Abstract
Objective
To investigate the effect of capecitabine on the sensitivity of oxaliplatin and on the level of transcription factor forkhead box P1 (FOXP1) and gamma-glutamyl transpeptidase (GGT) in patients with intermediate and advanced gastric cancer.
Methods
A total of 152 Patients with advanced gastric cancer who were continuously diagnosed and treated in our hospital were selected as the study objects. The general data were retrospectively analyzed. The patients in the control group received oxaliplatin, while the patients in the study group received capecitabine on the basis of the control group. The FOXP1 expression level was detected using immunohistochemistry. Serum levels of GGT were measured by chemiluminescence. Protein levels were detected by Western blot. The prognostic factors were analyzed by the COX regression model. The Kaplan–Meier survival curve was used to analyze the survival of gastric cancer.
Results
The effective rates (complete response, partial response, and stability) of the study group and the control group were 94.74% and 76.32%, respectively. Compared with adjacent normal tissues, the expression level of FOXP1 in gastric cancer tissues was lower (P < 0.05). After treatment, the average expression level of FOXP1 in the gastric cancer tissue of the study group was higher than the control group (P < 0.05). Moreover, lower FOXP1 expression was associated with lower overall survival (OS) (1-year survival and 3-year survival were 75.76% and 53.03%, respectively) (P < 0.05). Further analysis showed that capecitabine combined with oxaliplatin down-regulated the expression of DNA repair related-proteins and up-regulated the expression of key molecules of the apoptosis pathway, thus enhancing the killing effect of oxaliplatin on gastric cancer cells (P < 0.05). Both the 1-year and 3-year survival rates of the study group were higher than that in the control group (P < 0.05). The 1-year survival rate of 152 patients with gastric cancer was 84.87% (129/152) and the 3-year survival rate was 63.17% (96/152). Age, tumor-node-metastasis (TNM) stage, lymph node metastasis, chemotherapy regimen, FOXP1, and GGT levels were important factors in determining OS.
Conclusion
Capecitabine effectively enhanced the sensitivity of intermediate and advanced gastric cancer to oxaliplatin, improved the therapeutic effect and ameliorated the prognosis of patients.
Graphical Abstract
Keywords: Capecitabine, Advanced gastric cancer, Oxaliplatin, Sensitivity, FOXP1, GGT
Introduction
Gastric cancer is one of the most common malignant tumors of the digestive tract. According to the 2020 Global Cancer Burden Data, the incidence rate of gastric cancer in China ranks second in the incidence rate of all malignant tumors, and the mortality rate ranks third in the mortality rate of malignant tumors, indicating a high incidence and mortality rates of gastric cancer in China [1]. However, the incidence of gastric cancer in developed countries is gradually decreasing. The prognosis of gastric cancer is closely related to the timing of diagnosis and treatment. The five-year survival rate of advanced gastric cancer is still less than 30% even after comprehensive treatment with surgery [2, 3]. Moreover, the quality of life of patients is low, which brings a heavy burden to the families and country. However, the 5-year survival rate of most early-stage gastric cancers can exceed 90% after treatment [4], and even achieve a curative effect. Therefore, early diagnosis of gastric cancer is undoubtedly the key to improving the survival rate and survival time of gastric cancer patients. The pathogenesis of gastric cancer is complex. Its early onset is relatively insidious, only manifested as nausea and vomiting, and the symptoms are not obvious and lack certain specificity. As a result, most patients are in the intermediate and advanced stages at the time of diagnosis, missing the optimal time for treatment [5]. Surgery combined with radiotherapy and chemotherapy is the main method for the treatment of intermediate and advanced gastric cancer. Among them, chemotherapy based on oxaliplatin is considered as the first-line treatment against gastric cancer, which has achieved good results [6]. However, some tumor cells are less sensitive to chemotherapy drugs, so the effect of conventional chemotherapy is not satisfactory, especially for the treatment of advanced colorectal cancer. The emergence of chemoresistance poses a threat to long-term clinical benefits and may be an important factor contributing to treatment failure in patients with advanced gastric cancer [7]. Therefore, finding a safe and effective method that can enhance the sensitivity of tumor cells to oxaliplatin plays a momentous role in improving the treatment effect and prognosis of patients.
Capecitabine is an oral fluorouracil antitumor drug that inhibits the growth of tumor cells by inhibiting the activity of thymidine phosphatase (TPP) and hindering deoxyribonucleic acid (DNA) synthesis [8]. Capecitabine has a small gastrointestinal reaction, activates in liver and tumor tissues after oral administration, and is converted into 5-FU to exert anti-tumor effects, which is mainly used for the treatment of colorectal cancer, breast cancer, and gastric cancer, and can also be used as a rescue therapy for breast cancer after anthracycline and taxane therapy failure [9]. Oxaliplatin is a platinum-based antitumor drug that can hinder DNA replication and transcription of tumor cells through cross-linking with DNA strands, leading to tumor cell death [10]. The combined application of the two drugs can exert a synergistic anti-tumor effect and improve the efficacy of gastric cancer patients. However, it is not clear whether it can enhance the sensitivity of oxaliplatin and improve the therapeutic effect on patients with intermediate and advanced gastric cancer.
Transcription factor Fork-head box prote1 (FOXP1) belongs to the Fox family and participates in many processes, such as cell cycle, embryonic development, apoptosis, and differentiation [11]. FOXP1 is found to be abnormally expressed in ovarian cancer, renal cancer, prostate cancer, and other malignant tumors, and is closely related to the malignant degree [12]. In addition, the study has found that the FOXP1 transcription factor can promote epithelial-mesenchymal transition by directly affecting gastric tumor cells or indirectly affecting the tumor microenvironment, which may accelerate the invasion and metastasis of cancer [13]. γ- Glutamyl transpeptidase (GGT) is an enzyme bound to the cell membrane, which enables to hydrolyze glutathione and effectively maintains the homeostasis of glutathione and cysteine. It has been found that serum GGT can be used as a marker for the progression of colorectal cancer and other malignant tumors [14]. In addition, the study has shown that the effects of elevated GGT further increase the risk of gastric and colorectal cancer in diabetic patients, and that elevated GGT levels are associated with an increased risk of gastrointestinal cancer, regardless of the location of the cancer [15]. At present, there have been studies on the expression of FOXP1 and GGT in gastric cancer and their relationship with prognosis, but it is not known whether capecitabine can affect the treatment of gastric cancer patients with FOXP1 and GGT levels.
Thus, this study aimed to explore the effects of capecitabine on oxaliplatin sensitivity and the levels of FOXP1 and GGT in patients with intermediate and advanced gastric cancer.
