Abstract
The distribution and function of aquaporins (AQPs) have not previously been defined in sweat glands. In this study, AQP1, AQP3, and AQP5 mRNA were demonstrated in rat paw by reverse transcription (RT)–PCR, but AQP2 and AQP4 were not. AQP1, AQP3, and AQP5 protein were confirmed in these tissues by immunoblotting. AQP1 was identified in capillary endothelial cells by immunohistochemical labeling, but not in sweat glands or epidermis. Abundant AQP3 expression was seen in basal levels of epidermis, but not in sweat glands. AQP2 and AQP4 were not observed in either skin or sweat glands. Immunohistochemical labeling revealed abundant AQP5 in secretory parts of rat and mouse sweat glands, where immunoelectron microscopy demonstrated abundant AQP5 labeling in the apical plasma membrane. AQP5 immunolabeling of human sweat glands yielded a similar pattern. To establish the role of AQP5 in sweat secretion, we tested the response of adult mice to s.c. injection of pilocarpine, as visualized by reaction of secreted amylase with iodine/starch. The number of active sweat glands was dramatically reduced in AQP5-null (−/−) mice compared with heterozygous (+/−) and wild-type (+/+) mice. We conclude that the presence of AQP5 in plasma membranes of sweat glands is essential for secretion, providing potential insight into mechanisms underlying mammalian thermoregulation, tactile sensitivity, and the pathophysiology of hyperhidrosis.
Aquaporins (AQPs) are a family of membrane water channel proteins. Eleven mammalian AQPs are known, and several have been implicated in physiological processes and human diseases as predicted by the sites of expression. AQP1 resides in the plasma membranes of renal proximal tubules and descending thin limbs (1), and humans with AQP1 mutations exhibit defective urinary concentration (2). AQP1 is also abundant in capillary endothelium (3), facilitating movement of water between lumen and interstitium (4). AQP0 is abundant in lens fiber cells, and families with AQP0 mutations suffer congenital cataracts (5). AQP2 is expressed in renal collecting duct principal cells where it is shuttled from intracellular sites to the plasma membrane in response to vasopressin activation of adenylyl cyclase (6), and humans with AQP2 mutations have severe nephrogenic diabetes insipidus (7). AQP5 is usually expressed in apical plasma membranes of secretory glands (8), and transgenic mice lacking AQP5 are deficient in pilocarpine-induced salivation (9). Although AQP5 expression is not known to be impaired, humans suffering from Sjögren's syndrome experience decreased tear and saliva formation, and AQP5 is retained in intracellular sites (10, 11).
Sweating is essential for thermoregulation in many species, and sweat glands in the footpads of rodents are believed to enhance tactile sensitivity (12, 13). It is yet unknown whether specific aquaporins are present in sweat glands. Patients with Sjögren's syndrome also have difficulty in perspiring (hypohidrosis/anhidrosis) and exhibit markedly decreased sweating in response to methacholine stimulation (14, 15). Thus, AQP5 may play a significant role in sweat secretion, and the pathogenesis of hypohidrosis, observed in patients with Sjögren's syndrome, could be related to disregulation of AQP5 expressed in sweat glands.
This study was undertaken to define the localization of AQPs 1 through 5 in rat and mouse skin and sweat glands and to investigate the potential roles of AQPs in sweat secretion. Our studies demonstrate that AQP5 resides in plasma membranes of sweat secretory cells and is essential for sweat secretion.
Methods
Experimental Animals.
Male Wistar rats (180–260 g) and male Naval Medical Research Institute mice (20–30 g, M & B, Ry, Denmark) were maintained on standard rodent diet (Altromin, Lage, Germany) with free access to water. AQP5-null (−/−) mice, heterozygous (+/−), and wild-type (+/+) littermates have been described (16).
RNA Extraction and RT-PCR.
