Abstract
Background
Diarrhoea remains a leading cause of death in children. An intestinal adsorbent may reduce diarrhoea duration and severity.
Methods
Randomised controlled feasibility trial with two phases: phase 1 (0–4 hours and double-blind) and phase 2 (up to 5 days and open-label). 50 children aged 6–59 months with acute diarrhoea presenting with no or some dehydration to the emergency paediatric unit and outpatient clinic at Edward Francis Small Teaching Hospital, Banjul, The Gambia were randomised to either standard treatment (oral rehydration fluid and zinc) or standard treatment with polymethylsiloxane polyhydrate for up to 5 days.
Results
Recruitment was completed in 7 months. All but one child completed the study. There were no major protocol deviations although patient-held diaries did not collect reliable information. Time from randomisation to the last watery stool (primary outcome) was shorter in the intervention than control arm (mean difference −19.3 hours, 95% CI −30.9 to −7.8). Stool frequency was lower in the intervention arm on days 2 (95% CI −0.8 to −1.3 to −0.3) and 3 (95% CI −0.8; −1.3 to −0.3). One serious event (death) occurred in the control arm.
Conclusions
A randomised, controlled trial is feasible. Further clinical trials are warranted to confirm the efficacy of polymethylsiloxane polyhydrate in acute diarrhoea and inform management guidelines.
Trial registration number
PACTR202302683128875.
Keywords: Child Health, Gastroenterology, Low and Middle Income Countries
WHAT IS ALREADY KNOWN ON THIS TOPIC
Diarrhoea remains a leading cause of death particularly for children under 5 in low-resource settings where it can be challenging to undertake clinical trials. Intestinal adsorbents which bind harmful substances in the gastrointestinal tract without crossing the mucosal wall show potential as an adjunct to current treatment though clinical trial data remain limited.
WHAT THIS STUDY ADDS
A randomised, controlled efficacy trial of an intestinal absorbent is feasible in vulnerable children in this low-income and middle-income country setting. Polymethylsiloxane polyhydrate, in addition to oral rehydration fluid and zinc, may be safe and reduce the duration and severity of acute diarrhoea.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings from this study will inform the design of large-scale clinical trials to determine the efficacy of polymethylsiloxane polyhydrate in acute diarrhoea in low-resource settings.
Introduction
Diarrhoea remains a leading cause of death in children accounting for 9.1% (95% uncertainty range 7.9–9.9) of the 5.3 m under 5 deaths in 2019 with the highest mortality rates occurring in west and central Africa.1 Diarrhoea is caused by a range of infectious pathogens.2 To prevent and address dehydration and electrolyte imbalance, in addition to oral rehydration fluid (ORF), WHO has recommended routine zinc supplementation for 10–14 days for children 6 months and older since 2005.3 In a systematic review (9 trials; 2581 children), zinc had a modest effect on diarrhoea in children older than 6 months, reducing duration by 11.5 hours (95% CI −19.7 to −3.2). No benefit was observed in younger infants.4 However, diarrhoea may persist for several days despite recommended management and the use of ORF by parents and also health professionals has remained low over many years.5 More concerning still, curtailment of fluids during diarrhoea was widespread in six sub-Saharan African countries.6 In studies of failure to adhere to recommended treatment, caregivers perceived that increasing fluids does not stop diarrhoea and may even increase loose stools, and that additional treatments are needed.7 8
Although several additional interventions reduce the duration and frequency of diarrhoea,9 all have limitations regarding their use in vulnerable populations with limited access to healthcare. Overall, meta-analysis does not support the use of probiotics.10 Although Saccharomyces boulardii alone11 or combined with zinc9 may be effective, there are concerns regarding fungaemia, especially in immunocompromised and critically ill patients.12 For intestinal adsorbents, there is low certainty of evidence that smectite, a medicinal clay, may ameliorate diarrhoea.13 However, it is licensed only from age 2 years and is administered mixed with water which may compromise the amount of ORF that children take.
Polymethylsiloxane polyhydrate (trade name Enterosgel) is an intestinal adsorbent that binds bacterial toxins and viruses in the gastrointestinal tract, followed by complete removal from the body.14 15 It was CE certified in Europe in 2011 as a medical device (as it is not absorbed through the intestinal mucosa) with an indication for diarrhoea including acute diarrhoea and irritable bowel syndrome with diarrhoea in children and adults. The gel is easily suspended in water, is colourless and has no taste. Independent laboratory analysis has confirmed that polymethylsiloxane polyhydrate mixed with ORF does not absorb electrolytes, glucose or zinc (online supplemental 1). In in vitro studies, polymethylsiloxane polyhydrate adsorbed toxins of Escherichia coli, Clostridioides difficile, Shigella spp.,14 and human and animal strains of rotavirus16 and inhibited the growth of staphylococci, reduced production of staphylococcal enterotoxins A and B and absorbed staphylococcal toxins from biological substrates.17 It significantly reduced the duration of acute diarrhoea in adults18 and also symptoms and diarrhoea in irritable bowel syndrome with diarrhoea19 suggesting amelioration of diarrhoea through the absorption of bile acids and immune and inflammatory mediators in addition to bacterial toxins and viruses.14 In all clinical studies of polymethylsiloxane polyhydrate, no serious adverse events have been reported and there are no reported adverse reactions in Europe since polymethylsiloxane polyhydrate was certified in 2011 (http://www.adrreports.eu/en/index.html).
