Abstract
Dorsal anterior cingulate cortex (dACC) is a brain region that subserves cognition and motor control, but the mechanisms of these functions remain unknown. Human neuroimaging and monkey electrophysiology studies have provided valuable insights, but it has been difficult to link the two literatures. Based on monkey single-unit recordings, we hypothesized that human dACC is comprised of a mixture of functionally distinct cells that variously anticipate and detect targets, indicate novelty, influence motor responses, encode reward values, and signal errors. As an initial test of this conceptualization, the current event-related functional MRI study used a reward-based decision-making task to isolate responses from a subpopulation of dACC cells sensitive to reward reduction. As predicted, seven of eight subjects showed significant (P < 10−4) dACC activation when contrasting reduced reward (REDrew) trials to fixation (FIX). Confirmatory group analyses then corroborated the predicted ordinal relationships of functional MRI activation expected during each trial type (REDrew > SWITCH > CONrew ≥ FIX). The data support a role for dACC in reward-based decision making, and by linking the human and monkey literatures, provide initial support for the existence of heterogeneity within dACC. These findings should be of interest to those studying reward, cognition, emotion, motivation, and motor control.∥
Anterior cingulate cortex (ACC) lies on the medial surfaces of the brain's frontal lobes and encompasses subdivisions that play key roles in cognitive, motor, and emotional processing (1). Dorsal ACC (dACC) in humans includes cortex on the dorsal and ventral banks of the cingulate sulcus, and overlaps the territory occupied by the rostral cingulate motor area (CMAr) in monkeys (2, 3), which has projections directly to the spinal cord (4) and motor and limbic cortices (5). Convergent data (6, 7) has established that dACC specifically subserves cognition (8) and motor control (9), but the mechanisms by which this region operates have not been elucidated. Based primarily on work in humans, different functions have been ascribed to this area, including attention-for-action/target selection (10, 11), motivational valence assignment (12), motor response selection (13–15), error detection/performance monitoring (16, 17), competition monitoring (18), anticipation (19), working memory (20), novelty detection (21), and reward assessment (22), but no single unifying model explains the diverse results from neuroimaging and electrophysiological studies (1). In addition to the intrinsic importance of providing new information about normal cognition and motor control, determining how dACC works is essential because abnormalities of different ACC subdivisions have been implicated in the pathophysiology of many neuropsychiatric disorders (23).
Single-unit recording studies have confirmed heterogeneity in monkey CMAr. Niki and Watanabe (24) identified timing (stimulus anticipation) units, and others sensitive to targets, motor responses, rewards, or errors. Nishijo et al. (25) found anticipatory, stimulus-related, response-related, and reward ACC cells—adding that subsets responded to novelty, whereas others could discriminate rewarding, aversive, and neutral objects. Procyk et al. (26) recorded dACC cells that reacted to targets, rewards or error cues, and others involved in routine and nonroutine motor sequencing behaviors in macaques.
Although it has been established in monkeys that dACC is comprised of many different functional cell types, the problem of how to link the monkey literature to humans remains. Fortunately, a single-unit recording study provides a key piece of information. Shima and Tanji (27) recorded from dACC (CMAr) cells in Macaca fuscata during a reward-based decision-making task. The monkeys performed one of two movements (i.e., to push or turn a handle) to get a constant reward (CONrew) of apple juice. They were taught to persist with the same movement until they received a reduced reward (REDrew, less juice in this case) or heard a nonspecific switch signal (an auditory tone)—either signaled the monkey to do the alternate movement to get the full reward. As before, different cell populations responded to target detection, motor response, CONrew, and REDrew. The nonspecific SWITCH command (auditory tone) rarely caused REDrew-sensitive cells to fire. Critically, Shima and Tanji (27) also reported the proportions of each cell type were not equal in dACC—five times as many cells responded specifically to movement selection based on REDrew (37%) as opposed to CONrew (7%).
Thus, the combined evidence from human neuroimaging and monkey electrophysiology studies suggests that dACC is comprised of many morphologically and phenotypically unique neurons (see Table 1), and this leads directly to the expectation of functional heterogeneity. As an initial test of this conceptualization of dACC, the current event-related functional MRI (fMRI) study used a reward-based decision-making task that controlled for fMRI responses from heterogeneous cell types, thereby isolating responses from dACC cells sensitive to REDrew.
Table 1.
