Abstract
Adult second language learning seems to be more difficult and less efficient than first language acquisition during childhood. By using event-related brain potentials, we show that adults who learned a miniature artificial language display a similar real-time pattern of brain activation when processing this language as native speakers do when processing natural languages. Participants trained in the artificial language showed two event-related brain potential components taken to reflect early automatic and late controlled syntactic processes, whereas untrained participants did not. This result challenges the common view that late second language learners process language in a principally different way from native speakers. Our findings demonstrate that a small system of grammatical rules can be syntactically instantiated by the adult speaker in a way that strongly resembles native-speaker sentence processing.
The acquisition of certain basic cognitive functions seems to depend on appropriate input during so-called critical periods (1, 2). Rare cases of children who grew up without language input during their first years demonstrate that perfect mastery of a language cannot be acquired in later periods (3). It has been suggested that second language learning is subject to similar restrictions (4, 5).
Although the age of exposure during language acquisition seems to have a dramatic impact on the subsequent real-time processing of sentences, the mechanisms underlying this critical-period effect remain unclear. Although some researchers assume that the critical period effect can be explained by the earlier-is-better hypothesis (e.g., refs. 1 and 3; see also ref. 6), which holds that maturational constraints determine language learning, others assume the less-is-more hypothesis (e.g., refs. 7 and 8), which claims that differences in early and late language learning are a by-product of children's processing capacity limitations, providing a more focused approach to the language input.
The employment of brain imaging and electrophysiological techniques (9–11) has shed light on the neural bases of the well established behavioral differences between first (L1) and second language (L2) acquisition (12). Lexical-semantic processing of word meanings is relatively similar for native speakers and L2 learners. In contrast, syntactic real-time analyses of a sentence's grammatical information seem to differ considerably for late learners versus native speakers. In native speakers, severe syntactic violations elicit a characteristic biphasic response in the event-related brain potential (ERP) consisting of an early negativity and a late positivity. This early negativity was found often with a left anterior maximum (13–15) but sometimes with a more bilateral frontocentral distribution (16, 17). The factor determining this variation, however, has not yet been identified. Functionally, the early negativity occurring immediately after encountering the violating word (100–200 ms) is taken to reflect the interruption of a highly automatic parsing process (13, 14). The automaticity of this early process has been demonstrated by showing that this early negativity is not influenced by the proportion of correct and syntactically incorrect sentences in the experimental set (15). The late positivity, i.e., the second syntax-related component, normally appears about 600 ms after a syntactic anomaly (P600) and is associated with controlled processes of structural reanalysis and repair (18, 19). The specific type of revision process required seems to modulate the topographical distribution of the P600 component in native speakers (20); repair processes after outright syntactic violations are reflected usually by a centroparietal positivity (P600; refs. 19, 21, and 22), processes of syntactic reanalysis by a rather frontocentral P600 (18). Late learners do not display the early negativity and show only a reduced P600 in response to syntactic violations even if exposed to an L2 for several years. Instead they demonstrate posterior and right hemispheric negativities for syntactic violations, suggesting the possible involvement of compensatory conceptual-semantic processes (9, 11). These findings have been taken as evidence of fundamentally different and less automated syntactic sentence processing by learners who acquired an L2 after puberty (23).
The present study provides electrophysiological evidence that even the syntax of a language learned in adulthood can be processed fully automatically. To demonstrate this, we trained adults in a carefully constructed artificial language by using a computer-implemented learning paradigm. We then registered their ERPs while listening to syntactic errors and correct control sentences in the learned miniature language. We ensured that the adults reached a high level of proficiency and fluency in the miniature language. This way, we could show that with a high degree of language proficiency the brain mechanisms involved in L1 and L2 processing are similar even when the L2 has been learned late.
Proficiency of language use is the end product of a learning process that may be influenced by factors explicable in terms of either lexical or syntactic rules. First, a large but poorly established vocabulary could delay the fast availability of syntactic word category information crucial in building up syntactic structures on-line (24). Second, the syntactic rules may be very different between the L1 and L2, causing interference between the two grammar systems (25). Third, phonological patterns such as prosody have been shown to influence syntactic processing of more complex structures considerably (26). Thus prosodic variations between the languages also may affect proficiency in the L2, at least with complex sentences. To minimize the influence of these three factors, we examined the processing of syntax violations in a simple and highly controlled artificial grammar.
