Abstract
Rationale & Objective
There are likely over 42 million patients with hypertension taking thiazides in the United States and many more worldwide. Hyponatremia is a common complication of thiazide therapy. It is not currently known if thiazide-associated hyponatremia is also associated with increased mortality. The objective of this study was to determine if outpatients who start thiazide diuretic treatment and develop early hyponatremia are at increased risk of mortality when compared with those who do not develop hyponatremia after starting a thiazide.
Study Design
A retrospective cohort study.
Setting & Participants
This study used data from the TriNetX federated health research network comprising deidentified electronic medical records of ∼93 million patients from 76 health care organizations located primarily in the United States. The study population was adult patients 40-90 years old, with essential hypertension and who started on a thiazide diuretic between January 1, 2010, and December 31, 2021. The patients were then subdivided into a hyponatremia cohort and a control cohort. 22,057 patients met the inclusion criteria for the hyponatremia cohort, and 234,466 patients met the inclusion criteria for the control cohort. After propensity score matching, 22,052 remained in both cohorts. The primary outcome is one-year mortality.
Exposure
The hyponatremia cohort developed early hyponatremia defined as a serum sodium ≤ 135 mmol/L within 6 months after initiation of thiazide versus a control that had a serum sodium 136-144 mmol/L after initiation of thiazide.
Outcomes
Primary outcome is mortality. Secondary outcomes include development of sepsis, pneumonia, urinary tract infection, cellulitis, myocardial infarction, stroke, congestive heart failure, ataxia, fall, and hip fracture.
Analytical Approach
The design is a retrospective cohort study, propensity score matched.
Results
Patients in the hyponatremia cohort had a higher hazard of mortality than patients in control, HR 1.96 (95% CI, 1.72-2.28; P < 0.001). In addition, patients in the hyponatremia cohort had higher hazard of developing sepsis, pneumonia, urinary tract infection, cellulitis, myocardial infarction, stroke, congestive heart failure, ataxia, and hip fracture.
Limitations
The study had a retrospective design.
Conclusions
Patients who develop early hyponatremia (serum sodium ≤ 135 mmol/L) following initiation of a thiazide diuretic have a higher risk of mortality when compared with those who do not develop hyponatremia after initiation of a thiazide diuretic.
Index Words: Hyponatremia, thlazide diuretics, mortality, sodium
There are ∼120 million patients with hypertension in the United States.1 It is estimated that 35.6% of patients with hypertension take a thiazide diuretic as part of an antihypertensive pharmacological regimen.2 Therefore, there are likely over 42 million patients taking thiazides in the United States and many more worldwide. Hyponatremia is a common complication of thiazide therapy because thiazide diuretics impair the ability of the kidney to dilute the urine but not the ability to concentrate the urine.3, 4, 5, 6 Thiazide diuretics have been found to lead to sudden complications, such as hyponatremic encephalopathy, which is associated with severe neurological manifestations and possibly death.6, 7, 8 However, thiazide diuretics can also cause chronic, less symptomatic hyponatremia.3 A recent report shows that hyponatremia associated with thiazide diuretic use is more common than once thought, with the cumulative incidence of serum sodium of < 130 mmol/L up to 4% over a 2-year period in patients taking thiazides.9
Extensive literature over the past 2 decades has shown that hyponatremia of mild degrees (serum sodium ≤ 135 mmol/L) has been shown to be associated with increased mortality risk in a variety of clinical settings, including inpatient and outpatient cohorts and in the presence of medical comorbid conditions such as cardiac disease, liver disease, and chronic kidney disease and in community-based cohorts representative of the general population.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Chronic hyponatremia has also been found to be associated with increased risk of infections, cardiovascular disease, and a risk factor for hip fracture.12,14,27, 28, 29, 30 What is not clearly understood is whether mild chronic hyponatremia per se increases mortality risk or if it is the presence of comorbid conditions or other potentially unmeasured covariates that explains the association of mild hyponatremia with poor outcomes. It is generally presumed that mild chronic hyponatremia related to the use of thiazide diuretics is safe. However, the recognition that decreases of the serum sodium of even a modest degree (≤ 135 mmol/L) in observational cohort studies are associated with increased mortality calls into question the safety of mild, thiazide-associated hyponatremia.
Currently, there are no studies in the literature that address whether or not thiazide-associated hyponatremia (≤ 135 mmol/L) is linked to increased mortality when compared with patients on thiazides who do not become hyponatremic. In this study, a retrospective cohort analysis was performed to test the hypothesis that thiazide-induced hyponatremia is associated with increased mortality risk and infectious and cardiovascular outcomes.
Methods
Setting
To select cohorts of patients that meet the inclusion criteria for this study, we used data from the TriNetX federated health research network. TriNetX is an analytics platform for a large network of health care institutions that allow their electronic health record data to be used for research purposes to other members of the network. Currently, TriNetX contains deidentified electronic medical records of ∼93 million patients from 76 health care organizations located primarily in the United States. The data include demographics, diagnoses, laboratory values, medications, procedures, and outcomes, among other clinical variables searchable through standardized terminologies on TriNetX’s platform. We used search functions of TriNetX and the criteria listed in the paragraph below to define a cohort of patients that developed thiazide-associated hyponatremia and a control cohort. Analyses were performed on the TriNetX cloud-based analytics platform.
