process occurs to consume protons, leading to an increased pH. Importantly, this clinical condition highlights the mechanism underlying hypochloremic metabolic alkalosis as well as the rationale behind chloride loading for repair – the Cl-therapeutically reduces the plasma excess positive charge and the proton concentration in tandem. This process is unassociated with mortality, reflects the common use of loop diuretics for volume management, and will not be further explored
A central tenet of the Stewart methodology identifies the three independent control mechanisms for pH: SID, pCO2 and ATOT. Bicarbonate is a dependent variable, and as such does not determine the pH. This key concept aids in constructing acid–base repair strategies in the critical care environment. By way of example, patients with hyperchloremic metabolic acidosis may be corrected by altering their intravenous fluid prescription. An ideal strategy reduces plasma Cl- while preserving plasma Na+. This may be achieved by prescribing D5W plus a variable amount of NaHCO3 as the maintenance fluid, with the amount of NaHCO3 dependent on the desired amount of Cl- and pH change. This prescription provides a strong cation (Na+) without a strong anion, resulting in an expected increase in SID as Na+ is maintained but Cl- falls; the increased SID drives proton consumption and produces an increased pH.
Importantly, only changes in strong ions drive changes in proton concentration. There are, however, readily identifiable and compensatory changes in dependent ions such as bicarbonate. Understanding the physiologic mechanisms underpinning acid–base abnormalities thus provides a rationale for therapeutic intervention. Indeed, a recent comparison of traditional methods of acid–base interpretation to guide therapy with Stewart's physical–chemical method has championed the latter as an ideal means of determining the mechanism, and of uncovering acid–base abnormalities that were unappreciated using traditional classification and interpretation schemes [4].
Lactic acidosis and hyperlactatemia
The most common acid–base abnormality in trauma patients is lactic acidosis from hypovolemic shock and hypoperfusion. Lactic acidosis is a gap metabolic acidosis that is a labile feature of an evolving disease process. As such, lactic acidosis is a final common feature of a variety of processes that engender hypoperfusion, including diabetic ketoacidosis, septic shock, cardiogenic shock, and a variety of intoxications. These entities will therefore not be discussed separately; the discussion will instead focus on the consequences and implications of lactic acidosis regardless of etiology.
Lactate generated from hypoperfusion generates acidosis as the vast amount of lactate produced contributes a strong anion, decreases the SID, and generates protons. In contrast, lactate from lactated Ringer's (LR) solution is in small quantities (28 mmol/l) and is readily consumed, leaving behind Na+ as a strong cation; alkalinization results from the more positive SID leading to proton consumption.
Resolution of lactic acidosis correlates well with survival in a time-dependent fashion [5]. Moreover, resolving occult hypoperfusion (normal vital signs, but a persistent lactic acidosis) directly relates to infection risk as well as to mortality [6,7]. Reduced infectious events (principally respiratory complications) were realized using a protocol to clear lactate, whether overt or occult, as an arbiter of underlying hypoperfusion and systemic infection risk.
In order to avoid inappropriate therapy, it is important to differentiate lactic acidemia from hyperlactatemia (normal pH, elevated lactate level, constant lactate/pyruvate ratio). The former indicates a condition that merits therapy (volume expansion, inotropic support, septic source control), while hyperlactatemia frequently stems from exogenous medications, or as an endogenous accompaniment to persistently elevated endogenous catecholamines after shock or trauma [8].
Lactic acidosis has long been utilized as an outcome predictor with regard to survival after trauma, both blunt and penetrating, as well as intra-abdominal catastrophe [5-7,9,10]. However, lactate also performs quite well in the intensive care unit (ICU) as a mortality gauge [11]. The presence of this potent predictor of outcome is readily identifiable in the ICU setting with physical examination using extremity temperature as an arbiter (exclusive of patients with peripheral occlusive vascular disease) [12].
Lactic acidosis, but not hyperlactatemia [13], closely correlates with mortality risk and serves as a window into cell-level oxygen-dependent processes. Moreover, clearance of lactic acidemia portends an excellent likelihood of survival. In one convenience sampling of surgical ICU patients (general surgery and trauma) comparing lactate and base excess, lactate appears superior in predicting mortality and morbidity [14]. Relatedly, a separate study (prospective, consecutive, mixed medical–surgical patients) found that the combination of the two variables appeared superior to either lactate or base excess alone in predicting survival [15].