Although clinical studies have evaluated polymethylsiloxane polyhydrate in infants and children with diarrhoea in Eastern Europe,20,23 none have been double-blind randomised trials with an appropriate comparison group. Polymethylsiloxane polyhydrate has not been tested in children with acute diarrhoea in a low-income and middle-income country setting where both the burden of disease and the challenges of undertaking high-quality clinical trials are greatest.
We aimed to determine the feasibility of undertaking a clinical trial of polymethylsiloxane polyhydrate in reducing the severity and frequency of acute diarrhoea in children in a low-resource health facility. Specific objectives were to assess recruitment, random allocation, blinding and data collection, retention and the acceptability of ORF+polymethylsiloxane polyhydrate among the study participants and generate pilot data to identify appropriate outcomes for a subsequent clinical trial.
Methods
This study was undertaken in The Gambia where diarrhoea accounts for 14% of deaths in children 1–59 months.24 The study was carried out at the emergency paediatric unit (EPU) and paediatric outpatient department (OPD), Edward Francis Small Teaching Hospital (EFSTH), Banjul. Blood sample analyses were performed at the EFSTH Severe Malaria in Children Clinical Laboratory and the preparation of study treatments by the paediatric pharmacy. EFSTH is the only tertiary-referral hospital in Gambia and serves highly disadvantaged urban and rural populations but has limited experience of clinical trials in children.
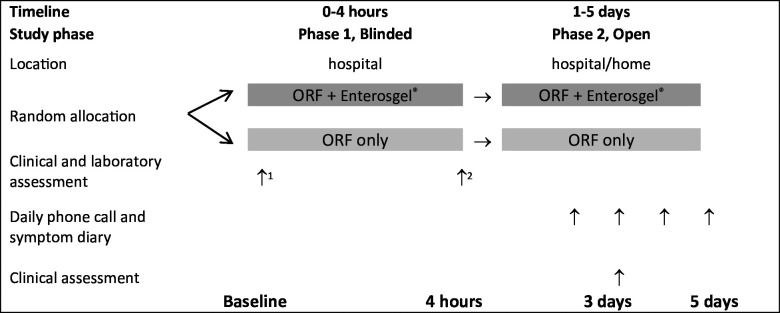
This randomised controlled feasibility trial was divided into phase 1 (double blind; 0–4 hours in the hospital), and phase 2 (open-label; 4 hours up to 5 days in hospital/at home; figure 1). The protocol for the INTestinal Adsorbent in childhood diarrhoea in The GAMbia study is available.25 The trial was registered with the Pan African Clinical Trial Registry (PACTR202302683128875; https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=24332).
Figure 1. Schematic study design. 1 Blood samples for electrolytes, kidney function and full blood count. 2 Blood sample for electrolytes and kidney function. ORF, oral rehydration fluid.
Participants and enrolment
Children aged 6–59 months presenting to the EPU or OPD with acute diarrhoea (three or more loose or watery stools in 24 hours3) for <4 days and with either no or some dehydration based on Integrated Management of Childhood Illness criteria26 were invited to join the study by the health staff on duty who had been trained on the study procedures. Eligibility was reassessed by a member of the study team. Exclusion criteria were severe dehydration, severe acute malnutrition (defined as mid-upper arm circumference <11.5 cm or weight-for-length/height z-score <−3 and/or nutritional oedema), significant concomitant illnesses such as malaria, sepsis, dysentery with systemic disturbance, suspected intestinal obstruction and those using anti-diarrhoeal medications, probiotic supplements, intestinal adsorbents or modified-release medications were excluded. To enable consistent follow-up, participants without access to a mobile phone or landline were also excluded. Following the provision of written and verbal study information, signed or thumbprint informed consent was obtained from parents/carers by a member of the research team. For parents/carers who could not read English, consent was confirmed by an independent witness.