Violations of nonlinguistic artificial grammars have been shown to elicit a monophasic positive ERP component distinct from the biphasic ERP patterns of real language violations (27). Therefore, we designed an artificial language called BROCANTO, the miniature grammar of which (Fig. 1) meets universal requirements of natural language. Thus, the current version of BROCANTO can be viewed as a small but expandable section of a full-fledged language comparable to natural languages. That is, the current limitations of both BROCANTO's vocabulary and grammar are by no means inherent but rather explicitly intended to probe the following controversial hypotheses. The critical period hypothesis predicts that L2 learning results in different processing patterns from native languages. The less-is-more hypothesis, on the other hand, predicts that L2 processing could be similar to L1 processing if the grammar to be learned is small. The latter proposal coincides with the idea that language proficiency rather than the age of acquisition as such may be a relevant factor in determining the pattern of language processing.
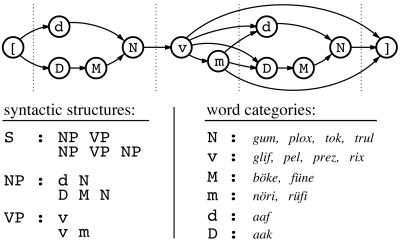
Figure 1.
Schematic representation of the artificial grammar of BROCANTO. Single letters represent the different word classes: N, noun; v, verb; M, noun modifier (adjective); m, verb modifier (adverb); d and D, determiner. Combined letters denote phrases: NP, noun phrase; VP, verb phrase. The letter S stands for the whole sentence. (Upper) The nodes specify word classes (nouns, verbs, etc.), and the arrows indicate valid transitions between nodes. Every sequence of transitions from the beginning node ([) to the end node (]) constitutes a well formed sentence. (Lower) Each colon specifies a rule, according to which valid phrases are formed. For example, a noun phrase in an utterance is either the sequence dN or DMN. The vocabulary items are printed in italics at the lower right and were chosen to be nonwords in the languages known to the subjects.
Materials and Methods
Participants.
Two groups of subjects participated in the experiment: a group trained in BROCANTO and an untrained group. The trained group (TG) consisted of 28 subjects (15 female, mean age 24.1 years) who had undergone a complete training program. The untrained control group (CG) consisted of 31 participants (18 female, mean age 23.3 years) who only received vocabulary training before the ERP session.
Stimulus Material.
The miniature artificial grammar used in the present study is displayed in Fig. 1. The stimulus material consisted of 488 spoken sentences in BROCANTO, half of which contained severe syntactic word category violations. Sentences were relatively simple main clauses comprising 5–8 words, and each represented a subject-verb-[object] structure that related to the pieces and moves in the computer game used to train subjects in the language (see below). Note, however, that the grammatical rules of BROCANTO did not pertain in any specific way to the rules of the game and vice versa. To derive violations of the legitimate phrase structure rules of BROCANTO (Fig. 1), tokens of one word category in a correct sentence were replaced by tokens of a different word category (the asterisk indicates the incorrect word). Examples of sentences are aaf trul prez nöri aak füne plox, which literally meant the trul-piece captures horizontally the round plox-piece, and the violated counterpart aaf trul prez nöri *rix füne plox literally meant the trul-piece captures horizontally *buy round plox-piece. Because of the large variety of violation types, words of all categories occurred almost equally often in the violation and corresponding correct conditions.
The auditory sentence stimuli were generated by rotating the same 14 basic word samples (recorded by a male speaker who pronounced the words without knowing their meaning or word category) in the sequences and inserting a 50-ms silence between words. This allowed for relatively natural-sounding sentence material with acoustically identical baseline periods and no coarticulation across word boundaries. To provide a good baseline for the ERP analyses, the correct and incorrect sentences were kept acoustically identical up to the point of violation. The position of the violation was distributed approximately equally over the word categories and sentence positions, but the violation never appeared as the first word.
Training Program of the TG.
