Abstract
With a better understanding of the susceptibility to atrial fibrillation (AF) and the thrombogenicity of the left atrium, the concept of atrial cardiomyopathy (ACM) has emerged. The conventional viewpoint holds that AF-associated hemodynamic disturbances and thrombus formation in the left atrial appendage are the primary causes of cardiogenic embolism events. However, substantial evidence suggests that the relationship between cardiogenic embolism and AF is not so absolute, and that ACM may be an important, underestimated contributor to cardiogenic embolism events. Chronic inflammation, oxidative stress response, lipid accumulation, and fibrosis leading to ACM form the foundation for AF. Furthermore, persistent AF can exacerbate structural and electrical remodeling, as well as mechanical dysfunction of the atria, creating a vicious cycle. To date, the relationship between ACM, AF, and cardiogenic embolism remains unclear. Additionally, many clinicians still lack a comprehensive understanding of the concept of ACM. In this review, we first appraise the definition of ACM and subsequently summarize the noninvasive and feasible diagnostic techniques and criteria for clinical practice. These include imaging modalities such as echocardiography and cardiac magnetic resonance imaging, as well as electrocardiograms, serum biomarkers, and existing practical diagnostic criteria. Finally, we discuss management strategies for ACM, encompassing “upstream therapy” targeting risk factors, identifying and providing appropriate anticoagulation for patients at high risk of stroke/systemic embolism events, and controlling heart rhythm along with potential atrial substrate improvements.
Keywords: atrial cardiomyopathy, atrial fibrillation, atrial remodeling, cardiogenic embolism
1. Introduction
Atrial fibrillation (AF) is one of the most common cardiac arrhythmias in clinical practice, affecting over 50 million individuals worldwide [1, 2, 3]. With the aging population and increasing life expectancy, the incidence and prevalence of AF are rising and are expected to more than double over the next 30 years [2, 4]. Due to its serious health implications and significant economic burden on families and society, AF has become a serious global public health issue [2, 4]. Over the last few decades, considerable progress has been made in understanding the pathogenesis of AF. The mechanism of AF is a complex process involving multiple factors and molecular mechanisms. Current research generally agrees that the occurrence and maintenance of AF are due to the combined effect of triggers [5] and substrates [6, 7]. In recent years, the concept of atrial cardiomyopathy (ACM) has been increasingly recognized, highlighting abnormal structural and functional changes in the atria that may be associated with the occurrence and maintenance of AF. The pathophysiological processes of ACM may include atrial fibrosis, atrial conduction abnormalities and atrial contractile dysfunction [8]. These changes can promote the atrial electrical and structural remodeling, providing the triggers and substrates for the onset and maintenance of AF [9]. Additionally, the persistent presence of AF may further exacerbate ACM, creating a vicious cycle [10]. Despite its importance, ACM still remains a scientific concept that has not yet been integrated into routine clinical practice. Many clinical practitioners still have gaps in their understanding and management of ACM. This review aims to provide clinical practitioners with a deeper understanding of the definition, diagnosis, and management of ACM, and to offer patients more specialized treatment approaches and management strategies.
2. Definition of Atrial Cardiomyopathy
In 1972, Williams et al. [11] described a familial disease characterized by atrial arrhythmias and atrioventricular block and first used the term “atrial cardiomyopathy” to define this condition. Over the past half century, with the deepening understanding of ACM, this concept has evolved from an initial descriptive definition to a well-recognized medical term. According to the EHRA/HRS/APHRS/SOLAECE expert consensus, ACM is defined as “any complex of structural, architectural, contractile or electrophysiological changes affecting the atrium with the potential to produce clinically relevant manifestations” [12]. This definition remains relatively vague and lacks clear diagnostic criteria. It is important to distinguish ACM from “atrial remodeling”. Atrial remodeling refers to the adaptive regulation of cardiomyocytes in response to external stressors (e.g., hypertension, heart failure, AF, and others), to maintain homeostasis [13]. These changes, encompassing atrial wall thickening, atrial dilation, and alterations in atrial electrophysiological characteristics, initially serve to compensate for cardiac function, but they may gradually evolve into nonadaptive changes, leading to progressive atrial pump failure and atrial arrhythmias [13]. The concept of ACM emphasizes the pathological changes within the atrial myocardium, which may serve as either the direct cause or the consequence of atrial remodeling. From this perspective, atrial remodeling can be considered a specific phase of ACM, particularly as a manifestation of ACM progression. However, ACM is a broader concept, encompassing not only atrial remodeling but also other inherent pathological changes in the atrial myocardium, including genetically related ACM [14] as well as systemic diseases (e.g., amyloidosis) involving the atria [15].
3. Diagnosis of Atrial Cardiomyopathy
A key reason why clinical practitioners have an insufficient understanding of ACM is the lack of clear diagnostic criteria. According to the EHRA/HRS/APHRS/SOLAECE expert consensus, the classification of ACM relies on histopathological findings and the presence of fibrosis and/or non-collagen deposits [12]. This pathological diagnosis method is impractical for clinical application. Here, we briefly introduce commonly used auxiliary inspection methods and feasible diagnostic criteria for assessing ACM. Effective assessment and characterization of ACM progression could enable a more informed and individualized strategy for addressing modifiable risk factors, as well as for the primary prevention of ACM complications.
3.1 Imaging Examination for Atrial Cardiomyopathy
Due to the widespread use of echocardiography, it has become the primary method for assessing ACM. Left atrial (LA) dilation is the most prominent feature in the remodeling process of ACM and the visual representation of LA diastolic dysfunction [16]. It is a significant predictor of various cardiovascular diseases, such as AF, stroke, and heart failure [17]. The measurement of LA anteroposterior diameter through M-mode and subsequent two-dimensional echocardiography is simple and reproducible, previous studies have used LA anteroposterior diameter as one of the morphological parameters for ACM [12, 18]. However, due to the irregular three-dimensional structural characteristics of the atrium and the heterogeneity of atrial remodeling, measuring the atrial volume is more accurate for reflecting and assessing the true state of the atria than atrial diameter. In the case of LA enlargement, the increase in LA anteroposterior diameter is not directly proportional to the increase in the total LA volume, which becomes the main source of error in the assessment of LA volume by two-dimensional echocardiography [19]. LA volumes assessed by two-dimensional echocardiography are generally less than those calculated by cardiac computed tomography (CCT) or by cardiac magnetic resonance imaging (CMRI) [17]. On the contrary, the accuracy of three-dimensional echocardiography in evaluating LA volume is closer to CCT or CMRI [10]. The previous view was that LA maximum volume is the most definitive parameter of LA morphology and reflects LA diastolic function to a certain extent [20]. However, the latest research supports the important role of minimal LA volume in assessing atrial function and disease [21]. Atrial fibrosis can lead to atrial structural remodeling, which is closely related to changes in atrial volume [22]. Another study has shown that LA enlargement is associated with adverse clinical outcomes, independent of AF, such as stroke and heart failure [23, 24]. However, in addition to intrinsic LA pathology, LA volume is also associated with a variety of factors, including left ventricular filling pressure, valvular heart disease, and so on, therefore it is difficult to separate the impact of LA dilatation from left ventricular (diastolic) dysfunction [17]. Moreover, changes in LA volume are reversible, thus LA volume does not necessarily reflect LA function independently [25].
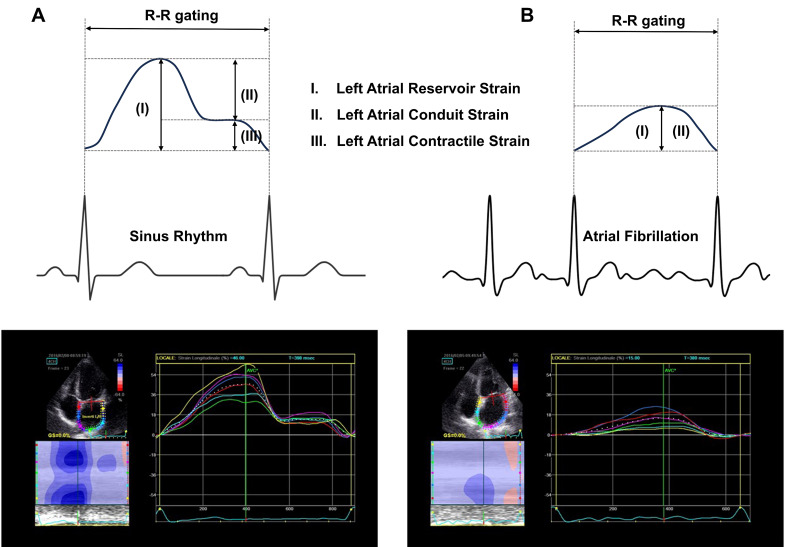
In addition to changes in LA structure, LA dysfunction also provides important diagnostic clues for ACM. In two prospective studies, after adjusting for baseline risk factors, a decreased left atrial emptying fraction (LAEF) was identified as a significant risk factor for new-onset AF or atrial flutter, independent of LA volume and left ventricular ejection fraction (LVEF) [26, 27]. Furthermore, a decreased LAEF was also associated with low-voltage zones in electroanatomical mapping [28] and the recurrence of AF after radiofrequency ablation [29]. Eichenlaub et al. [30] used a low-voltage zone (0.5 mV) greater than 2 cm2 detected by electroanatomical mapping as the diagnostic criterion for ACM and assessed the value of LAEF in diagnosing ACM. Their results indicated that a LAEF cutoff point of less than 34% could predict ACM effectively (area under the curve [AUC] 0.846, sensitivity 69.2%, specificity 76.5%) and was associated with a higher risk of AF recurrence after radiofrequency ablation [30]. Furthermore, LA flow status can also serve as a predictive indicator for ACM, with low left atrial appendage (LAA) peak flow velocity being associated with the recurrence of AF after ablation [31] and multiple infarctions in patients with cryptogenic stroke [32]. Another echocardiographic parameter that has gained attention is the LA strain parameter. Compared to conventional echocardiographic measurements, LA strain obtained through two-dimensional speckle-tracking echocardiography offers several advantages: it is free from angle alignment issues and is less influenced by loading conditions [33]. Additionally, LA strain can detect functional abnormalities even in the absence of visible LA dilation, making it a valuable predictor for ACM. The LA function consists of three primary components: the reservoir, the conduit, and the active pump. During a normal cardiac cycle, approximately 75% of the stroke volume flows into the left ventricle while the LA volume decreases during the early- and mid-conduit phases. In the pump phase, the LA actively contracts to expel the remaining 25% of the blood [34]. In the case of AF, the absence of effective atrial contraction typically reduces stroke volume by about 25%. The terminology of “LA strain” varies across different studies and appears as peak atrial longitudinal strain or atrial global strain. In this review, as shown in Fig. 1 (Ref. [35]), we use the terms LA reservoir strain (LASr), conduit strain (LAScd), and contractile strain (LASct) based on a consensus statement [36]. Previous studies have confirmed that LASr is inversely correlated with LA fibrosis confirmed histologically (R –0.55~–0.82, p 0.001) [37, 38]. Furthermore, LASr has a predictive value for newly developed AF and stroke in the general population [39]. LASr was also independently associated with stroke/systemic emboli (S/SE) events in patients with AF (OR 0.74; 95% CI 0.67–0.82; p 0.001), providing an incremental predictive value over the CHA2DS2-VASc (congestive heart failure, hypertension, age 75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65 to 74 years, sex category) score [40]. In a retrospective study, transesophageal echocardiography was utilized to determine the presence of LAA thrombosis in patients with non-valvular AF scheduled for electrical cardioversion. Additionally, transthoracic echocardiography and LA strain parameter measurements were routinely performed. The results of the multivariate logistic regression analysis indicated that LVEF, E/e’ (early diastolic velocity of mitral flow/early velocity of diastolic mitral annulus motion) ratio, and LASr were all independently associated with LAA thrombosis. Among these, LASr (cutoff value 9.1%, AUC 0.95) demonstrated the best diagnostic performance [41]. Another study found LA strain to be a significant predictor of AF recurrence after ablation over a 6-month follow-up period: patients who maintained sinus rhythm showed greater improvement in LASr than those who relapsed [42]. However, similar to three-dimensional cardiac ultrasound, LA strain is not widely used yet in current medical facilities, which limits its value in screening and evaluating ACM.
