Abstract
Introduction
Newer incretin-based therapies for type 2 diabetes (T2D) have the potential to substantially reduce glycated hemoglobin (HbA1c) and weight with a low associated risk of hypoglycemia. This study aimed to assess the percentage of participants randomized to tirzepatide or comparator who achieved the composite endpoint of HbA1c ≤ 6.5% and weight reduction ≥ 10% without hypoglycemia across prespecified baseline characteristics: T2D duration (≤ 5, > 5–10, or > 10 years), sex, HbA1c (≤ 8.5% or > 8.5%), age (< 65 or ≥ 65 years), and body mass index (< 30, 30 to < 35, or ≥ 35 kg/m2).
Methods
This post hoc analysis of SURPASS-1 through -5 evaluated adult study participants with T2D treated with tirzepatide 5, 10, or 15 mg versus placebo or active comparator. Missing HbA1c and weight values were imputed from mixed models for repeated measures. Logistic regression was used to compare tirzepatide versus comparators for the percentage of participants reaching the composite endpoint.
Results
Across subgroups, the composite endpoint was achieved by a median of approximately 30%, 45%, and 54% of participants who received tirzepatide 5, 10, and 15 mg, respectively; this was consistent across baseline subgroups, except that a greater percentage of women than men achieved the composite endpoint. The most common treatment-emergent adverse events were gastrointestinal in nature.
Conclusions
In this post hoc analysis, tirzepatide achieved the composite outcome of glycemic control and weight loss with no hypoglycemia, irrespective of baseline characteristics. This may help clinicians as they select suitable treatment in diverse populations.
Trial Registration
ClinicalTrials.gov: NCT03954834, NCT03987919, NCT03882970. NCT03730662, and NCT04039503.
Keywords: Baseline characteristics, Body weight, Composite endpoint, HbA1c, Hypoglycemia, Tirzepatide
Key Summary Points
| Why carry out this study? |
| Tirzepatide, a long-acting GIP/GLP-1 receptor agonist, demonstrated robust and clinically meaningful reductions in HbA1c and body weight in the SURPASS-1 through -5 clinical trial program. |
| This post hoc analysis assessed the likelihood of participants taking tirzepatide to meet the clinically relevant composite endpoint of HbA1c ≤ 6.5% and weight loss ≥ 10% from baseline without hypoglycemia with respect to prespecified baseline characteristics. |
| What was learned from the study? |
| In all five SURPASS trials, across all three tirzepatide doses (5, 10, 15 mg) and within each respective participant subgroup, the percentage of participants achieving the composite endpoint (HbA1c ≤ 6.5%, weight loss ≥ 10% from baseline without hypoglycemia) was greater among participants treated with tirzepatide than among those who received placebo or an active comparator; female participants were consistently more likely to achieve the composite endpoint than male participants. |
| Efficacy and safety of tirzepatide were generally consistent across a broad cross-section of adults with type 2 diabetes regardless of baseline characteristics. |
Introduction
Diabetes continues to be a major public health concern and affects over 537 million adults (20–79 years old) worldwide as of 2021; its prevalence is predicted to increase to more than 780 million people by 2045 [1]. Despite clinical guidelines and a broad range of pharmacotherapy options, in the USA, the Centers for Disease Control and Prevention estimates glycemic control to be inadequate: 47.4% of adults with T2D had glycated hemoglobin (HbA1c) values ≥ 7.0% and 67.4% had HbA1c ≥ 6.5% [2]. Further, weight management remains a problem in US adults with T2D, as 89.8% are classified as having overweight (body mass index [BMI] ≥ 25 kg/m2) or obesity (BMI ≥ 30 kg/m2) [2]. Only 11.1% of US adults with T2D achieve HbA1c < 7%, blood pressure < 130/80 mmHg, and non-high-density lipoprotein < 130 mg/dL, and do not smoke [2]. Similarly, the DISCOVER study highlighted that the mean HbA1c was 8.3% in an international cohort of participants with T2D who initiated second-line glucose-lowering therapy, and only 17.6% achieved HbA1c < 7.0% [3]. The low percentage of participants achieving glycemic control is unsurprising in light of the study’s objective of evaluating second-line therapy; however, more than 50% of the participants had HbA1c ≥ 8.0%, which demonstrates a significant need to improve glycemic control [3]. Postulated reasons for this failure to achieve guideline-recommended targets include therapeutic inertia, suboptimal adherence, cost, and hypoglycemia avoidance [4].
To guide clinicians in the management of diabetes and its complications and comorbidities, the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) consensus report [5] and the American Association of Clinical Endocrinology (AACE) practice guideline [6] both emphasize engaging a multidisciplinary team in a comprehensive, holistic, person-centered approach that allows participants to achieve goals related to glycemic targets, weight reduction, and cardiovascular/chronic kidney disease mitigation, while minimizing the risk of adverse reactions and hypoglycemia. The AACE recommends an HbA1c target of ≤ 6.5% for most nonpregnant adults, if it can be achieved safely, to diminish long-term microvascular disease [6]. The ADA standards of care and the ADA/EASD consensus statement emphasize that > 10% weight loss has a disease-modifying effect beyond the improved glycemic control and cardiometabolic effects seen with lower weight loss goals [5, 7]. The ADA standards of care further recommend that the effect of therapy on weight should be a consideration when choosing diabetes therapy [7]. Diabetes treatment guidelines increasingly emphasize achieving HbA1c goals and weight reduction without increased risk of hypoglycemia [7, 8], and a composite endpoint of HbA1c ≤ 6.5% with body weight reduction ≥ 10% without hypoglycemia represents a clinically meaningful endpoint to inform clinical decision-making. In contrast to the currently available data for the composite endpoint of HbA1c < 7% without weight gain or hypoglycemia [9–13], there is a paucity of data demonstrating the ability of a glucagon-like peptide 1 (GLP-1) receptor agonist (RA) or glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 RA to help participants achieve a composite endpoint that addresses the current standard of care recommendations.
Tirzepatide, a long-acting GIP/GLP-1 RA, has been approved in the USA for the treatment of T2D and obesity [14, 15]. In five phase 3 registration clinical trials (SURPASS-1 through -5), treatment with tirzepatide resulted in robust and clinically meaningful reductions in HbA1c and body weight and normalization of blood glucose in up to 62% of trial participants [16–21]. Composite outcomes of various HbA1c reduction and weight loss thresholds without hypoglycemia have previously been assessed for the participant populations of each of these studies [16]. The current post hoc analysis assesses a composite according to baseline characteristics to provide clinicians with information on how to manage various subpopulations reflecting differences in baseline glycemic control and weight, differences in severity/duration of disease, and biological characteristics that may affect response to therapy, consistent with comparable studies completed for GLP-1 RAs [22, 23].
The ADVANCE clinical trial set a primary outcome target of HbA1c ≤ 6.5%, an endpoint associated with significant reduction in microvascular and major adverse cardiovascular events primarily driven by the reduction in microvascular outcomes [24]. Furthermore, significant reduction in the risk of new or worsening albuminuria was associated with attainment of a median HbA1c of 6.3% versus 7.0% [24]. This is a post hoc analysis of the SURPASS-1 through -5 trials to assess the percentage of participants randomized to tirzepatide or comparator who achieved the composite endpoint of HbA1c ≤ 6.5% and weight reduction ≥ 10% without hypoglycemia (blood glucose < 54 mg/dL or severe hypoglycemia, defined as an episode of hypoglycemia requiring assistance to administer therapy) in alignment with current guidelines. This composite endpoint was assessed for five subgroups of participant characteristics at baseline that are clinically relevant when setting treatment goals: duration of T2D (≤ 5, > 5–10, or > 10 years), sex, HbA1c (≤ 8.5% or > 8.5%), age (< 65 or ≥ 65 years), and BMI (< 30, 30 to < 35, and ≥ 35 kg/m2).
Methods
Study Design
Details of the trial designs, objectives, and endpoints of SURPASS-1 through -5 have been published previously [17–21]. Briefly, all five trials were international, multicenter, randomized, parallel-group, phase 3 trials designed to evaluate the efficacy and safety of three doses of subcutaneous weekly tirzepatide (5, 10, and 15 mg) in adult participants with T2D. SURPASS-1 and SURPASS-5 were double-blind, placebo-controlled trials; SURPASS-2, -3, and -4 were open-label, active-controlled trials of tirzepatide versus semaglutide 1 mg, insulin degludec, and insulin glargine, respectively. The trials included participants who were newly diagnosed with T2D treated with diet and exercise only, inadequately controlled on up to three oral antihyperglycemic medications (OAMs; including in participants with increased cardiovascular risk), or inadequately controlled on basal insulin with/without metformin.
The SURPASS trials were conducted in accordance with consensus ethical principles, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation Good Clinical Practice guidelines, and applicable laws and regulations and were approved by the relevant ethics committee/review board at each site. All participants in all primary trials provided written informed consent. Each trial was registered with ClinicalTrials.gov: identifiers NCT03954834, NCT03987919, NCT03882970, NCT03730662, and NCT04039503.
Study Population
SURPASS-1 through -5 enrolled adults aged ≥ 18 years with inadequately controlled T2D. Across the five trials, eligible participants had a baseline HbA1c ranging from 7.0% to 10.5%, BMI of either ≥ 23 kg/m2 (SURPASS-1, and -5) or ≥ 25 kg/m2 (SURPASS-2, -3, and -4), and stable weight (± 5%) in the previous 3 months. Key exclusion criteria were type 1 diabetes, history of pancreatitis, and history of proliferative diabetic retinopathy, diabetic maculopathy, or nonproliferative diabetic retinopathy requiring acute treatment. Full eligibility criteria for each of the SURPASS trials have been published previously [17–21].