Cohorts
The cohort selection scheme is presented in Fig 1. The inclusion criteria are as follows: patients aged 40-90 years old, diagnosed with essential hypertension (ICD-10CM code I10) with thiazide diuretic start between January 1, 2010, and December 31, 2021, and a normal serum sodium value (136-144 mmol/L) measured within 6 months of thiazide initiation. Thiazide diuretic start was defined as evidence of thiazide diuretic use appearing in the electronic medical record preceded by a period of no less than 2 years with no thiazide diuretic use. In addition, patients must have had at least one outpatient serum sodium measurement between 100 mmol/L and 180 mmol/L during the qualifying period (more than 5 days after and within 6 months of thiazide start). To be included in the hyponatremia cohort, there must be at least one serum sodium ≤ 135 mmol/L during the qualifying period (Table S1). To be included in the control cohort, there must be no serum sodium measurements of ≤ 135 mmol/L during the qualifying period and at least one outpatient serum sodium of 136-144 mmol/L during the qualifying period. The index time for survival analysis was the date of the serum sodium value taken during the qualifying period, which could occur within 5 days and 6 months of thiazide initiation. In the hyponatremia cohort, the index date was the date of the first serum sodium of ≤ 135 mmol/L during the qualifying period. The index date in the comparison cohort, was the date of the last serum sodium taken during the qualifying period (recall that no serum sodiums of ≤ 135 mmol/L occurred during the qualifying period in the comparison cohort).
Figure 1.
Cohort selection scheme.
Patients were excluded if they had one or more of the following comorbid conditions that are associated with the development of hyponatremia: malignant neoplasms of ill-defined, other secondary and unspecified sites (ICD-10CM codes C76-C80), malignant neoplasms of digestive organs (ICD-10CM codes C15-C26), malignant neoplasms of lymphoid, hematopoietic and related tissue (ICD-10CM codes C81-C96), malignant neoplasms of respiratory and intra-thoracic organs (ICD-10CM codes C30-C39), heart failure (ICD-10CM codes I50), other pulmonary heart diseases (ICD-10CM codes I27), acute kidney failure and chronic kidney disease (ICD-10CM codes N17-N19), fibrosis and cirrhosis of the liver (ICD-10CM codes K74), other chronic obstructive pulmonary disease (ICD-10CM codes J44), within the 2 years before the thiazide start. Patients were also excluded if they had a previous diagnosis of hyponatremia (ICD-10CM code E87.1), if they had a serum sodium of ≤ 135 mmol/L within the 2 years before the thiazide start, or if they were hospitalized during the qualifying period (Fig 1).
Patients in the hyponatremia cohort were matched 1 to 1 with controls using propensity score matching based on the following baseline factors: age at index event, gender, race, ethnicity, medical comorbid conditions (presence or absence of the following conditions): ischemic heart disease, diabetes mellitus, hyperlipidemia, antihypertensive medication use (loop diuretics, potassium sparing diuretics, angiotensin converting enzyme inhibitors, β blockers, calcium channel blockers, angiotensin II inhibitors, α blockers, and other antihypertensives), serum sodium, serum creatinine, hemoglobin, albumin, total cholesterol, low density lipoprotein cholesterol, hemoglobin A1C, serum glucose, body mass index, systolic blood pressure, and diastolic blood pressure (Table 1). Propensity scores were calculated using logistic regression analysis, and 1:1 matching was performed using greedy nearest neighbor algorithms with a caliper width of 0.1 pooled standard deviations.
Table 1.