Materials and methods
General materials
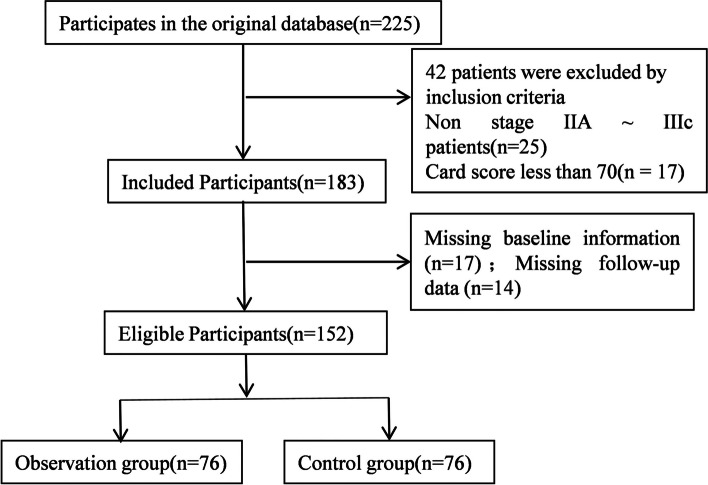
This study was approved by the hospital Ethics Committee. Patients with advanced gastric cancer diagnosed and treated in our hospital from April 2018 to May 2019 were selected as the research objects, and the general information on the front page of the hospitalization medical record was retrieved through the medical record information management system for retrospective analysis. Inclusion criteria: (1) All patients met the diagnosis and treatment criteria for gastric cancer [16]. (2) All patients with inoperable or postoperative recurrence and metastasis were confirmed by pathological examination. Before enrollment, all patients confirmed by pathological examination were in TNM stage IIIb ~ IV. (3) All patients had not received radiotherapy and chemotherapy one year before the chemotherapy in the study. The patients were aged over 18 years, and the expected survival was more than 6 months before treatment. (4) All subjects signed the informed consent form and they were willing to cooperate with this study. (5) The patient's clinicopathological data were complete and could cooperate with the study. (6) At least 1 measurable lesion by imaging examination with vital organs function normally (The function indexes of each organ were within ± 20% of the normal range [17]). (7) The score of the patient in the Eastern Cooperative Group (ECoG) of the United States was less than 2 points, and the card score was more than 70 points. Exclusion criteria: (1) Patients combined with diabetes, metabolic syndrome, and other diseases. (2) Patients with severe impairment of liver and kidney function or heart function. (3) The patient who was seriously allergic to the drugs used in the treatment process. (4) Patients with abnormal coagulation and hematopoiesis. (5) Pregnant or lactating patients. (6) Patients who were intolerant to chemotherapy. (7) Patients with mixed tumors. These objects were randomly divided into the study group and the control group according to the random number table method, with 76 cases in each group. The selection process of general materials is shown in Fig. 1.
Fig. 1.
The selection process of general materials
Methods
The control group: The patients in the control group were treated with oxaliplatin. Oxaliplatin injection with a dose of 130 mg/m2 (purchased from Shenzhen Haiwang Pharmaceutical Co., Ltd., production batch No.: 20171048, specification: 20 ml: 40 mg) was mixed with 5% glucose solution. 250 ml mixture was infused intravenously once every 3 weeks. Calcium folinate solution (purchased from Jiangsu Hengrui Pharmaceutical Co., Ltd., production batch No.: 20170584, specification: 10 ml: 0.1 g) was infused intravenously at a dose of 250 mg/m2, once a day. Fluorouracil (purchased from Hainan Sinochem United Pharmaceutical Industry Co., Ltd., production batch No.: 20171627, specification: 0.5 g) was infused intravenously at the dose of 300 mg/m2, and each intravenous infusion time should not be less than 8 h.
The study group: Patients in the study group were treated with capecitabine combined with oxaliplatin. Patients received capecitabine (purchased from Shanghai Roche Pharmaceutical Co., Ltd., production batch No.: 20173024, specification: 0.5 g * 12 s) orally, once every 30 min after breakfast and dinner at the dose of 1000 mg/m2. After 2 weeks of treatment, the drug was stopped for one week, with 3 weeks as a course of treatment.
All patients were treated for two consecutive courses.
Immunohistochemistry [18]
The tumor tissues of the gastric cancer patient were taken and placed in a 60 ℃ oven for 15 min. After dewaxing with xylene, hydrating with gradient alcohol, and washing with phosphate buffer, a citric acid repair solution was used for antigen repair. Endogenous peroxidase was used to block hydrogen peroxide. The slices were incubated with FOXP1 primary antibody in a refrigerator at 4℃ overnight. After rewarming at room temperature, horseradish peroxidase-labeled secondary antibody was added and incubated with the slices at room temperature for 60 min. Then the slices were washed with phosphate buffer solution and were administrated with DAB color development and hematoxylin counterstaining. After differentiation with hydrochloric acid and alcohol, the slices were sealed with neutral gum. Yellow, brown yellow, or brown staining in the nucleus, cytoplasm, and cell membrane of gastric cancer tissues was considered as the positive staining. Four high-power fields were randomly selected, among which 0, 1, 2, and 3 points were scored respectively if the percentage of positive cells was ≤ 5%, 6% ~ 25%, 26% ~ 75%, and ≥ 76%. According to the staining intensity of positive cells, no staining, light yellow, yellow, and brown-yellow were recorded as 0, 1, 2, and 3 points respectively. If the sum of the two scores was lower than 1 (including 1), it was regarded as a low expression, otherwise it was a high expression.
Outcome measures
Efficacy analysis
The treatment effect of patients after two courses of treatment was evaluated according to the evaluation criteria for the efficacy of solid tumors [19], including progressive disease, stable disease, partial response, and complete response. Among them, the lesion volume increased by more than 35% or new lesions appeared after treatment was regarded as progressive disease. Partial response was considered when the volume of the lesion decreased by more than 30% after treatment. It is considered as stable disease when it is between progressive disease and partial response. The complete disappearance of the lesion after treatment was regarded as a complete response. The effective rate = (complete response + partial response + stable disease) / total number of cases × 100%. All patients were evaluated after 6 weeks of treatment.
Expression of FOXP1 in gastric cancer tissues and GGT in serum
The expression of FOXP1 in gastric cancer tissues before treatment and after two courses of treatment was detected by immunohistochemistry. Serum GGT level was detected by chemiluminescence. 50 μL of the standard and the sample to be measured were placed in a row of microwells and labeled. An appropriate amount of enzyme markers was added to the standard and the wells of the sample to be measured, fully mixed, and incubated at 37 °C, and then 50 μL of substrate was added to fully mix, incubated for 20 min at room temperature in the dark, and stopped the reaction by adding a stop solution. The absorbance value at 470 nm was detected by a microplate reader. All steps were strictly in accordance with the detection steps of the chemiluminescence analyzer (Beijing Bell Bioengineering Co., Ltd., model: VI-200), and the results were measured at least 3 times to reduce errors.