Hypothesized factors affecting fMRI tasks
| Factor | FIX | CONrew | SWITCH | REDrew |
|---|---|---|---|---|
| Anticipation | x | x | x | x |
| Target detection | x | x | x | |
| Constant reward | x | x | x | |
| Motor response | x | XX | XX | |
| Novelty detection | x | x | ||
| Reduced reward | XX | |||
| Error detection* |
Factors hypothesized to affect fMRI responses (first column) have established single-neuron representations from monkey studies. The x marks indicate a hypothesized contribution to the condition above. The presence of XX denotes a particularly large hypothesized influence (i.e., much more processing is needed to overcome a prepotent motor output tendency in SWITCH and REDrew trials, and REDrew-specific cells represent 37% of dACC cells; ref. 27). Error detection units, though part of the model, are indicated here with an asterisk, as the current study could not assess this (because error rates were equivalent among the three tasks).
Specifically, we predicted that during a reward-based motor decision task modeled after the Shima and Tanji task (27) (Fig. 1), fMRI signal within human dACC would be (i) higher in both the REDrew and SWITCH trials as compared with FIX; (ii) higher in both the REDrew and SWITCH trials than during the CONrew condition, because both would recruit more cells (novelty detection, motor response selection) than would the CONrew condition; and (iii) higher for REDrew trials as compared with the nonspecific SWITCH command, because a large proportion (37%) of dACC cells in monkey respond vigorously to REDrew-based motor selection. A schematic representation of the predicted time-locked fMRI responses appears in Fig. 2.
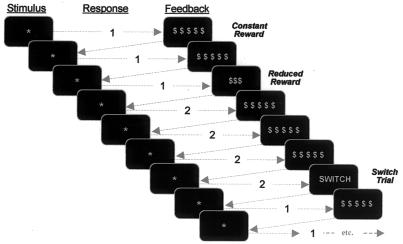
Figure 1.
Reward-based decision-making task. Subjects were instructed to respond to each stimulus (*) by pressing one of two buttons based on the feedback from the previous trial (i.e., to repeat the same button press after a CONrew, and to change buttons after either a REDrew or SWITCH signal).
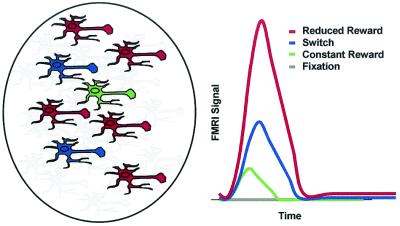
Figure 2.
Schematized predicted fMRI response. Schematized representation of components most relevant to the fMRI results. A CONrew cell is depicted in green, cells responsible for the additional demands of performing SWITCH trials are depicted in blue, and cells specific to REDrew trials are depicted in red. Following Shima and Tanji (27), REDrew and CONrew cells are depicted at an approximate 5:1 ratio. Gray cells represent cells that support all trial types (e.g., anticipation, target detection) but are not the subject of immediate focus because they do not serve to differentiate fMRI responses. Qualitative predictions for fMRI responses appear on the right. FIX was predicted to produce no activation, CONrew was predicted to produce only minimal activation, and SWITCH trials (recruiting novelty detection cells and placing greater demands on response selection) were predicted to produce significantly greater activation. REDrew trials, recruiting cells involved in all previous trial types plus the very numerous REDrew sensitive cells, were predicted to produce the greatest activation.
Materials and Methods
Eight healthy young adult volunteers (four male, four female) performed a reward-based motor decision task during event-related fMRI. Informed consent was obtained per Massachusetts General Hospital Subcommittee on Human Subjects guidelines.
Experimental Paradigm.
The task (Fig. 1) was modeled after the one used by Shima and Tanji (27). Subjects were given a button box with two buttons and instructed to use their right index and middle fingers to press buttons 1 and 2, respectively. The sequence and timing of stimuli were pseudorandomized and counterbalanced by schedule optimization (28). Trials began with the display of a central asterisk. On the first trial, subjects were asked to guess and press button 1 or 2. Immediately after this, they were given feedback displayed on the screen as follows. (i) CONrew trials: $ $ $ $ $ meant they had gotten the correct answer and would receive 15¢. Subjects were explicitly informed that CONrew trials would constitute the vast majority of trials (80%, in fact) and were told that on getting this feedback they should persist in pressing the same button on subsequent trials until they received one of the other two forms of feedback. (ii) REDrew trials: Ten percent of the time subjects saw $$$, indicating they had received a REDrew of 9¢ and should press the alternate button on the next trial to receive the full reward again. (iii) SWITCH trials: To eliminate the possibility that the dACC response might solely be produced by either the appearance of an oddball (rare) stimulus or nonspecifically by any command that instructed subjects to switch buttons, on 10% of the trials they saw the command switch, indicating that they should switch and press the other button on the following trial to receive the full reward again. Each of these trial types lasted 3 s. The central asterisk “go-signal” would appear for a maximum of 1.5 s if no response was given. Immediately on responding, feedback (CONrew/REDrew/SWITCH signal) was displayed and remained on the screen for the balance of the initial 1.5-s period. A minimum 1.5-s blank screen fixation (FIX) followed feedback. To minimize expectancy effects and provide a low-level baseline for comparison purposes, periods of blank screen FIX (in the range of 1.5 to 6 s in 1.5-s increments) were interspersed pseudorandomly (28) among these trial types—these additional FIX periods totaled 90 s (20%) of each 450-s long scan. Anticipating that subjects might become anxious if they missed a REDrew or SWITCH signal and “got off track” (i.e., invalidating all subsequent answers), subjects were told that there was no absolute right answer (i.e., 1 or 2) for any trial, but what was important was that they correctly continue with the same strategy when given the CONrew and change to the other button when given either the REDrew or SWITCH feedback. Subjects performed eight 450-s long scans with 1-min breaks between scans. Before scanning, subjects practiced the task for 90 s (27 trials).