Statistical Analyses
Post hoc analyses for attainment of the composite endpoint (defined as HbA1c ≤ 6.5%, weight reduction ≥ 10%, and no hypoglycemia [blood glucose < 54 mg/dL or severe hypoglycemia, defined as an episode of hypoglycemia requiring assistance to administer therapy]) at the primary endpoint (week 40 for SURPASS-1, -2, and -5 and week 52 for SURPASS-3 and -4) were performed on the following subgroups of selected baseline characteristics for each of the five SURPASS trials separately because of differences in trial design: duration of T2D (≤ 5 years, > 5 to 10 years, or > 10 years), sex, HbA1c (≤ 8.5% or > 8.5%), age (< 65 years or ≥ 65 years), and BMI (< 30, 30 to < 35, or ≥ 35 kg/m2). To evaluate each component of the composite endpoint concurrently, events of hypoglycemia were counted up to the respective primary endpoints and did not include the protocol-specified 4-week safety follow-up period. The analysis population included all randomized participants who took at least one dose of study drug and excluded those who discontinued study drug as a result of inadvertent enrollment. Data collected after initiation of rescue medication or permanent discontinuation of study drug were excluded (efficacy analysis set). The baseline is defined as the last measurement (including unscheduled visits) collected prior to or on the first dose day. A post hoc analysis for attainment of individual endpoints (HbA1c ≤ 6.5% and weight reduction ≥ 10%) stratified by baseline subgroup across the SURPASS trials was also included.
Missing HbA1c and weight endpoint measures were imputed by predictions using observed data in the efficacy analysis set from the same treatment group through mixed models for repeated measures for post-baseline measures with the respective baseline value, pooled country, treatment, visit, and treatment-by-visit interaction as factors. In addition, the following covariates were included: prior use of OAMs (yes/no) in SURPASS-1, baseline OAM use (metformin, metformin plus sodium-glucose co-transporter 2 inhibitor [SGLT2i]) in SURPASS-3, baseline SGLT2i use (yes/no) in SURPASS-4, and baseline metformin use (yes/no) in SURPASS-5. For weight, baseline HbA1c stratum (≤ 8.5% or > 8.5%) was also included in the imputation model factors. For the hypoglycemic endpoint, no imputation was done and documented hypoglycemic or severe hypoglycemic events recorded in the electronic case report form were used for the analyses. The percentage of participants achieving the composite endpoint was assessed between each of the tirzepatide doses versus comparators using logistic regression at the primary endpoint at week 40 (SURPASS-1, -2, and -5) or week 52 (SURPASS-3 and -4) on the imputed data. The following factors were included in the logistic regression model: respective baseline value, pooled country, and treatment. Treatment-by-subgroup interaction was also assessed with this interaction term added to the logistic regression.
Safety endpoints of gastrointestinal treatment-emergent adverse events (TEAEs) by subgroup and incidence of hypoglycemic events (with glucose < 54 mg/dL or severe hypoglycemia) by subgroup were evaluated on the basis of the safety analysis set. All available data from the entire trial period for each trial were included in the safety analysis: 40-week treatment plus 4-week safety follow-up period for SURPASS-1, -2, and -5; 52-week treatment plus 4-week safety follow-up period for SURPASS-3; and 52-week treatment (primary) plus variable treatment period (to 104 weeks) plus 4-week safety follow-up for SURPASS-4.
Statistical analyses for all trials were conducted using SAS version 9.4, unless otherwise noted.
Results
Baseline Demographics and Characteristics
Overall clinical and demographic characteristics at baseline in each trial are summarized in Table 1 and were similar between treatment groups within trials [17–21]. A total of 6263 participants were randomized and received at least one dose of study drug in the five SURPASS trials, of whom 85–89% completed treatment and 90–95% completed the trials.
Table 1.
Baseline demographics and clinical characteristics of participants with T2D in the SURPASS phase 3 trials (modified intent-to-treat population)
| SURPASS-1 N = 478 |
SURPASS-2 N = 1878 |
SURPASS-3 N = 1437 |
SURPASS-4 N = 1995 |
SURPASS-5 N = 475 |
|
|---|---|---|---|---|---|
| Mean age, years | 54.1 (11.9) | 56.6 (10.4) | 57.4 (10.0) | 63.6 (8.6) | 60.6 (9.9) |
| Age categories | |||||
| < 65 years | 373 (78.0%) | 1420 (75.6%) | 1058 (73.6%) | 1047 (52.5%) | 283 (59.6%) |
| ≥ 65 years | 105 (22.0%) | 458 (24.4%) | 379 (26.4%) | 948 (47.5%) | 192 (40.4%) |
| Duration of T2D, years | 4.7 (5.4) | 8.6 (6.5) | 8.4 (6.2) | 11.8 (7.5) | 13.3 (7.3) |
| Duration categories | |||||
| ≤ 5 years | 323 (67.6%) | 643 (34.2%) | 502 (35.0%) | 368 (18.4%) | 58 (12.2%) |
| > 5 to 10 years | 89 (18.6%) | 599 (31.9%) | 465 (32.4%) | 569 (28.5%) | 111 (23.4%) |
| > 10 years | 66 (13.8%) | 636 (33.95) | 469 (32.7%) | 1058 (53.0%) | 306 (64.4%) |
| HbA1c, % | 7.9 (0.9) | 8.3 (1.0) | 8.2 (0.9) | 8.5 (0.9) | 8.3 (0.9) |
| HbA1c categories | |||||
| ≤ 8.5% | 378 (79.1%) | 1192 (63.5%) | 1005 (69.9%) | 1131 (56.7%) | 296 (62.3%) |
| > 8.5% | 100 (20.9%) | 686 (36.5%) | 432 (30.1%) | 864 (43.3%) | 178 (37.5%) |
| BMI, kg/m2 | 31.9 (6.6) | 34.2 (6.9) | 33.5 (6.1) | 32.6 (5.5) | 33.4 (6.1) |
| BMI categories | |||||
| < 30 kg/m2 | 224 (46.9%) | 552 (29.4%) | 446 (31.0%) | 718 (36.0%) | 152 (32.0%) |
| 30 to < 35 kg/m2 | 123 (25.7%) | 636 (33.9%) | 496 (34.5%) | 721 (36.1%) | 143 (30.1%) |
| ≥ 35 kg/m2 | 131 (27.4%) | 690 (36.7%) | 495 (34.4%) | 556 (27.9%) | 180 (37.9%) |
| Sex | |||||
| Female | 231 (48.3%) | 996 (53.0%) | 635 (44.2%) | 749 (37.5%) | 211 (44.4%) |
| Male | 247 (51.7%) | 882 (47.0%) | 802 (55.8%) | 1246 (62.5%) | 264 (55.6%) |
| Weight (kg) | 85.9 (19.8) | 93.7 (21.9) | 94.3 (20.1) | 90.3 (18.7) | 95.2 (21.6) |
| Diabetes medication at randomization | |||||
| Metformina | – | 1878 (100%) | 1437 (100%) | 1893 (94.9%) | 394 (82.9%) |
| Sulfonylurea | – | – | 1086 (54.4%) | – | |
| SGLT2i | – | 458 (31.9%) | 501 (25.1%) | – | |
| Insulin glargineb | – | – | – | 475 (100.0%) | |
Data are mean (SD) or n (%) unless otherwise indicated
BMI body mass index, HbA1c hemoglobin A1c, T2D type 2 diabetes, SD standard deviation, SGLT2i sodium-glucose co-transporter 2 inhibitor
aMetformin doses ≥ 1500 mg/day
bInsulin glargine doses 37.6 U/day (SD 22.7) or 0.40 U/kg/day (SD 0.2)
The differences in characteristics among participants reflect differences in eligibility criteria among the trials. SURPASS-1 was a placebo-controlled, monotherapy trial with lower HbA1c eligibility criteria which resulted in a younger cohort (mean age 54.1 years) with a shorter duration of T2D (mean duration 4.7 years). Correspondingly, the eligibility criteria for SURPASS-4 (a high-risk cardiovascular population) and -5 (placebo-controlled, add-on to basal insulin) resulted in an older cohort (mean age 60.6–63.6 years) with a longer duration of T2D (mean duration 11.8–13.3 years). Baseline antihyperglycemic therapy also varied across the SURPASS trials according to enrollment criteria. These differences in trial design limited the ability to pool populations and account for some variation in the results among individual subgroups.
Composite Endpoint Achievement
Graphical plots of the percentage of participants in each treatment arm achieving the composite endpoint (defined as HbA1c ≤ 6.5%, weight reduction ≥ 10%, and no hypoglycemia [blood glucose < 54 mg/dL or severe hypoglycemia]) stratified by subgroup (duration of T2D, baseline HbA1c, baseline BMI, age, and sex) are depicted in Figs. 1, 2, 3, 4, and 5. Baseline HbA1c and weight for each trial–subgroup combination are presented in Table 2. In all five SURPASS trials, across all three tirzepatide doses (5, 10, 15 mg) and within each respective participant subgroup, the percentage of participants achieving the composite endpoint was greater among participants treated with tirzepatide than among those who received placebo or an active comparator and was evident even in trials that included concomitant antihyperglycemic medications that are associated with weight gain and increased risk of hypoglycemia. As depicted in Figs. 1, 2, 3, 4, and 5, there was generally a dose-dependent increase in the percentage of tirzepatide-treated participants who achieved the composite endpoint regardless of subgroup. In the subgroup analysis of SURPASS-1 duration of diabetes, > 10 years, SURPASS-5 duration of diabetes ≤ 5 years, SURPASS-1 BMI 30 to < 35 kg/m2 and BMI ≥ 35 kg/m2, SURPASS-5 age ≥ 65 years, SURPASS-1 and -5 male participants, and SURPASS-4 female participants, a dose-dependent increase in the percentage of participants who achieved the composite endpoint was not observed; however, the number of participants in each of the dose groups within these subgroups also tended to be smaller.
Fig. 1.