Total RNA was extracted by a conventional method (17). Reverse transcription of total RNA used Superscript (Life Technologies, Tåstrup, Denmark). PCR was performed for 30 cycles with sequence-specific sense and antisense oligonucleotide primers: AQP1: GGCCATGACCCTCTTCGTCT, GAGCAGAAGCCCCAGTGTGA; AQP2: AGCAGCATGTGGGAACTCAGATCC, AGCTCAGGCCTTGCTGCCGCGAGG; AQP3: CCATCTACACACTGGCACAGAC, ACATTCTCTGCCTCAGTGGAAG (18); AQP4: CATCGCCAAGTCTGTCTTCTACA, ACCTCTCCAGACGAGTCCTTCC (18); AQP5: CAAGGCGGTGTTCGCAGAGTTCC, CCTCTCGATGATCTTCCCAGTCC (18); β-actin: GAGTACAACCTCCTTGCAGCTC, TTGTAGAAAGTGTGGTGCCAAA.
Negative PCR controls included omission of reverse transcriptase or omission of cDNA. Products were confirmed by high-stringency Southern blotting with sequence-specific cDNA probes (data not shown).
Primary Antibodies.
Immune serum- or affinity-purified antibodies included: AQP1 (CHIP serum or LL266AP) (1); AQP2 (LL127 serum or LL127AP) (6, 19); AQP3 (LL178AP) (20); AQP4 (LL182 serum or LL182AP) (21); AQP5 (22, 23). Affinity-purified polyclonal antibodies were also raised against the C-terminal region (amino acids 248–267) of rat and mouse AQP1 (RA3391/2353AP) and the C-terminal region (amino acid 261–285) of rat AQP3 (RA3040/1592AP).
Membrane Fractionation and Immunoblotting.
Tissues were homogenized for 20–30 sec and centrifuged at 4,000 × g for 15 min at 4°C. The resulting supernatant was centrifuged at 200,000 × g for 1 h to produce a pellet containing plasma membranes and intracellular vesicles. Samples (2% SDS) were run on SDS-12% polyacrylamide Laemmli minigels, transferred to nitrocellulose membranes, incubated overnight with rat primary antibodies, and visualized with horseradish peroxidase-conjugated secondary antibody (P0448, DAKO, Glostrup, Denmark) by enhanced chemiluminiscence (Amersham Pharmacia) (6).
Immunohistochemistry.
Experimental animals were fixed by cardiac perfusion with 3% paraformaldehyde in 0.1 M cacodylate buffer, and tissues were extracted for paraffin embedding and sectioning. Paraffin sections (2 μm) were incubated overnight at 4°C with primary antibodies and visualized with horseradish peroxidase-conjugated secondary antibody (above). Punch biopsies of human tissues were incubated in 25 units/ml dispase (Roche Molecular Biomedicals, Hvidovre, Denmark) overnight on ice. The epidermis including intact sweat glands was gently removed from the dermis and fixed in neutral buffered formalin, rinsed, and kept in PBS with sodium azide. For staining, tissues were blocked in PB buffer (PBS, 0.3% Triton, 1% skimmed milk powder) and incubated overnight with human AQP5-antibody. Labeling was visualized with Alexa Fluor488 (Molecular Probes). Confocal microscopy used a Leica TCR2 (Leica, Deerfield, IL).
Immunoelectron Microscopy.
Ultrathin Lowicryl HM20 sections (40–80 nm) were incubated overnight at 4°C with rat anti-AQP5 antibodies (11, 22, 23) and visualized with goat anti-rabbit IgG conjugated to 10-nm colloidal gold particles at 1:50 (GAR.EM10, BioCell Research Laboratories, Cardiff, U.K.). Sections stained with uranyl acetate and lead citrate were examined with a Philips CM100 or Philips 208 electron microscope (Philips, Eindhoven, The Netherlands).
In Vivo Sweat Response.
Determination of pilocarpine-induced sweating was quantitated by a slightly modified standard method (24), in which secreted amylase was detected by its reaction with iodine and starch. Adult AQP5-null (−/−) mice, heterozygous (+/−), and wild-type (+/+) were anesthetized (0.66 ml ketamine/0.22 ml xylazine in 2.5 ml isotonic NaCl; dose 0.625 ml per 100 g body weight). Right hind paws were painted with 3.5% iodine in ethanol and coated with 10% starch solution in mineral oil. Pilocarpine (50 μg) was injected s.c. into the paws, and digital photos taken every 15 sec for up to 5 min. Black spots were counted 2 min after pilocarpine injection. Results are indicated as fraction of controls (wild type).
Results
Detection of AQP5 mRNA by RT-PCR and Immunoblotting.