The amounts of money used were not totally arbitrary. Although impossible to define truly comparable rewards for humans and monkeys, we tried to follow the spirit of Shima and Tanji's experiment (27) [i.e., given the current United States economic conditions and subjects of this approximate socioeconomic status (middle class young adults), these amounts represent small but not trivial rewards]. Subjects had been told from the outset that they would do many hundreds of trials and were instructed that the amount they would be paid (between $60 and $100) would be based on performance (although at the end, all were paid $100 and the justification for the ruse was explained). Thus, if subjects were to make any rough approximations, using 15¢ and 9¢ as the values would make the final “performance-based” totals plausible. Lastly, as we were instructing subjects to press buttons referred to as 1 and 2, we specifically avoided using 1¢ and 2¢ rewards.
fMRI Procedures.
Event-related fMRI (28) was performed in a Siemens 3.0 Tesla Allegra high-speed echoplanar imaging device (Munich) using a quadrature head coil. Subjects lay on a padded scanner couch in a dimly illuminated room and wore foam earplugs and earphones. Foam padding stabilized the head. Stimuli were generated by macstim 2.5 (West Melbourne, Australia) on a Macintosh 250 MHz Powerbook and projected with a Sharp XG-NV6XU Notevision 6 projector (Osaka), through a collimating lens onto a screen secured to the head coil. Subjects viewed images on a tilted mirror placed directly in front of their eyes. Stimuli subtended ≈1° of the visual angle vertically.
Either during the functional scanning session or in a separate session, high-resolution (1.0 × 1.0 × 1.3 mm) structural images were obtained for three-dimensional reconstruction. Structural images magnetization-prepared rapid acquisition with gradient echoes (MP-RAGE), 128 slices, 256 × 256 matrix, echo time (TE) = 3.3 ms; repetition time (TR) = 30 ms; flip = 40°] were collected on either a 1.5-T or 3-T Siemens MR scanner. Functional sessions began with an initial sagittal localizer scan, followed by autoshimming to maximize field homogeneity. To register functional data to the three-dimensional reconstructions, a set of high-resolution (16 coronal slices, perpendicular to the anterior commissure–posterior commissure line and extending posteriorly from the genu of the corpus callosum, 1.5 × 1.5 mm in-plane ×4 mm thick, no skip) inversion time T1-weighted echo-planar images [TE = 29 ms; TI = 1,200 ms; TR = 6,000 ms; number of excitations (NEX) = 4] was acquired, along with T2 conventional high-resolution anatomical scans (256 × 256 matrix, TE = 104 ms; TI = 1,200 ms; TR = 11 s, NEX = 2). The coregistered functional series (TR = 1,500 ms, 300 images per slice, TE = 30 ms, flip angle 90°, FOV = 20 × 20 cm, matrix = 64 × 64, in-plane resolution 3.125 mm2) lasted 450 s, and every subject completed eight scans for a total of 2,400 total images per subject. Data sets were motion-corrected by using afni (Medical College of Wisconsin, Milwaukee; http://afni.nimh.nih.gov/afni/index.shtml) and normalized to represent percent signal change from the mean activation of the FIX condition. Selective averaging of epoch time points corresponding to each of the conditions (REDrew, CONrew, and SWITCH) was done by using a trial averaging window of 20 s, beginning 3 s before the start of each trial.