Hyponatremic (N = 22,052) and Control (N = 22,052) Cohort Characteristics After Propensity Score Matching at Time of Index Event
| Demographics | ||||||
|---|---|---|---|---|---|---|
| Mean ± SD |
N (% of Cohort) |
P | Std diff. | |||
| Hyponatremic | Control | Hyponatremic | Control | |||
| Age at Index | 62.9 ± 13.2 | 62.8 ± 12.7 | 22,052 (100) | 22,052 (100) | 0.329 | 0.009 |
| Black or African American | 3,186 (14.4) | 3,230 (14.6) | 0.552 | 0.006 | ||
| White | 15,153 (68.7) | 15,089 (68.4) | 0.512 | 0.006 | ||
| Female | 11,778 (53.4) | 11,848 (53.7) | 0.504 | 0.006 | ||
| Male | 9,505 (43.1) | 9,423 (42.7) | 0.430 | 0.008 | ||
| Hispanic or Latino | 1,184 (5.4) | 1,188 (5.4) | 0.933 | 0.001 | ||
| Not Hispanic or Latino | 15,243 (69.1) | 15,228 (69.1) | 0.877 | 0.001 | ||
| Asian | 640 (2.9) | 694 (3.1) | 0.133 | 0.014 | ||
| Comorbid condition status | ||||||
| Hyponatremic | Control | |||||
| Essential (primary) hypertension | 22,052 (100) | 22,052 (100) | -- | -- | ||
| Ischemic heart disease | 3,235 (14.7) | 3,232 (14.7) | 0.968 | <0.001 | ||
| Diabetes mellitus | 7,081 (32.1) | 7,076 (32.1) | 0.959 | <0.001 | ||
| Hyperlipidemia | 12,824 (58.2) | 12,930 (58.6) | 0.306 | 0.010 | ||
| Medications | ||||||
| Hyponatremic | Control | |||||
| Thiazides/related diuretics | 22,052 (100) | 22,052 (100) | -- | -- | ||
| Loop diuretics | 2,356 (10.7) | 2,358 (10.7) | 0.975 | <0.001 | ||
| Potassium sparing/combination diuretics | 2,090 (9.5) | 2,103 (9.5) | 0.833 | 0.002 | ||
| ACE inhibitors | 10,643 (48.3) | 10,678 (48.4) | 0.739 | 0.003 | ||
| β Blockers/related | 9,170 (41.6) | 9,201 (41.7) | 0.765 | 0.003 | ||
| Calcium channel blockers | 7,902 (35.8) | 7,864 (35.7) | 0.706 | 0.004 | ||
| Angiotensin II inhibitors | 6,397 (29.0) | 6,361 (28.8) | 0.705 | 0.004 | ||
| α Blockers/related | 1,512 (6.9) | 1,497 (6.8) | 0.777 | 0.003 | ||
| Other antihypertensives | 3,803 (17.2) | 3,796 (17.2) | 0.930 | 0.001 | ||
| Antidepressants | 5,702 (25.9) | 5,651 (25.6) | 0.579 | 0.005 | ||
| Anticonvulsants | 3,641 (16.5) | 3,657 (16.6) | 0.838 | 0.002 | ||
| Laboratory | ||||||
| Hyponatremic | Control | Hyponatremic | Control | |||
| Sodium (mmol/L) | 139.2 ± 2.1 | 139.2 ± 2.0 | 22,053 (100) | 22,053 (100) | <0.001 | 0.191 |
| < 140 (mmol/L) | 17,198 (78.0) | 17,364 (78.7) | 0.055 | 0.018 | ||
| ≥ 140 (mmol/L) | 10,832 (49.1) | 10,798 (49.0) | 0.746 | 0.003 | ||
| Creatinine (mg/dL) | 1.0 ± 0.5 | 0.9 ± 0.3 | 21,788 (98.8) | 21,832 (99.0) | <0.001 | 0.060 |
| <0.90 mg/dL | 14,701 (66.7) | 14,717 (66.7) | 0.872 | 0.002 | ||
| ≥0.90 mg/dL | 14,636 (66.4) | 14,635 (66.4) | 0.992 | <0.001 | ||
| Hemoglobin (g/dL) | 13.0 ± 2.2 | 13.3 ± 1.8 | 18,847 (85.5) | 18,857 (85.5) | <0 .001 | 0.143 |
| Hemoglobin < 13.50 g/dL | 11,552 (52.4) | 11,525 (52.3) | 0.797 | 0.002 | ||
| Hemoglobin ≥ 13.50 g/dL | 11,876 (53.9) | 11,868 (53.8) | 0.939 | 0.001 | ||
| Albumin (g/dL) | 4.0 ± 0.6 | 4.1 ± 0.5 | 17,634 (80.0) | 17,644 (80.0) | <0 .001 | 0.096 |
| Albumin < 4 g/dL | 9,787 (44.4) | 9,738 (44.2) | 0.639 | 0.004 | ||
| Albumin ≥ 4 g/dL | 13,321 (60.4) | 13,183 (59.8) | 0.180 | 0.013 | ||
| Total cholesterol (mg/dL) | 184.0 ± 45.9 | 182.4 ± 44.2 | 15,880 (72.0) | 15,885 (72.0) | 0.002 | 0.035 |
| Total cholesterol < 200 mg/dL | 11,284 (51.2) | 11,248 (51.0) | 0.732 | 0.003 | ||
| Total Cholesterol ≥ 200 mg/dL | 6,643 (30.1) | 6,641 (30.1) | 0.983 | <0.001 | ||
| Cholesterol in LDL (mg/dL) | 103.3 ± 37.6 | 103.8 ± 37.1 | 15,638 (70.9) | 15,712 (71.2) | 0.181 | 0.015 |
| Cholesterol in LDL < 130 mg/dL | 12,717 (57.7) | 12,671 (57.5) | 0.658 | 0.004 | ||
| Cholesterol in LDL ≥ 130 mg/dL | 4,616 (20.9) | 4,621 (21.0) | 0.953 | 0.001 | ||
| Hemoglobin A1c (%) | 6.9 ± 1.9 | 6.7 ± 1.5 | 11,315 (51.3) | 11,076 (50.2) | <0.001 | 0.120 |
| Hemoglobin A1c < 7 % | 8,484 (38.5) | 8,484 (38.5) | 1 | <0.001 | ||
| Hemoglobin A1c ≥ 7 % | 4,275 (19.4) | 4,269 (19.4) | 0.942 | 0.001 | ||
| Glucose (mg/dL) | 135.2 ± 73.7 | 118.9 ± 41.5 | 21,699 (98.4) | 21,676 (98.3) | <0.001 | 0.272 |