Percentage of participants reaching the composite endpoint by duration of T2D (modified intent-to-treat, efficacy analysis set): subgroup-by-treatment interaction not statistically significant. Participants with T2D treated with tirzepatide achieved the composite endpoint (HbA1c ≤ 6.5%, ≥ 10% weight reduction, no hypoglycemia) irrespective of duration of T2D at baseline. In all five SURPASS trials, across all three tirzepatide doses (5 mg, light blue; 10 mg, medium blue; 15 mg, dark blue) and within each participant subgroup (duration of T2D ≤ 5, > 5–10, or > 10 years at baseline), the percentage of participants who achieved the composite endpoint was greater for tirzepatide than for placebo (SURPASS-1 and SURPASS-5) or active comparator (SURPASS-2, SURPASS-3, and SURPASS-4). Data are LSM. mITT population (efficacy analysis set). Data labels are percentage of participants achieving the composite endpoint. HbA1c glycated hemoglobin, LSM least squares mean, mITT modified intent-to-treat, MMRM mixed model repeated measures, T2D type 2 diabetes. Note 1: Missing endpoint measures (HbA1c and weight) were imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures. No imputation was done for hypoglycemic events. Note 2: The percentage of participants reaching the composite endpoint target was analyzed with a logistic regression model using imputed data. The treatment-by-subgroup interaction was assessed with a full model by including treatment-by-subgroup interaction as an additional factor
Fig. 2.
Percentage of participants reaching the composite endpoint by baseline HbA1c (modified intent-to-treat, efficacy analysis set): subgroup-by-treatment interaction not statistically significant. Participants with T2D treated with tirzepatide achieved the composite endpoint (HbA1c ≤ 6.5%, ≥ 10% weight reduction, no hypoglycemia) irrespective of baseline HbA1c, and overall greater percentages of participants with baseline HbA1c ≤ 8.5% achieved the composite endpoint versus those with baseline HbA1c > 8.5%. In all five SURPASS trials, across all three tirzepatide doses (5 mg, light blue; 10 mg, medium blue; 15 mg, dark blue) and within each participant subgroup (HbA1c ≤ 8.5% or > 8.5% at baseline), the percentage of participants who achieved the composite endpoint was greater for tirzepatide than for placebo (light gray) or active comparator (dark gray). Data are LSM. mITT population (efficacy analysis set). Data labels are percentage of participants who achieved the composite endpoint. HbA1c glycated hemoglobin, LSM least squares mean, mITT modified intent-to-treat, MMRM mixed model repeated measures, T2D type 2 diabetes. Note 1: Missing endpoint measures (HbA1c and weight) were imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures. No imputation was done for hypoglycemic events. Note 2: The percentage of participants reaching the composite endpoint target was analyzed with a logistic regression model using imputed data. The treatment-by-subgroup interaction was assessed with a full model by including treatment-by-subgroup interaction as an additional factor
Fig. 3.
Percentage of participants reaching the composite endpoint by baseline BMI (modified intent-to-treat, efficacy analysis set): subgroup-by-treatment interaction not statistically significant with the exception of SURPASS-1 (p = 0.041). Participants with T2D treated with tirzepatide achieved the composite endpoint (HbA1c ≤ 6.5%, ≥ 10% weight reduction, no hypoglycemia) irrespective of BMI at baseline. In all five SURPASS trials, across all three tirzepatide doses (5 mg, light blue; 10 mg, medium blue; 15 mg, dark blue) and within each participant subgroup (BMI < 30, 30 to < 35, or ≥ 35 kg/m2 at baseline), the percentage of participants achieving the composite endpoint was greater for tirzepatide than for placebo (light gray) or active comparator (dark gray). Data are LSM. mITT population (efficacy analysis set). Data labels are percentage of participants who achieved the composite endpoint. BMI body mass index, HbA1c glycated hemoglobin, LSM least squares mean, mITT modified intent-to-treat, MMRM mixed model repeated measures, T2D type 2 diabetes. Note 1: Missing endpoint measures (HbA1c and weight) were imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures. No imputation was done for hypoglycemic events. Note 2: The percentage of participants reaching the composite endpoint target was analyzed with a logistic regression model using imputed data. The treatment-by-subgroup interaction was assessed with a full model by including treatment-by-subgroup interaction as an additional factor
Fig. 4.
Percentage of participants reaching the composite endpoint by age (modified intent-to-treat, efficacy analysis set): subgroup-by-treatment interaction not statistically significant. Participants with T2D treated with tirzepatide achieved the composite endpoint (HbA1c ≤ 6.5%, ≥ 10% weight reduction, no hypoglycemia) irrespective of age at baseline. In all five SURPASS trials, across all three tirzepatide doses (5 mg, light blue; 10 mg, medium blue; 15 mg, dark blue) and within each participant subgroup (age < 65 or ≥ 65 years at baseline), the percentage of participants achieving the composite endpoint was greater for tirzepatide than for placebo (light gray) or active comparator (dark gray). Data are LSM. mITT population (efficacy analysis set). Data labels are percentage of participants achieving the composite endpoint. HbA1c glycated hemoglobin, LSM least squares mean, mITT modified intent-to-treat, MMRM mixed model repeated measures, T2D type 2 diabetes. Note 1: Missing endpoint measures (HbA1c and weight) were imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures. No imputation was done for hypoglycemic events. Note 2: The percentage of participants reaching the composite endpoint target was analyzed with a logistic regression model using imputed data. The treatment-by-subgroup interaction was assessed with a full model by including treatment-by-subgroup interaction as an additional factor
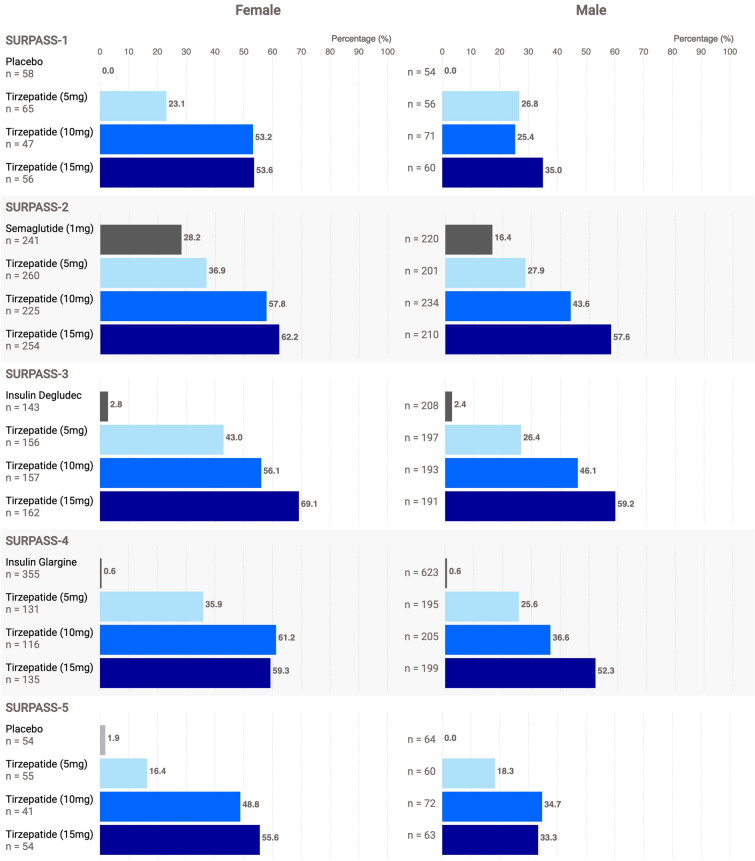
Fig. 5.
Percentage of participants reaching the composite endpoint by sex (modified intent-to-treat, efficacy analysis set): subgroup-by-treatment interaction statistically significant (SURPASS-1 to -4: p < 0.02 for each trial; SURPASS-5: p = 0.164). Participants with T2D treated with tirzepatide achieved the composite endpoint (HbA1c ≤ 6.5%, ≥ 10% weight reduction, no hypoglycemia) irrespective of sex; however, overall, a greater percentage of women than men achieved the composite endpoint. In all five SURPASS trials, across all three tirzepatide doses (5 mg, light blue; 10 mg, medium blue; 15 mg, dark blue) and among both men and women, the percentage of participants achieving the composite endpoint was greater for tirzepatide than for placebo (light gray) or active comparator (dark gray). In individual trials, with the exception of tirzepatide 5 mg in SURPASS-1 and SURPASS-5, greater percentages of women than men achieved the composite endpoint. Data are LSM. mITT population (efficacy analysis set). Data labels are percentage of participants achieving the composite endpoint. HbA1c glycated hemoglobin, LSM least squares mean, mITT modified intent-to-treat, MMRM mixed model repeated measures, T2D type 2 diabetes. Note 1: Missing endpoint measures (HbA1c and weight) were imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures. No imputation was done for hypoglycemic events. Note 2: The percentage of participants reaching the composite endpoint target was analyzed with a logistic regression model using imputed data. The treatment-by-subgroup interaction was assessed with a full model by including treatment-by-subgroup interaction as an additional factor
Table 2.