The initial analysis phase used a voxelwise t test to test whether greater activation occurred in dACC during REDrew trials versus FIX. The dACC region of interest (ROI) was defined based on a modification of a metaanalysis of 64 imaging studies that reported ACC activation during cognitively demanding tasks (1). The modification (added to better assess homology, based on the monkey electrophysiology studies and anatomical work in humans; refs. 2–5 and 24–27) was that only ACC cortex superior to the corpus callosum between y = 0 and +30 mm and within the cingulate sulcus (or between the cingulate and paracingulate sulci inclusive in cases displaying double parallel cingulate sulci) was considered (see Fig. 3). This modification was done for each subject on his/her anatomical scan. For this a priori-defined dACC region encompassing ≈250 voxels, statistical significance (of P < 0.05 corrected for multiple comparisons) was defined as P < 1.0 × 10−4. Resultant statistical maps were displayed in pseudocolor, scaled according to significance, and projected onto the high-resolution anatomical scan slices in native and Talairach space (23, 29). Seven of eight individuals displayed significant dACC activity during REDrew trials as compared with FIX (comparisons made in the 3- to 4.5-s poststimulus time window to allow for an approximate 4-s hemodynamic delay).
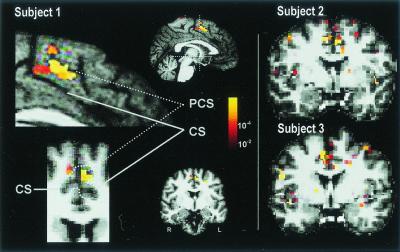
Figure 3.
dACC fMRI Response and ROI. Activation of dACC in response to REDrew trials (vs. FIX) is shown in three subjects. Pseudocolor-scaled statistical maps are displayed superimposed on the medial surface of the left hemisphere (sagittal view) and a coronal slice (y = 12 mm) in radiological convention (r = L) for subject 1. These areas are enlarged on the left. The dACC ROI included ACC between y = 0 and y = +30 within the cingulate sulcus (CS) for cerebral hemispheres with a single CS (Subject 1, Right), and ACC between the paracingulate sulcus (PCS) and CS, inclusive, for cerebral hemispheres with a double parallel cingulate sulcal pattern (Subject 1, Upper Left). It refers to the same cortical region that has been called the anterior cingulate cognitive division (ACcd) (1), rostral cingulate zone (9), or midcingulate cortex (3). The dACC ROI is indicated in aqua on the coronal slice enlargement (Subject 1, Lower Left). Coronal slices are shown for subjects 2 and 3.
Confirmatory group analyses were then done to test for the predicted ordinal relationships of fMRI activation expected during each trial type (REDrew > SWITCH > CONrew ≥ FIX). These comparisons were restricted to the t = 4.5- to 6-s time window (again allowing for an approximate 4-s hemodynamic delay). In the first confirmatory analysis, ordinal relationships were tested on fMRI data averaged from the maximally activated voxel from each subject showing significant activation in the REDrew minus FIX comparison (n = 7 of 8; see Fig. 4). Averaging was done after normalizing each time course to the mean of the three (pretrial) time points. Planned comparisons (paired one-tailed t tests, P < 0.05) were performed to ensure that (i) REDrew was higher than CONrew, (ii) SWITCH was higher than CONrew, and (iii) REDrew was also higher than SWITCH.
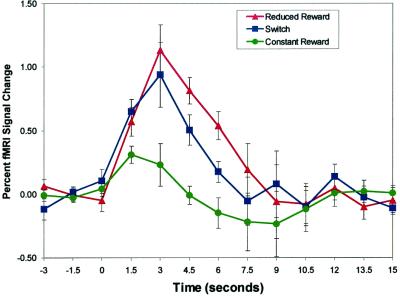
Figure 4.
dACC fMRI response. Group-averaged (n = 7), time-locked dACC activity for REDrew, SWITCH, and CONrew trials plotted as percent change from mean MRI signal during the first three images for each condition. Error bars indicate the SEM. As predicted, at t = 4.5 and 6 s (accounting for hemodynamic delay), REDrew > SWITCH trials > CONrew.
Although testing for these hypothesized ordinal relationships within voxels significantly activated in a REDrew-minus-FIX comparison is valid (as each trial type is assumed to be independent), it potentially introduces two confounding variables (i.e., by preselecting voxels with high activity during REDrew trials, it potentially biases the crucial REDrew versus SWITCH comparison, and it effectively prevented data from one subject, who did not activate, from being included). Therefore, to make the key REDrew versus SWITCH comparison in an unbiased manner, we tested for the predicted relationship (REDrew > SWITCH) in all dACC voxels significantly active in the SWITCH-minus-FIX comparison. If anything, this analysis was biased against finding significant activation for REDrew, as we were preselecting voxels known to have high activity during the SWITCH condition.
Finally, we conducted a group analysis based on commonly activated voxels in Talairach atlas space by constructing anatomically based group averages for each condition and testing to confirm the presence of the predicted ordinal relationships in an average of all dACC voxels activated in both the REDrew and SWITCH conditions.