Standard base excess (base deficit)
A companion acid–base variable, base excess (commonly presented as base deficit) has also been touted as a prognostic variable in assessing outcome in the critically ill. Base excess indicates metabolic acidosis or alkalosis, but does not help place the acidosis into one or another category with regard to genesis. It is, however, commonly and readily assessed and is therefore the focus of a host of studies. A plethora of studies present a mixed picture in the analysis of base excess since the data derive from two distinct time frames: Emergency Department arrival versus some time after resuscitation. It is in the interpretation of base excess that the Stewart principles are vital to guide interpretation. Indeed, it has been demonstrated that the base excess may be manipulated by fluid resuscitation. Generating a hyperchloremic metabolic acidosis will create a spuriously more negative base deficit (or increased base excess) as the Cl-decreases the pH unaccompanied by hypoperfusion and lactic acidemia [16]. Prognostication dependent on post-resuscitation standard base excess (SBE) values must therefore be interpreted with caution.
Nonetheless, presentation or pre-resuscitation base excess values reliably indicate the degree of acid production following injury [17]. Interestingly, in this large cohort analysis of presentation SBE, the 50% lethal dose for the acid load indicated by base deficit shifted to a substantially lower level for a given age when combined with a traumatic brain injury; it is unknown whether this is true for other injuries in isolation or combination. The interpretation of SBE must therefore incorporate the injury complex into decision-making, perhaps limiting its utility. A recent study of salvageable trauma patients who underwent arterial blood gas analysis identified that SBE utility was greatest in predicting the outcome of patients sustaining gunshot wounds and blunt injury versus those with stab wounds or lacerations [18]. Mortality was lower for stab/laceration patients at any given base deficit, rendering interpretation in this subgroup problematic. Similar to lactate, the rate of clearance of base deficit to normal, rather than the absolute value, correlates better with survival than do changes in pH [19].
It is important to note that, using an ex vivo model, base excess values are CO2 invariate (unlike pH), potentially aiding in their initial utility and interpretation [20]. However, the clinical milieu includes multiple elements that may impact base excess, rendering the CO2–base excess relationship difficult to appreciate. Nonetheless, base excess correlates with transfusion requirements and with length of stay [21].
In patients with major hepatic trauma, base deficit (50% lethal dose, -11.8 mmol/l) and 24-hour transfusion requirement (50% lethal dose, 5.4 l packed red blood cells) surfaced as the strongest predictors of the risk of death, outperforming arterial lactate [22]. Importantly, these observations and the model were then tested on a different cohort with only pelvic fractures, with excellent performance. Smaller studies in pediatric trauma patients found that a base deficit less negative than -5 predicted uniform survival since all study group deaths occurred in patients with more negative base deficit values [23]. It thus appears that pre-resuscitation base excess or deficit correlates with survival and serves as another indicator of an underlying disease (hypoperfusion), but interpretation must be tempered by age and the mechanism of injury.
Hyperchloremic acidosis
While we touched upon hyperchloremic acidosis earlier, this common iatrogenically induced entity deserves further exploration. As already noted, the genesis of hyperchloremic metabolic acidosis stems from excess chloride administration relative to sodium, commonly as 0.9% normal saline solution, 0.45% normal saline solution, and even LR solution in large quantities [24-26]. This entity is thus an iatrogenic metabolic acidosis of the nongap variety. Hyperchloremia has been identified in up to 80% of patients admitted to a mixed medical–surgical ICU [26]. While not a predictor of outcome, hyperchloremic metabolic acidosis may contribute to morbidity and resource utilization. ICU admission for an 'unexpected acidosis', increased and perhaps mechanically supported minute ventilation to compensate for acidosis, and more complex intravenous fluid prescriptions (especially when utilizing hyperalimentation for nutritional support) are but a few ICU care elements impacted by hyperchloremic metabolic acidosis. While these events are probably insignificant for the young and otherwise physiologically sound patients, they may be significantly physiologically challenging for the elderly or for those with physiologic decompensation following significant trauma and hemorrhagic or septic shock.