Baseline HbA1c and weight by trial and subgroup
| SURPASS-1 N = 478 |
SURPASS-2 N = 1878 |
SURPASS-3 N = 1437 |
SURPASS-4 N = 1995 |
SURPASS-5 N = 475 |
|
|---|---|---|---|---|---|
| Duration of T2D | |||||
| ≤ 5 years | 7.9% | 8.1% | 8.1% | 8.4% | 8.2% |
| 87.1 kg | 98.3 kg | 97.8 kg | 95.4 kg | 105.3 kg | |
| > 5 to 10 years | 7.9% | 8.4% | 8.2% | 8.5% | 8.3% |
| 83.2 kg | 94.5 kg | 95.2 kg | 92.2 kg | 100.0 kg | |
| > 10 years | 8.1% | 8.4% | 8.2% | 8.6% | 8.3% |
| 83.5 kg | 88.4 kg | 89.6 kg | 87.4 kg | 91.5 kg | |
| Baseline HbA1c | |||||
| ≤ 8.5% | 7.6% | 7.6% | 7.7% | 7.9% | 7.8% |
| 86.1 kg | 94.4 kg | 94.1 kg | 90.0 kg | 94.7 kg | |
| > 8.5% | 9.3% | 9.4% | 9.3% | 9.3% | 9.2% |
| 85.0 kg | 92.5 kg | 94.6 kg | 90.6 kg | 96.0 kg | |
| Baseline BMI | |||||
| < 30 kg/m2 | 8.0% | 8.4% | 8.2% | 8.5% | 8.3% |
| 72.1 kg | 75.7 kg | 77.5 kg | 75.4 kg | 74.6 kg | |
| 30 to < 35 kg/m2 | 8.0% | 8.3% | 8.2% | 8.6% | 8.3% |
| 87.6 kg | 89.0 kg | 91.8 kg | 90.5 kg | 94.6 kg | |
| ≥ 35 kg/m2 | 7.9% | 8.2% | 8.2% | 8.5% | 8.4% |
| 107.8 kg | 112.5 kg | 112.3 kg | 109.4 kg | 113.2 kg | |
| Baseline age | |||||
| < 65 years | 8.0% | 8.3% | 8.2% | 8.6% | 8.4% |
| 86.6 kg | 95.9 kg | 97.1 kg | 93.9 kg | 98.4 kg | |
| ≥ 65 years | 7.8% | 8.1% | 8.1% | 8.5% | 8.1% |
| 83.4 kg | 86.9 kg | 86.5 kg | 86.2 kg | 90.5 kg | |
| Sex | |||||
| Female | 7.9% | 8.3% | 8.2% | 8.5% | 8.3% |
| 82.4 kg | 87.6 kg | 88.1 kg | 83.6 kg | 88.9 kg | |
| Male | 8.0% | 8.3% | 8.1% | 8.5% | 8.3% |
| 89.1 kg | 100.6 kg | 99.2 kg | 94.3 kg | 100.2 kg | |
Baseline HbA1c data are presented as %. Baseline weight data are presented in kg
BMI body mass index, HbA1c glycated hemoglobin, T2D type 2 diabetes
There was no statistically significant subgroup-by-treatment interaction among participant subgroups when stratified by duration of T2D (Fig. 1), baseline HbA1c (Fig. 2), baseline BMI (Fig. 3), or age (Fig. 4). Visual differences in the achievement of the composite endpoint based on baseline HbA1c (Fig. 2) are likely driven by a greater percentage of participants in the ≤ 8.5% subgroup who achieved the individual HbA1c and weight components. Achievement of the individual composite components (HbA1c ≤ 6.5% and weight reduction ≥ 10%) is shown in Tables 3 and 4. In all cases, regardless of tirzepatide dose or subgroup, composite endpoint achievement was greater for tirzepatide than for active comparator or placebo. Notably, a statistically significant (p = 0.041) treatment-by-subgroup interaction was observed for the SURPASS-1 BMI subgroup.
Table 3.
Percentage of participants who achieved an HbA1c goal of ≤ 6.5% in each trial and subgroup
| Trial/treatment arm | Participants achieving an HbA1c goal of ≤ 6.5%, N (%) | |||||
|---|---|---|---|---|---|---|
| Duration of T2D (years) | Baseline HbA1c (%) | |||||
| ≤ 5 | > 5–10 | > 10 | ≤ 8.5% | > 8.5% | ||
| SURPASS-1 | Placebo | 10 (12.2%) | 0 (0%) | 1 (7.1%) | 9 (10.7%) | 2 (7.1%) |
| Tirzepatide 5 mg | 67 (81.7%) | 17 (81.0%) | 15 (83.3%) | 82 (86.3%) | 17 (65.4%) | |
| Tirzepatide 10 mg | 63 (82.9%) | 21 (77.8%) | 12 (80.0%) | 78 (80.4%) | 18 (85.7%) | |
| Tirzepatide 15 mg | 70 (92.1%) | 20 (87.0%) | 10 (58.8%) | 82 (88.2%) | 18 (78.3%) | |
| SURPASS-2 | Semaglutide 1 mg | 119 (75.8%) | 104 (68.0%) | 82 (54.3%) | 226 (75.6%) | 79 (48.8%) |
| Tirzepatide 5 mg | 132 (83.5%) | 96 (70.1%) | 113 (68.1%) | 235 (82.2%) | 106 (60.6%) | |
| Tirzepatide 10 mg | 141 (92.2%) | 137 (85.1%) | 99 (68.3%) | 254 (88.8%) | 123 (71.1%) | |
| Tirzepatide 15 mg | 151 (88.8%) | 121 (91.0%) | 132 (82.0%) | 274 (91.6%) | 130 (78.8%) | |
| SURPASS-3 | Insulin degludec | 69 (52.7%) | 41 (36.9%) | 46 (42.2%) | 130 (52.6%) | 26 (25.0%) |
| Tirzepatide 5 mg | 81 (72.3%) | 80 (66.7%) | 91 (75.2%) | 198 (80.8%) | 54 (50.0%) | |
| Tirzepatide 10 mg | 101 (82.1%) | 98 (79.0%) | 82 (79.6%) | 209 (86.7%) | 72 (66.1%) | |
| Tirzepatide 15 mg | 115 (91.3%) | 84 (81.6%) | 102 (82.3%) | 225 (91.5%) | 76 (71.0%) | |
| SURPASS-4 | Insulin glargine | 72 (41.1%) | 94 (35.3%) | 144 (26.8%) | 220 (39.5%) | 90 (21.4%) |
| Tirzepatide 5 mg | 47 (75.8%) | 69 (64.5%) | 99 (63.1%) | 144 (75.8%) | 71 (52.2%) | |
| Tirzepatide 10 mg | 48 (85.7%) | 71 (76.3%) | 125 (72.7%) | 136 (82.4%) | 108 (69.2%) | |
| Tirzepatide 15 mg | 62 (91.2%) | 73 (79.4%) | 136 (78.2%) | 169 (89%) | 102 (70.8%) | |
| SURPASS-5 | Placebo | 3 (15.0%) | 4 (15.4%) | 13 (18.1%) | 16 (22.9%) | 4 (8.3%) |
| Tirzepatide 5 mg | 14 (87.5%) | 14 (82.4%) | 64 (78.1%) | 64 (84.2%) | 28 (71.8%) | |
| Tirzepatide 10 mg | 9 (100%) | 34 (97.1%) | 64 (92.8%) | 65 (94.2%) | 42 (95.5%) | |
| Tirzepatide 15 mg | 12 (92.3%) | 29 (100%) | 67 (89.3%) | 70 (93.3%) | 38 (90.5%) | |
| Trial/treatment arm | Participants achieving an HbA1c goal of ≤ 6.5%, N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline BMI (kg/m2) | Age (years) | Sex | ||||||
| < 30 | 30 to < 35 | ≥ 30 | < 65 | ≥ 65 | Female | Male | ||
| SURPASS-1 | Placebo | 3 (5.8%) | 5 (17.9%) | 3 (9.4%) | 11 (12.8%) | 0 (0%) | 7 (12.1%) | 4 (7.4%) |
| Tirzepatide 5 mg | 49 (84.5%) | 23 (76.7%) | 27 (81.8%) | 73 (77.7%) | 26 (96.3%) | 52 (80.0%) | 47 (83.9%) | |
| Tirzepatide 10 mg | 50 (80.7%) | 20 (87.0%) | 26 (78.8%) | 72 (81.8%) | 24 (80.0%) | 38 (80.9%) | 58 (81.7%) | |
| Tirzepatide 15 mg | 42 (85.7%) | 31 (81.6%) | 27 (93.1%) | 84 (88.4%) | 16 (76.2%) | 50 (89.3%) | 50 (83.3%) | |
| SURPASS-2 | Semaglutide 1 mg | 96 (69.1%) | 100 (64.1%) | 109 (65.7%) | 228 (66.7%) | 77 (64.7%) | 167 (69.3%) | 138 (62.7%) |
| Tirzepatide 5 mg | 104 (76.5%) | 124 (75.6%) | 113 (70.2%) | 262 (74.0%) | 79 (73.8%) | 190 (73.1%) | 151 (75.1%) | |
| Tirzepatide 10 mg | 102 (78.5%) | 127 (83.0%) | 148 (84.1%) | 283 (82.8%) | 94 (80.3%) | 188 (83.6%) | 189 (80.8%) | |
| Tirzepatide 15 mg | 107 (80.5%) | 137 (88.4%) | 160 (90.9%) | 323 (88.7%) | 81 (81.0%) | 215 (84.7%) | 189 (90.0%) | |
| SURPASS-3 | Insulin degludec | 53 (47.3%) | 49 (41.5%) | 54 (44.6%) | 6 (43.0%) | 45 (48.4%) | 63 (44.1%) | 93 (44.7%) |
| Tirzepatide 5 mg | 80 (78.4%) | 97 (72.4%) | 75 (64.1%) | 191 (71.8%) | 61 (70.1%) | 108 (69.2%) | 144 (73.1%) | |
| Tirzepatide 10 mg | 93 (83.0%) | 89 (78.8%) | 99 (79.2%) | 214 (81.1%) | 67 (77.9%) | 128 (81.5%) | 153 (79.3%) | |
| Tirzepatide 15 mg | 89 (81.7%) | 103 (87.3%) | 109 (86.5%) | 217 (86.5%) | 84 (82.4%) | 138 (85.2%) | 163 (85.3%) | |
| SURPASS-4 | Insulin glargine | 109 (29.6%) | 108 (32.1%) | 93 (33.9%) | 158 (31.5%) | 152 (31.9%) | 98 (27.6%) | 212 (34.0%) |
| Tirzepatide 5 mg | 76 (63.9%) | 85 (72.0%) | 54 (60.7%) | 126 (68.1%) | 89 (63.1%) | 90 (68.7%) | 125 (64.1%) | |
| Tirzepatide 10 mg | 75 (69.4%) | 98 (79.0%) | 71 (79.8%) | 128 (78.1%) | 116 (73.9%) | 96 (82.8%) | 148 (72.2%) | |
| Tirzepatide 15 mg | 86 (78.9%) | 105 (79.6%) | 80 (86.0%) | 149 (83.7%) | 122 (78.2%) | 107 (79.3%) | 164 (82.4%) | |
| SURPASS-5 | Placebo | 8 (19.5%) | 4 (12.5%) | 8 (17.8%) | 15 (18.8%) | 5 (13.2%) | 9 (16.7%) | 11 (17.2%) |
| Tirzepatide 5 mg | 29 (80.6%) | 31 (81.6%) | 32 (78.1%) | 49 (80.3%) | 43 (79.6%) | 46 (83.6%) | 46 (76.7%) | |
| Tirzepatide 10 mg | 33 (94.3%) | 30 (90.9%) | 44 (97.8%) | 64 (95.5%) | 43 (93.5%) | 38 (92.7%) | 69 (95.8%) | |
| Tirzepatide 15 mg | 34 (94.4%) | 34 (94.4%) | 40 (88.9%) | 66 (94.3%) | 42 (89.4%) | 50 (92.6%) | 58 (92.1%) | |
Only subjects with non-missing baseline value and at least one non-missing post-baseline value of the response variable were included in the analysis
Imputed data include observed value and imputed value if endpoint measure is missing. Missing endpoint measures are imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures: HbA1c = Baseline HbA1c + Pooled Country + Baseline oral antihyperglycemic medication use (Metformin, Metformin plus SGLT2i) + Treatment + Visit + Visit * Treatment HbA1c = baseline HbA1c, pooled country, treatment, visit, and treatment-by-visit interaction as factors. In addition, the following covariates were included: prior use of OAMs (yes/no) in SURPASS-1, baseline OAM use (metformin, metformin plus SGLT2i) in SURPASS-3, baseline SGLT2i use (yes/no) in SURPASS-4, and baseline metformin use (yes/no) in SURPASS-5
BMI body mass index, HbA1c glycated hemoglobin, SGLT2i sodium-glucose co-transporter 2 inhibitor, T2D type 2 diabetes
Table 4.