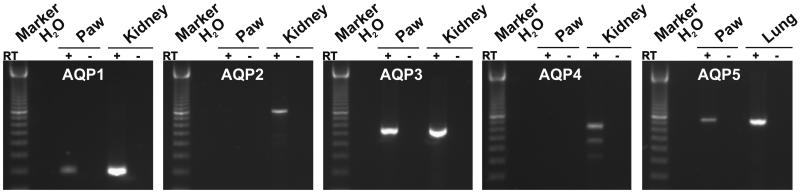
RT-PCR was performed on total RNA from rat paws (Fig. 1) by using specific primers. Rat kidneys and lungs were positive controls. Consistent with evidence that AQP1 is present in capillary endothelia (3, 25) and AQP3 in the basal layer of the skin epithelium (26), AQP1 and AQP3 primers produced bands in samples from rat paws and kidneys. AQP5 primers also produced a band in rat paws, identifying a previously unrecognized site for AQP5 expression. AQP2 and AQP4 primers failed to amplify mRNA from rat paws, but bands were produced from positive controls. Thus, AQP1, AQP3, and AQP5 mRNA were all detected in rat paws.
Figure 1.
RT-PCR analysis of rat paws. Products of the predicted sizes were generated by RT-PCR of rat paw AQP1 (199 bp), AQP3 (539 bp), and AQP5 (733 bp). Sequence specificity was confirmed by Southern blotting (not shown). Products were not generated by RT-PCR of rat paws for AQP2 (822 bp) or AQP4 (624 and 459 bp). Rat kidney and lung were positive controls. Negative controls were omission of reverse transcriptase (RT−) and omission of cDNA (H2O). PCR of β-actin confirmed the presence of cDNA in all samples (not shown).
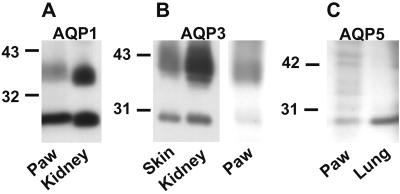
Immunoblots prepared from rat paws were reacted with specific antibodies to AQP1–5. Membrane samples prepared from rat kidney inner medulla and lung were positive controls. Anti-AQP1 specifically reacted with 29-kDa (nonglycosylated) and 35- to 40-kDa (glycosylated) forms of AQP1 in rat paws and kidney (Fig. 2A). Anti-AQP3 antibodies specifically reacted with 27-kDa (nonglycosylated) and 33- to 40-kDa (glycosylated) forms of AQP3 in rat skin and kidney (Fig. 2B). Anti-AQP5 specifically reacted with a 27-kDa band in rat paws and lung (Fig. 2C). Anti-AQP2 and anti-AQP4 failed to react with samples prepared from rat paws (not shown). Thus, the expression of AQP1, AQP3, and AQP5 were confirmed in rat paws.
Figure 2.
Immunoblot analysis of rat paws, skin, kidney (inner medulla), and lung. (A) Anti-AQP1 immunoblot of paw and kidney showed 29-kDa (nonglycosylated) and 35- to 40-kDa glycosylated forms of AQP1. (B) Anti-AQP3 immunoblot of rat skin, kidney, and paws showed 27-kDa (nonglycosylated) and 33- to 40-kDa (glycosylated) forms of AQP3. (C) Anti-AQP5 immunoblot of paws and lung revealed 27-kDa reaction.
Immunohistochemistry and Immunoelectron Microscopy.