To create a more natural environment for language learning that was characterized by the need to communicate rather than to explicitly learn rules, a special computer board game was developed. In the game, all necessary moves could be expressed unambiguously with the very limited 14-word vocabulary of BROCANTO (Fig. 2). During several training sessions, subjects in the TG played pairwise against each other for up to 5 h per session. Subjects were required to communicate all their moves verbally in BROCANTO. For example, once a sentence was uttered, the opponent player had to update the new game constellation on his/her screen according to this information. In the case of incorrect utterances or misunderstandings, the computer automatically corrected the moves, auditorily presented the correct sentence in BROCANTO, and offered help on the vocabulary. Language errors and additional help requests resulted in penalty scores. Thus, language mastery in both production and perception was necessary to win the game. Language performance was tested before and after each session, and training was continued until each subject reached a 95% accuracy criterion, which indicated high proficiency in BROCANTO.
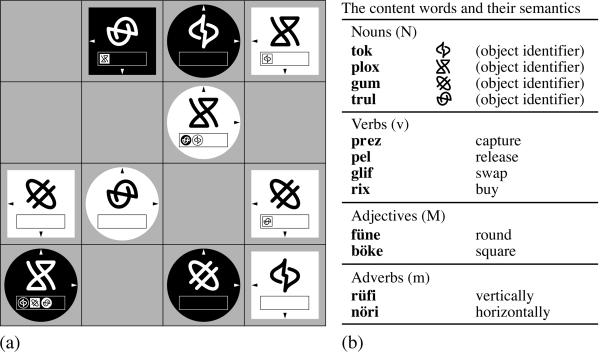
Figure 2.
(a) The board game used for language acquisition in the TG. The board consisted of 4 × 4 fields on which pieces, represented by abstract symbols, could be moved according to certain rules. The rules of the game had no direct relation to the syntactic rules of the grammar. At the word level, however, there was correspondence between nouns and game pieces. (b) List of the words and their referents. Nouns were represented by pieces, verbs by movements, adjectives by form, and adverbs by orientation. According to the rules of the grammar (see Fig. 1), the words could be assembled to form sentences. Each grammatically correct sentence referred to a specific move of a specific piece, and each valid move could be described by a statement in the artificial language BROCANTO.
Training Session of the CG.
To assure that the expected ERP differences between subject groups were actually caused by differences in syntactic processing, the CG received BROCANTO vocabulary training only. Here, the subjects learned symbol-word pairings until they reached a 95% accuracy criterion and were not given training on the grammar rules.
Experimental Session.
In the actual ERP experiment, the participants were presented with 488 spoken sentences. The subjects' task was to judge the sentence's grammatical correctness or to decide whether a visually displayed probe symbol (e.g., representing a game piece; see Fig. 2) corresponded to a word in the preceding sentence. The task to be performed was indicated after each sentence was presented. Participants from the CG were allowed to base their grammatical-correctness judgments on purely aesthetic considerations.
Electroencephalogram Recording and Analysis.
Brain potentials (electroencephalograms) were recorded continuously during the whole experimental session from 59 cap-mounted tin electrodes (Electro-Cap International, Eaton, OH) according to the international 10–10 system. Eye movements and blink artifacts were monitored by means of two bipolarly recorded electrooculograms. Electrode impedances were kept between 1 and 5 kΩ. Electroencephalogram and electrooculogram signals were amplified by using NeuroScan (Sterling, VA) DC amplifiers with a 50-Hz (−3 dB cut-off, −6 dB per octave) low-pass filter setting. All signals were digitized on-line (sampling frequency 250 Hz, 16-bit analog/digital resolution) and stored for later analysis.
Off-line-separated ERPs were averaged for each participant at each electrode site in each condition from trials free of electrooculogram artifacts. ERPs were time-locked to the onset of the critical violating item and its corresponding correct item. A 300-ms prestimulus interval was taken to compute the baseline. ERPs were quantified in four different time windows: 1, 100–200 ms; 2, 200–350 ms; 3, 350–550 ms; 4, 650–900 ms. All ERP data were analyzed separately for midline and lateral electrode sites. Where interactions with topographic variables are concerned, the effects were confirmed by ANOVAs for normalized data. To avoid excessive type 1 errors resulting from violations of sphericity, the Huynh and Feldt correction was applied when evaluating effects with more than one degree of freedom in the numerator. In these cases we report the original degrees of freedom for ease of interpretation and the corrected probability level.