Percentage of participants who achieved a weight reduction goal of ≥ 10% in each trial and subgroup
| Trial/treatment arm | Participants who achieved a weight reduction goal of ≥ 10%, N (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of T2D (years) | Baseline HbA1c (%) | Baseline BMI (kg/m2) | Age (years) | Sex | |||||||||
| ≤ 5 | > 5–10 | > 10 | ≤ 8.5% | > 8.5% | < 30 | 30 to < 35 | ≥ 35 | < 65 | ≥ 65 | Female | Male | ||
| SURPASS-1 | Placebo | 0 (0%) | 0 (0%) | 1 (7.1%) | 1 (1.2%) | 0 (0%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.9%) | 0 (0%) | 1 (1.9%) |
| Tirzepatide 5 mg | 22 (26.8%) | 7 (33.3%) | 8 (44.4%) | 31 (32.6%) | 6 (23.1%) | 18 (31.0%) | 6 (20.0%) | 13 (39.4%) | 27 (28.7%) | 10 (37.0%) | 17 (26.2%) | 20 (35.7%) | |
| Tirzepatide 10 mg | 32 (42.1%) | 11 (40.7%) | 4 (26.7%) | 40 (41.2%) | 7 (33.3%) | 25 (40.3%) | 11 (47.8%) | 11 (33.3%) | 34 (38.6%) | 13 (43.3%) | 26 (55.3%) | 21 (29.6%) | |
| Tirzepatide 15 mg | 42 (55.3%) | 10 (43.5%) | 3 (17.7%) | 46 (49.5%) | 9 (39.1%) | 24 (49.0%) | 15 (39.5%) | 16 (55.2%) | 44 (46.3%) | 11 (52.4%) | 31 (55.4%) | 24 (40.0%) | |
| SURPASS-2 | Semaglutide 1 mg | 38 (24.2%) | 35 (22.9%) | 49 (32.5%) | 88 (29.4%) | 34 (21%) | 41 (29.5%) | 43 (27.6%) | 38 (22.9%) | 84 (24.6%) | 38 (31.9%) | 79 (32.8%) | 43 (19.6%) |
| Tirzepatide 5 mg | 59 (37.3%) | 44 (32.1%) | 65 (39.2%) | 119 (41.6%) | 49 (28%) | 49 (36.0%) | 63 (38.4%) | 56 (34.8%) | 126 (35.6%) | 42 (39.3%) | 109 (41.9%) | 59 (29.4%) | |
| Tirzepatide 10 mg | 98 (64.1%) | 77 (47.8%) | 67 (46.2%) | 170 (59.4%) | 72 (41.6%) | 75 (57.7%) | 78 (51.0%) | 89 (50.6%) | 181 (52.9%) | 61 (52.1%) | 136 (60.4%) | 106 (45.3%) | |
| Tirzepatide 15 mg | 112 (65.9%) | 84 (63.2%) | 102 (63.4%) | 206 (68.9%) | 92 (55.8%) | 82 (61.7%) | 99 (63.9%) | 117 (66.5%) | 233 (64.0%) | 65 (65.0%) | 171 (67.3%) | 127 (60.5%) | |
| SURPASS-3 | Insulin degludec | 4 (3.1%) | 3 (2.7%) | 3 (2.8%) | 10 (4.1%) | 0 (0%) | 2 (1.8%) | 4 (3.4%) | 4 (3.3%) | 8 (3.1%) | 2 (2.2%) | 4 (2.8%) | 6 (2.9%) |
| Tirzepatide 5 mg | 39 (34.8%) | 48 (40.0%) | 46 (38%) | 98 (40.0%) | 35 (32.4%) | 34 (33.3%) | 55 (41.0%) | 44 (37.6%) | 96 (36.1%) | 37 (42.5%) | 79 (50.6%) | 54 (27.4%) | |
| Tirzepatide 10 mg | 69 (56.1%) | 64 (51.6%) | 58 (56.3%) | 140 (58.1%) | 51 (46.8%) | 65 (58.0%) | 60 (53.1%) | 66 (52.8%) | 147 (55.7%) | 44 (51.2%) | 95 (60.5%) | 96 (49.7%) | |
| Tirzepatide 15 mg | 84 (66.7%) | 71 (68.9%) | 88 (71%) | 177 (72.0%) | 66 (61.7%) | 80 (73.4%) | 83 (70.3%) | 80 (63.5%) | 170 (67.7%) | 73 (71.6%) | 119 (73.5%) | 124 (64.9%) | |
| SURPASS-4 | Insulin glargine | 3 (1.7%) | 4 (1.5%) | 8 (1.5%) | 11 (2.0%) | 4 (1.0%) | 5 (1.4%) | 3 (0.9%) | 7 (2.6%) | 5 (1.0%) | 10 (2.1%) | 6 (1.7%) | 9 (1.4%) |
| Tirzepatide 5 mg | 26 (41.9%) | 35 (32.7%) | 55 (35.0%) | 85 (44.7%) | 31 (22.8%) | 39 (32.8%) | 45 (38.1%) | 32 (36.0%) | 64 (34.6%) | 52 (36.9%) | 60 (45.8%) | 56 (28.7%) | |
| Tirzepatide 10 mg | 32 (57.1%) | 44 (47.3%) | 94 (54.7%) | 97 (58.8%) | 73 (46.8%) | 60 (55.6%) | 62 (50.0%) | 48 (53.9%) | 87 (53.1%) | 83 (52.9%) | 79 (68.1%) | 91 (44.4%) | |
| Tirzepatide 15 mg | 47 (69.1%) | 53 (57.6%) | 119 (68.4%) | 127 (66.8%) | 92 (63.9%) | 73 (67%) | 87 (65.9%) | 59 (63.4%) | 117 (65.7%) | 102 (65.4%) | 98 (72.6%) | 121 (60.8%) | |
| SURPASS-5 | Placebo | 0 (0%) | 1 (3.9%) | 0 (0%) | 1 (1.4%) | 0 (0%) | 1 (2.4%) | 0 (0%) | 0 (0%) | 1 (1.3%) | 0 (0%) | 1 (1.9%) | 0 (0%) |
| Tirzepatide 5 mg | 1 (6.3%) | 7 (41.2%) | 18 (22.0%) | 20 (26.3%) | 6 (15.4%) | 12 (33.3%) | 9 (23.7%) | 5 (12.2%) | 16 (26.2%) | 10 (18.5%) | 11 (20.0%) | 15 (25.0%) | |
| Tirzepatide 10 mg | 5 (55.6%) | 17 (48.6%) | 31 (44.9%) | 36 (52.2%) | 17 (38.6%) | 18 (51.4%) | 15 (45.5%) | 20 (44.4%) | 30 (44.8%) | 23 (50.0%) | 25 (61.0%) | 28 (38.9%) | |
| Tirzepatide 15 mg | 7 (53.9%) | 16 (55.2%) | 37 (49.3%) | 42 (56.0%) | 18 (42.9%) | 20 (55.6%) | 20 (55.6%) | 20 (44.4%) | 36 (51.4%) | 24 (51.1%) | 32 (59.3%) | 28 (44.4%) | |
Only subjects with non-missing baseline value and at least one non-missing post-baseline value of the response variable were included in the analysis
Imputed data include observed value and imputed value if endpoint measure is missing. Missing endpoint measures are imputed by predictions using observed data in the efficacy analysis set from the same treatment group through an MMRM analysis model for post-baseline measures: Weight = Baseline Weight + Baseline HbA1c Group (≤ 8.5%, > 8.5%) + Pooled Country + Treatment + Visit + Visit * Treatment + Baseline oral antihyperglycemic medication use (Metformin Weight = Baseline Weight + Baseline HbA1c group (≤ 8.5%, > 8.5%) + Pooled Country + Treatment + Visit + Visit * Treatment. The following additional covariate was included: prior use of OAMs (yes/no) in SURPASS-1, baseline OAM use (metformin, metformin plus SGLT2i) in SURPASS-3, baseline SGLT2i use (yes/no) in SURPASS-4, and baseline metformin use (yes/no) in SURPASS-5
BMI body mass index, HbA1c glycated hemoglobin, SGLT2i sodium-glucose co-transporter 2 inhibitor, T2D type 2 diabetes
In contrast, the treatment-by-sex interaction was only statistically significant for SURPASS-1 to -4 (p < 0.02 for SURPASS-1 to -4; p = 0.164 for SURPASS-5, Fig. 5). This treatment-by-sex interaction is quantitative and not qualitative in nature because treatment effects were in the same direction (greater percentages of participants treated with tirzepatide achieved the composite target than those in the placebo/comparator arms). Female participants assigned to either tirzepatide or semaglutide 1 mg achieved the composite endpoint more frequently than male participants (Fig. 5). This effect is likely driven by the greater percentage of female participants who achieved the weight reduction target of ≥ 10% (Table 4), as female participants had a lower baseline weight and greater mean weight reduction than male participants [25].