The relationship between hyperchloremia and renal dysfunction is well known [27,28]. Moreover, ICU survival has been linked to Acute Pathophysiology and Chronic Health Evaluation II/III scores and multiple organ dysfunction syndrome, of which acute renal failure is a major element [29]. Controversy has long surrounded whether patients die from their renal failure or whether they die from the disease process. Recent data strongly suggest that acute renal failure is an independent risk factor for death despite renal replacement therapy [30]. In this study of acute renal failure, patients requiring renal replacement therapy suffered an accelerated mortality (62.8%) compared with those without renal failure (15.6%). The mortality differences remained unexplained by differences in the severity of illness, thus helping establish acute renal failure as an independent risk factor for mortality. Moreover, complicated acidosis/alkalosis was independently associated with death.
The deleterious impact of acute renal failure is thus potentially minimized by avoiding iatrogenic hyperchloremia and its attendant compromise of renal function. Further studies are needed to ascertain the impact of this entity upon current arbiters of morbidity including the ICU length of stay, ventilator days, acute lung injury/acute respiratory distress syndrome, and ventilator-associated pneumonia. Moreover, virtually no research addresses hyperchloremia avoidance strategies and their impact on morbidity such as acute renal failure in at-risk populations, nor addresses mortality.
Both animal and human data identify a linearly decreased pH and an increased SID with progressive chloride loading [31-33]. Interestingly, metabolic acidosis induced by chloride from normal saline solution loading is associated with impaired coagulation and the need for bicarbonate buffering of the induced acidosis, while resuscitation with comparable amounts of LR solution required no such therapy [31,33]. Hyperchloremic acidosis, while not a predictor of outcome, may therefore serve as a sentinel for hemorrhage risk, for component transfusion therapy, and for accelerated resource utilization. Importantly, one ex vivo study noted the induction of a SIG with crystalloid-induced hyperchloremic acidosis; no SIG was induced by adding comparable amounts of large molecular weight hydroxyethyl starch [31]. In a related provocative study, sepsis survival was enhanced by resuscitation with a large molecular weight hydroxyethyl starch molecule suspended in a balanced salt solution compared with LR solution or saline, and was unassociated with hyperchloremic metabolic acidosis [34].
Immune effects of acidosis
The effects of metabolic acidosis span more than one system. Immune activation has been intimately linked to the presence of acidosis, and SIG generation may be but one feature. Crystalloid resuscitation serves as a potent trigger for human white blood cell count activation, manifested as an oxidative burst and the expression of cell surface adhesion molecules [35]. Activation of T-cell protein kinases has been demonstrated with hypertonic saline, an effect whose downstream cell-specific responses carry an uncertain significance [36]. More certainly, intravascular acid infusion reliably creates acute lung injury and increases exhaled nitric oxide concentration in a rat model [37]. This effect has been demonstrated to stem from acidosis-stimulated expression of inducible nitric oxide synthase, and was associated with elaboration of the proinflammatory cytokine IL-6, also in a rat preparation [38]. Importantly, this work suggests that correction of acidosis may ameliorate inducible nitric oxide synthase expression and reduce lung injury.
Relatedly, acidosis included by lactate, pyruvate or HCl has been recently demonstrated to increase whole blood viscosity at both high and low shear rates of flow. During acidosis induction, hematocrit increases reflecting red blood cell swelling were also observed. Most importantly, these rheologic changes were reversible with the correction of acidosis. These data lend support to the notion that correcting acidosis represents more than 'treating numbers' and instead addresses important cellular and subcellular events. It is possible that the increased viscosity and hematocrit is responsible, in part, for regional hypoperfusion despite normal or supranormal systemic flow. Clearly further study is warranted, but one must consider that the time-honored endpoint of mortality is not well suited to assess the interventions targeting acid–base balance. Measures of morbidity or resource utilization may be more appropriate instead.
Strong ion gap
There are several studies that either support [39,40] or decry the utility of the Stewart methodology in evaluating ICU patients [26,41,42]. The SIG, as determined by Stewart's physico-chemical method, is strongly associated with metabolic acidosis, but is an independent entity that is probably a labile feature of an evolving disease process. One element that has surfaced from these studies is that the Stewart methodology is a precise and readily utilizable means of identifying the nature of the metabolic aberration; a calculator to determine the individual components is downloadable from the Internet [43]. How may one resolve the seeming disparity of SIG utility identified in some studies that is conspicuously lacking in others? The answer may be found in the timing. Much like base excess, the value of the SIG may be related to the time of assay. Since the natural history of the SIG and its clearance value remains unknown (similar to the early lactate observations), we must look to pre-resuscitation SIG analysis as a more controlled evaluation scheme.