Results and Discussion
Behavioral Data.
The behavioral measures indicated that the TG showed equally high accuracy in both lexical tasks (probe detection: 89%, SD = 14) and syntactic tasks (grammatical correctness judgments: 93%, SD = 4). The CG also performed well in the lexical task (probe detection: 86%, SD = 5), but their syntactic performance differed significantly from that of the TG and was only slightly above chance (grammatical correctness judgments: 58%, SD = 5). These differences were reflected statistically by a highly significant interaction between group and task (P < 0.0001) and a task main effect in the CG (P < 0.0001) but not in the TG (P > 0.1).
ERPs.
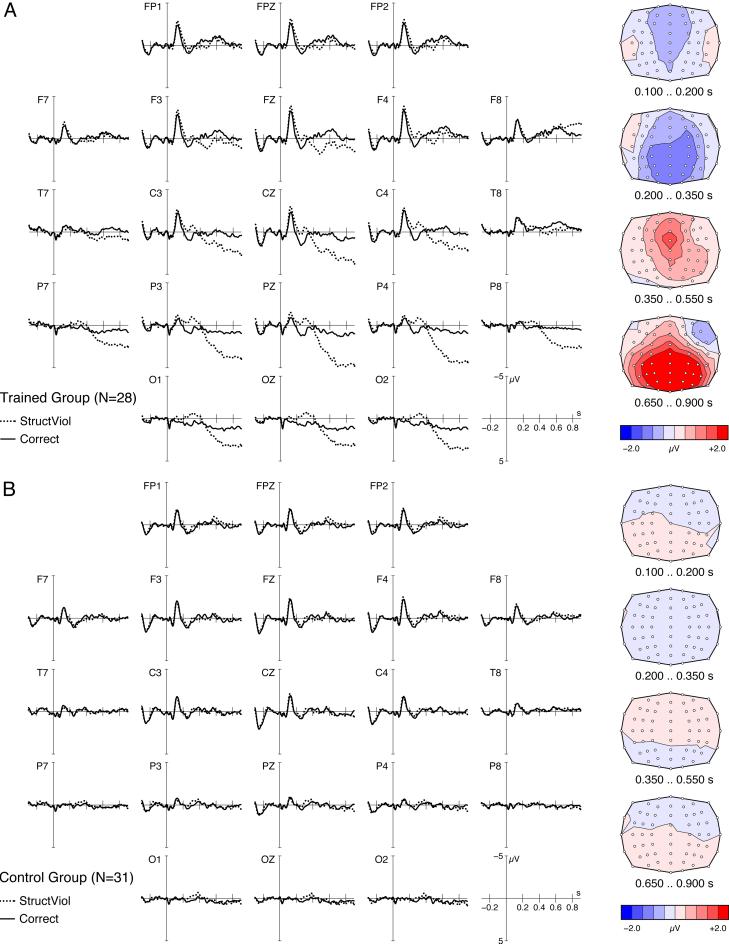
The ERP patterns for critical words comparing syntax violations and grammatically correct conditions are displayed in Fig. 3. They reveal a striking group difference. A clear amplitude difference between correct and grammatically incorrect sentences can be observed in the TG (Fig. 3), whereas virtually no effect of grammaticality is present for the CG. The effects were analyzed statistically in the four consecutive time windows. In proficient users of BROCANTO, syntax violations elicited an early frontocentrally distributed negativity between 100 and 200 ms in the first time window (P < 0.001). This was followed by a more posterior effect in the second time window between 200 and 350 ms (P < 0.0001). Finally, the TG showed a long-lasting and broadly distributed positivity for the violation condition. This positivity started frontocentrally between 350 and 550 ms (time window 3, P < 0.0004) and displayed a centroparietal distribution between 650 and 900 ms (time window 4, P < 0.0001). No such effect was found for the CG. Statistical analyses directly contrasting the two groups revealed significant interactions between group and grammaticality for each interval (all P values < 0.01). More detailed topographic analyses within the TG confirmed that the early negativity for syntax violations was most prominent at anterior electrode sites in the first time window. The late positive component had the typical centroparietal maximum of a P600 component elicited by grammatical violations (P values < 0.0005 for topographic interactions and the posterior main effect of grammaticality). Thus, these two components correspond precisely to the biphasic ERP pattern that is commonly thought to reflect automatic syntax parsing in healthy native speakers of natural languages.