Results
Behavioral Measures.
Subjects performed 960 trials (80% CONrew, 10% REDrew, 10% SWITCH command). Accuracy was virtually identical for all trial types (98.1% ± 2.4% SD for CONrew trials, 98.8% ± 1.1% for REDrew trials, and 98.8% ± 1.7% for SWITCH trials).
Reaction times (RT) were measured in response to the go-signal asterisks after each form of feedback. These were minimally (albeit significantly) faster after REDrew feedback (359 ms ± 85 ms) versus those after CONrew feedback (396 ms ± 106 ms) (P < 0.01, paired two-tailed t tests), and for those after a SWITCH command (368 ms ± 100 ms) versus those after CONrew (P < 0.01, paired two-tailed t test), but RTs to go-signals after REDrew were not significantly faster than those after a SWITCH command (P = 0.4).
fMRI Measures.
As predicted, in seven of eight individuals, significant activation (P < 1 × 10−4) was indeed observed in dACC when contrasting REDrew trials to FIX (Fig. 3). In these individuals, activity was uniformly highest for REDrew trials. Also, in five of these seven subjects, SWITCH trial activation was higher than that for CONrew.
Our initial anatomical definition of dACC, although rigorous, still potentially covered a relatively large area (x = 3 cm laterally, y = 3 cm anteriorly–posteriorly, and z = ≈2 cm superiorly–inferiorly, totaling 18 cm3). In point of fact, though, all seven subjects produced dACC activity in a tightly clustered area [x = 6 to −7 mm (within 7 mm of midline), y = 4–17 mm (13 mm total), z = 41–48 mm (7 mm total)]—representing a total volume of only 1.2 cm3.
Confirmatory analysis of the group-averaged data (from the maximally activated dACC voxel in the seven subjects displaying significant activation in the REDrew minus FIX comparison) confirmed that fMRI signal was higher for both the REDrew (P < 0.001) and SWITCH trials (P < 0.01) as compared with the CONrew condition. Also, activity during REDrew trials was higher than during SWITCH trials—showing that REDrew activity was indeed specific, and not caused by the nonspecific act of changing behavior (i.e., pressing the alternate button). Comparisons here were made approximately 4 s after feedback, in the t = 4.5- to 6-s time after stimulus period (P < 0.05). Ordinal relationships were also as predicted in voxels significantly activated in the SWITCH minus FIX comparison (i.e., REDrew > SWITCH > CONrew; P < 0.05 for each comparison).
Group analysis of dACC voxels in Talairach space commonly activated by the REDrew and SWITCH conditions (eight voxels) also showed the predicted ordinal relationships (in the t = 4.5- to 6-s period; P < 0.05).
Group averaged results for REDrew trials versus FIX revealed bilateral dACC (areas 32′/24c′) foci (right [x/y/z] = [6/4/46]; left = [−2/12/48]), and individual analysis showed bilateral dACC activity in five of the seven subjects that activated. Although our study focused on dACC, for completeness, we noted that activation meeting or exceeding the same significance threshold (P < 1 × 10−4) was also observed in left anterior cingulate gyrus (area 24b′) [−2/23/20], right lateral prefrontal cortex (middle frontal gyrus, area 45 [42/15/36]; inferior frontal sulcus, area 45/46 [42/30/13]; and premotor area 6 [29/−3/58]), left precentral cortex (motor area 4) [−37/−18/63], left postcentral gyrus (area 40) [−60/−17/14], and multiple areas in the inferior parietal lobule (area 40) [51/−40/48; −38/−40/63; and −47/−25/36]. However, none of these other regions showed significant activation in a REDrew-minus-SWITCH comparison, and only the right middle frontal gyrus showed even a trend at P = 6.5 × 10−4 (whereas a significance threshold for post hoc regions, properly corrected for whole-brain multiple comparisons, is on the order of P < 1 × 10−7). These other regions, which were not predicted a priori, are provided merely to assist future hypothesis generation and must be confirmed in future prospective studies.
Discussion
The data indicate that dACC plays a role in reward-based decision making. These observations provide initial support for the hypothesis that dACC is comprised of a heterogeneous admixture of functional cell types. This conceptualization of dACC is rooted within the monkey electrophysiology literature, providing it with a strong neuroanatomical foundation.
Theoretical Considerations.