Randomisation, allocation and blinding
Children were randomly assigned in a 1:1 ratio to either ORF or ORF+polymethylsiloxane polyhydrate according to a computer-generated random sequence using blocks of random size and allocated a unique identification number. The random sequence was prepared by the study statistician and restricted to the pharmacy staff and not accessible to other study staff. During phase 1 of the study (0–4 hours), research staff, participants and parents/carers were blinded to the allocated treatment.
Interventions
Study interventions were identical in taste and appearance and prepared by dedicated hospital pharmacy staff according to the allocation sequence. The volume and frequency of ORF were prescribed according to body weight and degree of dehydration.26 Polymethylsiloxane polyhydrate was added to the recommended volume of ORF as per a standardised protocol (online supplemental 2). Children were treated under supervision in hospital during phase 1.
Data collection and follow-up
Baseline demographic and clinical information including diarrhoea duration, stool consistency and frequency, symptoms such as vomiting, nutritional and hydration status were recorded on standard forms. Vesikari score was calculated at baseline.27 Serum and EDTA blood samples were collected to evaluate full-blood count (Medonics M-series; Boule, Sweden), electrolytes and kidney function (iSTAT 1; Abbott, UK). The intake of the allocated treatment, episodes of diarrhoea, symptoms and adverse events were recorded.
After 4 hours, clinical examination was repeated and another blood sample was collected for electrolytes and kidney function. The pharmacy provided the same treatment that the child had received in phase 1 for parents/carers to continue for up to 5 days or until the first formed stool in phase 2. As ORF±polymethylsiloxane polyhydrate treatment is intended to be taken within 24 hours of preparation, research staff instructed parents/carers on home preparation and provided guidance on the volume of ORF±polymethylsiloxane polyhydrate to give after each loose stool and to give additional ORF if the child will drink more (online supplemental 2). All children were treated with zinc.
Having observed how symptoms and the amount of study treatment taken was recorded during phase I, parents/carers were provided with a pictorial diary to record the same information at home (online supplemental 3). Intake of study interventions, symptoms and adverse events were also monitored through daily phone calls until the first formed stool or up to day 5. A clinical visit was arranged on day 3 to evaluate the clinical status including nutrition and hydration status.
Outcomes
The feasibility of the trial procedures was evaluated. Regarding the diarrhoea intervention, the primary outcome was the duration of diarrhoea defined as time from randomisation (in hours) to last loose or watery stool (takes the shape of the container). Secondary outcomes included feasibility of study procedures (recruitment to target, adherence to the random allocation sequence and the study protocol including administration of allocated treatments and follow-up procedures) and clinical outcomes (stool frequency, volume of study intervention taken, symptoms and the incidence of adverse events).
Data analysis
Categorical variables were summarised as number (%) and continuous variables as mean (SD). For continuous outcomes, the difference in means (95% CI) adjusted for age, sex, time from diarrhoea onset to randomisation, and hydration status was reported. Categorical variables were compared using the χ2 or Fisher’s exact tests. We considered that a total of 50 children (approximately 25 per study arm) would be sufficient to evaluate the feasibility of the study procedures. In order to meet the approval requirements of the Medical Control Agency of The Gambia, we undertook a formal sample size calculation which determined that 50 participants would be sufficient to demonstrate that the intervention reduced the duration of diarrhoea by 24 hours with 90% power and α=0.05 (online supplemental 4). The analysed population was defined as the intention-to-treat population, which included all randomised patients. Significance tests were two sided at the 5% significance level.28
Results
Participant flow and baseline data
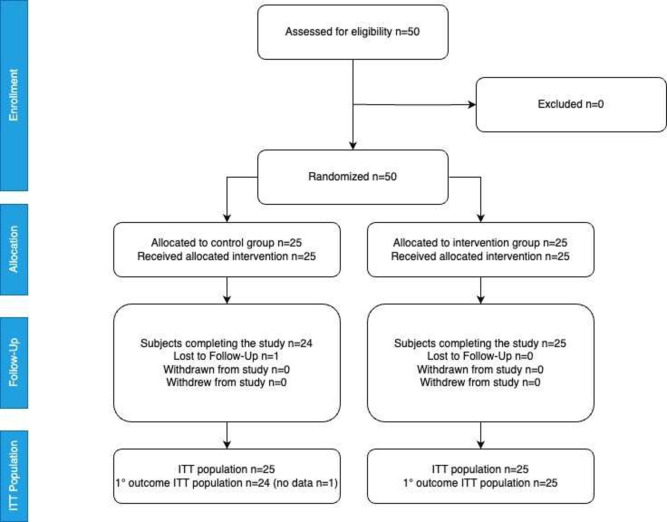
50 children with diarrhoea of duration <4 days were successfully recruited between August 2023 and February 2024. Research staff confirmed eligibility for all of the children identified by trained OPD and EPU health staff and none met any of the exclusion criteria; therefore, all were included and successfully randomised. One participant in the control group provided an incorrect phone number and failed to attend the clinic visit on day 3 and, therefore, was lost to follow-up (figure 2).