The expression changes of DNA repair-related proteins (PARP, ATM, etc.) and key molecules of the apoptosis pathway (Bax, Bcl-2) were detected by Western blot
Western blot: Gastric cancer tissue samples were collected from patients with advanced gastric cancer treated with capecitabine combined with oxaliplatin and oxaliplatin alone. The sample was homogenized or cleaved to extract the total protein. A BCA protein quantification kit was used to quantify the extracted proteins to ensure that the protein content of each group of samples was consistent. The quantitative protein samples were subjected to SDS-PAGE electrophoresis to separate proteins of different molecular weights. The proteins on the gel after electrophoresis were transferred to the PVDF membrane. PVDF membranes were sealed with a sealing solution to reduce non-specific binding. PVDF membrane was incubated with specific antibodies (PARP, ATM antibody) to detect the expression of a target protein. After incubation, unbound antibodies were washed away using a washing solution. PVDF membrane was incubated with a secondary antibody matching the primary antibody to enhance the signal. ECL agent was used to develop the PVDF membrane, and the results were photographed using an imaging system. According to the photographic results, the differences in the expression levels of target proteins in the samples of each group were analyzed.
Patients were followed up for 4 years
The survival rate of patients at 1 year and 3 years after treatment was recorded.
The occurrence of adverse reactions during and after treatment was recorded in the two groups
In this study, we classified adverse reactions to chemotherapy drugs into three levels based on the World Health Organization's (WHO) grading criteria: mild, moderate, and severe. Mild adverse reactions refer to patients with mild symptoms that do not affect their daily lives; Moderate adverse reactions refer to patients with obvious symptoms that have a certain impact on daily life, but are tolerable; Severe adverse reactions refer to patients with severe symptoms that are intolerable and require cessation of chemotherapy or special treatment.
Statistical analysis
SPSS 21.0 statistics software package was used to calculate different observation indicators and data. Enumeration data such as gender, curative effect, complications and overall incidence, survival rate, and low expression rate of FOXP1 in gastric cancer tissues were expressed in [cases (%)] and compared using χ2 test or Fisher's exact test. The expression rate of FOXO1 in each group before and after treatment was analyzed by the McNerma paired test. Grading data, such as clinical effects, were compared using the nonparametric Wilcoxon rank test. Serum GGT level and other measurement data were tested for normal distribution, and all of them were in line with normal distribution and expressed in the form of (). Bonferroni correction, repeated measures data, two-way (treatment mode, treatment time) ANOVA, and post-hoc test were used to compare the GGT levels of the two groups before and after treatment. The prognostic factors were analyzed by the COX regression model. The relationship between the influencing factors and the survival time of gastric cancer was analyzed using the Kaplan–Meier survival curve. P < 0.05 was indicated as the statistical significance.
In addition, we performed a statistical efficacy analysis to ensure that the sample size was sufficient to detect meaningful differences between the treatment groups. We found that the sample size in this study had sufficient statistical power to reliably evaluate the effect of capecitabine combined with oxaliplatin in the treatment of advanced gastric cancer by calculating the minimum sample size of each group and comparing it with the actual included sample size.
Results
Analysis of capecitabine enhancing the sensitivity of intermediate and advanced gastric cancer to oxaliplatin
A total of 225 patients with advanced gastric cancer who were continuously diagnosed and treated in our hospital from April 2018 to May 2019 were selected for retrospective analysis of clinical data. 152 cases were finally selected as the study subjects after screening according to inclusion and exclusion criteria. The general information on the front page of the hospitalization medical record was retrieved through the medical record information management system. Among them, there were 83 males and 69 females, aged from 29 to 75 years, with an average age of (50.37 ± 8.32) years. The effective rates of the study group and the control group were 94.74% and 76.32%, respectively. The effective rates in the study group were much higher than those in the control group. Capecitabine significantly enhanced the sensitivity of intermediate and advanced gastric cancer to oxaliplatin (P < 0.05, Table 1).
Table 1.
Analysis of capecitabine enhancing the sensitivity of intermediate to advanced gastric cancer to oxaliplatin [cases (%)]
| Groups | The study group (n = 76) | The control group (n = 76) | Z/Fisher test | P |
|---|---|---|---|---|
| Clinical outcome | 3.859 | < 0.001a | ||
| Complete remission | 8 (10.53) | 2 (2.63) | ||
| Partial remission | 28 (36.84) | 16 (21.05) | ||
| Stability | 36 (47.37) | 40 (52.63) | ||
| Deterioration | 4 (5.26) | 18 (23.68) | ||
| Effective rate | 72 (94.74) | 58 (76.32) | – | 0.003b |
arepresented the difference in the clinical outcome (ranked variables) between the two groups using the Wilcoxon rank-sum test
brepresented the difference in the effective rate (binary variable) with the use of Fisher's exact test
Expression of FOXP1 in gastric cancer tissues and GGT level in serum
The results of immunohistochemical detection showed that FOXP1 was mainly localized in the cytoplasm and nucleus in gastric cancer tissues, with a brown-yellow staining (Fig. 2). Compared with adjacent normal tissues, the expression level of FOXP1 in gastric cancer tissues was decreased (P < 0.05). Before treatment, both the low expression of FOXP1 and the level of GGT had no significant difference between the two groups (P > 0.05). After treatment, the average expression of FOXP1 in gastric cancer tissues of the study group was significantly higher than that of the control group (P < 0.05). After treatment, serum GGT levels were significantly decreased, and the study group was significantly lower than the control group (P < 0.05). There were 40 cases with FOXP1 expression level scores of 0, 46 cases with 1, 32 cases with 2, and 34 cases with 3 (Tables 2 and 3).
Fig. 2.
Detection of FOXP1 expression by immunohistochemistry (× 200). A FOXP1 expression in the control group before treatment; B FOXP1 expression in the study group before treatment; C FOXP1 expression in the control group after treatment; D: FOXP1 expression in the study group after treatment
Table 2.
Expression of FOXP1 in gastric cancer tissues [cases (%)], ()
| Groups | Cases | FOXP1 positive rate (%) | FOXP1 low expression [Cases(%)] | P1 | ||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |||
| The study group | 76 | 10.88 ± 5.89 | 33.05 ± 8.56 | 34 (44.74) | 4 (5.26) | < 0.001a |
| The control group | 76 | 10.92 ± 5.94 | 29.26 ± 7.06 | 32 (42.11) | 16 (21.05) | 0.005b |
| χ2/Fisher test | 0.042 | 2.978 | 0.107 | – | ||
| P2 | 0.967 | 0.003 | 0.743c | 0.020d | ||
aMcNemar test was used to compare the differences before and after treatment in the study group
bMcNemar test was used to compare the differences before and after treatment in the control group
cχ2 test was used to compare the differences between the study group and the control group before treatment
dFisher exact test was used to compare the differences between the study group and the control group after treatment
P1 is the comparison of before and after treatment data for the same group of patients; P2 showed the comparison of data between the study and control groups at the same time point
Table 3.