Fig. 1.
Schematic of left atrial strain in sinus rhythm (A) and atrial fibrillation (B). (A) During sinus rhythm, LASr = –(LAScd + LASct); (B) During atrial fibrillation, LASr = –LAScd due to the absence of LASct. LASr, left atrial reservoir strain; LAScd, left atrial conduit strain; LASct, left atrial contractile strain. Partially adapted from Cameli et al. [35].
CMRI is precise and highly reproducible and has now become the gold standard for evaluating LA volume and function. Late gadolinium enhancement (LGE) imaging is a non-invasive, well-established technique for detecting atrial fibrosis. The imaging agent gadolinium is taken up and released into the bloodstream quickly by healthy myocardial tissue, but it dissipates more slowly from diseased tissue with interstitial fibrosis. This property allows visualization of the amount of contrast agent retained in the myocardium at a delayed time (10–30 minutes) after contrast injection. Therefore, parametric T1-mapping indices offer high diagnostic accuracy for identifying diffuse interstitial fibrosis [43]. LA remodeling characterized by LGE-CMRI shows a significant correlation with atrial volume, AF type, and recurrence risk [44, 45]. Additionally, LGE-CMRI also contributes for predicting stroke risk in AF patients, LA fibrosis detected through LGE-CMRI was an independent predictor of stroke events and significantly increased the predictive power of the CHA2DS2-VASc score [46]. McGann et al. [47] attempted to identify LA remodeling and stratify individuals who would benefit from AF catheter ablation through LGE-CMRI. They found that extensive LGE (30% LA wall enhancement) predicted a poor response to catheter ablation therapy for AF [47]. In recent years, the application of CMRI in catheter ablation procedures for AF has received significant attention. During a two-year follow-up, Quinto et al. [48] found that identifying anatomical veno-atrial gaps using LGE-CMRI in repeat catheter ablation procedures could shorten ablation duration (161 52 minutes vs. 195 72 minutes) and decreased the incidence of recurrent atrial tachycardia (30% vs. 61%). Akoum et al. [49] utilized LGE-CMRI to assess the baseline level of atrial fibrosis in patients with AF and the level of residual fibrosis outside the scar tissue three months post-ablation. Their findings indicated that the level of residual fibrosis could serve as a predictor for AF recurrence [49]. However, results from the DECAAF II trial, published in the Journal of the American Medical Association (JAMA), found no statistically significant difference in atrial arrhythmia recurrence rates between CMRI-guided fibrosis ablation plus pulmonary vein isolation (PVI) and PVI catheter ablation alone in patients with persistent AF [50]. One potential explanation is that the ablation strategy for fibrotic atria has not been standardized, lacking a definitive ablation endpoint, making it susceptible to subjective influence from the operator. Moreover, several factors further limit the applicability of LGE-CMRI. For instance, the atrial wall is relatively thin compared to the resolution of CMRI, making its imaging signal susceptible to interference from adjacent tissues or “volume effect”. Additionally, the post-processing of acquired data remains non-standardized. Current algorithms for quantifying atrial fibrosis typically rely on signal intensity comparisons with the patient’s own normal atrial tissue or atrial blood pool signal-to-noise ratio, which lack uniform comparability among different individuals. Furthermore, the imaging quantification algorithms currently in use have not yet been correlated with low-voltage zones identified by electroanatomical mapping or with histological fibrosis detection [51].
3.2 Electrocardiogram Examination for Atrial Cardiomyopathy
Electrocardiogram (ECG) examination also played a routine and crucial role in assessing ACM. The P-wave is the summation of the depolarization of the right atria (first third of the P-wave) and the LA (last third of the P-wave). Several P-wave indices (PWIs) in ECG are currently thought to be associated with ACM, such as P-wave dispersion (PWd), the P-wave terminal force in lead V1 (PTFV1), P-wave axis (PWA), P-wave duration (PWD) and the burden of premature atrial contractions (PACs) [52].
PWd refers to the variation between the maximum and minimum P-wave durations measured simultaneously across the 12 leads of a standard ECG [53]. The durations in different leads of the ECG reflect regional heterogeneity in atrial depolarization. Inhomogeneous and discontinuous atrial conductions contribute to increased PWd based on the anisotropic distribution of connections between atrial myocardial fibers [54]. Collagen deposition in the LA myocardium results in decreased LA compliance, which is manifested as prolongation of the PWd on ECG [55]. Additionally, increased PWd is also considered to be connected with compromised mechanical function and enlargement of the LA [56]. Therefore, increased PWd suggests the formation of an atrial substrate conducive to AF and multiple studies have demonstrated a strong correlation between increased PWd and AF in cases of cryptogenic stroke [57, 58].
PTFV1 is defined as the product of the amplitude and duration of the terminal negative deflection of the P-wave in lead V1. Increased PTFV1 has been demonstrated to be correlated with LA abnormalities, such as enlargement, dysfunction and delayed interatrial conduction [59, 60]. Tiffany Win et al. [60] conducted a more detailed analysis of the two components of PTFV1 (amplitude and duration of the terminal negative deflection of P-wave), revealing their distinct predictive value. The duration correlates with the degree of atrial fibrosis, whereas the amplitude is tied to the LA mechanical functions such as volume and LA strain [60].
The PWA is defined as the net direction of electrical forces in the atrium, and its normal value is typically considered to be in the range of 0° and 75°. Previous studies have indicated that abnormal PWA is an important predictor of AF [61] and is associated with an increased risk of ischemic stroke [62] and all-cause mortality [63]. Maheshwari et al. [64] also found that abnormal PWA was the only PWI linked to an elevated risk of S/SE (HR [hazard ratio] 1.84; 95% CI 1.33–2.55) independent of CHA2DS2-VASc scores based on two large prospective cohort studies (Atherosclerosis Risk in Communities [ARIC] and Multi-Ethnic Study of Atherosclerosis [MESA]). They also created the P2-CHA2DS2-VASc score, which is a better prediction tool for AF-related ischemic stroke compared to the CHA2DS2-VASc score [64]. However, a long-term study showed that among subjects with abnormal PWA at baseline, less than 40% continued to have abnormal PWA at follow-up 11 years later [65], suggesting that the measurement of the PWA is quite unstable, limiting its clinical application. Furthermore, only the frontal PWA is routinely reported on a 12-lead ECG, atrial depolarization occurs in three-dimensional space, therefore a three-dimensional PWA loop can more accurately simulate the actual directionality of the atrial depolarization vector in vivo [66]. Spatial P loop vectors and morphology and the association between three-dimensional depolarization vector changes and ACM still need further research and demonstration.
In addition to the above PWIs, the prolonged PWD and frequent PACs are also considered to be related to ACM. The prolonged PWD (120 ms) indicates the presence of interatrial conduction block, which is caused by an altered cardiac conduction due to fibro-fatty transformation of the atrium PWD [59, 67]. Several previous studies have demonstrated that prolonged PWD increases the risk of AF [68] and ischemic stroke [69]. Amplified P-wave duration (aPWD) (40 to 50 mm/mV amplification and 100 to 200 mm/s sweep speed) can provide more valuable information. Jadidi et al. [70] found that the aPWD of LA (from -dV/dt to the end of the P-wave in lead V1) is closely related to the LA activation time and low-voltage substrate. Similarly, frequent PACs detected on ECG are also associated with AF development [71] and ischemic stroke [72]. The prolonged P-R interval [73, 74] and P-wave area (PWa) [75], which is the total geometric area under the P-wave in the 12-lead ECG, have also been considered related to ACM. However, their predictive performance and clinical application value still require further evaluation.
Artificial intelligence (AI)-based ECG analysis has become a promising direction for studying and diagnosing ACM. Verbrugge et al. [76] used AI-based ECG analysis to identify the presence of underlying ACM in patients with heart failure with preserved ejection fraction (HFpEF), and the results were further evidenced by structural, functional, hemodynamic abnormalities, and long-term risk for incident AF. Similarly, another study showed AI-based ECG analysis can effectively quantify the risk of AF and this predictive capability is complementary to traditional clinical risk factor models [77]. In a retrospective study published in The Lancet, Attia et al. [78] trained a convolutional neural network on ECG features of sinus rhythm in patients with a history of AF or atrial flutter, achieving good validation in the test group. However, whether these findings can be generalized to screening high-risk populations for AF still requires large-scale prospective studies.
3.3 Serum Biomarkers for Atrial Cardiomyopathy
Although not specific, various serum biomarkers characterizing myocyte injury, inflammation, and fibrosis have been linked to the occurrence and outcomes in ACM and can be used as an auxiliary means to evaluate and diagnose ACM.