Figure 2. CONSORT diagram showing patient flow. CONSORT, Consolidated Standards of Reporting Trials; ITT, intention to treat.
Baseline demographic, anthropometric and clinical variables are shown in table 1 and were generally well balanced across the groups. Overall, mean (SD) age of the participants was 23 (12) months and 22 (44%) were female. Undernutrition was common with 24 (48%) children with moderate acute malnutrition. Most children had watery stools (43; 86%) and without blood. Some dehydration occurred in about one in three children. These variables and mean Vesikari score were similar in the two study arms except for duration of diarrhoea before recruitment which was shorter in the intervention than control arm. In the intervention arm, 14 children (56%) reported symptoms started yesterday and 11 (44%) 2 days ago compared with 4 (16%) and 21 (84%), respectively, in the control group.
Table 1. Baseline demographic and clinical variables according to intervention arm.
| Variables | Control group | Intervention group |
| N=25 | N=25 | |
| Demography | ||
| Age (months); mean (SD) | 1.8 (1.1) | 2.0 (0.9) |
| 6–11 | 5 (20) | 2 (8) |
| 12–23 | 13 (52) | 13 (52) |
| 24–59 | 7 (28) | 10 (40) |
| Sex: female | 10 (40) | 12 (48) |
| Ethnicity | ||
| Fula | 7 (28) | 8 (32) |
| Mandinka | 9 (36) | 6 (24) |
| Wollof | 4 (16) | 7 (28) |
| Other | 5 (20) | 4 (16) |
| Anthropometry | ||
| Weight (kg); mean (SD) | 9.7 (2.5) | 10.0 (2.1) |
| 6 to <10 kg | 17 (68) | 12 (48) |
| 10 to <12 kg | 4 (16) | 9 (36) |
| 12 to 19 kg | 4 (16) | 4 (16) |
| Length (cm); mean (SD) | 86 (25) | 83 (10) |
| Weight-for-length/height z score; mean (SD) | −1.48 (1.2) | −1.12 (1.9) |
| Length/height for age z score; mean (SD) | −0.4 (1.8) | −0.8 (1.9) |
| Moderate acute malnutrition* | 12 (48) | 12 (48) |
| Clinical | ||
| Pulse rate (beats per minute) | 121 (14) | 125 (18) |
| Respiratory rate (breaths per minute) | 34 (4) | 33 (4) |
| When diarrhoea started | ||
| Yesterday | 4 (16) | 14 (56) |
| 2 days ago | 21 (84) | 11 (44) |
| Stool consistency | ||
| Watery | 22 (88) | 21 (84) |
| Semiliquid | 3 (12) | 4 (16) |
| Stool frequency in last 24 hours; mean (SD) | 4 (1) | 4 (1) |
| 2 | 1 (4) | 0 (0) |
| 3 | 9 (36) | 10 (40) |
| 4 | 15 (60) | 15 (60) |
| Mucus in stool | 13 (52) | 12 (48) |
| Blood in stool (streaks) | 1 (4) | 3 (12) |
| Some dehydration | 9 (36) | 8 (32) |
| Vesikari score; mean (SD) | 7.5 (2.1) | 7.2 (1.9) |
Data are presented as No. (%) unless stated otherwise.
dDefined as weight-for-length/height z score <-−2.
Baseline blood samples were collected from all children except for one child in the intervention arm. Full blood count was performed in all samples but in-date biochemistry test kits were available to test only 21 samples in each arm. Mean values for haematological indices and plasma electrolytes were similar in each arm (online supplemental 5). Hyponatraemia (plasma sodium concentration <135 mmol/L) occurred in 43% (9/21) of children in the control arm and 29% (6/21) in the intervention arm. Hypokalaemia (plasma potassium concentration <3.5 mmol/L) occurred in 29% (6/21) controls and 57% (12/21) children in the intervention arm.
In general, the acceptability of the study medication during phase 1 (0–4 hours) was high. The intake of study treatment and change in weight were similar in both study arms (table 2). Vomiting occurred in 3/25 (12%) control and 5/25 (20%) intervention children (OR 3.1, 95% CI 0.3 to 29.1; p=0.33). Some dehydration had resolved at 4 hours in all children (100%; 25/25) in the control arm and 87.5% (24/25) in the intervention arm. No child deteriorated clinically and required intravenous fluids or a nasogastric tube. Change in haematological and biochemical indices were similar in both arms. The frequency of hyponatraemia at 4 hours was similar in the control (19%, 4/21) and intervention arms (29%, 6/21; OR 2.9, 95% CI 0.3 to 27.2; p=0.36). The frequency of hypokalaemia at 4 hours was also similar in the control (29%, 6/21) and intervention arms (48%, 10/21; OR 0.7, 95% CI 0.0 to 8.7; p=0.75). All children were assessed to be well enough to continue treatment at home.