| < 125 mg/dL | 18,700 (84.8) | 18,756 (85.1) | 0.456 | 0.007 | ||
| ≥ 125 mg/dL | 11,534 (52.3) | 11,528 (52.3) | 0.954 | 0.001 | ||
| Body mass index (kg/m2) | 30.7 ± 6.7 | 31.3 ± 6.6 | 8,169 (37.0) | 8,097 (36.7) | <0.001 | 0.088 |
| < 30 kg/m2 | 4,465 (20.2) | 4,476 (20.3) | 0.896 | 0.001 | ||
| ≥ 30 kg/m2 | 4,532 (20.6) | 4,580 (20.8) | 0.572 | 0.005 | ||
| Blood pressure, systolic (mm Hg) | 133.2 ± 21.1 | 135.4 ± 19.8 | 15,129 (68.6) | 15,279 (69.3) | <0.001 | 0.108 |
| < 140 (mm Hg) | 13,026 (59.1) | 13,041 (59.1) | 0.884 | 0.001 | ||
| ≥ 140 (mm Hg) | 12,853 (58.3) | 12,913 (58.6) | 0.562 | 0.006 | ||
| Blood pressure, diastolic (mm Hg) | 76.3 ± 13.6 | 77.8 ± 12.8 | 15,169 (68.8) | 15,304 (69.4) | <0.001 | 0.110 |
| < 80 (mm Hg) | 12,078 (54.8) | 12,080 (54.8) | 0.985 | <0.001 | ||
| ≥ 80 (mm Hg] | 12,957 (58.8) | 12,981 (58.9) | 0.816 | 0.002 | ||
Outcome
The primary outcome is overall survival. Secondary outcomes are the development of sepsis (ICD-10 CM code A41.9), pneumonia (ICD-10 CM code J18), urinary tract infection (ICD-10 CM code N39.0), cellulitis (ICD-10 CM code L03), myocardial infarction (ICD-10 CM code I21), stroke (ICD-10 CM code I63), congestive heart failure (ICD-10 CM code I50), ataxia (ICD-10 CM code R27), fall (ICD-10 CM code W19), and hip fracture (ICD-10 CM code S72.009A). The study horizon was 1 day to 1 year after the index event.
Statistical Analyses
At baseline, differences in demographics and laboratory markers between the hyponatremia cohort and control cohorts were evaluated after propensity score matching with t test for the continuous variables (ie, age, hemoglobin, and etc.) and χ2 test for dichotomous variables (eg, ethnicity, gender, and etc.). For continuous variables, we report mean and standard deviation. For dichotomous variables, we report patient counts. The time-to-event outcome was evaluated using the Kaplan-Meier method and summarized as hazard ratio along with 95% CIs. The difference in survival across compared groups for time-to-event outcomes was assessed using the Log rank test. The P-value of < .05 was considered statistically significant.
All analyses were performed on the TriNetX’s cloud-based data analytics platform. These data were deidentified per the deidentification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. This research has been determined to not constitute human subject research as defined by the United States Department of Health and Human Services and FDA regulations and has been determined to not require approval by the institutional review board of the University of South Florida.
Results
Baseline Characteristics
We identified 22,057 patients who met the inclusion criteria for the hyponatremia cohort and 234,466 patients who met the inclusion criteria for the control cohort (Fig 1). After propensity score matching, 22,052 patients remained in each cohort. The 22,057 with sodium ≤ 135 out of the total patient population of 256,523 yields an incidence rate of 8.6% of hyponatremia within 6 months of thiazide initiation. Table 1 shows demographic and laboratory information for both cohorts after propensity score matching. There were no statistically significant differences in age at index, gender, ethnicity, and race. There were no statistically significant differences in rates of ischemic heart disease, diabetes mellitus, and hyperlipidemia. There were no statistically significant differences in rates of diuretic, calcium channel blockers, angiotensin converting enzyme inhibitors, α blockers, β blockers, angiotensin II blockers, other antihypertensives, antidepressants, or anticonvulsants (Table 1).
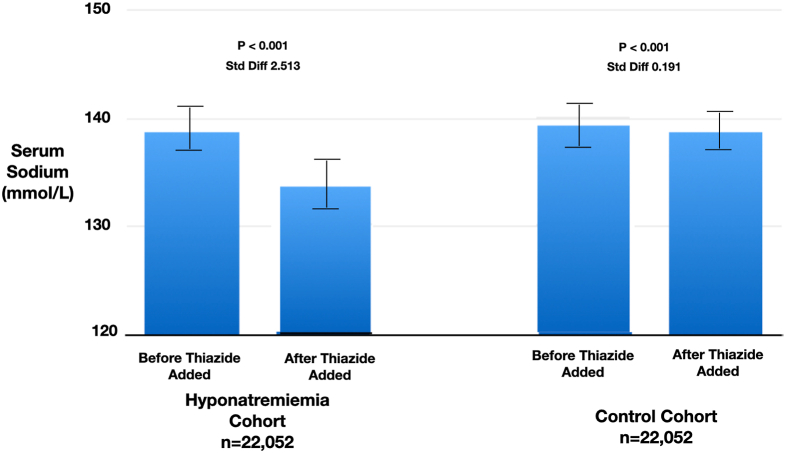
Within the first 6 months after initiation of thiazide diuretic, patients in the hyponatremia cohort had serum sodium levels of 133.8 ± 2.2 (SD) mmol/L, and the patients in the control cohort had 138.7 ± 1.9 (SD) mmol/L (P < 0.001) (Fig 2).