Safety
TEAEs
Consistent with the overall SURPASS trial population and across the five reported subgroups of baseline characteristics, the most common TEAEs of the gastrointestinal system organ class were nausea, diarrhea, dyspepsia, decreased appetite, vomiting, and constipation. Gastrointestinal TEAEs occurred in all subgroups and trials: 22.2–66.7% for all doses of tirzepatide, 7.7–27.5% for placebo, 5.3–13.5% for insulin degludec, 12.8–16.5% for insulin glargine, and 33.3–48.4% for semaglutide 1 mg (Table 5). The number of participants with ≥ 1 gastrointestinal TEAE was consistent across participant subgroups stratified by baseline HbA1c, baseline duration of T2D, baseline age, sex, or baseline BMI.
Table 5.
Gastrointestinal TEAEs (safety analysis set)
| Trial/treatment arm | Participants with ≥ 1 gastrointestinal treatment-emergent adverse event, n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of T2D (years) | Baseline HbA1c (%) | Baseline BMI (kg/m2) | Age (years) | Sex | |||||||||
| ≤ 5 | > 5–10 | > 10 | ≤ 8.5% | > 8.5% | < 30 | 30 to < 35 | ≥ 35 | < 65 | ≥ 65 | Female | Male | ||
| SURPASS-1 | Placebo | 17 (20.2) | 3 (18.8) | 2 (13.3) | 17 (19.5) | 5 (17.9) | 8 (15.4) | 5 (17.2) | 9 (26.5) | 20 (22.5) | 2 (7.7) | 16 (27.1) | 6 (10.7) |
| Tirzepatide 5 mg | 29 (35.4) | 7 (33.3) | 10 (55.6) | 34 (35.8) | 12 (46.2) | 25 (43.1) | 10 (33.3) | 11 (33.3) | 35 (37.2) | 11 (40.7) | 30 (46.2) | 16 (28.6) | |
| Tirzepatide 10 mg | 30 (38.5) | 13 (48.1) | 7 (43.8) | 41 (41.8) | 9 (39.1) | 28 (45.2) | 13 (54.2) | 9 (25.7) | 40 (44.0) | 10 (33.3) | 20 (40.8) | 30 (41.7) | |
| Tirzepatide 15 mg | 31 (39.2) | 11 (44.0) | 8 (47.1) | 42 (42.9) | 8 (34.8) | 26 (50.0) | 12 (30.0) | 12 (41.4) | 39 (39.4) | 11 (50.1) | 29 (50.0) | 21 (33.3) | |
| SURPASS-2 | Semaglutide 1 mg | 75 (47.5) | 58 (37.2) | 60 (38.7) | 124 (41.1) | 69 (41.3) | 49 (34.0) | 73 (46.5) | 71 (42.3) | 141 (40.8) | 52 (42.3) | 118 (48.4) | 75 (33.3) |
| Tirzepatide 5 mg | 73 (45.6) | 55 (39.0) | 60 (35.5) | 125 (42.7) | 63 (35.6) | 50 (35.7) | 71 (42.3) | 67 (41.4) | 138 (38.3) | 50 (45.5) | 109 (41.1) | 79 (38.5) | |
| Tirzepatide 10 mg | 83 (53.5) | 84 (50.3) | 49 (33.3) | 143 (48.6) | 73 (41.7) | 61 (45.9) | 66 (42.6) | 89 (49.2) | 170 (48.9) | 46 (38.0) | 117 (50.6) | 99 (41.6) | |
| Tirzepatide 15 mg | 79 (46.5) | 59 (43.7) | 73 (44.2) | 141 (46.5) | 70 (41.95) | 55 (40.7) | 74 (47.4) | 82 (45.8) | 169 (46.2) | 42 (40.4) | 129 (50.4) | 82 (38.3) | |
| SURPASS-3 | Insulin degludec | 13 (9.6) | 9 (7.8) | 10 (9.2) | 18 (7.0) | 14 (13.5) | 11 (9.4) | 9 (7.5) | 12 (9.8) | 27 (10.2) | 5 (5.3) | 18 (12.2) | 14 (6.6) |
| Tirzepatide 5 mg | 43 (37.7) | 38 (31.3) | 40 (33.1) | 85 (34.3) | 36 (32.7) | 39 (37.5) | 45 (33.1) | 37 (31.4) | 96 (35.6) | 25 (28.4) | 59 (37.3) | 62 (31.0) | |
| Tirzepatide 10 mg | 46 (37.1) | 58 (45.7) | 50 (45.9) | 105 (42.2) | 49 (44.1) | 59 (50.9) | 53 (44.5) | 42 (33.6) | 114 (42.4) | 40 (44.0) | 76 (46.1) | 78 (40.0) | |
| Tirzepatide 15 mg | 56 (43.8) | 48 (45.7) | 69 (54.8) | 124 (49.2) | 49 (45.8) | 62 (56.9) | 54 (44.6) | 57 (44.2) | 120 (47.2) | 53 (50.5) | 98 (59.4) | 75 (38.7) | |
| SURPASS-4 | Insulin glargine | 28 (15.6) | 38 (14.0) | 80 (14.6) | 85 (14.8) | 61 (14.4) | 52 (13.7) | 51 (14.9) | 43 (15.4) | 66 (12.8) | 80 (16.5) | 57 (15.7) | 89 (14.0) |
| Tirzepatide 5 mg | 19 (30.6) | 35 (32.1) | 54 (34.2) | 62 (32.1) | 46 (33.8) | 40 (33.6) | 39 (32.2) | 29 (32.6) | 59 (31.9) | 49 (34.0) | 47 (35.9) | 61 (30.8) | |
| Tirzepatide 10 mg | 20 (35.1) | 37 (39.4) | 81 (45.8) | 74 (43.5) | 64 (40.5) | 55 (50.5) | 52 (41.3) | 31 (33.3) | 69 (41.6) | 69 (42.6) | 54 (45.4) | 84 (40.2) | |
| Tirzepatide 15 mg | 37 (53.6) | 49 (52.1) | 94 (53.7) | 112 (58.0) | 68 (46.9) | 67 (60.4) | 63 (47.7) | 50 (52.6) | 95 (52.8) | 85 (53.8) | 74 (54.8) | 106 (52.2) | |
| SURPASS-5 | Placebo | 3 (15.0) | 5 (19.2) | 18 (24.3) | 15 (20.8) | 11 (22.9) | 10 (23.8) | 5 (15.6) | 11 (23.9) | 15 (18.8) | 11 (27.5) | 13 (24.1) | 13 (19.7) |
| Tirzepatide 5 mg | 5 (31.3) | 4 (22.2) | 33 (40.2) | 25 (32.9) | 17 (42.5) | 11 (30.6) | 16 (42.1) | 15 (35.7) | 23 (37.1) | 19 (35.2) | 25 (45.5) | 17 (27.9) | |
| Tirzepatide 10 mg | 6 (66.7) | 10 (27.0) | 29 (39.7) | 21 (29.6) | 24 (51.1) | 17 (44.7) | 16 (45.7) | 12 (26.1) | 29 (41.4) | 16 (32.7) | 20 (42.6) | 25 (34.7) | |
| Tirzepatide 15 mg | 8 (61.5) | 13 (43.3) | 34 (44.2) | 32 (41.6) | 23 (53.5) | 18 (50.0) | 18 (47.4) | 19 (41.3) | 34 (47.9) | 21 (42.9) | 30 (54.5) | 25 (38.5) | |
Safety evaluation included all available data from the entire trial period for each trial: 40-week treatment + 4-week safety follow-up period for SURPASS-1, -2, and -5; 52-week treatment + 4-week safety follow-up period for SURPASS-3; and 52-week treatment (primary) + variable treatment period (to 104 weeks) + 4-week safety follow-up for SURPASS-4
BMI body mass index, HbA1c glycated hemoglobin, SGLT2i sodium-glucose co-transporter 2 inhibitor, T2D type 2 diabetes, TEAE treatment-emergent adverse event
Hypoglycemia
As noted in the primary trials, tirzepatide treatment was generally associated with low rates of hypoglycemia < 54 mg/dL or severe hypoglycemia (0–0.03 aggregated rate per year) when used on its own [18–20]. However, when tirzepatide was used in combination with agents known to increase the risk of hypoglycemia (SURPASS-4 [add-on to any combination of metformin, sulfonylurea, and/or SGLT2i] and SURPASS-5 [add-on to insulin glargine ± metformin]), the aggregated rate of hypoglycemia per year ranged from 0.14 to 0.16 and from 0.43 to 0.64, respectively [17, 21]. Within each trial, the incidence of hypoglycemic events (consistent with presentation of the composite endpoint) was similar among participant subgroups stratified by baseline HbA1c, baseline duration of T2D, baseline age, sex, or baseline BMI (Table 6).
Table 6.