Table 2. Clinical and laboratory outcomes according to intervention arm.
| Variable | Control groupN=24 | Intervention groupN=25 | Difference in means (95% CI) | P value |
| Duration of diarrhoea; time to (hours)* | ||||
| Last watery/loose stool | 24; 38.7 (22.0) | 25; 19.6 (15.5) | −19.3 (−30.9 to −7.8) | 0.0016 |
| First formed stool | 24; 49.3 (23.1) | 24; 31.1 (14.5) | −16.6 (−28.5 to −4.7) | 0.0075 |
| No. watery stools | ||||
| 0–4 hours | 23; 0.7 (0.9) | 24; 0.6 (0.7) | 0.0 (−0.5 to 0.5) | >0.99 |
| Day 2 | 23; 2.4 (1.2) | 24; 1.6 (0.9) | −0.8 (−1.3 to −0.3) | 0.0022 |
| Day 3 | 23; 1.3 (1.0 | 24; 0.5 (0.8) | −0.8 (−1.3 to −0.3) | 0.0014 |
| Day 4 | 13; 0.9 (0.9) | 2; 0.0 (0.0) | −1.0 (−2.3 to 0.3) | 0.13 |
| Day 5 | 1; 2.0 (−) | 0; − | – | – |
| Volume of study treatment taken 0–4 hours* | ||||
| mL/kg | 25; 26.7 (14.7) | 25; 25.6 (14.0) | 1.2 (−4.2 to 6.6) | 0.65 |
| % prescribed | 25; 92.3 (18.7) | 25; 93.0 (30.6) | 4.7 (−9.0 to 18.4) | 0.49 |
| Change in weight 0–4 hours (kg) | 25; 0.20 (0.44) | 25; 0.57 (0.65) | 0.29 (−0.23 to 0.80) | 0.28 |
| Volume of study treatment taken in phase 2 (cups) | ||||
| Day 2 | 23; 3.1 (0.8) | 25; 3.3 (0.7) | 0.2 (−0.3 to 0.7) | 0.51 |
| Day 3 | 24; 1.8 (1.1) | 25; 2.3 (0.9) | 0.5 (0.0 to 1.0) | 0.07 |
| Day 4 | 13; 1.8 (0.8) | 2; 1.0 (0.0) | −0.8 (−2.1 to 0.5) | 0.23 |
| Day 5 | 1; 3.0 (−) | 0; − | – | – |
| Laboratory indices: change between baseline and 4 hours* | ||||
| Haemoglobin (g/L) | 21; −0.3 (1.0) | 21; −0.2 (0.9) | 0.2 (−0.3 to 0.8) | 0.43 |
| Sodium (mmol/L) | 21; 1.3 (3.2) | 21; 0.2 (2.0) | −1.3 (−3.0 to 0.3) | 0.11 |
| Potassium (mmol/L) | 21; 0.1 (1.5) | 21; 0.0 (0.4) | −0.4 (−1.0 to 0.2) | 0.20 |
| Creatinine (μmol/L) | 21; −0.4 (2.1) | 20; −4.2 (19.9) | 0.3 (−0.7 to 1.4) | 0.52 |
All values are number; mean (SD).
Statistically significant differences are shown in bold type.
aAdjusted for age, sex, time from diarrhoea onset to randomisation, and hydration status.
During phase 2, (2–5 days), review of pictorial diaries indicated that they were either not completed well by parents/careers or were not brought back to clinic on day 3. To minimise missing follow-up assessments, symptoms and amount of the interventions taken were mainly based on verbal information provided by the caretakers during the daily follow-up calls. The time to last watery or loose stool was shortened in the intervention group compared with the controls with a mean difference of 19.3 hours (95% CI −30.9 to −7.8 hours; p=0.0016) taking into account baseline variables including duration of diarrhoea before recruitment (table 2). Similarly, the time to first formed stool was shortened (p=0.0075). Additionally, daily stool frequency was lower in the intervention than the control group and this reached statistical significance on days 2 (p=0.0022) and 3 (p=0.0014). Diarrhoea had resolved by day 3 in 96% (23/24) of children in the control group and all (100%; 25/25) in the intervention group.