N-terminal pro-B-type natriuretic peptide (NT-proBNP) and B-type natriuretic peptide (BNP) are well-known indicators of congestive heart failure due to volume overload and myocardial damage. However, NT-proBNP also shows a strong correlation with echocardiographic parameters of LA remodeling and dysfunction [79]. Patel et al. [80] found that higher levels of NT-proBNP and troponin I were independently associated with lower LASr and LASct (p 0.05). Furthermore, increased NT-proBNP may serve as a predictor of LA fibrosis and has been utilized as one of the diagnostic criteria for ACM in some investigations [81, 82, 83]. Elevated BNP and NT-proBNP levels have been linked to an increased risk of cardioembolic stroke independently of AF [84], the biomarker-based ABC (age, biomarkers [high-sensitivity (hs-) troponin and NT-proBNP], and clinical history of prior stroke/transient ischemic attack) stroke score was well applicable and generally more suitable than the CHA2DS2-VASc and ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) stroke scores in anticoagulated patients with AF [85]. Furthermore, a meta-analysis by Xu and Tang [86] demonstrated that baseline BNP/NT-proBNP levels can serve as predictive indicators for AF recurrence following successful electrical cardioversion.
In contrast to NT-proBNP and BNP, which are generated by both the atria and ventricles, atrial natriuretic peptide (ANP) is predominantly produced by the atria, theoretically making it a more precise biomarker for assessing ACM. Midregional pro-A-type natriuretic peptide (MR-proANP) is more stable than ANP, leading to its widespread use in clinical settings. Similar to NT-proBNP, MR-proANP levels are independently linked to LA enlargement [87]. A study involving 190 patients demonstrated that MR-proANP is a stronger biomarker than NT-proBNP for indicating the LA volume index and atrial volume overload in individuals with HFpEF [88]. Seewöster and colleagues [89] developed an innovative biomarker-based score (consisting of age 65 or above, NT-proANP levels above the 75th percentile in peripheral blood [17 ng/mL], and persistent AF), which was found to be a noticeable predictor of low-voltage zones in patients with AF who were undergoing catheter ablation.
Biomarkers of fibrosis and inflammation are also implicated in atrial inflammation, matrix remodeling, and atrial fibrosis, significantly associated with the progression of ACM. In a sub-analysis of ARTEMIS trail, the soluble isoform of suppression of tumorigenicity-2 (sST2) and hs-C-reactive protein (CRP), retained significant power in predicting new-onset AF after correcting for other risk factors [90]. Additionally, ST-2 is also associated with increased LA volume index [91]. A study including 63 patients showed a negative correlation between galectin-3 and LA related echocardiographic parameters, including LA volume and LA strain rate [92]. Serum levels of carboxyl terminal peptide from pro-collagen I (PICP) and amino terminal peptide from pro-collagen III (PIIINP) have been reported to be associated with the existence of LA fibrosis and may serve as predictors for post-operative AF even in patients without previous AF history [93]. Furthermore, there is also a linear correlation between serum PICP levels and the extent of LA fibrosis [93]. However, the translational value of biomarkers of fibrosis and inflammation in assessing and diagnosing ACM needs further validation.
3.4 Electrophysiological Examination for Atrial Cardiomyopathy
Electroanatomical mapping has become a common method for assessing the atrial substrate during AF radiofrequency ablation. This technique involves mapping thousands of bipolar voltage points (i.e., the voltage difference between two adjacent unipolar electrodes) onto a three-dimensional model of the atrium. Current views suggest that low bipolar atrial voltage may serve as an alternative marker for atrial fibrosis. Physiologically, low-voltage zones in electroanatomical mapping result from the anisotropic distribution of connections between cardiomyocytes and fibroblasts, as well as the low coupling between different cell types [94, 95]. Therefore, decreased conduction velocity may also coexist with low-voltage zones. Compared to paroxysmal AF, patients with persistent AF have a longer atrial activation time, which is negatively correlated with LA voltage [96]. Additionally, a reduction in atrial voltage often accompanies increased atrial volume and dysfunction, indicating that low voltage is closely related to changes in LA morphology and atrial wall stress. In an ex vivo experiment using Langendorff-perfused canine hearts, acute LA dilation led to a significant decrease in LA voltage, especially in the posterior wall [97]. Another clinical study found that patients with low-voltage zones in the LA typically had larger LA volumes and a higher incidence of persistent AF [98]. Some perspectives suggest that low-voltage zones are also associated with AF triggers. For instance, patients with AF originating from the pulmonary veins often have lower voltages in the pulmonary vein antra, while those with AF triggered from the superior vena cava tend to have lower mapping voltages in the right atrium [99]. Therefore, substrate modification guided by low-voltage zones may be a promising strategy for AF ablation. Rolf et al. [100] first reported on this in 2014. In their non-randomized study, AF patients with low-voltage zones who underwent PVI combined with tailored substrate modification achieved a higher 12-month AF-free survival rate compared to those who received PVI alone [100]. Although subsequent studies have yielded conflicting conclusions on whether modification of low-voltage zones benefits patients [101, 102], a meta-analysis concluded that ablation of low-voltage zones can generally improve the long-term effectiveness of AF ablation [103].
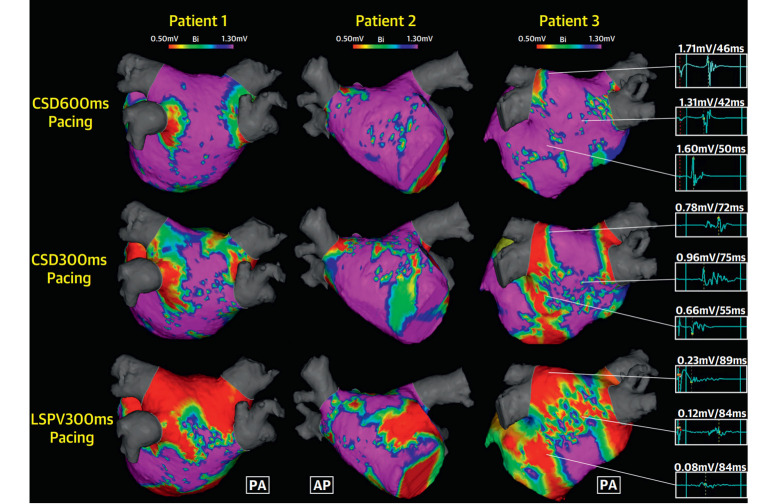
However, electrophysiological examination also has certain limitations in diagnosing ACM. Firstly, as an invasive procedure, it is limited in its widespread application in clinical settings, especially as it cannot serve as a routine screening method for ACM. Secondly, electroanatomical mapping technology has inherent shortcomings in accurately reflecting atrial fibrosis and electrical remodeling. For instance, atrial tissue thickness varies among different patients and different regions of the atrium (such as the pectinate muscles compared to the smooth atrial wall), leading to significant differences in normal voltage levels. Therefore, it is not feasible to simply use a uniform cutoff point (e.g., 0.5 mV) to define low-voltage zones. Furthermore, the distribution of low-voltage zones in electroanatomical mapping is significantly influenced by the direction and velocity of wavefront propagation. Research by Wong et al. [104] published in the Journal of the American College of Cardiology (JACC): Clinical Electrophysiology indicates that changes in pacing site and pacing cycle length can significantly affect the low-voltage zones, conduction velocity, and complex fractionation in LA electroanatomical mapping (Fig. 2, Ref. [104]). Additionally, heart rhythm status significantly impacts the presentation of bipolar electrograms. Two early studies revealed that some low-voltage zones detected by electroanatomical mapping during AF episodes unexpectedly displayed normal voltage after the restoration of sinus rhythm [105, 106]. A study by Qureshi et al. [107] published in Heart Rhythm, found that with sufficient sampling time, the spatial distribution of low-voltage zones detected during AF episodes correlated more closely with myocardial fibrosis zones identified by LGE-CMRI than those detected during sinus rhythm. The lower voltage observed in bipolar electrograms during AF may be attributed to changes in wavefront direction relative to electrode orientation, as well as the effects of complex fractionation and collision. Compared to bipolar techniques, omnipolar electrograms can extract the maximum atrial voltage during AF episodes without being influenced by these factors [108]. Therefore, the next step should focus on standardizing the pacing site, pacing cycle length, and atrial rhythm as a priority. Based on this standardization, high-quality clinical studies should be conducted to further characterize the auxiliary value of electroanatomical mapping in the diagnosis of ACM and the process of AF ablation. Additionally, we should actively explore and implement new technologies, including omnipolar mapping and dynamic voltage mapping.
Fig. 2.
Marked variation in the mapped atrial substrate was observed under different pacing sites and pacing cycle lengths among three patients. The red areas represent low-voltage zones (0.5 mV), which show significant differences with changes in pacing site and cycle length, even within the same patient. In patient 3, a 72-year-old male with persistent atrial fibrillation, the complex fractionated electrograms were longer and accompanied by lower atrial voltages under each identical pacing strategy compared to the other two patients. CSD distal coronary sinus; LSPV, left superior pulmonary vein; PA, posteroanterior; AP, anteroposterior. Adapted from Wong et al. [104].
3.5 Feasible Diagnostic Criteria for Atrial Cardiomyopathy
The inclusion criteria of the ARCADIA trial defined ACM as P-wave terminal force greater than 5000 µVms in ECG lead V1, a serum NT-proBNP level greater than 250 pg/mL, or LA diameter index of 3 cm/m2 or greater on the echocardiogram [83]. The ATTICUS trial compared the effectiveness of apixaban versus aspirin in preventing the recurrence of ischemic stroke. Patients were included based on ECG and echocardiogram parameters indicative of ACM, which included at least one of the following criteria: LA diameter 45 mm (measured at parasternal axis), spontaneous echo contrast in the LAA, LAA flow velocity 0.2 m/s, atrial high-rate episodes and CHA2DS2-VASc score 4 [109].