Incidence of hypoglycemia with blood glucose < 54 mg/dL or severe hypoglycemia (safety analysis set)
| Trial/treatment arm | Participants with hypoglycemia with glucose < 54 mg/dL or severe hypoglycemia, n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration of T2D (years) | Baseline HbA1c (%) | Baseline BMI (kg/m2) | Age (years) | Sex | |||||||||
| ≤ 5 | > 5–10 | > 10 | ≤ 8.5% | > 8.5% | < 30 | 30 to < 35 | ≥ 35 | < 65 | ≥ 65 | Female | Male | ||
| SURPASS-1 | Placebo | 1 (1.2) | 0 | 0 | 1 (1.2) | 0 | 1 (1.9) | 0 | 0 | 0 | 1 (3.9) | 1 (1.7) | 0 |
| Tirzepatide 5 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tirzepatide 10 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tirzepatide 15 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SURPASS-2 | Semaglutide 1 mg | 1 (0.6) | 0 | 1 (0.7) | 2 (0.7) | 0 | 1 (0.7) | 0 | 1 (0.6) | 2 (0.6) | 0 | 2 (0.8) | 0 |
| Tirzepatide 5 mg | 0 | 2 (1.4) | 2 (1.2) | 3 (1.0) | 1 (0.6) | 1 (0.7) | 2 (1.2) | 1 (0.6) | 3 (0.8) | 1 (0.9) | 3 (1.1) | 1 (0.5) | |
| Tirzepatide 10 mg | 0 | 0 | 1 (0.7) | 1 (0.3) | 0 | 1 (0.8) | 0 | 0 | 0 | 1 (0.8) | 1 (0.4) | 0 | |
| Tirzepatide 15 mg | 3 (1.8) | 2 (1.5) | 3 (1.8) | 5 (1.7) | 3 (1.8) | 2 (1.5) | 1 (0.6) | 5 (2.8) | 8 (2.2) | 0 | 4 (1.6) | 4 (1.9) | |
| SURPASS-3 | Insulin degludec | 11 (8.2) | 6 (5.2) | 9 (8.3) | 18 (7.1) | 8 (7.7) | 6 (5.2) | 13 (10.8) | 7 (5.7) | 17 (6.5) | 9 (9.5) | 10 (6.9) | 16 (7.6) |
| Tirzepatide 5 mg | 2 (1.8) | 1 (0.8) | 2 (1.7) | 4 (1.6) | 1 (0.9) | 1 (1.0) | 2 (1.5) | 2 (1.7) | 2 (0.8) | 3 (3.4) | 3 (1.9) | 2 (1.0) | |
| Tirzepatide 10 mg | 3 (2.4) | 1 (0.8) | 0 | 2 (0.8) | 2 (1.8) | 3 (2.6) | 0 | 1 (0.8) | 4 (1.5) | 0 | 1 (0.6) | 3 (1.5) | |
| Tirzepatide 15 mg | 3 (2.3) | 0 | 5 (4.0) | 7 (2.8) | 1 (0.9) | 4 (3.7) | 4 (3.3) | 0 | 4 (1.6) | 4 (3.8) | 3 (1.8) | 5 (2.6) | |
| SURPASS-4 | Insulin glargine | 21 (11.7) | 40 (14.7) | 130 (23.7) | 112 (19.5) | 79 (18.6) | 91 (24.0) | 54 (15.8) | 46 (16.5) | 98 (19.0) | 93 (19.2) | 81 (22.3) | 110 (17.3) |
| Tirzepatide 5 mg | 3 (4.8) | 7 (6.4) | 19 (12.0) | 21 (10.9) | 8 (5.9) | 13 (10.9) | 8 (6.6) | 8 (9.0) | 14 (7.6) | 15 (10.4) | 13 (9.9) | 16 (8.1) | |
| Tirzepatide 10 mg | 2 (3.5) | 6 (6.4) | 12 (6.8) | 16 (9.4) | 4 (2.5) | 6 (5.5) | 8 (6.4) | 6 (6.5) | 15 (9.0) | 5 (3.1) | 10 (8.4) | 10 (4.8) | |
| Tirzepatide 15 mg | 4 (5.8) | 4 (4.3) | 19 (10.9) | 20 (10.4) | 7 (4.8) | 11 (9.9) | 10 (7.6) | 6 (6.3) | 15 (8.3) | 12 (7.6) | 11 (8.2) | 16 (7.9) | |
| SURPASS-5 | Placebo | 2 (10.0) | 3 (11.5) | 10 (13.5) | 7 (9.7) | 8 (16.7) | 8 (19.1) | 1 (3.1) | 6 (13.0) | 6 (7.5) | 9 (22.5) | 8 (14.8) | 7 (10.6) |
| Tirzepatide 5 mg | 2 (12.5) | 1 (5.6) | 15 (18.3) | 11 (14.5) | 7 (17.5) | 4 (11.1) | 8 (21.1) | 6 (14.3) | 10 (16.1) | 8 (14.8) | 11 (20.0) | 7 (11.5) | |
| Tirzepatide 10 mg | 0 | 8 (21.6) | 15 (20.6) | 15 (21.1) | 8 (17.0) | 9 (24.3) | 5 (14.3) | 9 (19.6) | 16 (22.9) | 7 (14.3) | 10 (21.3) | 13 (18.1) | |
| Tirzepatide 15 mg | 1 (7.7) | 5 (16.7) | 11 (14.3) | 9 (11.7) | 8 (18.6) | 4 (11.1) | 11 (29.0) | 2 (4.4) | 8 (11.3) | 9 (18.4) | 6 (10.9) | 11 (16.9) | |
Severe hypoglycemia was defined as an episode of hypoglycemia requiring assistance to administer therapy. Safety evaluation included all available data from the entire trial period for each trial: 40-week treatment + 4-week safety follow-up period for SURPASS-1, -2, and -5; 52-week treatment + 4-week safety follow-up period for SURPASS-3; and 52-week treatment (primary) + variable treatment period (to 104 weeks) + 4-week safety follow-up for SURPASS-4
BMI body mass index, HbA1c glycated hemoglobin, SGLT2i sodium-glucose co-transporter 2 inhibitor, T2D type 2 diabetes
Discussion
To minimize complications and improve overall health, ADA/EASD and AACE have identified both HbA1c reduction and weight loss as important treatment objectives for people with T2D [5, 6]. Many therapies target single goals and necessitate polypharmacy, but comprehensive disease management commonly requires that multiple treatment goals be addressed simultaneously. More participants randomized to tirzepatide than the respective comparator achieved the composite endpoint of HbA1c ≤ 6.5% and weight reduction ≥ 10% without hypoglycemia, a key holistic outcome conceptually identified by ADA/EASD and AACE guidelines [5, 6], as well as the ADA standards of care [7, 8]. This analysis stratified selected baseline characteristics and demonstrated the ability of tirzepatide-treated participants to consistently achieve the composite endpoint more frequently than those who received the comparator in a generally dose-dependent manner in all subgroups across all SURPASS trials. Importantly, achieving near-normal glycemic levels while minimizing the occurrence of hypoglycemia was shown to be possible with tirzepatide. Even in SURPASS-4 and SURPASS-5, which included concomitant use of antihyperglycemic medications known to cause weight gain and associated with higher risk of hypoglycemia, many participants treated with tirzepatide were able to achieve the composite outcome of this analysis.
Across subgroups, the composite endpoint was achieved by a median of approximately 30%, 45%, and 54% of participants receiving 5-, 10-, and 15-mg doses of tirzepatide, respectively. In comparison, treatment with long-acting insulin and placebo rarely (e.g., ≤ 3.9%) resulted in achievement of the composite endpoint, while treatment with semaglutide 1 mg resulted in achievement of the composite endpoint in a median of 22% of participants across all subgroups. Outlier results in some tirzepatide subgroups appeared to be largely related to small sample sizes in individual groups. It is generally accepted that more stringent therapeutic goals can be attempted for younger people with a shorter duration of T2D, but this analysis suggests that similar composite outcome achievement may be safely possible regardless of diabetes duration or age. This is especially relevant for older adults with T2D, who often have other comorbidities, to be able to improve glycemic outcomes without increased risk of hypoglycemia. In addition, similar achievement of the composite endpoint was also seen in participants across a range of BMI values and baseline HbA1c.
Female participants in the five trials were consistently more likely to achieve the composite endpoint than male participants, and this treatment-by-subgroup interaction was significant, likely driven by the higher percentage of female participants than male participants who achieved a weight loss target of ≥ 10%. In contrast, no discernible difference was observed in the percentage of male or female participants who achieved HbA1c ≤ 6.5%. Of note, this trend was also observed in the comparator arm in SURPASS-2, where a greater percentage of female participants assigned to semaglutide 1 mg achieved both the composite endpoint and the weight loss target of ≥ 10%, a trend not observed with the other non-incretin comparators. The effect of sex on weight loss has been reported elsewhere for participants with T2D treated with liraglutide, exenatide, dulaglutide, or semaglutide [26–29]. This effect may be explained by a higher exposure of medication among female participants than among male participants, potentially because the lower mean body weight of female participants compared to male participants may increase exposure (or plasma concentration) of treatment with known weight reduction capability [30]; however, this hypothesis should be evaluated in future studies.
The use of composite outcome measures in addition to individual treatment targets to assess overall disease control has become common practice, particularly for T2D. Previous studies of GLP-1 RAs in the LEAD, AWARD, and SUSTAIN clinical trial programs including liraglutide, dulaglutide and semaglutide, respectively, have analyzed the composite outcomes of HbA1c < 7.0% without weight gain or hypoglycemia versus active comparators [11, 12, 31]. In each program, the GLP-1 RA was able to help participants achieve the composite outcome more frequently than other non-GLP-1 RA comparators. The present analysis uses a stricter composite outcome definition (HbA1c ≤ 6.5%, weight reduction ≥ 10%, and no hypoglycemia) to assess tirzepatide, a novel GIP/GLP-1 RA, which has not been reported previously, to our knowledge. Although stricter HbA1c criteria have been proposed previously because of potential benefit to long-term outcomes, implementation has been limited because of hypoglycemia risk [32–36] and the inability of those medications to attain this level of glycemic control. Additionally, few non-surgical therapies have been able to consistently result in ≥ 10% weight reduction. The ability of participants randomized to tirzepatide to achieve this stricter composite definition consistently across various participant subgroups highlights several intriguing questions that require additional research. For example, what would be the relationship between this stricter composite endpoint and long-term outcomes such as mortality or cardiovascular events when the hypoglycemia risk is low? How does this composite relate to adherence or participant-reported outcomes? How does the risk/benefit calculation for considering stricter targets in various participant subgroups change? This composite endpoint approach may help clinicians as they strive to make comprehensive decisions with their patients with T2D with various clinical characteristics consistent with the updated guideline recommendations [5–8, 37].