We noted that caretakers often gave more fluid than indicated by the number of loose stools consistent with the guidance provided to maximise oral fluid intake (online supplemental 2). The reported intake of study treatment on day 3 tended to be higher in the intervention than the standard treatment arm (p=0.07; table 2) and, when analysis was limited to participants without dehydration at baseline, intake was significantly greater in the intervention (n=17; mean (SD) 2.3 (0.8) cups) than the controls (n=16; 1.6 (1.1) cups; p=0.010). In general, participants tolerated the intestinal adsorbent well. Vomiting during phase 2 was reported to be uncommon and occurred at a similar frequency in the two study arms.
Adverse events
Abdominal pain was reported in 8% (2/24) children in the control group and none in the intervention group. One serious adverse event occurred in a child assigned to the control arm who was admitted to hospital on day 5 with recurrence of diarrhoea, severe dehydration and severe acute malnutrition and died the following day.
Discussion
The importance of undertaking feasibility studies ahead of definitive clinical trials is well established.29 30 This feasibility study provided important information to inform the design of a follow-on, larger clinical trial and also developed the capacity of staff in several hospital departments.
Overall, recruitment of the target number of participants within the study period was successful with recruitment completed in 7 months. Participant selection by clinicians and nurses trained in the study procedures proved to be effective as they applied the screening criteria reliably. A limitation of the screening process was that we did not record details of children who did not meet the eligibility criteria which may have been useful for planning follow-on studies.
Baseline demographic, anthropometric and clinical data were collected for all children during phase 1 of the study. Random allocation resulted in an equitable distribution between study arms except for a shorter duration of diarrhoea before recruitment in the intervention arm which likely occurred by chance and was accounted for in analysis. Blood samples were collected and analysed with quality control procedures in place. However, some samples could not be analysed due to unavailability of in-date sample cassettes for biochemistry samples which could be addressed by improved stock control for follow-up studies. This is particularly important in this setting as quality-controlled alternative assays either in the hospital or local private laboratories are not readily available as a back-up and often require out-of-pocket expenditure for patients if not recruited for a research study. Blinding of parents/carers and research staff to treatment allocation was maintained.
Adherence to study procedures was generally high and only one child in the control arm was lost to follow-up. Although procedures for collecting outcome data were effective during phase 2 of the study, the pictorial diaries designed to assist caretakers with recording symptoms and fluid intake at home were not completed well or not brought to clinic. Therefore, the additional burden of completing diaries in this setting proved inappropriate in collecting reliable data. However, to minimise the risk of missing follow-up assessments, we additionally conducted daily follow-up calls which seemed a feasible option for recording outcome data. Assessments included time to the last loose or watery stool which could be maintained as the primary outcome for a follow-on study. Further work is needed to assess whether paper-based or digital means of recording information by parents/carers have benefits over simple verbal reporting.
In the intervention group, fluid intake was higher compared with the control group and reached a statistically significant difference on day 3 among children with no dehydration. While engagement in the study likely encouraged fluid administration by caretakers in both study arms, our findings suggest that provision of an effective intervention that reduces the duration and severity of diarrhoea and is administered together with ORF may increase use of ORF. This was an encouraging finding given the well-established poor compliance with fluid management both by parents/carers and health staff that has been documented over many years.2431,33 The effects of combining an effective treatment for diarrhoea with ORF in increasing fluid intake should be assessed in future implementation studies.
We attempted to mitigate common biases in the collection of patient-reported outcomes including non-response bias, acquiescent response bias or inappropriate timing of surveys34 through rigorous daily assessments. However, these and other biases, such as positive expectations in reporting34 (eg, during the open-label phase), should be considered in the design of a follow-up trial. Extending the double-blinded phase of the study and providing ready-prepared medication to participants daily during home visits could improve the reliability of reporting symptoms and fluid intake.
Although all children received recommended treatment including zinc, the mean duration of diarrhoea in the intervention arm was reduced by about 19 hours compared with the controls. Stool frequency was also significantly lower in the intervention arm on days 2 and 3. These findings are consistent with previous studies of polymethylsiloxane polyhydrate in childhood diarrhoea35 36 but are of direct relevance to a follow-on clinical trial given that this is the only study conducted in a low-resource setting to date.
The recurrence of diarrhoea with severe dehydration and the development of severe acute malnutrition in one child in the control arm who subsequently died emphasises that diarrhoea remains a leading cause of death in under 5 and the need for additional interventions beyond ORF and zinc. Careful clinical follow-up including laboratory assessment did not identify any adverse events related to polymethylsiloxane polyhydrate but this should also be assessed in further trials in this vulnerable patient group in whom moderate acute malnutrition was common and several had persistent hyponatraemia and hypokalaemia after the initial 4 hours of management.