Expression of GGT in serum, ()
| Groups | Cases | GGT (U/L) | F | P2 | |
|---|---|---|---|---|---|
| Before treatment | After treatment | ||||
| The study group | 76 | 542.79 ± 186.16 | 292.16 ± 91.53ab | F time = 19.149 | P time < 0.001 |
| The control group | 76 | 552.38 ± 205.15 | 421.15 ± 135.76a | ||
| F | F treatment = 9.610 | F time×treatment = 16.552 | P time×treatment < 0.001 | ||
| P1 | P treatment = 0.001 | ||||
aP < 0.05 compared with the same group before treatment
bP < 0.05 compared with the control group after treatment
P1 indicates the comparison between the study group and the control group. P2 represents the comparison between the same group before and after treatment
Comparison of the incidence of adverse reactions between the two groups
There were 35 cases of adverse reactions in the study group, including 18 mild cases, 10 moderate cases, and 7 severe cases. There were 31 cases of adverse reactions in the control group, containing 9 mild cases, 14 moderate cases, and 8 severe cases. There was no difference in the incidence of adverse reactions between the two groups (P > 0.05, Table 4).
Table 4.
Comparison of the incidence of adverse reactions between the two groups [Case (%)]
| Group | The study group (n = 76) | The control group (n = 76) | χ2 | P |
|---|---|---|---|---|
| Complications | ||||
| Nausea and vomiting | 10 (13.16) | 13 (17.11) | ||
| Oral mucositi | 8 (10.53) | 6 (7.89) | ||
| Granulocytopenia | 10 (13.16) | 7 (9.21) | ||
| Peripheral neurotoxicity | 7 (9.21) | 5 (6.58) | ||
| Total incidence | 35 (46.05) | 31 (40.79) | 0.429 | 0.513 |
P indicates the comparison between the study group and the control group
Comparison of PARP, ATM, Bax, and Bcl-2 levels between the two groups after treatment
Capecitabine combined with oxaliplatin down-regulated the expressions of DNA repair related-proteins PARP and ATM, and up-regulated the expressions of Bax and Bcl-2 (the key molecules of the apoptosis pathway), thus enhancing the killing effect of oxaliplatin on gastric cancer cells (P < 0.001, Table 5).
Table 5.
Comparison of PARP, ATM, Bax and Bcl-2 levels between the two groups after treatment
| Group | Cases | PARP | ATM | Bax | Bcl-2 |
|---|---|---|---|---|---|
| Research group | 76 | 2.26 ± 0.89 | 3.65 ± 1.07 | 5.26 ± 2.07 | 6.74 ± 3.33 |
| Control group | 76 | 5.08 ± 1.74 | 7.90 ± 2.19 | 2.66 ± 1.74 | 3.62 ± 2.80 |
| t | 12.579 | 15.201 | 8.382 | 6.252 | |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Analysis of 1- and 3-year survival rate and recurrence rate of patients with intermediate to advanced gastric cancer after treatment
The 1-year survival rate of the study group and the control group was 90.79% and 78.95%, respectively. The 3-year survival rate of the study group and the control group was 75.00% and 51.32%, respectively. The 1-year and 3-year survival rates in the study group were higher than those in the control group (P < 0.05, Table 6).
Table 6.
Analysis of 1- and 3-year survival rate of patients with intermediate to advanced gastric cancer after treatment [cases (%)]
| Group | Cases | Survival rate | |
|---|---|---|---|
| 1 year | 3 years | ||
| The study group | 76 | 69 (90.79) | 57 (75.00) |
| The control group | 76 | 60 (78.95) | 39 (51.32) |
| χ2 (independent-unpaired) | 4.150 | 9.161 | |
| P | 0.042 | 0.002 | |
P indicates the comparison between the study group and the control group
Univariate analysis of prognostic factors in patients with gastric cancer
The 1-year survival rate of 152 patients with gastric cancer was 84.87% (129/152) and the 3-year survival rate was 63.16% (96/152). Age, tumor diameter, tumor-node-metastasis (TNM) stage, lymph node metastasis, chemotherapy regimen and the expression of FOXP1 and GGT had statistically significant effects on the survival rate (P < 0.05, Table 7).
Table 7.
Univariate analysis of prognostic factors in patients with gastric cancer [cases (%)]
| Factors | Cases | 1-year survival rate | χ2 | P | 3-year survival rate | χ2 | P | |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | 83 | 73 (87.95) | 1.354 | 0.245 | 55 (66.27) | 0.759 | 0.384 |
| Female | 69 | 56 (81.16) | 41 (59.42) | |||||
| Age | < 60 years old | 51 | 48 (94.12) | 5.113 | 0.024 | 40 (78.43) | 7.695 | 0.006 |
| ≥ 60 years old | 101 | 81 (80.20) | 56 (55.44) | |||||
| Tumor location | Upper 1/3 | 77 | 68 (88.31) | 1.441 | 0.230 | 48 (62.34) | 0.045 | 0.832 |
| Lower 1/3 | 75 | 61 (81.33) | 48 (64.00) | |||||
| Tumor diameter | < 5 cm | 112 | 105 (93.75) | 26.143 | < 0.001 | 85 (75.89) | 29.664 | < 0.001 |
| ≥ 5 cm | 40 | 24 (60.00) | 11 (27.50) | |||||