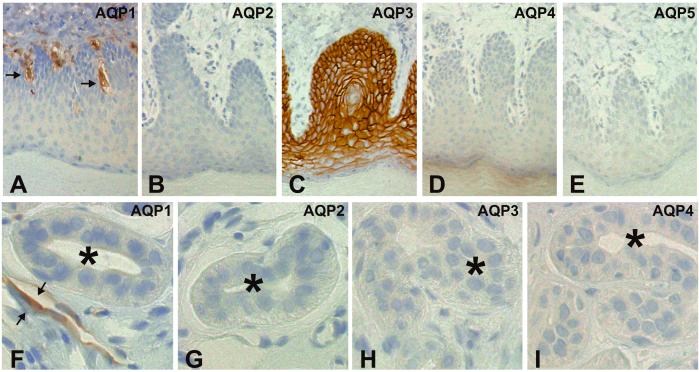
Cellular and subcellular localization of AQP1–5 were defined by immunoperoxidase labeling of 2-μm paraffin sections from rat paws (Fig. 3) and mouse paws (Fig. 4). The anti-AQP1 labeling was confined to capillary endothelial cells in rat paws (Fig. 3A), with no labeling of sweat glands or skin epithelium (Fig. 3F). As expected (26), basal levels in skin epithelium exhibited abundant anti-AQP3 labeling (Fig. 3C), but anti-AQP3 labeling was not observed in sweat glands (Fig. 3H). Moreover, anti-AQP2 and anti-AQP4 immunolabeling were not observed in skin (Figs. 3 B and D) or in rat sweat glands (Figs. 3 G and I). Anti-AQP5 did not label the skin epithelium (Fig. 3E). Identical anti-AQP1–5 labeling patterns were observed in mouse paws (not shown).
Figure 3.
Immunoperoxidase localization of AQP1–5 in 2-μm semithin paraffin sections of rat paws. (A and F) Anti-AQP1 labeled capillary structures but not epithelium (A) or sweat glands (F, *). (B and G) Anti-AQP2 labeling was not observed in epithelium (C) or sweat glands (G, *). (C and H) Anti-AQP3 labeled the plasma membranes of basal epithelium (C) but not sweat glands (H, *). (D and I) Anti-AQP4 labeling was not observed in epithelium (D) or sweat glands (I, *). (E) No Anti-AQP5 labeling was observed in epithelium. The same labeling patterns were observed in mouse paw (not shown). (Magnification: A–E, ×75; F–I, ×350.)
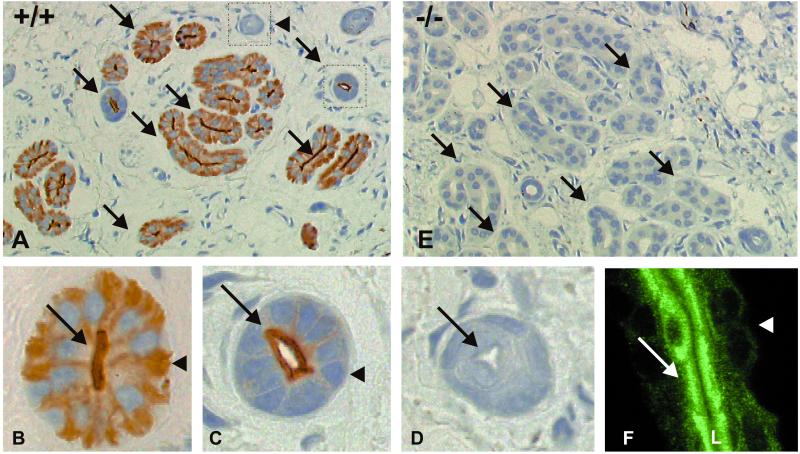
Figure 4.
Immunoperoxidase localization of AQP5 in 2-μm semithin paraffin sections of mouse paws and human sweat glands. (A–D) Anti-AQP5 labeling of a wild-type (+/+) mouse, revealed signal (arrows) over apical and basolateral membranes of the excretory part of the sweat glands. (E) Anti-AQP5 labeling was not detected on sections from AQP5-null (−/−) mouse (arrows). (C) Anti-AQP5 labeling was restricted to the apical membranes (arrow) in the first portion of the excretory duct; no anti-AQP 5 labeling was detected over the basolateral membrane (arrowhead). (D) No anti-AQP5 labeling was observed over the last portion of the excretory duct (arrow indicates apical). Identical anti-AQP5 labeling patterns were observed in rat paws and Naval Medical Research Institute mouse paws (not shown). (F) Confocal laser scanning microscopy reveals strong staining of AQP5 in sections of an uncoiled human sweat duct. (Magnification: A and E, ×175; B–D, ×670; F, ×900.
Immunohistochemical staining revealed strong anti-AQP5 labeling of both apical and basolateral plasma membrane domains of the secretory parts of mouse sweat glands (Fig. 4 A and B). The same pattern was found in rat sweat glands (not shown). The apical membranes of proximal excretory ducts of sweat gland were also prominently labeled by anti-AQP5 (Fig. 4C), whereas distal excretory ducts were not labeled (Fig. 4D). Sections of paw from AQP5-null (−/−) mice failed to react with the anti-AQP5 (Fig. 4E), establishing the specificity of the labeling pattern. Also strong anti-AQP5 labeling was seen in the apical plasma membrane domains of human sweat glands (Fig. 4F). No apparent changes occurred in the distribution or number of sweat gland profiles in the wild-type and AQP5 null mice.