Figure 3.
Grand average ERPs contrasting the grammatically incorrect words (StructViol, dotted lines) against the corresponding correct words (solid lines) in each group. Negative potentials are plotted upward. The vertical line at 0 s indicates the presentation onset of the critical word in the sentence. The four scalp maps in the right column illustrate the topographic distribution of the difference waves (violation minus correct) in four consecutive time intervals after word onset. (A) In the TG (n = 28), syntax violations elicit a biphasic ERP effect, i.e., an early frontal negativity and a late posterior positivity. The violation and correct conditions are composed of a total of 4,934 trials and 4,931 trials, respectively. (B) In the syntactically untrained CG (n = 31), ERPs for the violation condition (5,932 trials) and the correct condition (5,425 trials) do not differ.
An additional negativity in the second time window had a more posterior distribution. This pattern resembles two previously observed ERP effects. First, it displays a profile similar to the phonological mismatch negativity observed for target words that differ phonologically from the expected word (28). In our study, phonological expectations leading to phonological mismatch negativity effects could have resulted from the very limited vocabulary of only 14 words. Second, this negativity between 200 and 350 ms resembles that observed for syntactic violations in late L2 learners of a natural language (11). In the absence of an early anterior negativity, this later posterior negativity was interpreted to reflect conceptual compensatory strategies. The posterior negativity in the present study may reflect processes activated in addition to those purely structural processes indicated by the early frontal negativity. If so, one could conclude that although the automatic syntactic processes observed in native-speaking adults are present also in proficient late L2 learners, additional nonstructural processes may run in parallel. Future research will have to specify the exact conditions under which the second negativity is elicited.
Grammatical Rule Transfer.
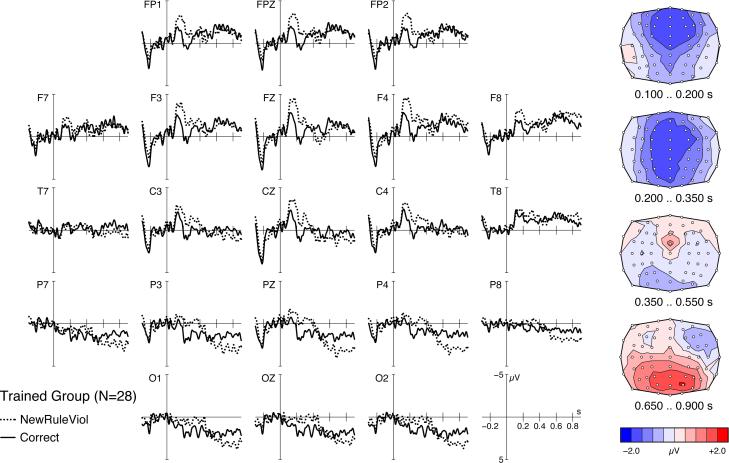
The absence of any effects in the CG strongly suggests that the components observed in the TG are related directly to syntactic processing. However, the current data do not yet provide definitive evidence as to whether the TG was processing newly acquired syntactic rules or transferred rules from their L1. Trained participants may have transferred syntactic rules from their German mother tongue to the artificial language, processing the new vocabulary of BROCANTO with their parsing system from German. This potential transfer was controlled for by introducing two grammatical rules into BROCANTO that do not exist either in German or in any other natural language known to the participants. BROCANTO has two different types of determiners: one determiner (aaf) requires a noun to follow directly, e.g., aaf plox (the plox-piece), and the other determiner (aak) requires an adjective to precede the noun, e.g., aak füne plox (the round plox-piece). The subtle replacement of aak by aaf or vice versa leads to a violation in BROCANTO, and these syntax rules had to be learned from scratch. Thus, if any observed brain response to syntax violations rested on a rule transfer from German to BROCANTO, violations of the new rules should not elicit this response. As illustrated in Fig. 4, violations of these rules in particular replicated precisely the biphasic ERP pattern in the TG (P values < 0.01), whereas once again no significant effect was found in the CG.