Conclusions
This feasibility study informs critical issues in the design of a subsequent efficacy study and provides pilot data to determine sample size. The positive findings regarding reduced duration and severity of diarrhoea and also increased fluid intake should encourage further studies of polymethylsiloxane polyhydrate for the management of diarrhoea in vulnerable populations.
supplementary material
Acknowledgements
We are grateful to the parents/carers and their children for participating in this trial and for assistance from EPU and outpatient staff for identifying potentially eligible children.
Footnotes
Funding: The study was funded by the manufacturer of Enterosgel in Europe, Bioline Product s.r.o. Enteromed Ltd., UK sponsored the study (award/grant number: n/a) and helped with the design and development of the documentation for the study. Sponsor staff were located in the UK while the study was run by the principal investigator SA and the study team in The Gambia. All study data was collected and uploaded by investigators on site to an independent database held at the LSTM before being sent directly to the independent statistician for analysis. The sponsor and funder did not have any contact with the data until after the results were analysed and the report written.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained from parent(s)/guardian(s).
Ethics approval: This study was conducted in accordance with the principles set forth in the ICH Harmonised Tripartite Guideline for Good Clinical Practice and the Declaration of Helsinki. The protocol and all participant materials were approved by The Gambia Government/MRC Joint Ethics Committee (reference 28237; 4 January 2023); EFSTH Research Ethics Committee (reference EFSTH_REC_2023_004; 16 January 2023) and the Medicine Controls Agency (reference MCA/AD/23/EM (11), 7 August 2023).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability free text: Anonymised study data are available from the corresponding author on reasonable request.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6:106–15. doi: 10.1016/S2352-4642(21)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Geneva: 2005. The treatment of diarrhoea. [Google Scholar]
- 4.Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016;12:CD005436. doi: 10.1002/14651858.CD005436.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sreeramareddy CT, Low YP, Forsberg BC. Slow progress in diarrhea case management in low and middle income countries: evidence from cross-sectional national surveys, 1985-2012. BMC Pediatr. 2017;17:83. doi: 10.1186/s12887-017-0836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perin J, Carvajal-Velez L, Carter E, et al. Fluid curtailment during childhood diarrhea: a countdown analysis. BMC Public Health. 2015;15:588. doi: 10.1186/s12889-015-1878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis AA, Winch P, Daou Z, et al. Home management of childhood diarrhoea in southern Mali--implications for the introduction of zinc treatment. Soc Sci Med. 2007;64:701–12. doi: 10.1016/j.socscimed.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Ansari M, Ibrahim MIM, Hassali MA, et al. Mothers’ beliefs and barriers about childhood diarrhea and its management in Morang district, Nepal. BMC Res Notes. 2012;5:576. doi: 10.1186/1756-0500-5-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Florez ID, Veroniki A-A, Al Khalifah R, et al. Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: A systematic review and network meta-analysis. PLoS ONE. 2018;13:e0207701. doi: 10.1371/journal.pone.0207701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collinson S, Deans A, Padua-Zamora A, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2020;12:CD003048. doi: 10.1002/14651858.CD003048.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu H, Li J, Xu X, et al. Effectiveness and Safety of Saccharomyces Boulardii for the Treatment of Acute Gastroenteritis in the Pediatric Population: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Comput Math Methods Med. 2022;2022:6234858. doi: 10.1155/2022/6234858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenplas Y, Brunser O, Szajewska H. Saccharomyces boulardii in childhood. Eur J Pediatr. 2009;168:253–65. doi: 10.1007/s00431-008-0879-7. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Gaxiola G, Cuello-García CA, Florez ID, et al. Smectite for acute infectious diarrhoea in children. Cochrane Database Syst Rev. 2018;4:CD011526. doi: 10.1002/14651858.CD011526.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell CA, Mikhalovsky SV, Markaryan EN, et al. Investigation of the adsorption capacity of the enterosorbent Enterosgel for a range of bacterial toxins, bile acids and pharmaceutical drugs. Sci Rep. 2019;9:5629. doi: 10.1038/s41598-019-42176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolaev V. Biodefence: Advanced Materials and Methods for Health Protection. 2011. Enterosgel: a novel organosilicon enterosorbent with a wide range of medical applications; pp. 199–221. [Google Scholar]