Figure 2.
Baseline outpatient serum sodium values before and after thiazide initiation.
Outcomes
Over the course of the study, in the hyponatremia cohort there were 657 deaths out of 22,052 patients, and in the control cohort there were 344 deaths out of 22,052 patients. The Kaplan-Meier survival curves comparing the survival probabilities between the cohorts are shown in (Fig 3; Table 2). Patients in the hyponatremia cohort had a higher hazard of mortality than patients in controls, HR 1.96 (95% CI, 1.72-2.28; P < 0.001) at any time during the study period. Secondary outcomes are presented in (Fig 4; Table 2). The hyponatremia cohort had a higher hazard of sepsis than patients in controls, HR 1.95 (95% CI, 1.61-2.37; P < 0.001) 298 events versus 156 events; pneumonia HR 1.35 (95% CI, 1.19-1.53; P < 0.001) 561 events versus 427 events; urinary tract infection HR 1.20 (95% CI, 1.11-1.31; P < 0.001) 1,193 events versus 1,018 events; cellulitis HR 1.20 (95% CI, 1.08-1.34; P < 0.001) 728 events versus 620 events; myocardial infarction HR 1.29 (95% CI, 1.11-1.50; P < 0.001) 393 events versus 311 events; stroke HR 1.21 (95% CI, 1.09-1.33; P < 0.001) 871 events versus 736 events; new-onset congestive heart failure HR 1.26 (95% CI, 1.13-1.40; P < 0.001) 712 events versus 580 events; ataxia HR 1.41 HR (95% CI,1.15-1.74; P < 0.001) 216 events versus 156 events; and hip fracture HR 1.95 (95% CI, 1.16-3.26; P < 0.001) 42 events versus 22 events (Fig 4; Table 2). Patients in the hyponatremia cohort did not have a higher hazard of falling than in controls, HR 1.05 (95% CI, 0.91-1.20; P = 0.199) 387 events versus 379 events (Fig 4; Table 2).
Figure 3.
Patient survival.
Table 2.
Primary and Secondary Outcomes
| Events in hyponatremia cohort (control) | Hazard ratio (95% CI) | P | |
|---|---|---|---|
| Primary outcome | |||
| Mortality | 657 (344) | 1.96 (1.72-2.28) | <0.001 |
| Secondary outcome | |||
| Sepsis | 298 (156) | 1.95 (1.61-2.37) | <0.001 |
| Pneumonia | 561 (427) | 1.35 (1.19-1.53) | <0.001 |
| Urinary tract infection | 1,193 (1,018) | 1.20 (1.11-1.31) | <0.001 |
| Cellulitis | 728 (620) | 1.20 (1.08-1.34) | <0.001 |
| Myocardial infarction | 393 (311) | 1.29 (1.11-1.50) | <0.001 |
| Stroke | 871 (736) | 1.21 (1.09-1.33) | <0.001 |
| New-onset congestive heart failure | 712 (580) | 1.26 (1.13-1.40) | <0.001 |
| Ataxia | 216 (156) | 1.41 (1.15-1.74) | <0.001 |
| Hip fracture | 42 (22) | 1.95 (1.16-3.26) | <0.001 |
| Fall | 387 (379) | 1.05 (0.91-1.20) | 0.199 |
Figure 4.
Secondary outcomes.
Discussion
This study demonstrates that thiazide-induced hyponatremia is associated with an increased risk of 1 year mortality and an increase in the risk of infection, cardiovascular events, and hip fracture relative to those taking thiazides who do not develop hyponatremia. Thiazide diuretics are commonly prescribed antihypertensive medications. Although the safety profile of thiazides is generally favorable, hyponatremia is a common complication and occurs at rates higher than once previously thought [9]. The incidence of the thiazide-related hyponatremia (≤ 135 mmol/L) hyponatremia as defined in this study is 8.6%; with an estimated 42 million thiazide users in the United States, the number of patients with thiazide-related hyponatremia is likely in excess of 3.6 million in the United States, with many times that number worldwide.
There is evidence over the past 2 decades from observational studies showing that mild hyponatremia is associated with increased mortality.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Whether the increased risk of mortality noted with mild hyponatremia seen in these observational studies signified that thiazide-induced hyponatremia would also lead to increased mortality had not been known. This study provides the first evidence that we are aware of that thiazide-associated hyponatremia is associated with increased risk of mortality relative to those taking thiazides who do not develop hyponatremia.