| TNM stage | I ~ II | 102 | 98 (96.08) | 30.343 | < 0.001 | 70 (68.63) | 3.987 | 0.046 |
| III ~ IV | 50 | 31 (62.00) | 26 (52.00) | |||||
| Degree of differentiation | Low differentiation | 69 | 58 (94.06) | 0.065 | 0.799 | 48 (62.34) | 2.229 | 0.135 |
| Medium and high differentiation | 83 | 71 (85.54) | 48 (64.00) | |||||
| Lymph node metastasis | N0 ~ N1 | 95 | 90 (94.74) | 19.211 | < 0.001 | 69 (72.63) | 9.771 | 0.002 |
| N2 ~ N3 | 57 | 39 (68.42) | 27 (47.37) | |||||
| Chemotherapy regimen | Oxaliplatin | 76 | 69 (90.79) | 4.150 | 0.042 | 41 (53.95) | 5.542 | 0.019 |
| Capecitabine + oxaliplatin | 76 | 60 (78.95) | 55 (72.37) | |||||
| FOXP1 | Low expression | 66 | 50 (75.76) | 7.540 | 0.006 | 36 (53.03) | 5.142 | 0.023 |
| High expression | 86 | 79 (91.86) | 61 (70.93) | |||||
| GGT | < 387.2 (U/L) | 58 | 55 (94.82) | 7.244 | 0.007 | 47 (81.03) | 12.881 | < 0.001 |
| ≥ 387.2 (U/L) | 94 | 74 (78.72) | 49 (52.13) | |||||
COX regression analysis of prognostic factors in patients with gastric cancer
After using patient survival as the dependent variable and age (1 = ≥ 60 years, 0 = < 60 years), TNM (1 = stage IV, 0 = stage III), lymph node metastasis (1 = N2 ~ N3, 0 = N0 ~ N1), FOXP1 (1 = low expression, 0 = high expression), GGT (1 = ≥ 387.2 U/L, 0 = < 387.2 U/L) as independent variables, we adjusted for factors such as patient gender in the COX regression model to control for potential confounding factors. We found that age (OR = 3.297, 95%CI: 1.367–5.252, P < 0.05), TNM stage (OR = 7.526, 95%CI), and TNM stage (OR = 6.952, 95%CI) for different stages: TNM III stage vs TNM IV stage, OR = 6.952, 95%CI: 1.632–10.336, P < 0.05), lymph node metastasis (OR = 4.297, 95%CI: 1.269 ~ 10.517, P < 0.05; N0N1 vs N2N3, OR = 3.662, 95%CI: 1.026 ~ 5.662, P < 0.05), chemotherapy regimen (combined chemotherapy vs single chemotherapy, OR = 3.964, 95%CI: 1.128 ~ 5.169, P < 0.05), FOXP1 (OR = 7.266, 95%CI: 2.169 ~ 9.259, P < 0.05), GGT (GGT < 387.2 vs GGT ≥ 387.2, OR = 4.697, 95%CI: 1.066 ~ 7.569, P < 0.05) were independent risk factors for the prognosis of patients with gastric cancer (Table 8).
Table 8.
COX regression analysis of prognostic factors in patients with gastric cancer
| Factors | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age ≥ 60 years | 2.046 | 0.436 | 4.045 | 0.014 | 3.297 | 1.367 ~ 5.252 |
| Tumor diameter ≥ 5 cm | 0.860 | 0.357 | 3.092 | 0.074 | 2.593 | 1.136 ~ 3.894 |
| TNM stage of III ~ IV | 2.160 | 0.936 | 4.297 | 0.023 | 7.526 | 3.684 ~ 12.412 |
| lymph node metastasis N2 ~ N3 | 1.988 | 0.126 | 7.236 | < 0.001 | 4.297 | 1269 ~ 10.517 |
| Combined drug chemotherapy | 0.967 | 0.412 | 5.161 | 0.002 | 3.964 | 1128 ~ 5.169 |
| Low expression of FOXP1 | 1.269 | 0.469 | 8.589 | < 0.001 | 7.266 | 2.169 ~ 9.259 |
| GGT ≥ 387.2 | 1.115 | 0.419 | 6.335 | < 0.001 | 4.697 | 1.066 ~ 7.569 |
Kaplan–Meier survival curve was used to analyze the relationship between the influencing factors and the survival time of gastric cancer
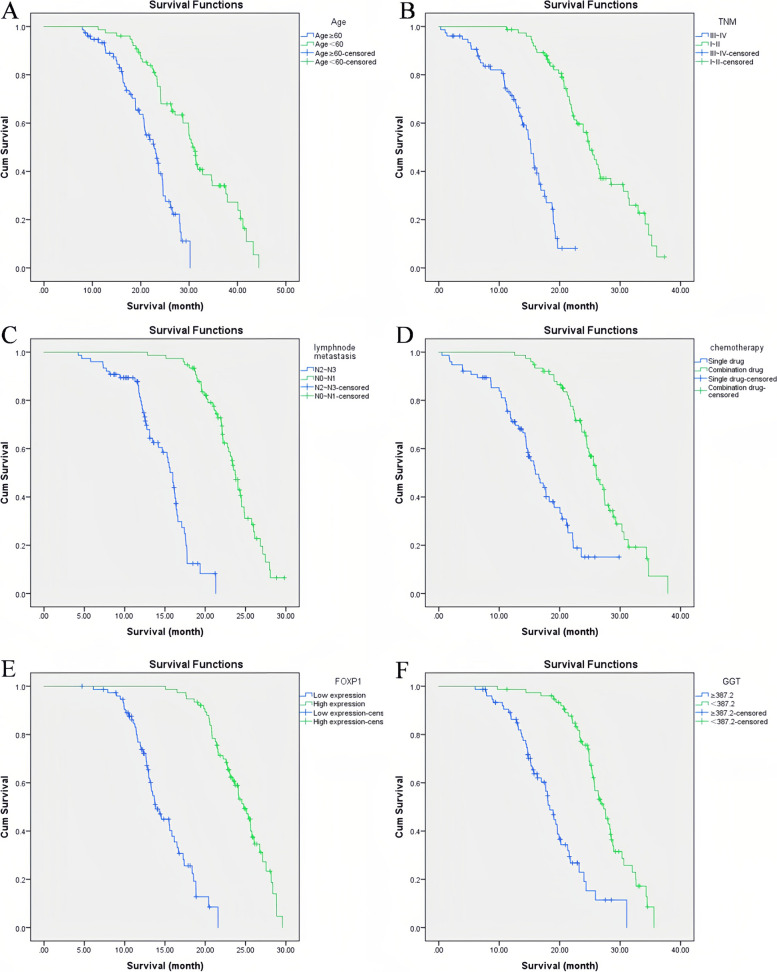
The results of the Kaplan–Meier survival curve analysis showed that the median survival of patients aged ≥ 60 years and < 60 years was 23 months and 33 months, respectively. The survival of patients aged ≥ 60 years was significantly lower than that of patients aged < 60 years. The median survival of patients with TNM stage III ~ IV and TNM I ~ II was 16 months and 27 months, respectively. The survival of patients with TNM stage III ~ IV was notably shorter compared with the patients with stage I ~ II. The median survival of patients with lymph node metastasis N2 ~ N3 and lymph node metastasis N0 ~ N1 was 17 months and 24 months, respectively. The survival of patients with lymph node metastasis N2 ~ N3 was markedly shorter than the patients with N0 ~ N1. The median survival of patients with combined-drug chemotherapy and single-drug chemotherapy was 28 months and 17.5 months, respectively. The survival of gastric cancer patients with single-drug chemotherapy was significantly lower than that of gastric cancer patients with combined-drug chemotherapy. The median survival of patients with low FOXP1 expression and high FOXP1 expression was 14.5 months and 25.5 months, respectively. The survival of patients with low FOXP1 expression was observably lower than that of patients with high FOXP1 expression. The median survival of patients with GGT ≥ 387.2 and GGT < 387.2 was 19.5 months and 29 months, respectively. The survival of patients with GGT ≥ 387.2 was notably shorter than the patients with GGT < 387.2 (P < 0.05, Fig. 3).