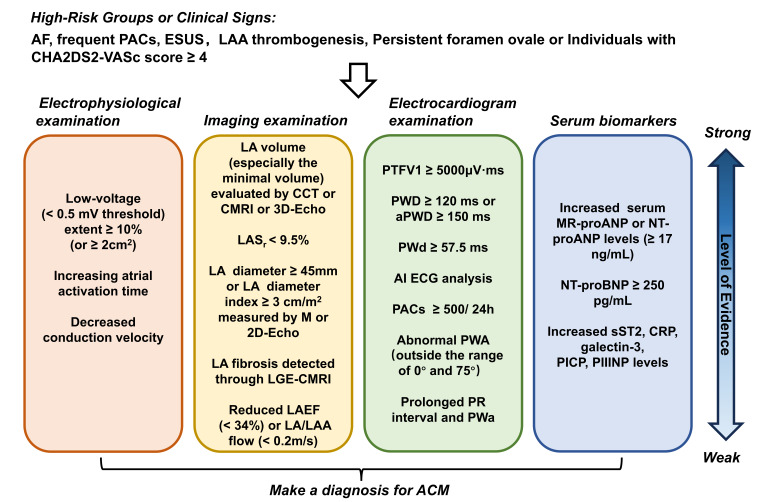
Johnson et al. [110] established another possible staging system for ACM in the absence of ongoing AF based on a large-scale population survey. Briefly, Johnson et al. classified the risk of ACM in individuals into four stages based on ECG and echocardiographic features: Stage 0: No evidence of atrial disease or arrhythmia; Stage 1: PACs 500/24 h OR LA volume index 34 mL/m2 OR P-wave abnormalities (P-wave duration 120 ms or terminal P-wave force in V14 mVs [We believe there is an error here, it should be changed to “mVms”]); Stage 2: Coexistence of 2 Stage 1 criteria; Stage 3: Coexistence of 3 Stage 1 criteria. Given the relative ease of obtaining ECG and echocardiographic indices, the stratification system proposed by Johnson et al. is not difficult to implement in clinical practice. These indices are derived from routine examinations for diagnosing and assessing heart diseases, and their ubiquity and non-invasive nature enable doctors to conduct risk assessments with ease. In the sample, 42% of individuals had at least one marker of ACM, but only 9% had two markers, and 0.3% had three markers. Regrettably, due to the lack of long-term follow-up studies, we are currently unable to accurately determine the risk of AF or other cardiovascular events in individuals at different stages. Based on Framingham Heart Study data, the lifetime risk of AF in the population is 37% [3], indicating that individuals in stage 2 or 3 should be given particular attention. The authors noted that the correlations among the ACM criteria analyzed were relatively weak, with limited overlap [110], indicating that the system required further refinement to enhance the accuracy and reliability of assessment to provide a more systematic and structured framework for the risk evaluation and management of ACM. Based on the above research, we made a summary of the diagnostic criteria and evidence for ACM, as shown in Fig. 3.
Fig. 3.
Diagnostic criteria and evidence levels for atrial cardiomyopathy. For high-risk individuals or patients with clinical signs of atrial cardiomyopathy, a comprehensive evaluation should be conducted based on the specific circumstances. This evaluation should include imaging examinations, electrocardiograms, serum biomarker assessments, and electrophysiological examinations to accurately diagnose atrial cardiomyopathy. AF, atrial fibrillation; ESUS, embolic stroke of undetermined source; LA, left atrial; LAA, left atrial appendage; CCT, cardiac computed tomography; CMRI, cardiac magnetic resonance imaging; 3D-Echo, three-dimensional echocardiography; LASr, left atrial reservoir strain; LGE, late gadolinium enhancement; LAEF, left atrial emptying fraction; PTFV1, P-wave terminal force in lead V1; PWD, P-wave duration; aPWD, amplified P-wave duration; PWd, P-wave dispersion; AI, artificial intelligence; ECG, electrocardiogram; PACs, premature atrial contractions; PWA, P-wave axis; PWa, P-wave area; NT-proBNP, N-terminal pro-B-type natriuretic peptide; MR-proANP, midregional pro-A-type natriuretic peptide; CRP, C-reactive protein; PICP, carboxyl terminal peptide from pro-collagen I; PIIINP, amino terminal peptide from pro-collagen III; sST2, soluble suppression of tumorigenicity-2; ACM, atrial cardiomyopathy; M, M-mode echocardiography.
4. Management Strategies of Atrial Cardiomyopathy
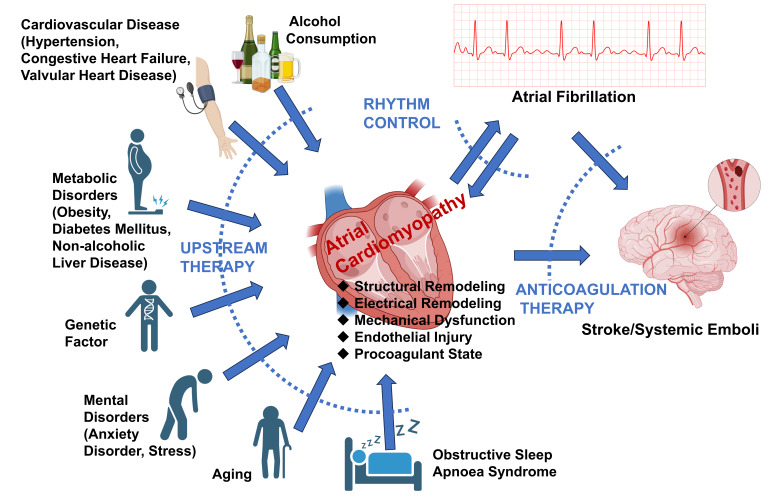
Although there are no unified guidelines or expert consensus specifically tailored to ACM, its management can be informed by relevant clinical practice and research. As shown in Fig. 4, the management of ACM typically includes the following aspects:
Fig. 4.
Major risk factors, complications, and treatment strategies for atrial cardiomyopathy. Under the influence of various risk factors, the heart may develop into atrial cardiomyopathy characterized by structural and electrical remodeling, mechanical dysfunction, endothelial injury, and a procoagulant state. The main complications of atrial cardiomyopathy include atrial fibrillation and stroke/systemic emboli. Moreover, persistent atrial fibrillation can further exacerbate the atrial substrate for cardiomyopathy. Therefore, the primary treatment strategies for atrial cardiomyopathy include “upstream therapy” to correct risk factors, rhythm control, and prevention of embolic events.
4.1 Upstream Treatments for Atrial Cardiomyopathy
There are many potential causes or risk factors of ACM, including genetic factors, aging, congestive heart failure, hypertension, valvular heart disease, obstructive sleep apnoea syndrome, alcohol consumption, metabolic disorders (e.g., obesity, diabetes mellitus [DM], and non-alcoholic liver disease), mental disorders (e.g., anxiety disorder, stress) [111]. “Upstream therapies”, including lifestyle changes and risk factor management, should be the cornerstone of ACM management [112]. Shen et al. [113] proposed a potential staging system for ACM based on the 2013 American College of Cardiology Foundation/American Heart Association guidelines for heart failure guidelines: Stage A (at risk of developing ACM), Stage B (asymptomatic but detectable ACM), Stage C (manifest disease but potentially reversible), and Stage D (irreversible). Without effective intervention, ACM will eventually progress to stage D. Identifying risk factors in the early stages ACM is crucial, as it allows for the timely initiation of upstream therapies to prevent or even reverse structural and electrophysiological changes in the atrium. Thus, early identification and intervention of reversible risk factors are essential for the primary prevention of ACM complications and represent one of the most important strategies in managing the condition.
Obesity is now widely recognized as being associated with the progression of ACM. The risk of new-onset AF increases by up to 8% with each unit increment in body mass index (BMI) [114, 115]. Although obesity is characterized by the expansion of subcutaneous fat, it is also accompanied by the accumulation of visceral adipose tissue. Epicardial adipose tissue (EAT), a unique visceral fat deposit located adjacent to the heart’s epicardial surface, is now considered a significant factor in the formation and progression of ACM [116]. Venteclef et al.’s experimental results [117] indicate that EAT promotes myocardial tissue fibrosis by secreting adipo-fibrokines, such as Activin A. The expansion of EAT also triggers a chronic inflammatory response, with activated adipokines, pro-inflammatory and pro-oxidant cytokines, along with metabolic changes and myocardial alterations, all contributing to the development of ACM [117, 118, 119, 120]. Furthermore, the accumulation of adipocytes within the myocardium can disrupt atrial conduction and facilitate the development and persistence of reentrant circuits [121]. Specifically, these models exhibited LA enlargement, biatrial conduction abnormalities, increased expression of profibrotic factors, exacerbated atrial interstitial fibrosis, and an increased propensity of spontaneous and inducible AF [122]. Existing evidence shows that weight loss can significantly improve symptoms and quality of life in patients with AF, and slow the progression of ACM by reducing EAT [123, 124]. It may also reduce the recurrence of AF after ablation and even reverse persistent AF to paroxysmal AF or normal sinus rhythm [125].
DM is another risk factor for ACM that requires special attention. DM promotes atrial interstitial fibrosis through inflammatory and oxidative stress responses, and it is also accompanied by electrical remodeling, including changes in ion channels and gap junctions [126, 127, 128]. Chao et al. [129] conducted a detailed analysis of electrophysiological characteristics using a three-dimensional electroanatomical mapping system in patients with paroxysmal AF who underwent their first catheter ablation. The results showed a significant reduction in atrial voltage in both the right and left atria of patients with diabetes and impaired glucose metabolism, indicating electrical remodeling and atrial fibrosis in individuals with DM. Moreover, the long-term follow-up revealed a significantly higher AF recurrence rate in patients with DM compared to the control group [129]. In addition to structural and electrical remodeling, DM also promotes the progression of ACM by inducing autonomic remodeling [127, 128]. Sprouting and hyperinnervation of the atrial autonomic nerves regulate AF initiation and maintenance through modulation of cardiac electricity working synergistically with electrical remodeling. Furthermore, DM may also contribute to thrombosis in ACM and a higher risk of stroke. Higher levels of Plasminogen activator inhibitor-1 and lower expression of anticoagulant molecules, such as thrombomodulin and protein C, have been described in DM patients [130, 131]. Additionally, DM increases the expression of fibrinogen and tissue factor, enhances thrombin sensitivity and early platelet activation, and induces endothelial damage [128, 132, 133]. Furthermore, effective insulin therapy may alleviate atrial fibrosis and the progression of atrial reentrant substrate [126]. As a novel class of hypoglycemic drugs, sodium-glucose cotransporter 2 inhibitors (SGLT2i) decrease the concentration of plasma glucose by inhibiting glucose reabsorption through the SGLT2 receptor in the proximal tubules of the kidney. Since the 2022 ACC/AHA/HFSA (American College of Cardiology/American Heart Association/Heart Failure Society of America)) Guideline for the Management of Heart Failure recommended SGLT2i for the treatment of chronic heart failure with reduced ejection fraction [134], current evidence also suggests their potential therapeutic value for ACM. In a sub-analysis of the DECLARE-TIMI 58 trial, compared to placebo, dapagliflozin reduced the risk of first AF/atrial flutter or the number of total AF/atrial flutter events by 19% and 23%, respectively, in patients with type 2 DM and cardiovascular injury [135]. A meta-analysis indicated that various SGLT2i could reduce the incidence of AF or atrial flutter, with dapagliflozin providing the greatest benefit [136]. Notably, the protective effects of SGLT2i on ACM are not solely dependent on glucose control. They may also mitigate atrial electrical and structural remodeling by improving mitochondrial function, exerting anti-inflammatory and antioxidant effects [137, 138, 139], and reducing left atrial dilatation through blood pressure reduction and increased natriuresis [138].