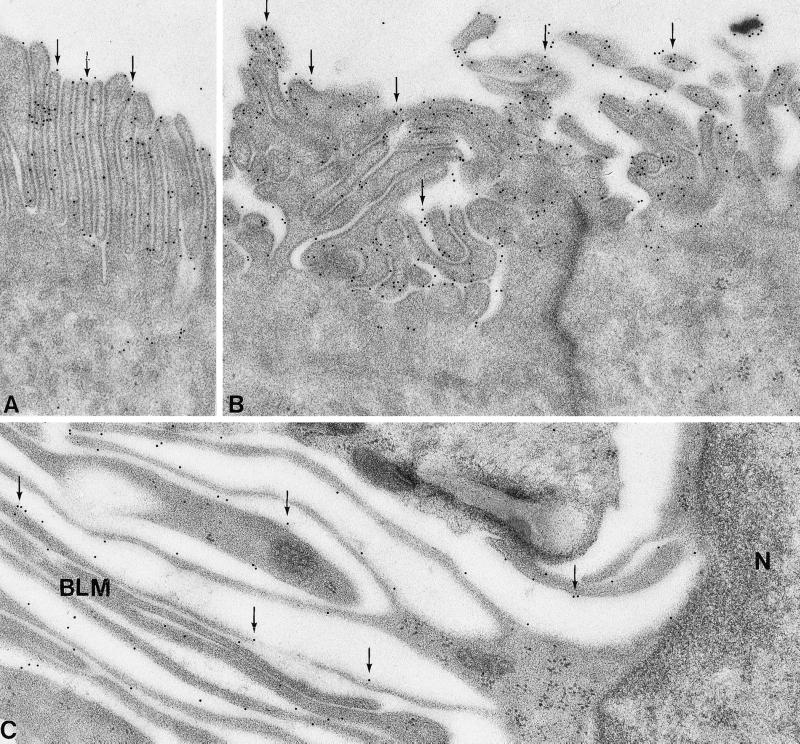
The subcellular localization of AQP5 in apical and basolateral plasma membranes of secretory cells in sweat glands was established by immunogold electron microscopy. Abundant anti-AQP5 labeling of apical plasma membranes was observed in secretory parts of mouse sweat glands (Fig. 5 A and B). Distinct labeling was also observed in basolateral plasma membranes (Fig. 5C), consistent with the immunohistochemical findings. Almost no intracellular labeling occurred.
Figure 5.
Immunoelectron microscopy of sweat gland secretory cells in Naval Medical Research Institute mouse paw. (A and B) Anti-AQP5 labeling was observed in the apical plasma membranes (arrows); intracellular compartments were not labeled. (C) Anti-AQP5 labeling of the basolateral plasma membranes was observed with only minor labeling of intracellular compartments (arrows). N, Nucleus; BLM, basolateral membrane folding. (Magnification: ×63,000.)
Pilocarpine-Induced Sweat Response.