Fig. 3.
The relationship between the influencing factors and the survival time of gastric cancer was analyzed using the Kaplan–Meier survival curve. A Survival curves of patients at different ages; B Survival curves of patients at different TNM stages; C Survival curve of patients with different lymph node metastasis; D Survival curve of patients with different chemotherapy regimens; E Survival curves of patients with different FOXP1 expression levels; F Survival curves of patients with different GGT levels
Discussion
Gastric cancer is a malignant tumor that seriously affects the lives of patients. Worldwide, mortality rates from gastric cancer are second only to those from lung cancer. Among the recently increased diagnoses of gastric cancer patients each year, more than half of cases occur in China [20]. Gastric cancer pathogenesis is relatively complex, and there is evidence that it may be the result of the combined actions of multiple factors, including genetic variations, environmental features, and eating habits [21, 22]. As gastric cancer is insidious in its early stages, most patients have mid- or late-stage disease at diagnosis.
Chemotherapy is the main method for the treatment of advanced malignant tumors and has an important role in reducing disease recurrence rates and prolonging patient survival. Oxaliplatin is a platinum-based chemotherapeutic drug, which blocks the replication and transcription of cellular DNA and has a mild myelosuppressive effect [23]. Oxaliplatin combined with cisplatin is effective for the treatment of gastric cancer [24]. Further, folinic acid combined with oxaliplatin can effectively improve the survival rate of patients with intermediate to advanced gastric cancer, and an oxaliplatin-based combination regimen is considered an effective method to treat gastric cancer; however, oxaliplatin often induces resistance to chemotherapeutic drugs, which is an important factor leading to treatment failure [25]. Therefore, it is important to identify methods that improve treatment efficacy, while minimizing adverse reactions. Capecitabine is a fluorouracil carbamate with a fast absorption rate that is selectively activated in tumors. Capecitabine can completely penetrate the liver and forms 5-fluorouracil under catalysis mediated by specific enzymes in the liver, thus exerting relevant anti-tumor effects. Capecitabine is established as effective for the treatment of advanced gastric cancer [26]. Further, in terms of efficacy, toxicity, and convenience, the combination of cisplatin and capecitabine is effective and well-tolerated in the treatment of patients with advanced gastric cancer and may be among standard first-line treatment regimens [27]. Further, chemoresistance is a major problem in the treatment of malignant tumors. In this study, to explore whether capecitabine could enhance sensitivity to oxaliplatin, patients received an oxaliplatin regimen, or capecitabine combined with oxaliplatin, for the treatment of advanced gastric cancer. Compared with the oxaliplatin regimen, capecitabine combined with oxaliplatin had higher clinical efficacy and led to a higher three-year survival rate, indicating that capecitabine can enhance sensitivity to oxaliplatin, reduce disease recurrence rates, and prolong patient survival.
FOXP1 is a transcription factor whose expression levels are greatly reduced in prostate cancer, breast cancer, and other malignant tumors, indicating that FOXP1 may act as a tumor suppressor gene [28]. FOXP1 is reported to have potentially important roles in diffuse large B-cell lymphoma occurrence and development, and high FOXP1 expression may be closely related to poor patient prognosis [29]. Further, FOXP1 expression levels are a potentially important factor affecting the survival of patients with gastric cancer, where those with high FOXP1 expression have higher survival rates than those with low expression [30].
GGT is a molecule located on the extracellular surface of cells and involved in glutathione metabolism. Since glutathione is the main water-soluble antioxidant in cells, GGT is often activated under oxidative stress, which can be induced by alcohol, drugs, or hepatitis-induced liver damage, and is widely used to monitor liver function [31]. GGT has been reported as a potentially important marker of colorectal cancer, esophageal cancer, and other diseases [32], and patients with high-level serum GGT have a much lower risk of morbidity than those with low-level serum GGT [10], which is comparable to the results of the present study. Here, we analyzed FOXP1 levels in gastric cancer tissues and serum GGT levels in the two groups of patients. Our results show that capecitabine combined with oxaliplatin can considerably reduce the proportion of patients with low FOXP1 expression, as well as serum GGT levels. This may be related to the direct killing effect of drugs on gastric cancer cells and the induction of cell apoptosis. Meanwhile, the increased FOXP1 expression level might also enhance the sensitivity of gastric cancer cells to drugs, thus improving the therapeutic effect. Specifically, FOXP1 might regulate drug response in the following ways: Regulation of drug-metabolizing enzymes [33]: FOXP1 might be involved in regulating the expression and activity of drug-metabolizing enzymes, thereby affecting drug metabolism and excretion in gastric cancer cells. By regulating the enzyme that metabolizes drugs, FOXP1 can change the concentration and duration of action of drugs in cells, which in turn affects the efficacy of drugs. Affecting drug targets [34]: FOXP1 might act as a regulator of some drug targets and affect drug binding and signal transduction by changing the expression or activity of the target. This regulatory effect can make gastric cancer cells more sensitive or resistant to specific drugs. Regulation of apoptosis and proliferation: As mentioned above, FOXP1 can induce apoptosis and inhibit cell proliferation. In the treatment of gastric cancer, these effects might cooperate with the action of drugs to jointly promote the death of gastric cancer cells and reduce recurrence. In this study, we found that capecitabine combined with oxaliplatin down-regulated the expression of DNA repair related-proteins such as PARP and ATM, which suggested that this treatment regimen might make gastric cancer cells more vulnerable to the killing effect of oxaliplatin by inhibiting DNA repair process. Cox regression and Kaplan–Meier survival curve analyses both indicated that FOXP1 and serum GGT levels were risk factors affecting the prognosis of patients with gastric cancer and that these two molecules may be effective biological markers of gastric cancer prognosis. The results of this study indicate that FOXP1 and GGT levels are related to the treatment of patients with gastric cancer; our data suggest that capecitabine combined with oxaliplatin can maintain low expression levels of FOXP1 and GGT in patients. Reducing FOXP1 expression can induce cell cycle G1/S phase arrest, resulting in inhibition of gastric cancer cell proliferation [35]. Further, decreasing GGT expression can reduce the