Figure 4.
ERP effects in the TG for words violating new BROCANTO-specific syntax rules. Compared with correct words (solid lines, 959 trials), incorrect words (NewRuleViol, dotted lines, 809 trials) elicited a biphasic ERP pattern similar to that presented in Fig. 3A. Here cross-linguistic rule transference cannot explain the effect.
With the possibility of transfer from German ruled out, we can conclude that the native speaker-like biphasic ERP pattern observed in the TG was caused by syntactic processing of the newly acquired but mastered miniature language.
Syntax Processing in L2.
Functionally, the two syntax-related ERP components, the early frontal negativity and the late positivity, are taken to reflect two stages of parsing. The first-pass stage assigns an initial syntactic structure, whereas the second stage involves syntactic integration (21, 29). This view is supported by evidence that shows that the early anterior negativity is only observed with violations of syntactic rules (13, 14, 19). In contrast, the late positivity is seen when processing grammatical violations (21, 30, 31) as well as with grammatically unusual or complex sentences (16, 32–34). Although most studies on native speakers found the anterior negativity with a left lateralization (13, 21, 31, 33), others reported a more bilateral distribution (16). The latter distribution is similar to the one observed here for trained users of BROCANTO.
Neuroanatomically, the early ERP component in native speakers has been shown to originate from the inferior frontolateral cortex in the vicinity of Broca's area and the anterior part of the superior temporal gyrus (35). These brain areas were shown to be involved in syntactic processes in some recent brain-imaging studies (36–40). Moreover, lesion studies indicate that both Broca's area and the anterior part of the superior temporal gyrus are linked to syntactic processes. Patients with lesions in Broca's area clearly demonstrate syntactic deficits during behavioral tasks (41) and do not show an early anterior negativity (17). Interestingly, patients with temporal lesions also display a similar syntactic deficit but only when the anterior portion of the superior temporal gyrus is involved (42).
Recent brain-imaging studies on the cortical representation of an L2 are not univocal. Although some studies argue for different neural tissue being involved in L1 and L2 processing (43, 44), others report a shared neural substrate (45). One attempt to unify these opposing results has been to propose that not only the age of acquisition but particularly the proficiency level determines the amount of cortical overlap involved in L1 and L2 processing (10). This view based on neurotopological findings is compatible with the present neurochronometric data. Both types of evidence suggest a close similarity in the temporal structure of the brain's activity during L1 and L2 processing when L2 is learned to a high degree of proficiency. One major task for future research will be to determine whether the topographical changes of brain activity in language processing can be linked directly to consecutive stages of proficiency in the same individual.
Prior neurophysiological studies on adult L2 processing of natural languages did not find the early syntactic negativity usually seen in adult native speakers (9, 46). The difference between the former and present ERP findings seems to be attributable to the “size” of the target language and, as a consequence, to the proficiency with which it could be learned. In contrast to natural languages, BROCANTO contains a small number of items in the different syntactic categories. The number of category members is relevant for first-pass parsing, because the initial grammaticality check depends on a word category check. Word category assignment can be accomplished more quickly and thus more proficiently in a grammar with a limited vocabulary size than in a full-fledged natural language. Therefore, proceduralization of initial syntactic processes reflected by the early negativity can be achieved quite quickly in a grammar such as BROCANTO, leading to a biphasic ERP pattern similar to adult native language users. As our data demonstrate, rule transfer from the L1 cannot account for this pattern.