Mild hyponatremia has previously been associated with disparate clinical outcomes, such as osteoporosis,31 hip fracture,29 and cardiovascular events.12 The secondary outcomes in this study provide some insight into why patients with thiazide-associated hyponatremia are at increased mortality risk, with the largest contributor of excess risk appears to be increases in infection. Both sepsis, pneumonia, and urinary tract infection saw increased risk in the hyponatremia group, especially sepsis (Fig 4). There have been previous reports linking hospitalization with hyponatremia and infectious risk.28,32 and increased risk of infection-related hospitalization.27 Bench research studies have also suggested that extracellular sodium concentration modulates TH-17 helper cell activation.33, 34, 35 Our results would seem to be in line with this previous literature on hyponatremia and infection risk.
Another contributor to excess mortality risk is the increase in cardiovascular events, both myocardial infarction and congestive heart failure (Fig 4). Studies in rats have shown that chronic hyponatremia leads to oxidative stress and activates intracellular signaling pathways in osteoclasts that lead to resorptive bone loss,36 and cardiomyopathy characterized by increased cardiac weight and perivascular and interstitial fibrosis.37 Finally, as has been seen in previous studies, 29 risk of hip fracture was increased in the hyponatremia cohort in our study (Fig 4). In summary, the results in the current article fit with previous literature linking hyponatremia with infection risk, cardiovascular disease, and osteoporosis.
There is also a previous study with relevant findings detailing the relationship between copeptin levels (which is a precursor peptide to vasopressin, the levels of which have been shown to mirror serum vasopressin levels) and mortality. In a prospective study of patients admitted to an acute care hospital with either normal serum sodium or hyponatremia (sodium ≤ 135 mmol/L), both copeptin levels and hyponatremia predicted mortality independently, with the association of hyponatremia persisting after adjustment for copeptin level.38 The implication of this result is that hyponatremia per se, not just serum vasopressin level predict mortality, and therefore hyponatremia, independent of the mechanism of how hyponatremia develop, predicts mortality. The findings of this study would seem to support this concept. Although copeptin (or vasopressin) levels are not known in our study, we excluded patients with pre-existing conditions predisposing to hyponatremia. Whether the higher levels of morbidity and mortality in the hyponatremia cohort are due to imbalances in higher risk patient characteristics that persisted after propensity score matching, or if thiazide-induced hyponatremia is indeed causal, it is still difficult to say. Very likely there is a combination of both at play. Those at risk for hyponatremia after a thiazide is initiated may in fact be intrinsically higher risk patients than those that do not. This however, does not rule out that mild hyponatremia after thiazide initiation is itself a causal factor. We suspect that it is likely a combination of these 2 and a cautious approach would be to take steps to mitigate thiazide-associated hyponatremia even when mild and to consider stopping the medication if these efforts fail (especially in those at risk for sepsis).
It is generally advised that patients be cautioned about excessive liquid drinking while taking a thiazide. Beyond this, however, there is little guidance about strategies for management of thiazide-associated hyponatremia, and there is an incomplete understanding of what degree of thiazide-associated hyponatremia is acceptable. It is common practice to continue to prescribe thiazide diuretics despite the development of mild chronic hyponatremia, and the results of this study challenge the safety of this practice. Furthermore, our results highlight the importance of closely following the serum electrolytes of patients on thiazide diuretics and counseling patients about the need to restrict fluid intake while taking thiazides. It is worth mentioning that patients who initiate a thiazide can have a dipsogenic response and they may be prone to hyponatremia and this can often manifest early on in the course of thiazide diuretic therapy.4 A simple and useful tactic that can be employed to identify patients at elevated risk of thiazide-associated hyponatremia is to ask patients to weigh themselves (or have them return to the clinic) 48 hours after the start of a thiazide diuretic. Because it is expected that a patient will lose a small amount of weight within 48 hours of initiating a thiazide diuretic, those in whom the weight is unchanged or increased will be at elevated risk of hyponatremia. This group of patients then may need counseling about fluid intake and close monitoring of the serum electrolytes or consideration of a different agent. Given that this study has shown that even modest degrees of thiazide-associated hyponatremia will increase mortality risk, we argue that this type of monitoring practice be recommend for all patients who initiate a thiazide diuretic.
Another key point is that the design of this study allowed the assembly of a large number of patients without pre-existing conditions that are typically associated with hyponatremia, such as cancer, congestive heart failure, acute and chronic kidney disease, and chronic lung disease as these were excluded from the analysis. This study does, however, have some important limitations that need mentioning. The first is the retrospective design and observational nature of the study. Patients with medical comorbid conditions were excluded from the analysis, so these results may not be generalizable to the general hypertension population. Although the design of our study allowed the selection of a control cohort that is closely matched in medical comorbid condition status to the hyponatremia cohort (Table 1) we cannot completely rule out that unmeasured confounders explain the differences in outcomes between the 2 groups. Also, patients who did not have sodium values within the first 6 months of thiazide initiation were not included in this study, this may have biased our results in an unpredictable way. Also, because of the design of this study, patients in the control group may have had a longer exposure to thiazide medication use as patients with hyponatremia may have entered follow-up closer in time to thiazide initiation than the control. Finally, it is important to note that this study is not a study of the overall safety of thiazide diuretic use and should not be construed as suggesting that alternative antihypertensive regimens are superior to thiazides. Our results leave some important questions that were beyond the scope of this study unanswered and provide future research questions. Are results different between the various thiazide and thiazide-like diuretics (eg, chlorthalidone, indapamide, or metolazone)? Is more severe thiazide-related hyponatremia associated with worse outcomes than with mild thiazide-related hyponatremia?