redox state in cancer cells, ameliorate inflammation, and thereby inhibit the spread of cancer cells, control cancer development, and achieve improved therapeutic effects [36]. The analysis may be related to the fact that capecitabine is an oral fluorouracil drug that inhibits the growth of tumor cells by suppressing the activity of deoxynucleotide translocation enzymes and blocking DNA synthesis. In the treatment of gastric cancer, capecitabine represses the proliferation of gastric cancer cells by inhibiting DNA replication and RNA synthesis and inducing apoptosis [37]. FOXP1 has the function of regulating cell growth, differentiation, and apoptosis in the occurrence and development of gastric cancer. Elevated expression of GGT may lead to increased glutathione synthesis and secretion, which in turn enhances the viability and drug resistance of gastric cancer cells. Therefore, it is believed that capecitabine may inhibit the growth and spread of gastric cancer cells by down-regulating the expression of FOXP1 and GGT, which provides a theoretical basis for further optimizing the treatment regimen of gastric cancer and is expected to improve the treatment effect and prolong the survival of patients. Based on the tumor-inhibitory properties of FOXP1, it can be speculated that the change in FOXP1 expression might affect the sensitivity of gastric cancer cells to drugs. When FOXP1 expression is reduced, gastric cancer cells might enhance drug resistance through some mechanism or change the drug metabolism pathway, thereby reducing the efficacy of drugs. GGT is mainly involved in the metabolism of glutathione, which is an important intracellular antioxidant and can protect cells from oxidative stress [38]. Capecitabine is converted to 5-fluorouracil in the liver and produces cytotoxic metabolites that may bind to DNA in liver cells and interfere with normal biochemical processes through a series of reactions, thereby triggering liver cell damage [39]. Oxaliplatin blocks DNA replication and transcription through the formation of platinum–DNA adduct, induces apoptosis, and might lead to neurotoxicity and other side effects, among which oxidative stress is one of the important mechanisms of oxaliplatin-induced neurotoxicity, and changes in GGT activity might affect the level of intracellular glutathione, thus affecting the resistance of cells to oxidative stress [40]. Therefore, GGT might theoretically affect the sensitivity of gastric cancer cells to capecitabine or oxaliplatin, but further experimental studies are needed to reveal the specific mechanism of this effect.
In summary, capecitabine can strongly enhance the sensitivity of intermediate and advanced gastric cancer to oxaliplatin, improve treatment efficacy, and reduce the proportions of patients with low FOXP1 expression and serum GGT levels, thereby lowering disease recurrence rates and improving patient prognosis. This study has some limitations. First, it was a single-center retrospective study with a limited sample size, which may have introduced bias in the results. Second, some predictive factors with potential significance, such as blood infiltration and Helicobacter pylori infection, were not included in our analysis because their influence is affected by numerous factors, such as tumor stage, grade, and pathology. The clinical effects of capecitabine combined with oxaliplatin warrant further exploration and confirmation in large-scale prospective clinical trials. In addition, although we analyzed several important factors, the study did not examine other potential factors that might influence drug sensitivity, such as genetic or epigenetic changes, tumor microenvironment factors, or changes in immune response, which are limitations of this manuscript. In future studies, we would incorporate these factors and use multi-omics techniques and immunoassay methods to fully explore their relationship with the efficacy of capecitabine and oxaliplatin combined therapy. We can further optimize gastric cancer treatment strategies, improve treatment outcomes, and extend patient survival by better understanding the impact of these potential factors.
Acknowledgements
None.
Authors’ contributions
Xy G and Y L confirmed the authenticity of all the raw data and edited the manuscript, Xy G collected data and processed the data. Xy G conducted the statistics. Y L participated in the acquisition of data, software and validation. Xy G and Y L reviewed and revised the article. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Funding
None.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This is a retrospective study, all data were analyzed retrospectively. This research was approved by the Ethics Review Committees of Fuwai Central China Cardiovascular Hospital (approval number: 2023-EC-015). Written Informed consent was obtained from participants for the participation in the study and all methods were carried out in accordance with relevant guidelines and regulations. All procedures performed in studies involving human participants were in accordance with the standards upheld with those of the 1964 Helsinki Declaration and its later amendments for ethical research involving human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Huang CM, Zheng CH, et al. Consideration of tumor size improves the accuracy of TNM predictions in patients with gastric cancer after curative gastrectomy. Surg Oncol. 2013;22(3):167–71. [DOI] [PubMed] [Google Scholar]
- 3.Huang ZN, Ma Y, Chen QY, et al. Potential survival benefits of open over laparoscopic radical gastrectomy for gastric cancer patients beyond three years after surgery: result from multicenter in-depth analysis based on propensity matching. Surg Endosc. 2022;36(2):1456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21(5):449–56. [DOI] [PubMed] [Google Scholar]
- 5.Horii N, Kosaka T, Fujiwara R, et al. Psoas muscle depletion during preoperative chemotherapy for advanced gastric cancer has a negative impact on long-term outcomes after gastrectomy. Asia Pac J Clin Oncol. 2021;18(1):61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Wang C, Lan L, et al. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability. Cell Mol Life Sci. 2022;79(3):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka M, Shibuya N, Takagi K, et al. Omentectomy does not affect the postoperative outcome of patients with locally advanced gastric cancer: a systematic review and meta-analysis. J Surg Res. 2021;264(1):287–95. [DOI] [PubMed] [Google Scholar]
- 8.Miao X, Wu H, Liu Y, et al. Clinical efficacy of acupuncture on neoadjuvant chemotherapy with capecitabine plus paclitaxel and radiotherapy in progressive gastric cancer. J Oncol. 2022;2022:6156585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camera S, Deleporte A, Bregni G, et al. MOMENTUM: a phase I trial investigating 2 schedules of capecitabine with aflibercept in patients with gastrointestinal and breast cancer. Clin Colorectal Cancer. 2020;19(4):311-318.e1. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Sakamoto N, Ukai S, et al. Establishment of oxaliplatin-resistant gastric cancer organoids: importance of myoferlin in the acquisition of oxaliplatin resistance. Gastric Cancer. 2021;24(6):1264–77. [DOI] [PubMed] [Google Scholar]