In patients with major vascular injury requiring operative repair, but prior to resuscitation, an increased SIG (> 5) is predictive of mortality [44]. Performance characteristics based on receiver–operator characteristic curve analysis indicated a SIG area of 0.991 for mortality (95% confidence interval, 0.972–0.998) and that for anion gap of 0.994 (95% confidence interval, 0.976–0.999), outperforming lactate (receiver–operator characteristic curve area, 0.981; 95% confidence interval, 0.957–0.993). Multivariate logistic regression analysis indicated that an increased SIG (odds ratio, 3.6; 95% confidence interval, 1.99–6.78), more strongly than injury severity score (odds ratio, 1.17; 95% confidence interval, 1.06–1.31), was predictive of mortality.
In a related study in unselected trauma patients, the SIG discriminated quite well between survivors and those who died within 72 hours of Emergency Department arrival, again outperforming lactate and base deficit [45]. While the absolute SIG levels were not identical, the import behind the elevated level remains unaltered. It may be that the degree of SIG elevation is disease specific. An increased SIG occurs in patients with hepatic dysfunction [46] and renal dysfunction [26], as well as during endotoxin-induced sepsis [47]. In a large retrospective database analysis of patients requiring ICU care, SIG > 2 was independently linked with mortality in patients evidencing metabolic acidosis [48].
Based on these studies, longitudinal assessments of changes in the SIG as a predictor of outcome are underway. Nonetheless, it seems prudent to incorporate the pre-resuscitation SIG into the mélange of information that guides outcome prognostication. These data may be incorporated into daily practice using a handheld calculator, or a computer-based macro utilizing the relevant data points from the clinical laboratory; automated abstraction is ideal but awaits the development of appropriate interfaces with existing laboratory devices. It is essential to note that no evaluation method besides the physico-chemical one of Stewart allows the clinician to ascertain the presence and magnitude of the SIG.
Conclusion
Traditional classification schemes of acid–base derangements are too broad to aid in prognostication. Individual acid–base element evaluation allows one to draw valid conclusions regarding the likelihood of survival. The Stewart physico-chemical approach to acid–base analysis readily lends itself to these determinations by precisely evaluating the independent determinants of pH as well as the important SIG. At present, lactate, pre-resuscitation base deficit and the SIG appear most predictive of outcome in the critically ill, and they should be incorporated into a prognostication method. Future studies of acid–base prediction of outcome should strongly consider including each of these variables in their methodology. Further evaluation of these and potentially other markers of morbidity and resource utilization is appropriate.
Abbreviations
ATOT = sum of weak acids and proteins in human plasma; ICU = intensive care unit; IL = interleukin; LR = lactated Ringer's; pCO2 = Partial pressure of carbon dioxide in arterial blood; SBE = standard base excess; SID = strong ion difference; SIDe = effective strong ion difference; SIG = strong ion gap.
Competing interests
The author(s) declare that they have no competing interests.