In addition, previous studies have shown that alcohol consumption contributes to myocardial fibrosis, left ventricle (LV) enlargement, and the development of AF [140, 141]. Latest research results also indicated that increased alcohol consumption was associated with LA dysfunction, including larger LA volume and lower LA reservoir and contractile strain [142, 143]. Despite the study results indicating a dose-dependent relationship between alcohol consumption and the deterioration of LA volume index and LA strain, it is disappointing to note that the study found no improvement in LA volume index and LV strain after reducing alcohol intake [142], suggesting that atrial remodeling caused by alcohol may be irreversible. However, this does not necessarily mean that limiting alcohol intake is not important for patients with ACM. Current evidence and clinical experience support that reducing alcohol intake can significantly alleviate the AF burden [140].
4.2 Predicting and Preventing Stroke/Systemic Embolism Events
The traditional view posits that during AF, the loss of effective contractile function of LA leads to sluggish blood flow, potentially resulting in LAA thrombogenesis and subsequent S/SE, particularly upon the return of atrial contraction with sinus rhythm restoration. However, this traditional perspective has been challenged in recent years. In the ASSERT [144] and TRENDS [145] studies, researchers included 2580 and 2486 patients with implanted monitoring devices, respectively. Among the patients who experienced a S/SE during the follow-up period, only 8% and 23% detected episodes of AF within the 30 days preceding the stroke. Furthermore, the IMPACT trial indicated that promptly initiating anticoagulation therapy upon the detection of atrial arrhythmias (mainly AF) through remote rhythm monitoring by implantable devices did not confer a greater reduction in stroke risk compared to conventional office-based follow-up methods (1.0 vs. 1.6 per 100 patient-years, RR –35.3% [95% CI –35.3%], p = 0.251). Moreover, there was no temporal relationship between atrial arrhythmias and S/SE [146]. The outcomes of the aforementioned clinical trials suggest that the relationship between AF and S/SE is not absolute.
The CHA2DS2-VASc score is currently widely used for S/SE risk identification and stratification in patients with AF [147]. The factors included in this score are precisely the risk factors or primary causes for ACM that are currently widely accepted [12, 148]. However, the intrinsic characteristics of AF (e.g., whether it is persistent, paroxysmal, or long-standing persistent; the frequency of AF episodes if it is paroxysmal; and the characteristics of the f-waves or ventricular rate during AF episodes) do not influence the decision to initiate anticoagulation therapy. In patients without risk factors indicative of ACM, the risk of S/SE in AF patients is similar to that in patients without AF [149]. Therefore, even in patients with persistent AF, anticoagulation therapy is not recommended if the CHA2DS2-VASc score is 0 [147]. From a different perspective, in patients with amyloidosis and cardiac involvement, the incidence of thromboembolic events can reach as high as 5–10% [15]. Even in sinus rhythm, these patients have a significantly increased risk of developing LA/LAA thrombus [150]. In fact, irrespective of the presence of AF, the inflammatory and fibrotic processes in the LA are the primary causes of impaired atrial conduit function and promote the thrombogenicity of the atrial endocardium [151]. Thus, various lines of evidence suggest that the presence of ACM may be an independent risk factor for S/SE, and early identification of ACM can help predict and prevent the occurrence of S/SE.
Embolic stroke of undetermined source (ESUS) is defined as a non-lacunar cerebral infarction without any large arterial stenoses 50% or identifiable cardioembolic causes and accounts for 15%–20% of all ischemic strokes [152]. Traditionally, ESUS was thought to be primarily associated with asymptomatic paroxysmal AF. However, recent research evidence indicates that ACM may be an underlying factor contributing to ESUS. A study comparing the prevalence of ACM among patients with different etiologies of ischemic stroke revealed that the incidence of ACM (defined by PTFV1 5000 µVms or severe left atrial enlargement) was significantly higher in patients with ESUS compared to those with strokes caused by large artery atherosclerosis or small vessel disease [153]. Based on the above facts, several clinical studies [154, 155] have compared the effectiveness of non-vitamin K antagonist oral anticoagulants (NOACs) and aspirin in preventing secondary ischemic events in patients with initial ESUS. The multicenter, randomized, controlled, double-blind clinical trials NAVIGATE ESUS [154] and RE-SPECT ESUS [155] concluded that rivaroxaban or dabigatran were not better than aspirin in preventing recurrent stroke after an initial ESUS and was associated with a higher risk of bleeding events. However, patients after ESUS benefited from anticoagulation if the LA diameter was 4.6 cm based on the secondary analysis of NAVIGATE ESUS [18]. Furthermore, the ARCADIA trial [83] compared anticoagulation with antiplatelet therapy for secondary stroke prevention in patients with ESUS and evidence of ACM. In this study, ACM was assessed using a composite criterion (PTFV1 5000 µVms, NT-proBNP 250 pg/mL, LA diameter index 3 cm/m2), and patients were randomly assigned to receive either aspirin or apixaban. The results, recently published in JAMA, showed that the rate of recurrent stroke during follow-up was 4.4% in both the apixaban and aspirin groups (HR 1.00; 95% CI 0.64–1.55) [83]. The results of the aforementioned studies indicate that in patients with clear evidence of ACM, the effectiveness of NOACs in preventing recurrent stroke is comparable to that of aspirin. Whether the combined use of aspirin and NOACs can further improve the prognosis of these patients without significantly increasing the risk of bleeding still requires further research to verify.
As previously mentioned, strokes can occur independently of AF. Therefore, accurately identifying individuals at high risk for stroke and initiating timely anticoagulant therapy is crucial. Notably, the CHA2DS2-VASc scoring system still remains effective in predicting the risk of ischemic stroke, even in the absence of a history of AF [156]. Furthermore, oral anticoagulants may provide benefits for ACM patients identified through abnormal imaging, ECG findings, and elevated inflammatory biomarkers, even those with low CHA2DS2-VASc scores [151]. For male patients with a CHA2DS2-VASc score of 1 and female patients with a score of 2, current AF clinical guidelines do not provide clear recommendations on anticoagulation therapy. Therefore, it is essential to consider whether the patient has concomitant ACM evidence when deciding whether to initiate anticoagulation. This consideration will help to more comprehensively weigh the benefits and risks of treatment.
4.3 Rhythm Control and “Reverse Remodeling” of Atrial Cardiomyopathy
Despite emerging evidence suggesting that stroke can occur in patients with AF even after sinus rhythm is restored [149], this does not imply that rhythm control is unimportant in the treatment of ACM. The EAST-AFNET 4 trial results indicated that early rhythm control therapy in early AF patients can reduce the risk of adverse cardiac outcomes including S/SE compared to usual treatment [157]. It is noteworthy that these patients received not only early rhythm control but also standardized anticoagulation therapy and comprehensive management of comorbid underlying cardiovascular diseases. These upstream treatments for ACM may be associated with better outcomes.
Furthermore, rhythm control itself may improve the substrate of ACM. In the early stages of AF, episodes typically originate from focal electrical activity mainly within muscle sleeves that extend into the pulmonary veins, usually manifesting as paroxysmal attacks. As the disease progresses, the atrial substrate undergoes continuous remodeling and deterioration, leading to a transition from paroxysmal to persistent AF, a process metaphorically referred to as “AF begets AF” [158, 159]. Therefore, maintaining sinus rhythm may be beneficial for atrial substrates. There is some evidence to suggest that the progression of ACM may be slowed or even reversed after effective catheter ablation. Sugumar et al. [160] published a subgroup analysis of the CAMERA-MRI study in the Journal of the American College of Cardiology: Clinical Electrophysiology, exploring the process of atrial electrical and structural remodeling after AF catheter ablation. They conducted detailed electroanatomical mapping of the right atrium using CARTO3 and contact force-sensing catheters on 15 patients who experienced a reduction of more than 90% in AF burden post-ablation. Over an average follow-up period of 23.4 11.9 months, transthoracic echocardiography and CMR imaging revealed an improvement in LVEF and decreases in both right and left atrial areas. Additionally, electroanatomical (EA) mapping indicated a rise in right atrium bipolar voltage. Myocardial tissue voltage increased in all regions, with statistical differences observed in the posterior and septal segments [160]. Similarly, research by Pump et al. [161] suggested that catheter ablation is effective in patients with non-paroxysmal AF and severe LA enlargement, leading to reverse remodeling of LA and an improvement in left ventricular function.
However, the detrimental impact of additional damage caused by catheter ablation on atrial substrate remains unknown [162]. Extensive radiofrequency ablation generates additional scar tissue in the LA, and while this scar tissue may terminate current episodes of AF, its long-term effects on the atrial substrate are still uncertain. For patients with chronic systemic inflammatory or metabolic disorders, simple AF ablation cannot eradicate the pathophysiological mechanisms leading to ACM. In fact, the additional injury from the ablation procedure may further impair the atrial conduit, reservoir, and systolic functions. For patients already exhibiting significant signs of ACM before surgery, maintaining normal sinus rhythm after ablation is particularly challenging. Some physicians may opt for multiple ablations to eliminate AF, but the additional injury can further reduce LA ejection fraction and atrial compliance [163], exacerbate atrial fibrosis, and potentially lead to “stiff LA syndrome” [164]. As described above, the DECAAFII trail attempted to use LGE-CMRI to guide AF ablation, and 2 patients died post-ablation in the fibrosis-guided ablation group. In one case, the cause of death was likely due to excessive ablation of LA posterior wall [50]. Targeted ablation of fibrotic regions in LA may be a promising therapeutic strategy. Although the DECAAFII trail did not achieve statistically significant results, this study provides valuable insights for AF ablation strategies [50]. In the ERASE-AF study, PVI combined with individualized ablation of atrial low-voltage myocardium significantly improved ablation success rates and outcomes compared to PVI alone in patients with persistent AF [165]. Therefore, identifying which patients may derive long-term benefits from ablation surgery is especially crucial. This necessitates further research and validation through large-scale clinical trials.