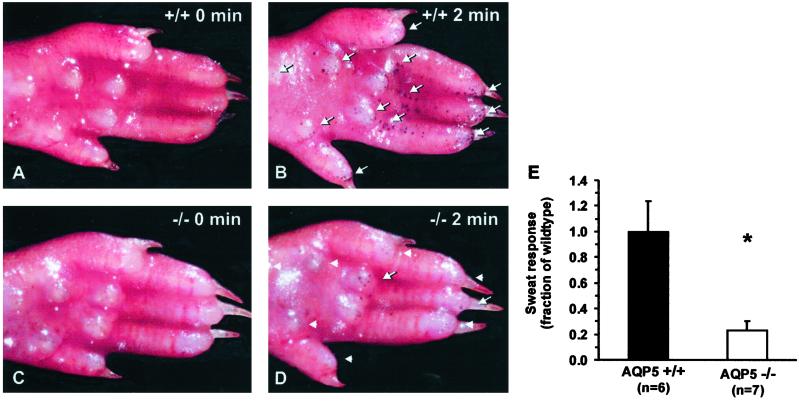
Sections of rat and mouse paws in wild-type (+/+) and AQP5-null (−/−) mice revealed no apparent differences in the number or morphological appearance of sweat glands (Fig. 4). The ability of AQP5-null (−/−) mice to secrete sweat was evaluated by painting hindpaws with iodine and starch solution, a standard method for detecting amylase, which causes the appearance of black spots over functional glands. Prior to pilocarpine injection (50 μg), few glands were observed (Fig. 6A), whereas after 2 min, wild-type (+/+) mice had a higher number of visibly functional glands (arrows, Fig. 6B) than did AQP5-null (−/−) mice (arrowheads, Fig. 6D). Quantification of functional glands from six wild-type (+/+) and seven AQP5-null (−/−) mice showed a consistently lower number of active glands (23 ± 7%) in the AQP5-null (−/−) mice when compared with normalized values for wild types (100 ± 24%; Fig. 6E). Heterozygous mice (n = 3) showed a response not significantly different from wild type (data not shown).
Figure 6.
Pilocarpine-induced sweat response monitored by amylase reaction with iodine/starch in mouse paw. (A) Adult AQP5 wild-type (+/+) mouse prior to (0 min) pilocarpine injection. (B) Adult AQP5 wild-type (+/+) mouse 2 min after pilocarpine injection. (C) Adult AQP5-null (−/−) mouse prior to (0 min) pilocarpine injection. (D) Adult AQP5-null (−/−) mouse 2 min after injection. Functional glands appear as black dots (arrows); lack of sweat response is indicated (arrowheads). (E) Sweat response estimated 2 min after pilocarpine injection. The AQP5-null (−/−) mice had a markedly reduced sweat response. *, P < 0.05.
Discussion
In this study, we demonstrate AQP5 expression in sweat glands by RT-PCR, immunoblotting, and immunocytochemistry of rat and mouse paws. Abundant AQP5 immunolabeling was observed in secretory cells and in the excretory duct of sweat glands. Immunoelectron microscopy demonstrated that AQP5 labeling is predominantly associated with apical and basolateral plasma membranes of secretory cells of mouse sweat glands with only minor labeling of intracellular structures. Pilocarpine-induced sweat responses in AQP5-null (−/−) mice were significantly reduced when compared with wild-type (+/+) and heterozygous (+/−) mice. These studies establish an essential role for AQP5 in sweat production that may be of importance for understanding thermoregulation and the pathophysiology of hyperhidrosis or hypohidrosis.
The localization of AQP5 in sweat glands and its possible role in sweat secretion had not previously been established. AQP1 is present in the microvasculature (3), and fluid secretion requires substantial fluxes of water from plasma to interstitium. AQP1 has a similar distribution in the vascular endothelial cells of rat exocrine pancreas as well as submandibular salivary glands (27) where it presumably serves a similar function. AQP3 is present at the basal levels of skin epidermis (26). Several rat exocrine glands, including lacrimal glands (28, 29), subepithelial glands of the upper airways (8), and submandibular and parotid salivary glands (8) contain AQP5 in the apical membranes of acinar cells (30). In this study, we showed that AQP5 is abundant in apical membranes and is also present in basolateral membranes of sweat glands in rat and mouse paws.
The sympathetic innervation of sweat glands elicits sweat secretion in rodents. It is not known, however, which steps are regulated by acetylcholine or catecholamines. Postganglionic fibers in sweat glands release acetylcholine, and catecholamines have been shown to act directly on gland cells to trigger their final differentiation for acquisition of secretory responsiveness (31). Muscarinic agonists, like pilocarpine, mimic the ability of nerve stimulation to induce sweating (32, 33), and sweating is blocked by muscarinic antagonists (34). Previous studies have suggested that multiple steps are involved in the coupling of muscarinic receptor stimulation to sweat secretion (32, 35). Similar to sweat glands, rat salivary glands have abundant apical AQP5 in serous gland cells where autonomic nerve systems are innervated (8). In parotid glands, stimulation of muscarinic receptors generates inositol 1,4,5-trisphosphate and diacylglycerol via phospholipase C activation and increased phosphoinositide turnover. Generation of inositol 1,4,5-trisphosphate leads to the increase in the intracellular calcium levels (36, 37) resulting in the rapid secretion of saliva (38). This outcome may (39) possibly require translocation of AQP5 water channel proteins to the apical plasma membrane or other forms of activation (36, 40). Consistent with this requirement, AQP5-null (−/−) mice exhibit marked reduction in pilocarpine-stimulated secretion of saliva (9).