Conclusions
The results from the present experiment provide neurophysiological data for the discussion on the critical period view that assumes that language acquisition in adulthood cannot rest on the same brain mechanisms used in processing a native language. Our data indicate that a late-learned language, in principle, can be processed in a native speaker-like way, as evidenced in the biphasic ERP pattern shown by the fully trained BROCANTO language users. The reported differences between the two groups in our study clearly do not depend on the age of acquisition, because this was similar for all participants. Thus, it seems that for L2 learning, the strong version of the age-related critical period hypothesis based on maturational neural constraints needs to be reconsidered. Both the level of proficiency and aspects of the language size available during learning must be taken into account more explicitly.
The employment of a carefully designed artificial language can help to examine these issues further. A particular advantage of artificial languages is their potential of “controlled expansion.” Varying type and size of the vocabulary may clarify the nature of the negativity between 200 and 350 ms. Including further linguistic features (e.g., prosodic patterns) may elucidate how the different levels of information contribute to the difficulties in L2 learning.
Abbreviations
- L1
first language
- L2
second language
- ERP
event-related brain potential
- TG
trained group
- CG
control group
References
- 1.Lenneberg E H. Biological Foundations of Language. New York: Wiley; 1976. [Google Scholar]
- 2.Hubel D H, Wiesel T N. Proc R Soc London. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- 3.Curtiss S. Genie: A Psycholinguistic Study of a Modern-Day Wild Child. New York: Academic; 1977. [Google Scholar]
- 4.Johnson J S, Newport E L. Cognit Psychol. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- 5.Harley B, Wang W. In: Tutorials in Bilingualism: Psycholinguistic Perspectives. de Groot A M B, Kroll J F, editors. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 19–51. [Google Scholar]
- 6.Birdsong D. In: Second Language Acquisition and the Critical Period Hypothesis. Birdsong D, editor. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 1–22. [Google Scholar]
- 7.Newport E L. Lang Sci. 1988;10:147–172. [Google Scholar]
- 8.Newport E L. Cogn Sci. 1990;14:11–28. [Google Scholar]
- 9.Weber-Fox C M, Neville H J. J Cogn Neurosci. 1996;8:231–256. doi: 10.1162/jocn.1996.8.3.231. [DOI] [PubMed] [Google Scholar]
- 10.Perani D, Paulesu E, Galles N S, Dupoux E, Dehaene S, Bettinardi V, Cappa S F, Fazio F, Mehler J. Brain. 1998;121:1841–1852. doi: 10.1093/brain/121.10.1841. [DOI] [PubMed] [Google Scholar]
- 11.Hahne A, Friederici A D. Bilingualism Lang Cognit. 2001;4:123–141. [Google Scholar]
- 12.Ritchie W C, Bhatia T K. Handbook of Second Language Acquisition. San Diego: Academic; 1996. [Google Scholar]
- 13.Neville H J, Nicol J, Barss A, Forster K I, Garrett M F. J Cogn Neurosci. 1991;3:151–165. doi: 10.1162/jocn.1991.3.2.151. [DOI] [PubMed] [Google Scholar]
- 14.Friederici A D, Pfeifer E, Hahne A. Cognit Brain Res. 1993;1:183–192. doi: 10.1016/0926-6410(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 15.Hahne A, Friederici A D. J Cogn Neurosci. 1999;11:194–205. doi: 10.1162/089892999563328. [DOI] [PubMed] [Google Scholar]
- 16.Knoesche T R, Maess B, Friederici A D. Brain Topogr. 2000;12:75–87. doi: 10.1023/a:1023442426799. [DOI] [PubMed] [Google Scholar]
- 17.Friederici A D, von Cramon D Y, Kotz S A. Brain. 1999;122:1033–1047. doi: 10.1093/brain/122.6.1033. [DOI] [PubMed] [Google Scholar]