References
- Gunnerson K, Kellum JA. Acid–base and electrolyte analysis in critically ill patients: are we ready for the new millennium? Curr Opin Crit Care. 2003;9:468–473. doi: 10.1097/00075198-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Stewart PA. Modern quantitative acid–base chemistry. Can J Physiol Pharm. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Miller L, Waters JH. Mechanism of hyperchloremic nonanion gap acidosis. Anesthesiology. 1997;87:1009–1010. doi: 10.1097/00000542-199710000-00050. [DOI] [PubMed] [Google Scholar]
- Fencl V, Jabor A, Kazda A, Figge J. Diagnosis of metabolic acid–base disturbances in critically ill patients. Am J Respir Crit Care Med. 2000;162:2246–2251. doi: 10.1164/ajrccm.162.6.9904099. [DOI] [PubMed] [Google Scholar]
- Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma. 1993;35:584–589. doi: 10.1097/00005373-199310000-00014. [DOI] [PubMed] [Google Scholar]
- Claridge JA, Crabtree TD, Pelletier SJ, Butler K, Sawyer RG, Young JS. Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J Trauma. 2000;48:8–14. doi: 10.1097/00005373-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Blow O, Magliore L, Claridge JA, Butler K, Young JS. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcomes from major trauma. J Trauma. 1999;47:964–969. doi: 10.1097/00005373-199911000-00028. [DOI] [PubMed] [Google Scholar]
- James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354:505–508. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- Jeng JC, Jablonski K, Bridgeman A, Jordan MH. Serum lactate, not base deficit, rapidly predicts survival after major burns. Burns. 2002;28:161–166. doi: 10.1016/S0305-4179(01)00098-5. [DOI] [PubMed] [Google Scholar]
- Mikulaschek A, Henry SM, Donovan R, Scalea TM. Serum lactate in not predicted by anion gap or base excess after trauma resuscitation. J Trauma. 1996;40:218–224. doi: 10.1097/00005373-199602000-00008. [DOI] [PubMed] [Google Scholar]
- Mizock BM, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992;20:80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- Kaplan L, McPartland K, Santora TA, Trooskin SZ. Start with the physical examination to identify hypoperfusion in ICU patients. J Trauma. 2001;50:620–628. doi: 10.1097/00005373-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Mizock BM. Significance of hyperlactatemia without acidosis during hypermetabloic stress. Crit Care Med. 1997;25:1780–1781. doi: 10.1097/00003246-199711000-00009. [DOI] [PubMed] [Google Scholar]
- Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictor of mortality and morbidity. Am J Surg. 2003;185:485–491. doi: 10.1016/S0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, Bennett ED. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Int Care Med. 2001;27:74–83. doi: 10.1007/s001340051352. [DOI] [PubMed] [Google Scholar]
- Brill SA, Stewart TR, Brundage SI, Schreiber MA. Base deficit does not predict mortality when secondary to hyperchloremic acidosis. Shock. 2002;17:459–462. doi: 10.1097/00024382-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Rutherford E, Morris JA, Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Feliciano DV, Rozycki G. Assessment of initial base deficit as a predictor of outcome: mechanism of injury does make a difference. Am Surg. 2002;68:689–693. [PubMed] [Google Scholar]
- Davis J, Kaups KL, Parks SN. Base deficit is superior to pH in evaluating clearance of acidosis after traumatic shock. J Trauma. 1998;44:114–118. doi: 10.1097/00005373-199801000-00014. [DOI] [PubMed] [Google Scholar]
- Morgan TJ, Clark C, Endre Z. Accuracy of base excess – an in vitro evaluation of the Van Slyke equation. Crit Care Med. 2000;28:2932–2936. doi: 10.1097/00003246-200008000-00041. [DOI] [PubMed] [Google Scholar]
- Davis JW, Parks SN, Kaups KL, Gladen HE, O'Donnell-Nicol S. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996;41:769–774. doi: 10.1097/00005373-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Rivkind AI, Dalal S, Godzari S. Early physiologic predictors of injury severity and death in blunt multiple trauma. Arch Surg. 1990;125:498–508. doi: 10.1001/archsurg.1990.01410160084019. [DOI] [PubMed] [Google Scholar]
- Randolph LC, Takacs M, Davis KA. Resuscitation in the pediatric trauma population: admission base deficit remains an important prognostic indicator. J Trauma. 2002;53:838–842. doi: 10.1097/00005373-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Bellomo R, Liu G, McNicol L, Buxton B. The aetiology and pathogenesis of cardiopulmonary bypass-associated metabolic acidosis using polygeline pump prime. Int Care Med. 1999;25:680–685. doi: 10.1007/s001340050930. [DOI] [PubMed] [Google Scholar]