- 16.Barbova A. Sorbtsiia rotavirusov cheloveka i zhivotnykh Enterosgelem [The sorption of human and animal rotaviruses by Enterosgel] Mikrobiol Z. 1995;57:52–7. [PubMed] [Google Scholar]
- 17.Fluer FS, Kudryavtseva AV, Titarev SI, et al. MEANS FOR INHIBITION OF PRODUCTION OF STAPHYLOCOCCUS ENTEROTOXINS AND THEIR ELIMINATION FROM BIOLOGICAL SUBSTRATES. Journal of Microbiology, Epidemiology and Immunobiology. 2017;94:71–7. doi: 10.36233/0372-9311-2017-3-71-77. [DOI] [Google Scholar]
- 18.Howell CA, Markaryan E, Allgar V, et al. Enterosgel for the treatment of adults with acute diarrhoea in a primary care setting: a randomised controlled trial. BMJ Open Gastroenterol. 2019;6:e000287. doi: 10.1136/bmjgast-2019-000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell CA, Kemppinen A, Allgar V, et al. Double-blinded randomised placebo controlled trial of enterosgel (polymethylsiloxane polyhydrate) for the treatment of IBS with diarrhoea (IBS-D) Gut. 2022;71:2430–8. doi: 10.1136/gutjnl-2022-327293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tunda I, editor. Rotavirus Infection in Newborns: Clinical Performance, Treatment and Prophylaxis (Dissertation. Bogomolets National Medical University; 2004. [Google Scholar]
- 21.Markovinović L, Knezović I, Kniewald T, et al. Enteroadsorbent Polymethylsiloxane Polyhydrate vs. Probiotic Lactobacillus reuteri DSM 17938 in the Treatment of Rotaviral Gastroenteritis in Infants and Toddlers, a Randomized Controlled Trial. Front Pediatr. 2020;8:553960. doi: 10.3389/fped.2020.553960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruzhentsova T, Gorelov A, Ploskireva A. Choice of an adequate therapy regimen for acute enteric infections in children: results of a randomized trial. Epidemiology and Infectious Diseases Current Items. 2016;4:70–4. [Google Scholar]
- 23.Tuychiev LN, Khudaykulova GK, Muminova MT. Experience of practical use of enterosorbents in the treatment of HIV-infected children with diarrhea syndrome. VIČ-infekc immunosupr. 2024;16:100–5. doi: 10.22328/2077-9828-2024-16-2-100-105. [DOI] [Google Scholar]
- 24.UNICEF Countdown 2030. https://data.unicef.org/countdown-2030/country/Gambia/2 Available.
- 25.Rahden P, Jobarteh M, Sallah A, et al. INTestinal Adsorbent in childhood diarrhoea in The GAMbia (INTAGAM): protocol for a double-blinded randomised controlled feasibility study. Researchgate. 2024 doi: 10.13140/RG.2.2.33335.33445. [DOI] [Google Scholar]
- 26.WHO . Pocket Book of Hospital Care of Children. 2nd. Geneva: 2013. edn. [Google Scholar]
- 27.Schnadower D, Tarr PI, Gorelick MH, et al. Validation of the modified Vesikari score in children with gastroenteritis in 5 US emergency departments. J Pediatr Gastroenterol Nutr. 2013;57:514–9. doi: 10.1097/MPG.0b013e31829ae5a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Medicines Agency ICH E6 (R2) good clinical practice - scientific guideline. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice-scientific-guideline2016 n.d. Available.
- 29.Pearson N, Naylor P-J, Ashe MC, et al. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud. 2020;6:167. doi: 10.1186/s40814-020-00634-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajadhyaksha V. Conducting feasibilities in clinical trials: an investment to ensure a good study. Perspect Clin Res. 2010;1:106–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Workneh BS, Mekonen EG, Ali MS, et al. Recommended homemade fluid utilization for the treatment of diarrhea and associated factors among children under five in sub-Saharan African countries: a multilevel analysis of the recent demographic and health survey. BMC Pediatr. 2024;24:322. doi: 10.1186/s12887-024-04810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha D, Akinsola A, Sharples K, et al. Health Care Utilization and Attitudes Survey: understanding diarrheal disease in rural Gambia. Am J Trop Med Hyg. 2013;89:13–20. doi: 10.4269/ajtmh.12-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deichsel EL, Keita AM, Verani JR, et al. Management of Diarrhea in Young Children in Sub-Saharan Africa: Adherence to World Health Organization Recommendations During the Global Enteric Multisite Study (2007–2011) and the Vaccine Impact of Diarrhea in Africa (VIDA) Study (2015–2018) Clin Infect Dis. 2023;76:S23–31. doi: 10.1093/cid/ciac926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd H, Jenkinson C, Hadi M, et al. Patient reports of the outcomes of treatment: a structured review of approaches. Health Qual Life Outcomes. 2014;12:5. doi: 10.1186/1477-7525-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usenko DG, Rudyk AV. Application of enteroscorbents in the treatment of intestinal infections in children with concomitant atopic dermatitis. Pharmateka. 2015;10:61–5. [Google Scholar]
- 36.Uchaykin V, Novokshonov А, Sokolova N, et al. Clinical Report: Study of clinical efficacy of gastrointestinal adsorbent Enterosgel in acute intestinal infections in children. 2001