- 11.Gu C, Luo Y, Zhang S, et al. MAb NJ001 inhibits lung adenocarcinoma invasiveness by directly regulating TIMPpromoter activity via FOXP1 binding sites. Thoracic Cancer. 2020;11(9):2630–26380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko Y, Varma S, Zhu CF, et al. Gene expression profiling of head and neck tumors identifies FOXP1 and SOX10 expression as useful for distinguishing ameloblastoma from basaloid salivary gland tumors. Am J Surg Pathol. 2020;44(5):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Y, Li Q, Liao S, et al. Epstein-Barr virus-encoded miR-BART11 promotes tumor-associated macrophage-induced epithelial-mesenchymal transition via targeting FOXP1 in gastric cancer. Virology. 2020;548:6–16. [DOI] [PubMed] [Google Scholar]
- 14.Ozcelik F. Prognostic value of gamma-glutamyl transpeptidase in liver cirrhosis and hepatocellular cancer regardless of other parameters. Gastroenterol Clin Biol. 2021;45(5):101708–101708. [DOI] [PubMed] [Google Scholar]
- 15.Hong SW, Lee HJ, Han K, et al. Risk of gastrointestinal cancer in patients with an elevated level of gamma-glutamyltransferase: a nationwide population-based study. PLoS One. 2021;16(2):e0245052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet. 2020;396(10251):635–48. [DOI] [PubMed] [Google Scholar]
- 17.Kell DB, Pretorius E. To what extent are the terminal stages of sepsis, septic shock, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome actually driven by a prion/amyloid form of fibrin? Semin Thromb Hemost. 2018;44(3):224–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo R, Ma L, Bai X, et al. A scoring method for immunohistochemical staining on Ki67. Appl Immunohistochem Mol Morphol. 2021;29(3):e20–8. [DOI] [PubMed] [Google Scholar]
- 19.Chung HC, Bang YJ, S Fuchs C, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Fut Oncol. 2021;17(5):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata Y, Hatta W, Ohara Y, et al. Predictors of early and late mortality after the treatment for early gastric cancers. Dig Endosc. 2022;34(4):816–25. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZK, Lin JX, Zheng CH, et al. ASO author reflections: Long-term efficacy of splenic hilar lymph node dissection for proximal gastric cancer. Ann Surg Oncol. 2021;28(11):6663–4. [DOI] [PubMed] [Google Scholar]
- 22.Seko-Nitta A, Nagatani Y, Murakami Y, et al. 18F-fluorodeoxyglucose uptake in advanced gastric cancer correlates with histopathological subtypes and volume of tumor stroma. Eur J Radiol. 2021;145(1):110048–110048. [DOI] [PubMed] [Google Scholar]
- 23.Kang L, Tian Y, Xu S, Chen H. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol. 2021;268(9):3269–82. [DOI] [PubMed] [Google Scholar]
- 24.Takahari D, Ohashi M, Takashima A, et al. Feasibility study of TAS-118 plus oxaliplatin as perioperative chemotherapy for patients with locally advanced gastric cancer (APOLLO-11). J Clin Oncol. 2021;39(3_suppl):205–205. [Google Scholar]
- 25.Ykk A, Kc B, Phcc C, et al. S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (SOLAR): a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(8):1045–56. [DOI] [PubMed] [Google Scholar]
- 26.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J, BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–73. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Qian Y, Yin Y. Comparison of S-1-based vs. capecitabine-based adjuvant chemotherapy for patients with gastric cancer: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2021;77(12):1791–804. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Liu C, Ji L, et al. The circACC1/miR-29c-3p/FOXP1 network plays a key role in gastric cancer by regulating cell proliferation. Biochem Biophys Res Commun. 2021;557(1):221–7. [DOI] [PubMed] [Google Scholar]
- 29.Wright WE, Li C, Zheng CX, et al. FOXP1 interacts with MyoD to repress its transcription and myoblast conversion. J Cell Signal. 2021;2(1):9–26. [PMC free article] [PubMed] [Google Scholar]
- 30.Neyroud D, Nosacka RL, Callaway CS, et al. FoxP1 is a transcriptional repressor associated with cancer cachexia that induces skeletal muscle wasting and weakness. J Cachexia Sarcopenia Muscle. 2021;12(2):421–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang CH, Ni XC, Chen BY, Qiu SJ, Zhu YM, Luo M. Combined preoperative albumin-bilirubin (ALBI) and serum γ-glutamyl transpeptidase (GGT) predicts the outcome of hepatocellular carcinoma patients following hepatic resection. J Cancer. 2019;10(20):4836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai T, Deng M, Ye L, et al. Prognostic value of combined preoperative gamma-glutamyl transpeptidase to platelet ratio and fibrinogen in patients with HBV-related hepatocellular carcinoma after hepatectomy. Am J Transl Res. 2020;12(6):2984–97. [PMC free article] [PubMed] [Google Scholar]
- 33.Ling S, Chen T, Wang S, Zhang W, Zhou R, Xia X, Yao Z, Fan Y, Ning S, Liu J, Qin L, Tucker HO, Wang N, Guo X. Deacetylation of FOXP1 by HDAC7 potentiates self-renewal of mesenchymal stem cells. Stem Cell Res Ther. 2023;14(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma H, Sukonina V, Zhang W, Meng F, Subhash S, Palmgren H, Alexandersson I, Han H, Zhou S, Bartesaghi S, Kanduri C, Enerbäck S. The transcription factor Foxp1 regulates aerobic glycolysis in adipocytes and myocytes. J Biol Chem. 2023;299(6):104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Sun J, Cui M, Zhao F, Ge C, Chen T, Yao M, Li J. Downregulation of FOXP1 inhibits cell proliferation in hepatocellular carcinoma by inducing G1/S phase cell cycle arrest. Int J Mol Sci. 2016;17(9):1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu F, Zhu Y, Liu Y, Qiu G, Shang X, Meng T, Yuan H, Hu F. Poly-γ-glutamic acid derived nanopolyplexes for up-regulation of gamma-glutamyl transpeptidase to augment tumor active targeting and enhance synergistic antitumor therapy by regulating intracellular redox homeostasis. Biomater Sci. 2020;8(21):5955–68. [DOI] [PubMed] [Google Scholar]
- 37.Yuan F, Shi H, Ji J, Cai Q, Chen X, Yu Y, Liu B, Zhu Z, Zhang J. Capecitabine metronomic chemotherapy inhibits the proliferation of gastric cancer cells through anti-angiogenesis. Oncol Rep. 2015;33(4):1753–62. [DOI] [PubMed] [Google Scholar]
- 38.Mitrić A, Castellano I. Targeting gamma-glutamyl transpeptidase: a pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic Biol Med. 2023;1(208):672–83. [DOI] [PubMed] [Google Scholar]
- 39.Cura Y, Pérez-Ramírez C, Sánchez-Martín A, Membrive-Jimenez C, Valverde-Merino MI, González-Flores E, Morales AJ. Influence of single-nucleotide polymorphisms on clinical outcomes of capecitabine-based chemotherapy in colorectal cancer patients: a systematic review. Cancers (Basel). 2023;15(6):1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Liu Z, He S, He S, Wang Y. Fighting against drug-resistant tumors by the inhibition of γ-glutamyl transferase with supramolecular platinum prodrug nano-assemblies. J Mater Chem B. 2021;9(22):4587–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.