Although it is true that heterogeneity is a common pattern observed in many areas of the brain, including lateral prefrontal cortex (24), the monkey data clearly indicate that the pattern is not identical for all regions. The proportions of different cells vary from region to region, and knowledge of these differential proportions may be used to predict the fMRI response for a given region. Specifically, Shima and Tanji (27) quantified cell types not only in CMAr (dACC homologue), but also in the caudal cingulate motor area (CMAc). Although 37% of CMAr cells responded to reward reduction, only 3% of CMAc cells did so. Predictably, we did not observe significant fMRI activity in cingulate cortex caudal to dACC when comparing the REDrew condition to any of the other conditions (SWITCH, CONrew, or FIX). Although the matter is obviously more complex, combined with the dACC findings, the negative result observed in the caudal cingulate motor zone supports the validity of the experimental construct (i.e., that human fMRI response can be predicted from the monkey electrophysiology work).
If used in their strict, narrowly defined forms, alternative unimodal theories of ACC function (such as response selection, novelty detection, competition monitoring, error detection, or task difficulty) cannot account for the fMRI results (i.e., significantly greater dACC activity to REDrew compared with SWITCH trials). The fMRI results could not be construed as simply caused by response selection or novelty detection in the narrow sense, because REDrew and SWITCH trials occurred with equal frequency and were counterbalanced for change direction (button 1 to 2 and button 2 to 1). Competition monitoring does not explain the results either, for although both the REDrew and SWITCH trials both call for overriding a prepotent response, there is no difference in the amount of competition between the two. There is not a strong reason to believe that subjects would interpret REDrew as an error (because humans have the cognitive capacity to know there was no way they could have anticipated when a change might come). Moreover, in the monkey's case, the equivalent of SWITCH signals were indicated by an auditory tone with no reward given—theoretically this might have been perceived as an even greater error, but such was not the case (i.e., monkey REDrew cells did not respond to the auditory tone). In any case, accuracy was uniformly high (≈98%) for all conditions, eliminating task difficulty and error detection as possibilities.
Even if unimodal theories could be construed to explain the imaging data from this single fMRI study, they cannot account for the sum total of data from the monkey electrophysiology and human neuroimaging studies. Monkey single-unit data (24) and human fMRI data (19) clearly indicate that dACC responds well in advance of stimuli—a fact other theories cannot adequately incorporate. Also, the fact that monkey single-unit studies (24–27) repeatedly show responses by different cells at different times (prestimulus, preresponse, peri-response, postresponse) presents a puzzle for alternative, unimodal theories. If dACC cells only responded to errors or rewards, then activity should not occur before the response (and certainly not before stimulus). If dACC cells were only involved in response selection or competition monitoring or indicating task difficulty, then one would not expect to observe activity before stimulus and after response).
However, the conceptualization of dACC as heterogeneous allows incorporation of these alternative theories in their broader senses. Although not directly addressed here, dACC would be expected to play an important role in error processing (16, 17) because single-unit recordings have confirmed the presence of error detecting cells in this region (24, 26). Similarly, although competition monitoring and task difficulty cannot explain all of the data, they are factors that would be predicted to yield increased dACC activity, because diverse dACC cell types would be recruited by cognitively difficult tasks such as those requiring the resolution of competition. Thus, in addition to readily explaining activity during many reward-based decision making and other complex response selection tasks (such as the Stroop, Stroop-like, Eriksen Flanker, divided attention, go-no-go, and verb generation tasks) (1), the heterogeneity concept has the unique capacity to explain anticipatory (prestimulus) activity (1, 19), error feedback activity (16), and reward-and-punishment activity (22)—observations that existing theories have difficulty accounting for (1).
Methodological Considerations.
The imaging results cannot be considered definitive, as the relationship between neuronal firing and fMRI's blood oxygenation level-dependent (BOLD) response is not precisely known (30–32), and human dACC might not be comprised of cells in the exact same proportions as seen in the monkey. However, the main conclusions do not depend on a linear neuronal-BOLD relationship, and an observed 5-fold differential in the number of cells responding (37% REDrew vs. 7% CONrew) in the monkey provides a large margin for error when testing for a differential BOLD response in human dACC. Of course, future work should be done in animals to examine the relationship between single-unit activity and the BOLD response.
Although RTs after REDrew and SWITCH signals were slightly faster than those after CONrew, this does not change the final result. First, the differences were minimal (37 ms and 28 ms, respectively), and there was no difference between RTs after REDrew and those after SWITCH commands. More importantly, functional imaging data from cognitive interference tasks (Stroop, Stroop-like, and Eriksen Flanker tasks; ref. 1) shows that increased RTs are correlated with increased dACC activity. Thus, if anything, faster RTs after REDrew and SWITCH trials (as compared with CONrew trials) would have acted to minimize any fMRI differences. Further, RTs after REDrew and SWITCH commands were not different, so this could not account for the significant fMRI difference between these two conditions.