Our studies demonstrate the presence of AQP5 in sweat glands and a marked reduction in pilocarpine-induced sweat response in AQP5-deficient mice, thus raising the possibility that dysregulation of AQP5 may be important in several clinical disorders. Thus, dysregulation of AQP5 in sweat glands may contribute to the pathogenesis of hypohidrosis observed in patients with Sjögren's syndrome in whom markedly decreased sweating was evident in response to methacholine stimulation (14, 15). In contrast, hyperhidrosis is a chronic idiopathic disorder of excessive sweating which may affect the palms, axillae, soles of the feet, and the face. It may be complicated by skin maceration as well as secondary microbial infections, and all the treatment modalities are associated with several complications (41). Our data suggest a key role of AQP5 in sweat production and raise the possibility that AQP5 inhibition may provide a new therapeutic option for reducing excessive sweating in this clinical disorder.
Acknowledgments
We thank R. James Turner and Luis Reuss for critical readings of the manuscript and helpful suggestions, and Inger Merete Paulsen, Helle Høyer, Zhila Nikrozi, Gitte Christensen, Mette Frank Vistisen, and Albert O. Meier for excellent technical assistance. The Water and Salt Research Center at the University of Aarhus is supported by the Danish National Research Foundation (Danmarks Grundforskningsfond). This work was supported by the Karen Elise Jensen Foundation, Novo Nordisk Foundation, Danish Medical Research Council, the Dongguk University, Helen and Ejnar Bjørnows Foundation, A.P. Møller and Spouse Chastine Mc-Kinney Møllers Foundation, Mrs. Ruth T.E. Konig–Petersens Research Foundation for Kidney Diseases, and The Research Foundation of the Danish Kidney Association, and National Institutes of Health Grants DE138283, HL61781, HL33991, HL48268, and EY11239.
Abbreviations
- AQP
aquaporin
- RT
reverse transcription
References
- 1.Nielsen S, Smith B, Christensen E I, Knepper M A, Agre P. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King L S, Choi M, Fernandez P C, Cartron J P, Agre P. N Engl J Med. 2001;345:175–179. doi: 10.1056/NEJM200107193450304. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen S, Smith B L, Christensen E I, Agre P. Proc Natl Acad Sci USA. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King, L. S., Nielsen, S., Agre, P. & Brown, R. H. (2002) Proc. Natl. Acad. Sci. USA 99, in press. [DOI] [PMC free article] [PubMed]
- 5.Berry V, Francis P, Kaushal S, Moore A, Bhattacharya S. Nat Genet. 2000;25:15–17. doi: 10.1038/75538. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Chou C L, Marples D, Christensen E I, Kishore B K, Knepper M A. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deen P M, Verdijk M A, Knoers N V, Wieringa B, Monnens L A, van-Os C H, van-Oost B A. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen S, King L S, Christensen B M, Agre P. Am J Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 9.Ma T, Song Y, Gillespie A, Carlson E J, Epstein C J, Verkman A S. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 10.Steinfeld S, Cogan E, King L S, Agre P, Kiss R, Delporte C. Lab Invest. 2001;81:143–148. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 11.Tsubota K, Hirai S, King L S, Agre P, Ishida N. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy W R, Sakuta M, Quick D C. Neuroscience. 1984;11:741–749. doi: 10.1016/0306-4522(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler H L, Fisher E R. Arch Dermatol. 1968;97:189–201. doi: 10.1001/archderm.97.2.189. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell J, Greenspan J, Daniels T, Whitcher J P, Maibach H I. J Am Acad Dermatol. 1987;16:233–235. doi: 10.1016/s0190-9622(87)80070-1. [DOI] [PubMed] [Google Scholar]