In summary, this study shows that the development of hyponatremia on initiation of a thiazide diuretic is linked to increased mortality compared with maintenance of a normal serum sodium level on initiation of a thiazide diuretic. It is likely that 3.6 million patients taking thiazide diuretics in the United States may be at elevated mortality risk. This increase in mortality risk is accompanied by an increase in risk of sepsis, infections, cardiovascular outcomes and ataxia, and hip fracture. These results also provide important information for physicians who prescribe thiazide diuretics when making decisions about the safest and most efficacious antihypertensive medication regimens for individual patients. Finally, the results also argue for increased efforts at educating patients about fluid intake during thiazide diuretic therapy and for steps to be taken to identify patients at risk very early on through monitoring of the body weight and to closely monitor the serum electrolytes in patients on thiazides diuretic and to intervene early when patients with a serum sodium of ≤ 135 mmol/L are identified.
Article Information
Authors’ Full Names and Academic Degrees
Steven G. Achinger, MD, Juan Carlos Ayus, MD, Ambuj Kumar, MD, MPH, and Athanasios Tsalatsanis, PhD
Authors’ Contributions
SGA: concept, hypothesis, study design, and data interpretation. JCA: data interpretation. AK: study design, analysis, and data interpretation. AT: study design, analysis, and data interpretation. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Data Sharing
This study was performed on the TriNetX research platform and all data are stored by TriNetX and is available on their platform.
Peer Review
Received May 28, 2024. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form October 29, 2024.
Footnotes
Complete author and article information provided before references.
Table S1: Hyponatremic (N = 22,057) and Control (N = 234,466) Cohort Characteristics Before Propensity Score Matching.
Supplementary Materials
Table S1.
References
- 1.Centers for Disease Control and Prevention; Atlanta G Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2017–2020. https://www.cdc.gov/nchs/products/databriefs/db364.htm
- 2.Singh H., Johnson M.L. Prescribing patterns of diuretics in multi-drug antihypertensive regimens. J Clin Hypertens (Greenwich) 2005;7(2):81–87. doi: 10.1111/j.1524-6175.2005.03922.x. quiz 88-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayus J.C., Arieff A.I. Abnormalities of water metabolism in the elderly. Sem Nephrol. 1996;16(4):277–288. [PubMed] [Google Scholar]
- 4.Ayus J.C., Arieff A.I. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281(24):2299–2304. doi: 10.1001/jama.281.24.2299. [DOI] [PubMed] [Google Scholar]
- 5.Achinger S.G., Moritz M.L., Ayus J.C. Dysnatremias: why are patients still dying? South Med J. 2006;99(4):353–362. doi: 10.1097/01.smj.0000209351.55330.76. [DOI] [PubMed] [Google Scholar]
- 6.Ayus J.C., Achinger S.G., Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Ren Physiol. 2008;295(3):F619–F624. doi: 10.1152/ajprenal.00502.2007. [DOI] [PubMed] [Google Scholar]
- 7.Ayus J.C., Krothapalli R.K., Arieff A.I. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190–1195. doi: 10.1056/NEJM198711053171905. [DOI] [PubMed] [Google Scholar]
- 8.Achinger S.G., Ayus J.C. Treatment of hyponatremic encephalopathy in the critically ill. Crit Care Med. 2017;45(10):1762–1771. doi: 10.1097/CCM.0000000000002595. [DOI] [PubMed] [Google Scholar]
- 9.Andersson N.W., Wohlfahrt J., Feenstra B., Hviid A., Melbye M., Lund M. Cumulative incidence of thiazide-induced hyponatremia: a population-based cohort study. Ann Intern Med. 2024;177(1):1–11. doi: 10.7326/M23-1989. [DOI] [PubMed] [Google Scholar]
- 10.Heuman D.M., Abou-Assi S.G., Habib A., et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatol. 2004;40(4):802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 11.Gill G., Huda B., Boyd A., et al. Characteristics and mortality of severe hyponatraemia—a hospital-based study. Clin Endocrinol (Oxf) 2006;65(2):246–249. doi: 10.1111/j.1365-2265.2006.02583.x. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg A., Hammerman H., Petcherski S., et al. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch Intern Med. 2006;166(7):781–786. doi: 10.1001/archinte.166.7.781. [DOI] [PubMed] [Google Scholar]
- 13.Kim W.R., Biggins S.W., Kremers W.K., et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilberberg M.D., Exuzides A., Spalding J., et al. Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulm Med. 2008;8:16. doi: 10.1186/1471-2466-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusinaru D., Buiciuc O., Leborgne L., Slama M., Massy Z., Tribouilloy C. Relation of serum sodium level to long-term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol. 2009;103(3):405–410. doi: 10.1016/j.amjcard.2008.09.091. [DOI] [PubMed] [Google Scholar]