5. Conclusions
Although several noninvasive tools exist to characterize ACM, clear diagnostic criteria are still lacking. Consequently, the concept of ACM remains largely in the realm of research and has not yet been translated into clinical practice guidelines. The pathogenesis of ACM is intricate and multifaceted, involving neural and humoral regulation, lipid accumulation, oxidative stress, and inflammatory responses. Given this complexity, interventional measures for ACM must be comprehensive. Future research should focus on developing multiple strategies to jointly intervene in the progression of ACM, particularly with targeted treatments that address the underlying mechanisms of the disease. Despite recent progress in ACM research, several key issues remain unresolved. These include establishing more precise and practical diagnostic criteria for ACM and determining whether anticoagulation therapy should be administered to individuals exhibiting characteristics of ACM but without AF. Additionally, further research is needed to identify which patients will benefit from AF ablation and whether patients with characteristics of ACM should continue long-term anticoagulation therapy after successful ablation. Addressing these issues is crucial for the clinical management of ACM and can help improve the overall prognosis of patients.
Acknowledgment
Special thanks to BioRender for providing drawing elements and to ChatGPT-4o for English polishing service.
Funding Statement
This research was supported by National Natural Science Foundation of China (82270243, 82270365) and Nature Science Foundation of Hubei Province (2024AFC155).
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
GW selected the topic. ZYL and TL designed and drafted the initial manuscript. ZYL edited the manuscript and creating the figures. GW and TL were instrumental in the initial conception of the manuscript, structured the article, and provided essential critical reviews. All authors have reviewed and approved the final version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research was supported by National Natural Science Foundation of China (82270243, 82270365) and Nature Science Foundation of Hubei Province (2024AFC155).
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation . 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baman JR, Passman RS. Atrial Fibrillation. JAMA . 2021;325:2218. doi: 10.1001/jama.2020.23700. [DOI] [PubMed] [Google Scholar]
- [3].Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ (Clinical Research Ed.) . 2018;361:k1453. doi: 10.1136/bmj.k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circulation Research . 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine . 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- [6].Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. Journal of the American College of Cardiology . 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- [7].Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation . 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- [8].Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation . 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- [9].Tubeeckx MRL, De Keulenaer GW, Heidbuchel H, Segers VFM. Pathophysiology and clinical relevance of atrial myopathy. Basic Research in Cardiology . 2024;119:215–242. doi: 10.1007/s00395-024-01038-0. [DOI] [PubMed] [Google Scholar]
- [10].Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology . 2016;18:1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams DO, Jones EL, Nagle RE, Smith BS. Familial atrial cardiomyopathy with heart block. The Quarterly Journal of Medicine . 1972;41:491–508. [PubMed] [Google Scholar]
- [12].Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm . 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qiu D, Peng L, Ghista DN, Wong KKL. Left Atrial Remodeling Mechanisms Associated with Atrial Fibrillation. Cardiovascular Engineering and Technology . 2021;12:361–372. doi: 10.1007/s13239-021-00527-w. [DOI] [PubMed] [Google Scholar]
- [14].Lukas Laws J, Lancaster MC, Ben Shoemaker M, Stevenson WG, Hung RR, Wells Q, et al. Arrhythmias as Presentation of Genetic Cardiomyopathy. Circulation Research . 2022;130:1698–1722. doi: 10.1161/CIRCRESAHA.122.319835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nicol M, Siguret V, Vergaro G, Aimo A, Emdin M, Dillinger JG, et al. Thromboembolism and bleeding in systemic amyloidosis: a review. ESC Heart Failure . 2022;9:11–20. doi: 10.1002/ehf2.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoit BD. Left atrial size and function: role in prognosis. Journal of the American College of Cardiology . 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- [17].Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. Journal of the American College of Cardiology . 2019;73:1961–1977. doi: 10.1016/j.jacc.2019.01.059. [DOI] [PubMed] [Google Scholar]
- [18].Healey JS, Gladstone DJ, Swaminathan B, Eckstein J, Mundl H, Epstein AE, et al. Recurrent Stroke With Rivaroxaban Compared With Aspirin According to Predictors of Atrial Fibrillation: Secondary Analysis of the NAVIGATE ESUS Randomized Clinical Trial. JAMA Neurology . 2019;76:764–773. doi: 10.1001/jamaneurol.2019.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vyas H, Jackson K, Chenzbraun A. Switching to volumetric left atrial measurements: impact on routine echocardiographic practice. European Journal of Echocardiography: the Journal of the Working Group on Echocardiography of the European Society of Cardiology . 2011;12:107–111. doi: 10.1093/ejechocard/jeq119. [DOI] [PubMed] [Google Scholar]
- [20].Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography . 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- [21].Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, et al. Evaluation of Left Atrial Size and Function: Relevance for Clinical Practice. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography . 2020;33:934–952. doi: 10.1016/j.echo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- [22].Ninni S, Algalarrondo V, Brette F, Lemesle G, Fauconnier J, Groupe de Reflexion sur la Recherche Cardiovasculaire (GRRC) Ninni S, Algalarrondo V, Brette F, Lemesle G, Fauconnier J, Groupe de Reflexion sur la Recherche Cardiovasculaire (GRRC). Left atrial cardiomyopathy: Pathophysiological insights, assessment methods and clinical implications. Archives of Cardiovascular Diseases . 2024;117:283–296. doi: 10.1016/j.acvd.2024.02.001. [DOI] [PubMed] [Google Scholar]
- [23].Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. Journal of the American College of Cardiology . 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- [24].Froehlich L, Meyre P, Aeschbacher S, Blum S, Djokic D, Kuehne M, et al. Left atrial dimension and cardiovascular outcomes in patients with and without atrial fibrillation: a systematic review and meta-analysis. Heart (British Cardiac Society) . 2019;105:1884–1891. doi: 10.1136/heartjnl-2019-315174. [DOI] [PubMed] [Google Scholar]
- [25].Camen S, Schnabel RB. Genetics, atrial cardiomyopathy, and stroke: enough components for a sufficient cause? European Heart Journal . 2021;42:4533–4535. doi: 10.1093/eurheartj/ehab523. [DOI] [PubMed] [Google Scholar]
- [26].Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, et al. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. American Journal of Cardiology . 2008;101:1626–1629. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- [27].Hirose T, Kawasaki M, Tanaka R, Ono K, Watanabe T, Iwama M, et al. Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. European Heart Journal . 2012;13:243–250. doi: 10.1093/ejechocard/jer251. Cardiovascular Imaging. [DOI] [PubMed] [Google Scholar]
- [28].Löbe S, Stellmach P, Darma A, Hilbert S, Paetsch I, Jahnke C, et al. Left atrial total emptying fraction measured by cardiovascular magnetic resonance imaging predicts low-voltage areas detected during electroanatomical mapping. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology . 2023;25:euad307. doi: 10.1093/europace/euad307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chou CC, Lee HL, Chang PC, Wo HT, Wen MS, Yeh SJ, et al. Left atrial emptying fraction predicts recurrence of atrial fibrillation after radiofrequency catheter ablation. PloS One . 2018;13:e0191196. doi: 10.1371/journal.pone.0191196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eichenlaub M, Mueller-Edenborn B, Minners J, Allgeier M, Lehrmann H, Allgeier J, et al. Echocardiographic diagnosis of atrial cardiomyopathy allows outcome prediction following pulmonary vein isolation. Clinical Research in Cardiology: Official Journal of the German Cardiac Society . 2021;110:1770–1780. doi: 10.1007/s00392-021-01850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang L, Zhang Y, Zhou W, Chen J, Li Y, Tang Q, et al. Relationship between left atrial appendage peak flow velocity and nonvalvular atrial fibrillation recurrence after cryoablation. Frontiers in Cardiovascular Medicine . 2023;10:1053102. doi: 10.3389/fcvm.2023.1053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tokunaga K, Hashimoto G, Mizoguchi T, Mori K, Shijo M, Jinnouchi J, et al. Left Atrial Appendage Flow Velocity and Multiple Infarcts in Cryptogenic Stroke. Cerebrovascular Diseases (Basel, Switzerland) . 2021;50:429–434. doi: 10.1159/000514672. [DOI] [PubMed] [Google Scholar]
- [33].Vieira MJ, Teixeira R, Gonçalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography . 2014;27:463–478. doi: 10.1016/j.echo.2014.01.021. [DOI] [PubMed] [Google Scholar]
- [34].Sun BJ, Park JH. Echocardiographic Measurement of Left Atrial Strain - A Key Requirement in Clinical Practice. Circulation Journal: Official Journal of the Japanese Circulation Society . 2021;86:6–13. doi: 10.1253/circj.CJ-21-0373. [DOI] [PubMed] [Google Scholar]
- [35].Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: A useful index in atrial fibrillation. International Journal of Cardiology . 2016;220:208–213. doi: 10.1016/j.ijcard.2016.06.197. [DOI] [PubMed] [Google Scholar]
- [36].Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. European Heart Journal. Cardiovascular Imaging . 2018;19:591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- [37].Her AY, Choi EY, Shim CY, Song BW, Lee S, Ha JW, et al. Prediction of left atrial fibrosis with speckle tracking echocardiography in mitral valve disease: a comparative study with histopathology. Korean Circulation Journal . 2012;42:311–318. doi: 10.4070/kcj.2012.42.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. The American Journal of Cardiology . 2013;111:595–601. doi: 10.1016/j.amjcard.2012.10.049. [DOI] [PubMed] [Google Scholar]