Tirzepatide was generally well tolerated in all five SURPASS trials. The most common TEAEs within the gastrointestinal system organ class were nausea, vomiting, and diarrhea. No pattern of elevated hypoglycemia risk was observed with respect to body weight subgroups or tirzepatide dose even in participants with longer duration of diabetes > 10 years, or BMI < 30 kg/m2 who may have less insulin resistance. However, a tendency toward hypoglycemia < 54 mg/dL or severe hypoglycemia was observed among participants enrolled in SURPASS-4 among whom concomitant sulfonylurea use was permitted or among participants taking insulin in SURPASS-5. Otherwise, tirzepatide treatment was generally associated with low rates of hypoglycemia.
This analysis should be interpreted in the context of several limitations. The SURPASS trials were neither designed nor powered to assess the differential efficacy of tirzepatide between these subgroups of participants. This was a post hoc analysis of five different SURPASS trials and the analysis used unpooled data with several differences in trial characteristics and assessed a composite that was not controlled for multiplicity. Moreover, in some cases, the numbers in the subgroups were small. The analysis also does not account for social determinants of health, which may impact the glycemic and weight outcomes in this population [38]. However, the large percentage of participants from SURPASS-1 through -5 included in the current analysis and in previously published studies provides reassurance that the efficacy and safety of tirzepatide are consistent regardless of T2D duration, sex, HbA1c, age, or BMI at baseline. Additionally, both ADA/EASD and AACE guidelines stress the importance of treating cardiovascular risk factors; the composite in this analysis did not focus on cardiovascular risk factors or endpoints because the completed SURPASS program was not designed to assess cardiovascular outcomes. The potential clinical relevance of reaching and maintaining near normoglycemia with accompanying clinically relevant weight loss in the context of T2D management and the effect of tirzepatide on cardiovascular outcomes remain to be elucidated.
Conclusion
In this post hoc analysis, a higher percentage of participants randomized to tirzepatide achieved a composite outcome of glycemic control and weight loss without hypoglycemia versus comparators. The percentage of participants randomized to tirzepatide who achieved the composite endpoint and the safety of tirzepatide were generally consistent across a broad cross-section of adults with T2D, regardless of baseline characteristics, including age and disease severity at treatment initiation.
Acknowledgements
We thank the study participants and their families for their involvement in the studies.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Regina E. Burris, PhD, at Syneos Health and funded by Eli Lilly and Company.
Author Contributions
Christophe De Block, Jennifer Peleshok, Anita Y.M. Kwan, Neda Rasouli, Juan M. Maldonado, and Brian D. Benneyworth contributed to the study conception and design. Data analysis was performed by Juan M. Maldonado, Minzhi Liu, and Brian D. Benneyworth. Data interpretation was performed by Christophe De Block, Anita Y.M. Kwan, Juan M. Maldonado, Carol Wysham, Grazia Aleppo, and Brian D. Benneyworth. Jennifer Peleshok, Juan M. Maldonado, and Brian D. Benneyworth participated in writing the first draft of the manuscript and all authors critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Eli Lilly and Company.
Data Availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Declarations
Conflict of Interest
Christophe De Block has received consulting fees and honoraria for speaking from: Abbott, A. Menarini Diagnostics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Medtronic, Novo Nordisk, and Roche. John P. H. Wilding is a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Napp, Novo Nordisk, Mundipharma, Rhythm Pharmaceuticals, Sanofi, and Saniona, a grant holder (University of Liverpool) for research grants for clinical trials from AstraZeneca and Novo Nordisk, and has received personal honoraria / lecture fees from AstraZeneca, Boehringer Ingelheim and Napp. Neda Rasouli receives research funding from Eli Lilly and Company and Novo Nordisk, and a consultant for Eli Lilly and Company, Novo Nordisk and Sanofi. Carol Wysham has received support from Novo Nordisk, Eli Lilly and Company, Biomea, Abbott, CeQur, Fractyl Health, and Abvance. Grazia Aleppo has received research support to her employer from Fractyl Health, Insulet, MannKind, Tandem Diabetes, and Welldoc; Grazia Aleppo has received consulting fees from Medscape, Dexcom, and Insulet. Jennifer Peleshok, Anita Y. M. Kwan, Juan M. Maldonado, and Brian D. Benneyworth are full-time employees of Eli Lilly and Company and are minority holders of company stock. Minzhi Liu reports no commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
The SURPASS trials were conducted in accordance with consensus ethical principles, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation Good Clinical Practice guidelines, and applicable laws and regulations and were approved by the relevant ethics committee/review board at each site. All participants in all primary trials provided written informed consent. Each trial was registered with ClinicalTrials.gov: identifiers NCT03954834, NCT03987919, NCT03882970, NCT03730662, and NCT04039503.
References
- 1.International Diabetes Federation. About diabetes: facts and figures. Brussels: International Diabetes Federation; 2023. https://idf.org/about-diabetes/diabetes-facts-figures/. Accessed Dec 6, 2023.
- 2.Centers for Disease Control and Prevention. National diabetes statistics report: risk factors for diabetes-related complications; 2022. Updated Sept 30, 2022. https://www.cdc.gov/diabetes/data/statistics-report/risks-complications.html.
- 3.Gomes MB, Rathmann W, Charbonnel B, et al. Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Diabetes Res Clin Pract. 2019;151:20–32. [DOI] [PubMed] [Google Scholar]
- 4.King A, Miller EM. Glucagon-like peptide 1 receptor agonists have the potential to revolutionize the attainment of target A1C levels in type 2 diabetes-so why is their uptake so low? Clin Diabetes. 2023;41(2):226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology clinical practice guideline: developing a diabetes mellitus comprehensive care plan-2022 update. Endocr Pract. 2022;28(10):923–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ElSayed NA, Aleppo G, Aroda VR, et al. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergenstal RM, Li Y, Porter TK, Weaver C, Han J. Exenatide once weekly improved glycaemic control, cardiometabolic risk factors and a composite index of an HbA1c < 7%, without weight gain or hypoglycaemia, over 52 weeks. Diabetes Obes Metab. 2013;15(3):264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonora E, Frias JP, Tinahones FJ, et al. Effect of dulaglutide 3.0 and 4.5 mg on weight in patients with type 2 diabetes: exploratory analyses of AWARD-11. Diabetes Obes Metab. 2021;23(10):2242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVries JH, Desouza C, Bellary S, et al. Achieving glycaemic control without weight gain, hypoglycaemia, or gastrointestinal adverse events in type 2 diabetes in the SUSTAIN clinical trial programme. Diabetes Obes Metab. 2018;20(10):2426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dungan KM, Raz I, Skrivanek Z, Sealls W, Fahrbach JL. Achieving the composite endpoint of glycated haemoglobin <7.0%, no weight gain and no hypoglycaemia in the once-weekly dulaglutide AWARD programme. Diabetes Obes Metab. 2016;18(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frías JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9(9):563–74. [DOI] [PubMed] [Google Scholar]
- 14.Eli Lilly and Company. MOUNJARO® (tirzepatide) Injection, for subcutaneous use. 2023.
- 15.Eli Lilly and Company. ZEPBOUND—tirzepatide injection, solution. 2023.
- 16.Lingvay I, Cheng AY, Levine JA, et al. Achievement of glycaemic targets with weight loss and without hypoglycaemia in type 2 diabetes with the once-weekly glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide: a post hoc analysis of the SURPASS-1 to -5 studies. Diabetes Obes Metab. 2023;25(4):965–74. [DOI] [PubMed] [Google Scholar]
- 17.Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–55. [DOI] [PubMed] [Google Scholar]
- 19.Ludvik B, Giorgino F, Jodar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–98. [DOI] [PubMed] [Google Scholar]
- 20.Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15. [DOI] [PubMed] [Google Scholar]
- 21.Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–24. [DOI] [PubMed] [Google Scholar]
- 22.Aroda VR, Capehorn MS, Chaykin L, et al. Impact of baseline characteristics and beta-cell function on the efficacy and safety of subcutaneous once-weekly semaglutide: a patient-level, pooled analysis of the SUSTAIN 1–5 trials. Diabetes Obes Metab. 2020;22(3):303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plat AW, Rasouli N, Peleshok J, Sapin H, Wilding J. 720-P: change in body weight from baseline with tirzepatide: sex subgroup analysis of the SURPASS studies. Diabetes. 2022. 10.2337/db22-720-P. [Google Scholar]
- 26.Mirabelli M, Chiefari E, Caroleo P, et al. Long-term effectiveness of liraglutide for weight management and glycemic control in type 2 diabetes. Int J Environ Res Public Health. 2019. 10.3390/ijerph17010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onishi Y, Oura T, Matsui A, Matsuura J, Iwamoto N. Analysis of efficacy and safety of dulaglutide 0.75 mg stratified by sex in patients with type 2 diabetes in 2 randomized, controlled phase 3 studies in Japan. Endocr J. 2017;64(5):553–60. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard RV, Hertz CL, Ingwersen SH, Navarria A, Drucker DJ. Levels of circulating semaglutide determine reductions in HbA1c and body weight in people with type 2 diabetes. Cell Rep Med. 2021;2(9): 100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rentzeperi E, Pegiou S, Koufakis T, Grammatiki M, Kotsa K. Sex differences in response to treatment with glucagon-like peptide 1 receptor agonists: opportunities for a tailored approach to diabetes and obesity care. J Pers Med. 2022;12(3):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilding JP, Overgaard RV, Jacobsen LV, Jensen CB, le Roux CW. Exposure-response analyses of liraglutide 3.0 mg for weight management. Diabetes Obes Metab. 2016;18(5):491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinman B, Schmidt WE, Moses A, Lund N, Gough S. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012;14(1):77–82. [DOI] [PubMed] [Google Scholar]
- 32.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. [DOI] [PubMed] [Google Scholar]
- 34.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 35.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. [DOI] [PubMed] [Google Scholar]
- 36.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unnikrishnan AG, Bhattacharyya A, Baruah MP, Sinha B, Dharmalingam M, Rao PV. Importance of achieving the composite endpoints in diabetes. Indian J Endocrinol Metab. 2013;17(5):835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2020;44(1):258–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.