- 16.Sajadieh A., Binici Z., Mouridsen M.R., Nielsen O.W., Hansen J.F., Haugaard S.B. Mild hyponatremia carries a poor prognosis in community subjects. Am J Med. 2009;122(7):679–686. doi: 10.1016/j.amjmed.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Waikar S.S., Mount D.B., Curhan G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857–865. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whelan B., Bennett K., O'Riordan D., Silke B. Serum sodium as a risk factor for in-hospital mortality in acute unselected general medical patients. QJM. 2009;102(3):175–182. doi: 10.1093/qjmed/hcn165. [DOI] [PubMed] [Google Scholar]
- 19.Tada Y., Nakamura T., Funayama H., et al. Early development of hyponatremia implicates short- and long-term outcomes in ST-elevation acute myocardial infarction. Circ J. 2011;75(8):1927–1933. doi: 10.1253/circj.cj-10-0945. [DOI] [PubMed] [Google Scholar]
- 20.Waikar S.S., Curhan G.C., Brunelli S.M. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med. 2011;124(1):77–84. doi: 10.1016/j.amjmed.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mc Causland F.R., Brunelli S.M., Waikar S.S. Dialysate sodium, serum sodium and mortality in maintenance hemodialysis. Nephrol, Dial, Transplant. 2012;27(4):1613–1618. doi: 10.1093/ndt/gfr497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gankam-Kengne F., Ayers C., Khera A., de Lemos J., Maalouf N.M. Mild hyponatremia is associated with an increased risk of death in an ambulatory setting. Kidney Int. 2013;83(4):700–706. doi: 10.1038/ki.2012.459. [DOI] [PubMed] [Google Scholar]
- 23.Mohan S., Gu S., Parikh A., Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med. 2013;126(12):1127–1137.e1. doi: 10.1016/j.amjmed.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturdik I., Adamcova M., Kollerova J., Koller T., Zelinkova Z., Payer J. Hyponatraemia is an independent predictor of in-hospital mortality. Eur J Intern Med. 2014;25(4):379–382. doi: 10.1016/j.ejim.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Rhee C.M., Ravel V.A., Ayus J.C., et al. Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol, Dial, Transplant. 2016;31(6):992–1001. doi: 10.1093/ndt/gfv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravel V.A., Streja E., Mehrotra R., et al. Serum sodium and mortality in a national peritoneal dialysis cohort. Nephrol, Dial, Transplant. 2017;32(7):1224–1233. doi: 10.1093/ndt/gfw254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandai S., Kuwahara M., Kasagi Y., et al. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol. 2013;14:276. doi: 10.1186/1471-2369-14-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayus J.C., Caputo D., Bazerque F., Heguilen R., Gonzalez C.D., Moritz M.L. Treatment of hyponatremic encephalopathy with a 3% sodium chloride protocol: a case series. Am J Kidney Dis. 2015;65(3):435–442. doi: 10.1053/j.ajkd.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Ayus J.C., Fuentes N.A., Negri A.L., et al. Mild prolonged chronic hyponatremia and risk of hip fracture in the elderly. Nephrol, Dial, Transplant. 2016;31(10):1662–1669. doi: 10.1093/ndt/gfw029. [DOI] [PubMed] [Google Scholar]
- 30.Wannamethee S.G., Shaper A.G., Lennon L., Papacosta O., Whincup P. Mild hyponatremia, hypernatremia and incident cardiovascular disease and mortality in older men: A population-based cohort study. Nutr Metab Cardiovasc Dis. 2016;26(1):12–19. doi: 10.1016/j.numecd.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbalis J.G., Barsony J., Sugimura Y., et al. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554–563. doi: 10.1359/jbmr.090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nzerue C.M., Baffoe-Bonnie H., You W., Falana B., Dai S. Predictors of outcome in hospitalized patients with severe hyponatremia. J Nat Med Assoc. 2003;95(5):335–343. [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinewietfeld M., Manzel A., Titze J., et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C., Yosef N., Thalhamer T., et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Meer J.W., Netea M.G. A salty taste to autoimmunity. New Engl J Med. 2013;368(26):2520–2521. doi: 10.1056/NEJMcibr1303292. [DOI] [PubMed] [Google Scholar]
- 36.Barsony J., Sugimura Y., Verbalis J.G. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011;286(12):10864–10875. doi: 10.1074/jbc.M110.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barsony J., Manigrasso M.B., Xu Q., Tam H., Verbalis J.G. Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age (Dordr) 2013;35(2):271–288. doi: 10.1007/s11357-011-9347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckart A., Hausfater P., Amin D., et al. Hyponatremia and activation of vasopressin secretion are both independently associated with 30-day mortality: results of a multicenter, observational study. J Intern Med. 2018;284(3):270–281. doi: 10.1111/joim.12764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.