- [39].Alhakak AS, Biering-Sørensen SR, Møgelvang R, Modin D, Jensen GB, Schnohr P, et al. Usefulness of left atrial strain for predicting incident atrial fibrillation and ischaemic stroke in the general population. European Heart Journal. Cardiovascular Imaging . 2022;23:363–371. doi: 10.1093/ehjci/jeaa287. [DOI] [PubMed] [Google Scholar]
- [40].Obokata M, Negishi K, Kurosawa K, Tateno R, Tange S, Arai M, et al. Left atrial strain provides incremental value for embolism risk stratification over CHA₂DS₂-VASc score and indicates prognostic impact in patients with atrial fibrillation. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography . 2014;27:709–716.e4. doi: 10.1016/j.echo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- [41].Sonaglioni A, Lombardo M, Nicolosi GL, Rigamonti E, Anzà C. Incremental diagnostic role of left atrial strain analysis in thrombotic risk assessment of nonvalvular atrial fibrillation patients planned for electrical cardioversion. The International Journal of Cardiovascular Imaging . 2021;37:1539–1550. doi: 10.1007/s10554-020-02127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shaikh AY, Maan A, Khan UA, Aurigemma GP, Hill JC, Kane JL, et al. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: a prospective study. Cardiovascular Ultrasound . 2012;10:48. doi: 10.1186/1476-7120-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. The New England Journal of Medicine . 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- [44].Paddock S, Tsampasian V, Assadi H, Mota BC, Swift AJ, Chowdhary A, et al. Clinical Translation of Three-Dimensional Scar, Diffusion Tensor Imaging, Four-Dimensional Flow, and Quantitative Perfusion in Cardiac MRI: A Comprehensive Review. Frontiers in Cardiovascular Medicine . 2021;8:682027. doi: 10.3389/fcvm.2021.682027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA . 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- [46].Peters DC. Association of Left Atrial Fibrosis Detected by Delayed Enhancement Magnetic Resonance Imaging and Risk of Stroke in Patients with Atrial Fibrillation. Journal of Atrial Fibrillation . 2011;4:384. doi: 10.4022/jafib.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circulation. Arrhythmia and Electrophysiology . 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Quinto L, Cozzari J, Benito E, Alarcón F, Bisbal F, Trotta O, et al. Magnetic resonance-guided re-ablation for atrial fibrillation is associated with a lower recurrence rate: a case-control study. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology . 2020;22:1805–1811. doi: 10.1093/europace/euaa252. [DOI] [PubMed] [Google Scholar]
- [49].Akoum N, Morris A, Perry D, Cates J, Burgon N, Kholmovski E, et al. Substrate Modification Is a Better Predictor of Catheter Ablation Success in Atrial Fibrillation Than Pulmonary Vein Isolation: An LGE-MRI Study. Clinical Medicine Insights . 2015;9:25–31. doi: 10.4137/CMC.S22100. Cardiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher L, et al. Effect of MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA . 2022;327:2296–2305. doi: 10.1001/jama.2022.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hopman LHGA, Bhagirath P, Mulder MJ, Eggink IN, van Rossum AC, Allaart CP, et al. Quantification of left atrial fibrosis by 3D late gadolinium-enhanced cardiac magnetic resonance imaging in patients with atrial fibrillation: impact of different analysis methods. European Heart Journal. Cardiovascular Imaging . 2022;23:1182–1190. doi: 10.1093/ehjci/jeab245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Acampa M, Lazzerini PE, Martini G. Atrial Cardiopathy and Sympatho-Vagal Imbalance in Cryptogenic Stroke: Pathogenic Mechanisms and Effects on Electrocardiographic Markers. Frontiers in Neurology . 2018;9:469. doi: 10.3389/fneur.2018.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Acampa M, Lazzerini PE, Martini G. How to Identify Patients at Risk of Silent Atrial Fibrillation after Cryptogenic Stroke: Potential Role of P Wave Dispersion. Journal of Stroke . 2017;19:239–241. doi: 10.5853/jos.2016.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Okutucu S, Aytemir K, Oto A. P-wave dispersion: What we know till now? JRSM Cardiovascular Disease . 2016;5:2048004016639443. doi: 10.1177/2048004016639443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Abou R, Leung M, Tonsbeek AM, Podlesnikar T, Maan AC, Schalij MJ, et al. Effect of Aging on Left Atrial Compliance and Electromechanical Properties in Subjects Without Structural Heart Disease. The American Journal of Cardiology . 2017;120:140–147. doi: 10.1016/j.amjcard.2017.03.243. [DOI] [PubMed] [Google Scholar]
- [56].Vural MG, Cetin S, Yilmaz M, Akdemir R, Gunduz H. Relation between Left Atrial Remodeling in Young Patients with Cryptogenic Stroke and Normal Inter-atrial Anatomy. Journal of Stroke . 2015;17:312–319. doi: 10.5853/jos.2015.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dogan U, Dogan EA, Tekinalp M, Tokgoz OS, Aribas A, Akilli H, et al. P-wave dispersion for predicting paroxysmal atrial fibrillation in acute ischemic stroke. International Journal of Medical Sciences . 2012;9:108–114. doi: 10.7150/ijms.9.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Acampa M, Guideri F, Tassi R, Dello Buono D, Celli L, di Toro Mammarella L, et al. P wave dispersion in cryptogenic stroke: A risk factor for cardioembolism? International Journal of Cardiology . 2015;190:202–204. doi: 10.1016/j.ijcard.2015.04.185. [DOI] [PubMed] [Google Scholar]
- [59].Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation . 2009;119:e251–e261. doi: 10.1161/CIRCULATIONAHA.108.191097. [DOI] [PubMed] [Google Scholar]
- [60].Tiffany Win T, Ambale Venkatesh B, Volpe GJ, Mewton N, Rizzi P, Sharma RK, et al. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Heart Rhythm . 2015;12:155–162. doi: 10.1016/j.hrthm.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rangel MO, O’Neal WT, Soliman EZ. Usefulness of the Electrocardiographic P-Wave Axis as a Predictor of Atrial Fibrillation. The American Journal of Cardiology . 2016;117:100–104. doi: 10.1016/j.amjcard.2015.10.013. [DOI] [PubMed] [Google Scholar]
- [62].Maheshwari A, Norby FL, Soliman EZ, Koene RJ, Rooney MR, O’Neal WT, et al. Abnormal P-Wave Axis and Ischemic Stroke: The ARIC Study (Atherosclerosis Risk In Communities) Stroke . 2065;48:2060–2065. doi: 10.1161/STROKEAHA.117.017226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li Y, Shah AJ, Soliman EZ. Effect of electrocardiographic P-wave axis on mortality. The American Journal of Cardiology . 2014;113:372–376. doi: 10.1016/j.amjcard.2013.08.050. [DOI] [PubMed] [Google Scholar]
- [64].Maheshwari A, Norby FL, Roetker NS, Soliman EZ, Koene RJ, Rooney MR, et al. Refining Prediction of Atrial Fibrillation-Related Stroke Using the P2-CHA2DS2-VASc Score. Circulation . 2019;139:180–191. doi: 10.1161/CIRCULATIONAHA.118.035411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lehtonen AO, Langén VL, Puukka PJ, Kähönen M, Nieminen MS, Jula AM, et al. Incidence rates, correlates, and prognosis of electrocardiographic P-wave abnormalities - a nationwide population-based study. Journal of Electrocardiology . 2017;50:925–932. doi: 10.1016/j.jelectrocard.2017.07.004. [DOI] [PubMed] [Google Scholar]
- [66].German DM, Kabir MM, Dewland TA, Henrikson CA, Tereshchenko LG. Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Annals of Noninvasive Electrocardiology: the Official Journal of the International Society for Holter and Noninvasive Electrocardiology, Inc . 2016;21:20–29. doi: 10.1111/anec.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Scott CC, Leier CV, Kilman JW, Vasko JS, Unverferth DV. The effect of left atrial histology and dimension on P wave morphology. Journal of Electrocardiology . 1983;16:363–366. doi: 10.1016/s0022-0736(83)80086-7. [DOI] [PubMed] [Google Scholar]
- [68].Thijs V. Atrial Fibrillation Detection: Fishing for An Irregular Heartbeat Before and After Stroke. Stroke . 2017;48:2671–2677. doi: 10.1161/STROKEAHA.117.017083. [DOI] [PubMed] [Google Scholar]
- [69].He J, Tse G, Korantzopoulos P, Letsas KP, Ali-Hasan-Al-Saegh S, Kamel H, et al. P-Wave Indices and Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke . 2072;48:2066–2072. doi: 10.1161/STROKEAHA.117.017293. [DOI] [PubMed] [Google Scholar]
- [70].Jadidi A, Müller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier J, et al. The Duration of the Amplified Sinus-P-Wave Identifies Presence of Left Atrial Low Voltage Substrate and Predicts Outcome After Pulmonary Vein Isolation in Patients With Persistent Atrial Fibrillation. JACC. Clinical Electrophysiology . 2018;4:531–543. doi: 10.1016/j.jacep.2017.12.001. [DOI] [PubMed] [Google Scholar]
- [71].Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke . 2015;46:936–941. doi: 10.1161/STROKEAHA.115.008714. [DOI] [PubMed] [Google Scholar]
- [72].O’Neal WT, Kamel H, Kleindorfer D, Judd SE, Howard G, Howard VJ, et al. Premature Atrial Contractions on the Screening Electrocardiogram and Risk of Ischemic Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Neuroepidemiology . 2016;47:53–58. doi: 10.1159/000448619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schumacher K, Dagres N, Hindricks G, Husser D, Bollmann A, Kornej J. Characteristics of PR interval as predictor for atrial fibrillation: association with biomarkers and outcomes. Clinical Research in Cardiology: Official Journal of the German Cardiac Society . 2017;106:767–775. doi: 10.1007/s00392-017-1109-y. [DOI] [PubMed] [Google Scholar]
- [74].Montalvo M, Tadi P, Merkler A, Gialdini G, Martin-Schild S, Navalkele D, et al. PR Interval Prolongation and Cryptogenic Stroke: A Multicenter Retrospective Study. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association . 2017;26:2416–2420. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.036. [DOI] [PubMed] [Google Scholar]