- 15.Huang C L, Kuo T T, Chan H L. J Am Acad Dermatol. 1996;35:350–352. doi: 10.1016/s0190-9622(96)90668-4. [DOI] [PubMed] [Google Scholar]
- 16.Krane C M, Melvin J E, Nguyen H V, Richardson L, Towne J E, Doetschman T, Menon A G. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Patil R V, Saito I, Yang X, Wax M B. Exp Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- 19.Marples D, Christensen B M, Frokiaer J, Knepper M A, Nielsen S. Am J Physiol. 1998;275:F400–F409. doi: 10.1152/ajprenal.1998.275.3.F400. [DOI] [PubMed] [Google Scholar]
- 20.Ecelbarger C A, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper M A. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 21.Terris J, Ecelbarger C A, Marples D, Knepper M A, Nielsen S. Am J Physiol. 1995;269:F775–F785. doi: 10.1152/ajprenal.1995.269.6.F775. [DOI] [PubMed] [Google Scholar]
- 22.Delporte C, O'Connell B C, He X, Ambudkar I S, Agre P, Baum B J. J Biol Chem. 1996;271:22070–22075. doi: 10.1074/jbc.271.36.22070. [DOI] [PubMed] [Google Scholar]
- 23.King L S, Nielsen S, Agre P. Am J Physiol. 1997;273:C1541–C1548. doi: 10.1152/ajpcell.1997.273.5.C1541. [DOI] [PubMed] [Google Scholar]
- 24.Tafari A T, Thomas S A, Palmiter R D. J Neurosci. 1997;17:4275–4281. doi: 10.1523/JNEUROSCI.17-11-04275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen S, Pallone T, Smith B L, Christensen E I, Agre P, Maunsbach A B. Am J Physiol. 1995;268:F1023–F1037. doi: 10.1152/ajprenal.1995.268.6.F1023. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. J Histochem Cytochem. 1999;47:1275–1286. doi: 10.1177/002215549904701007. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Nielsen S, Dai Y, Lazowski K W, Christensen E I, Tabak L A, Baum B J. Pflügers Arch. 1994;428:455–460. doi: 10.1007/BF00374565. [DOI] [PubMed] [Google Scholar]
- 28.Ishida N, Hirai S I, Mita S. Biochem Biophys Res Commun. 1997;238:891–895. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Cell Tissue Res. 1999;295:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- 30.He X, Tse C M, Donowitz M, Alper S L, Gabriel S E, Baum B J. Pflügers Arch. 1997;433:260–268. doi: 10.1007/s004240050276. [DOI] [PubMed] [Google Scholar]
- 31.Tian H, Habecker B, Guidry G, Gurtan A, Rios M, Roffler-Tarlov S, Landis S C. J Neurosci. 2000;20:7362–7369. doi: 10.1523/JNEUROSCI.20-19-07362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant M P, Landis S C. J Neurosci. 1991;11:3772–3782. doi: 10.1523/JNEUROSCI.11-12-03772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landis S C, Keefe D. Dev Biol. 1983;98:349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- 34.Stevens L M, Landis S C. Dev Biol. 1987;123:179–190. doi: 10.1016/0012-1606(87)90440-4. [DOI] [PubMed] [Google Scholar]
- 35.Landis S C. Life Sci. 1999;64:381–385. doi: 10.1016/s0024-3205(98)00578-5. [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa Y, Eguchi T, Skowronski M T, Ishida H. Biochem Biophys Res Commun. 1998;245:835–840. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 37.Dai Y S, Ambudkar I S, Horn V J, Yeh C K, Kousvelari E E, Wall S J, Li M, Yasuda R P, Wolfe B B, Baum B J. Am J Physiol. 1991;261:C1063–C1073. doi: 10.1152/ajpcell.1991.261.6.C1063. [DOI] [PubMed] [Google Scholar]
- 38.Foskett J K, Melvin J E. Science. 1989;244:1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- 39.Nauntofte B. Am J Physiol. 1992;263:G823–G837. doi: 10.1152/ajpgi.1992.263.6.G823. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa Y, Skowronski M T, Ishida H. FEBS Lett. 2000;477:253–257. doi: 10.1016/s0014-5793(00)01763-4. [DOI] [PubMed] [Google Scholar]
- 41.Stolman L P. Dermatol Clin. 1998;16:863–869. doi: 10.1016/s0733-8635(05)70062-0. [DOI] [PubMed] [Google Scholar]