- 18.Osterhout L, Holcomb P J. J Mem Lang. 1992;31:785–804. [Google Scholar]
- 19.Hagoort P, Brown C M, Groothusen J. Lang Cognit Proc. 1993;8:439–483. [Google Scholar]
- 20.Friederici, A. D., Hahne, A. & Saddy, D. (2001) J. Psycholing. Res., in press. [DOI] [PubMed]
- 21.Friederici A D, Hahne A, Mecklinger A. J Exp Psychol Learn Mem Cognit. 1996;22:1219–1248. doi: 10.1037//0278-7393.22.5.1219. [DOI] [PubMed] [Google Scholar]
- 22.Münte T F, Heinze H J, Mangun G R. J Cogn Neurosci. 1993;5:335–344. doi: 10.1162/jocn.1993.5.3.335. [DOI] [PubMed] [Google Scholar]
- 23.Neville H J, Mills D L, Lawson D S. Cereb Cortex. 1992;2:244–258. doi: 10.1093/cercor/2.3.244. [DOI] [PubMed] [Google Scholar]
- 24.Kersten A W, Earles J L. J Mem Lang. 2001;44:250–273. [Google Scholar]
- 25.Birdsong D, Molis M. J Mem Lang. 2001;44:235–249. [Google Scholar]
- 26.Steinhauer K, Alter K, Friederici A D. Nat Neurosci. 1999;2:191–196. doi: 10.1038/5757. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin K B, Kutas M. Psychophysiology. 1997;34:74–86. doi: 10.1111/j.1469-8986.1997.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 28.Connolly J F, Phillips N A. J Cogn Neurosci. 1994;6:256–266. doi: 10.1162/jocn.1994.6.3.256. [DOI] [PubMed] [Google Scholar]
- 29.Kaan E, Harris A, Gibson E, Holcomb P. Lang Cognit Proc. 2000;15:159–201. [Google Scholar]
- 30.Münte T F, Matzke M, Johannes S. J Cogn Neurosci. 1997;9:318–329. doi: 10.1162/jocn.1997.9.3.318. [DOI] [PubMed] [Google Scholar]
- 31.Coulson S, King J W, Kutas M. Lang Cognit Proc. 1998;13:21–58. [Google Scholar]
- 32.Mecklinger A, Schriefers H, Steinhauer K, Friederici A D. Mem Cognit. 1995;23:477–494. doi: 10.3758/bf03197249. [DOI] [PubMed] [Google Scholar]
- 33.Featherston S, Gross M, Münte T F, Clahsen H. J Psycholing Res. 2000;29:141–154. doi: 10.1023/a:1005188810604. [DOI] [PubMed] [Google Scholar]
- 34.Rösler F, Friederici A D, Pütz P, Hahne A. J Cogn Neurosci. 1993;5:345–362. doi: 10.1162/jocn.1993.5.3.345. [DOI] [PubMed] [Google Scholar]
- 35.Friederici A D, Wang Y, Herrmann C S, Maess B, Oertel U. Hum Brain Mapp. 2000;11:1–11. doi: 10.1002/1097-0193(200009)11:1<1::AID-HBM10>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Just M A, Carpenter P A, Keller T A, Eddy W F, Thulborn K R. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 37.Dapretto M, Bookheimer S Y. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 38.Friederici A D, Meyer M, von Cramon D Y. Brain Lang. 2000;74:289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- 39.Meyer M, Friederici A D, von Cramon D Y. Cognit Brain Res. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 40.Ni W, Constable R T, Mencl W E, Pugh K R, Fulbright R K, Shaywitz S E, Shaywitz B A, Gore J C, Shankweiler D. J Cogn Neurosci. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- 41.Caplan D, Waters G S. Behav Brain Sci. 1999;22:77–126. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- 42.Dronkers N E, Redfern B B, Ludy C A. Brain Lang. 1995;51:62–65. [Google Scholar]
- 43.Dehaene S, Dupoux E, Mehler J, Cohen L, Paulesu E, Perani D, VandeMoortele P-F, Lehéricy S, Le Bihan D. Neuroreport. 1997;8:3809–3815. doi: 10.1097/00001756-199712010-00030. [DOI] [PubMed] [Google Scholar]
- 44.Kim K H S, Relkin N R, Lee K M, Hirsch J. Nature (London) 1997;388:171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- 45.Klein D, Milner B, Zatorre R J, Zhao V, Nikelski J. Neuroreport. 1999;10:2841–2846. doi: 10.1097/00001756-199909090-00026. [DOI] [PubMed] [Google Scholar]
- 46.Hahne A. J Psycholing Res. 2001;30:251–266. doi: 10.1023/a:1010490917575. [DOI] [PubMed] [Google Scholar]