- Moviat M, van Haren F, van der Hoeven H. Conventional or physicochemical approach in intensive care unit patients with metabolic acidosis. Crit Care. 2003;7:R41–R45. doi: 10.1186/cc2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated ringer's solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Barie P, Hydo LJ, Fischer E. Utility of severity scoring for prediction of prolonged critical care. J Trauma. 1996;40:513–519. doi: 10.1097/00005373-199604000-00002. [DOI] [PubMed] [Google Scholar]
- Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Patterson T, Bailey H, Kaplan LJ. Hyperchloremia induces acidosis, increases the strong ion gap, and impairs coagulation [abstract] Crit Care Med. 2000;Suppl 28:A118. [Google Scholar]
- Healey MA, Davis RE, Liu FC, Loomis WH, Hoyt DB. Lactated ringers in superior to normal saline in a model of massive hemorrhage and resuscitation. J Trauma. 1998;45:894–899. doi: 10.1097/00005373-199811000-00010. [DOI] [PubMed] [Google Scholar]
- Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid–base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–305. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, Burris D, Ling G, Sun L. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28:74–78. doi: 10.1097/00003246-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Junger WG, Hoyt DB, Hamreus M, Liu FC, Herdon-Remelius C, Junger W, Altman A. Hypertonic saline activates protein kinases and mitogen-activated protein kinase p38 in T-cells. J Trauma. 1997;42:437–443. doi: 10.1097/00005373-199703000-00011. [DOI] [PubMed] [Google Scholar]
- Pedoto A, Caruso JE, Nandi J, Oler A, Hoffmann SP, Tassiopoulos AK, McGraw DJ, Camporesi EM, Hakim TS. Acidosis stimulates nitric oxide production and lung damage in rats. Am J Respir Crit Care Med. 1999;159:397–402. doi: 10.1164/ajrccm.159.2.9802093. [DOI] [PubMed] [Google Scholar]
- Haque IU, Huang CJ, Scumpia PO, Nasiroglu O, Skimming JW. Intravascular infusion of acid promotes intrapulmonary inducible nitric oxide synthase activity and impairs blood oxygenation in rats. Crit Care Med. 2003;31:1454–1460. doi: 10.1097/01.CCM.0000065678.24064.58. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Metabolic acidosis in the critically ill: lessons learned from physical chemistry. Kidney Int. 1998;53(Suppl 66):S81–S86. [PubMed] [Google Scholar]
- Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl–Stewart method predict mortality better than base excess, anion gap, and lactate in patients admitted in the pediatric intensive care unit. Crit Care Med. 1999;27:1577–1581. doi: 10.1097/00003246-199908000-00030. [DOI] [PubMed] [Google Scholar]
- Hatherill M, Waggie Z, Purves L, Reynolds L, Argent A. Mortality and the nature of metabolic acidosis in children with shock. Int Care Med. 2003;29:286–291. doi: 10.1007/s00134-002-1585-y. [DOI] [PubMed] [Google Scholar]
- Cusack RJ, Rhodes A, Lochhead P, Jordan B, Perry S, Ball JA, Grounds RM, Bennett ED. The strong ion gap does not have prognostic value in critically ill patients in a mixed medical/surgical adult ICU. Int Care Med. 2002;28:864–869. doi: 10.1007/s00134-002-1318-2. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Ed The Acid Base pHorum http://www.ccm.upmc.edu/education/resources/phorum.html accessed 10 December 2003.
- Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference and strong ion gap predicts outcome from major vascular injury. Crit Care Med. 2004;32:1120–1124. doi: 10.1097/01.CCM.0000125517.28517.74. [DOI] [PubMed] [Google Scholar]
- Kaplan LJ, Bailey H, Klein A, et al. Strong ion gap: a predictor of early mortality following blunt or penetrating trauma [abstract] Crit Care Med. 1999;Suppl 27:A42. doi: 10.1097/00003246-199912001-00076. [DOI] [Google Scholar]
- Kellum JA, Kramer DJ, Pinsky MR. Strong ion gap: a methodology for exploring unexplained anions. J Crit Care. 1995;10:51–55. doi: 10.1016/0883-9441(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Hepatic anion flux during acute endotoxemia. J Appl Physiol. 1995;78:2212–2217. doi: 10.1152/jappl.1995.78.6.2212. [DOI] [PubMed] [Google Scholar]
- Gunnerson KJ, Saul M, Kellum JA. Lactic versus nonlactic metabolic acidosis: outcomes in critically ill patients [abstract] Crit Care. 2003;7(Suppl 2):S8. doi: 10.1186/cc1906. [DOI] [PMC free article] [PubMed] [Google Scholar]