Although we attempted to roughly equate our use of secondary (monetary) rewards for our human subjects to the primary (juice) reward amounts used in the monkey study, the two are not equivalent. In this initial study, we elected to use monetary reward, as (i) we did not feel that we could necessarily equate primary rewards (33), and (ii) secondary rewards are logistically much simpler to employ in the fMRI environment. We suggest that future followup studies use primary rewards. Another experimental permutation worth examining in future studies would be to counterbalance the use of the $$$ and SWITCH stimuli as the indicators for the REDrew and SWITCH conditions, respectively.
A lack of significant activity during the CONrew condition, as opposed to FIX, might initially seem inconsistent with the hypothesis. However, CONrew trials were very simple, and Picard and Strick's metaanalysis of motor neuroimaging tasks concluded that the caudal cingulate motor zone (and not dACC) is responsible for assisting with such simple motor tasks (9). Second, the percentage of CONrew-sensitive cells in dACC is low (7%), and therefore unlikely to produce robust activation. Finally, even if the motor demands were significant at the start, subjects performed 760 CONrew trials, making this a highly overpracticed task. Thus, the data are consistent with neuroimaging (34–36) and monkey electrophysiology (37) work showing habituation of dACC activity with task practice. This last point (i.e., repeated observations of decreasing dACC activity with practice) is consistent with the work of Schultz and colleagues (42), who have shown temporal variations in dopaminergic reward signals with repetitive stimulus presentation, and Gabriel's work showing decreasing activity of ACC with learning (38).
dACC and Reward-Based Decision-Making.
Taken together, the data suggest that dACC may play a special role in reward circuitry—particularly in reward-based decision making, learning, and the performance of novel (nonautomatic) tasks—functions known to be substantially influenced by dopamine. The story is not simple though—although Schultz (39) reported that phasic dopaminergic responses occur in response to novel stimuli, the same review also stated that dopaminergic neurons are depressed by reward omission. Schultz and colleagues (39, 42) and others (33) also emphasize that the predictability of reward heavily influences reward-sensitive cells, illustrating the complexity of reward circuitry. Clearly, more study is needed to determine specifically how dACC contributes to reward processing. This line of inquiry may have clinical relevance, given the implication of dopaminergic pathways in the pathophysiology of attention deficit disorder (40) and observed dysfunction of dACC in this illness (23).
More speculatively, although much work needs to be done to establish the existence of an actual dACC “local intracortical network,” the framework for how such a network might contribute to reward-based decision making is straightforward and consistent with observed behavior. Signaling from anticipatory/timing cells would have predictive value and improve the processing of salient stimuli (41, 42). Novelty and target-detection cells would enhance attention to relevant stimuli. Motor-response cells in dACC contribute to complex motor behaviors (26). Of course, reward-and-error cells would provide invaluable feedback that would guide future actions based on experience.
Although the current study provides preliminary support for the existence of a heterogeneous dACC architecture, it leaves many important questions unanswered. Are these cells actually networked, and if so, what is the exact nature of the intranetwork communication patterns? Do certain “functional characteristics” (e.g., motivation) arise as epiphenomenona of network activity, and how is this represented? Finally, what is the nature of dACC's interaction with other brain regions in support of cognitive and motor processing?
Given the unique position of dACC in the larger distributed cortical network (dACC has strong interconnections with lateral prefrontal cortex, parietal cortex, striatum, and motor systems) (2–5, 43), and extrapolating from the current study and data in monkeys and humans, it appears likely that assisting with reward-based decision-making is only one facet of what dACC does. In humans, dACC may help guide behavior by integrating information from its own “internal network of cells” that evaluate motivation, anticipate events, detect targets, encode reward values, and signal errors, and then directly and/or indirectly influencing attention allocation, motor preparation and motor responses (1). More generally, it is likely that a “heterogeneous intracortical network pattern” is one repeated in other areas subserving cognitive and emotional processing. Thus, these findings should be of general interest to those studying reward, cognition, emotion, motivation, neuropsychiatric illnesses, and motor control.
Acknowledgments
We thank Mary Foley, Larry White, Lisa Shin, Rahul Desikan, Evelina Busa, David Salat, Larry Wald, and the anonymous reviewers. Special thanks go to our intrepid volunteers. Support was provided by National Institute of Mental Health Grant K01-MH01611, the National Alliance for Research on Schizophrenia and Depression, and the Forrest C. Lattner Foundation.
Abbreviations
- ACC
anterior cingulate cortex
- dACC
dorsal ACC, fMRI, functional MRI
- CMAr
rostral cingulate motor area
- REDrew
reduced reward
- CONrew
constant reward, FIX, fixation
- RT
reaction time
- ROI
region of interest
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
The results in this article were presented in preliminary form at the Seventh Annual Meeting of the Organization for Human Brain Mapping, June 10–14, 2001, Brighton, U.K.
References
- 1.Bush G, Luu P, Posner M I. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 2.Vogt B A, Nimchinsky E A, Vogt L J, Hof P R. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 3.Vogt B A, Vogt L J, Nimchinsky E A, Hof P R. In: Handbook of Chemical Neuroanatomy, The Primate Nervous System. Bloom F E, Björklund A, Hökfelt T, editors. Vol. 13. Amsterdam: Elsevier; 1997. , Part I, pp. 455–528. [Google Scholar]
- 4.Dum R P, Strick P L. In: Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Vogt B A, Gabriel M, editors. Boston: Birkhäuser; 1993. pp. 415–441. [Google Scholar]
- 5.Van Hoesen G W, Morecraft R J, Vogt B A. In: Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Vogt B A, Gabriel M, editors. Boston: Birkhäuser; 1993. pp. 249–284. [Google Scholar]
- 6.Vogt B A, Finch D M, Olson C R. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 7.Devinsky O, Morrell M J, Vogt B A. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 8.Drevets W C, Raichle M E. Cogn Emotion. 1998;12:353–385. [Google Scholar]
- 9.Picard N, Strick P L. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 10.Posner M I, Petersen S E, Fox P T, Raichle M E. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 11.Frith C D, Friston K J, Liddle P F, Frackowiak R S J. Proc R Soc London Ser B. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- 12.Mesulam M-M. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 13.Paus T, Petrides M, Evans A C, Meyer E. J Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- 14.Badgaiyan R D, Posner M I. NeuroImage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- 15.Turken AU, Swick D. Nat Neurosci. 1999;2:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- 16.Gehring W J, Knight R T. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 17.Luu P, Flaisch T, Tucker D M. J Neurosci. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter C S, Braver T S, Barch D M, Botvinick M M, Noll D, Cohen J D. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 19.Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. Hum Brain Mapp. 1996;4:103–112. doi: 10.1002/(SICI)1097-0193(1996)4:2<103::AID-HBM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Petit L, Courtney S M, Ungerleider L M, Haxby J V. J Neurosci. 1998;18:9429–9437. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark V P, Fannon S, Song L, Benson R, Bauer L. J Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- 22.Knutson B, Westdorp A, Kaiser E, Hommer D. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 23.Bush G, Frazier J A, Rauch S L, Seidman L J, Whalen P J, Jenike M A, Rosen B R, Biederman J. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 24.Niki H, Watanabe M. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- 25.Nishijo H, Yamamoto Y, Ono T, Uwano T, Yamashita J, Yamashima T. Neurosci Lett. 1997;227:79–82. doi: 10.1016/s0304-3940(97)00310-8. [DOI] [PubMed] [Google Scholar]
- 26.Procyk E, Tanaka Y L, Joseph J P. Nat Neurosci. 2000;3:502–508. doi: 10.1038/74880. [DOI] [PubMed] [Google Scholar]
- 27.Shima K, Tanji J. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 28.Burock M A, Dale A M. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme Medical Publishers; 1988. [Google Scholar]
- 30.Fox P T, Raichle M E. J Neurophysiol. 1984;51:1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- 31.Kwong K K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Kennedy D N, Hoppel B E, Cohen M S, Turner R, et al. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen M S. In: Brain Mapping: The Methods. Toga A W, Mazziotta J C, editors. San Diego: Academic; 1996. pp. 223–255. [Google Scholar]
- 33.Berns G S, McClure S M, Pagnoni G, Montague P R. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raichle M E, Fiez J A, Videen T O, MacLeod A M, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 35.Bush G, Whalen P J, Rosen B R, Jenike M A, McInerney S C, Rauch S L. Hum Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jueptner M, Stephan K M, Frith C D, Brooks D J, Frackowiak R S, Passingham R E. J Neurophysiol. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto T, Ogawa M, Tsukada H, Kakiuchi T, Sasaki K. Neurosci Lett. 2000;283:69–72. doi: 10.1016/s0304-3940(00)00913-7. [DOI] [PubMed] [Google Scholar]
- 38.Gabriel M. In: Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Vogt B A, Gabriel M, editors. Boston: Birkhäuser; 1993. pp. 478–523. [Google Scholar]
- 39.Schultz W. Nat. Rev. Neurosci. 2000; 2000. 1, 199–207. [DOI] [PubMed] [Google Scholar]
- 40.Biederman J, Spencer T. Biol Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 41.Posner M I, Snyder C R, Davidson B J. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- 42.Schultz W, Dayan P, Montague P R. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 43.Bates J F, Goldman-Rakic P S. J Comp Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]