- [75].Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke . 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Verbrugge FH, Reddy YNV, Attia ZI, Friedman PA, Noseworthy PA, Lopez-Jimenez F, et al. Detection of Left Atrial Myopathy Using Artificial Intelligence-Enabled Electrocardiography. Circulation. Heart Failure . 2022;15:e008176. doi: 10.1161/CIRCHEARTFAILURE.120.008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Khurshid S, Friedman S, Reeder C, Di Achille P, Diamant N, Singh P, et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrillation. Circulation . 2022;145:122–133. doi: 10.1161/CIRCULATIONAHA.121.057480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet (London, England) . 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- [79].Sieweke JT, Pfeffer TJ, Biber S, Chatterjee S, Weissenborn K, Grosse GM, et al. miR-21 and NT-proBNP Correlate with Echocardiographic Parameters of Atrial Dysfunction and Predict Atrial Fibrillation. Journal of Clinical Medicine . 2020;9:1118. doi: 10.3390/jcm9041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Patel RB, Alenezi F, Sun JL, Alhanti B, Vaduganathan M, Oh JK, et al. Biomarker Profile of Left Atrial Myopathy in Heart Failure With Preserved Ejection Fraction: Insights From the RELAX Trial. Journal of Cardiac Failure . 2020;26:270–275. doi: 10.1016/j.cardfail.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen J, Gao F, Liu W. Atrial cardiopathy in embolic stroke of undetermined source. Brain and Behavior . 2021;11:e02160. doi: 10.1002/brb3.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yaghi S, Boehme AK, Hazan R, Hod EA, Canaan A, Andrews HF, et al. Atrial Cardiopathy and Cryptogenic Stroke: A Cross-sectional Pilot Study. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association . 2016;25:110–114. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kamel H, Longstreth WT, Jr, Tirschwell DL, Kronmal RA, Marshall RS, Broderick JP, et al. Apixaban to Prevent Recurrence After Cryptogenic Stroke in Patients With Atrial Cardiopathy: The ARCADIA Randomized Clinical Trial. JAMA . 2024;331:573–581. doi: 10.1001/jama.2023.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fonseca AC, Coelho P. Update on Biomarkers Associated to Cardioembolic Stroke: A Narrative Review. Life (Basel, Switzerland) . 2021;11:448. doi: 10.3390/life11050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Oldgren J, Hijazi Z, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al. Performance and Validation of a Novel Biomarker-Based Stroke Risk Score for Atrial Fibrillation. Circulation . 2016;134:1697–1707. doi: 10.1161/CIRCULATIONAHA.116.022802. [DOI] [PubMed] [Google Scholar]
- [86].Xu X, Tang Y. Relationship between Brain Natriuretic Peptide and Recurrence of Atrial Fibrillation after Successful Electrical Cardioversion: an Updated Meta-Analysis. Brazilian Journal of Cardiovascular Surgery . 2017;32:530–535. doi: 10.21470/1678-9741-2017-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Truong QA, Siegel E, Karakas M, Januzzi JL, Jr, Bamberg F, Mahabadi AA, et al. Relation of natriuretic peptides and midregional proadrenomedullin to cardiac chamber volumes by computed tomography in patients without heart failure: from the ROMICAT Trial. Clinical Chemistry . 2010;56:651–660. doi: 10.1373/clinchem.2009.138586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cui K, Huang W, Fan J, Lei H. Midregional pro-atrial natriuretic peptide is a superior biomarker to N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure patients with preserved ejection fraction. Medicine . 2018;97:e12277. doi: 10.1097/MD.0000000000012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Seewöster T, Büttner P, Zeynalova S, Hindricks G, Kornej J. Are the atrial natriuretic peptides a missing link predicting low-voltage areas in atrial fibrillation? Introducing the novel biomarker-based atrial fibrillation substrate prediction (ANP) score. Clinical Cardiology . 2020;43:762–768. doi: 10.1002/clc.23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nortamo S, Ukkola O, Lepojärvi S, Kenttä T, Kiviniemi A, Junttila J, et al. Association of sST2 and hs-CRP levels with new-onset atrial fibrillation in coronary artery disease. International Journal of Cardiology . 2017;248:173–178. doi: 10.1016/j.ijcard.2017.07.022. [DOI] [PubMed] [Google Scholar]
- [91].Kreimer F, Gotzmann M. Left Atrial Cardiomyopathy - A Challenging Diagnosis. Frontiers in Cardiovascular Medicine . 2022;9:942385. doi: 10.3389/fcvm.2022.942385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wałek P, Grabowska U, Cieśla E, Sielski J, Roskal-Wałek J, Wożakowska-Kapłon B. Analysis of the Correlation of Galectin-3 Concentration with the Measurements of Echocardiographic Parameters Assessing Left Atrial Remodeling and Function in Patients with Persistent Atrial Fibrillation. Biomolecules . 2021;11:1108. doi: 10.3390/biom11081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Swartz MF, Fink GW, Sarwar MF, Hicks GL, Yu Y, Hu R, et al. Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation. Journal of the American College of Cardiology . 2012;60:1799–1806. doi: 10.1016/j.jacc.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nattel S. New ideas about atrial fibrillation 50 years on. Nature . 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- [95].Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovascular Research . 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- [96].Chang SL, Tai CT, Lin YJ, Wongcharoen W, Lo LW, Tuan TC, et al. Biatrial substrate properties in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology . 2007;18:1134–1139. doi: 10.1111/j.1540-8167.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- [97].Huang JL, Tai CT, Lin YJ, Ting CT, Chen YT, Chang MS, et al. The mechanisms of an increased dominant frequency in the left atrial posterior wall during atrial fibrillation in acute atrial dilatation. Journal of Cardiovascular Electrophysiology . 2006;17:178–188. doi: 10.1111/j.1540-8167.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- [98].Ikoma T, Naruse Y, Kaneko Y, Sakakibara T, Narumi T, Sano M, et al. Pre-procedural predictors of left atrial low-voltage zones in patients undergoing catheter ablation of atrial fibrillation. PloS One . 2022;17:e0266939. doi: 10.1371/journal.pone.0266939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Huang SY, Lin YJ, Tsao HM, Chang SL, Lo LW, Hu HF, et al. The biatrial substrate properties in different types of paroxysmal atrial fibrillation. Heart Rhythm . 2011;8:961–967. doi: 10.1016/j.hrthm.2011.01.046. [DOI] [PubMed] [Google Scholar]
- [100].Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circulation. Arrhythmia and Electrophysiology . 2014;7:825–833. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- [101].Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, et al. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas With Selective Activation Characteristics. Circulation. Arrhythmia and Electrophysiology . 2016;9:e002962. doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- [102].Cutler MJ, Johnson J, Abozguia K, Rowan S, Lewis W, Costantini O, et al. Impact of Voltage Mapping to Guide Whether to Perform Ablation of the Posterior Wall in Patients With Persistent Atrial Fibrillation. Journal of Cardiovascular Electrophysiology . 2016;27:13–21. doi: 10.1111/jce.12830. [DOI] [PubMed] [Google Scholar]
- [103].Blandino A, Bianchi F, Grossi S, Biondi-Zoccai G, Conte MR, Gaido L, et al. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing and Clinical Electrophysiology: PACE . 2017;40:199–212. doi: 10.1111/pace.13015. [DOI] [PubMed] [Google Scholar]
- [104].Wong GR, Nalliah CJ, Lee G, Voskoboinik A, Prabhu S, Parameswaran R, et al. Dynamic Atrial Substrate During High-Density Mapping of Paroxysmal and Persistent AF: Implications for Substrate Ablation. JACC. Clinical Electrophysiology . 2019;5:1265–1277. doi: 10.1016/j.jacep.2019.06.002. [DOI] [PubMed] [Google Scholar]
- [105].Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, et al. The relationship between complex fractionated electrograms and atrial low-voltage zones during atrial fibrillation and paced rhythm. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology . 2011;13:1709–1716. doi: 10.1093/europace/eur197. [DOI] [PubMed] [Google Scholar]
- [106].Jadidi AS, Duncan E, Miyazaki S, Lellouche N, Shah AJ, Forclaz A, et al. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circulation. Arrhythmia and Electrophysiology . 2012;5:32–42. doi: 10.1161/CIRCEP.111.964197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Qureshi NA, Kim SJ, Cantwell CD, Afonso VX, Bai W, Ali RL, et al. Voltage during atrial fibrillation is superior to voltage during sinus rhythm in localizing areas of delayed enhancement on magnetic resonance imaging: An assessment of the posterior left atrium in patients with persistent atrial fibrillation. Heart Rhythm . 2019;16:1357–1367. doi: 10.1016/j.hrthm.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Haldar SK, Magtibay K, Porta-Sanchez A, Massé S, Mitsakakis N, Lai PFH, et al. Resolving Bipolar Electrogram Voltages During Atrial Fibrillation Using Omnipolar Mapping. Circulation. Arrhythmia and Electrophysiology . 2017;10:e005018. doi: 10.1161/CIRCEP.117.005018. [DOI] [PubMed] [Google Scholar]
- [109].Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nägele T, et al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): Rationale and study design. International Journal of Stroke: Official Journal of the International Stroke Society . 2017;12:985–990. doi: 10.1177/1747493016681019. [DOI] [PubMed] [Google Scholar]
- [110].Johnson LS, Platonov PG, Conen D, Kennbäck C, Jujic A, Healey JS, et al. Markers of Atrial Myopathy in the General Population: Prevalence, Predictors, and Inter-Relations. JACC. Clinical Electrophysiology . 2023;9:2240–2249. doi: 10.1016/j.jacep.2023.07.012. [DOI] [PubMed] [Google Scholar]
- [111].Wang Z, Qin H, Chen G, Dai Y, Cai Y, Cheng X, et al. Anxiety is associated with increased risk for atrial cardiopathy. Acta Neurologica Belgica . 2020;120:1383–1388. doi: 10.1007/s13760-020-01335-0. [DOI] [PubMed] [Google Scholar]
- [112].Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology . 2011;13:308–328. doi: 10.1093/europace/eur002. [DOI] [PubMed] [Google Scholar]
- [113].Shen MJ, Arora R, Jalife J. Atrial Myopathy. JACC. Basic to Translational Science . 2019;4:640–654. doi: 10.1016/j.jacbts.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tsang TSM, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. European Heart Journal . 2008;29:2227–2233. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wang TJ, Parise H, Levy D, D’Agostino RB, Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA . 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- [116].Sanghai SR, Sardana M, Hansra B, Lessard DM, Dahlberg ST, Aurigemma GP, et al. Indexed Left Atrial Adipose Tissue Area Is Associated With Severity of Atrial Fibrillation and Atrial Fibrillation Recurrence Among Patients Undergoing Catheter Ablation. Frontiers in Cardiovascular Medicine . 2018;5:76. doi: 10.3389/fcvm.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. European Heart Journal . 2015;36:795–805a. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- [118].Gungor H, Ayik MF, Kirilmaz B, Ertugay S, Gul I, Yildiz BS, et al. Serum resistin level: as a predictor of atrial fibrillation after coronary artery bypass graft surgery. Coronary Artery Disease . 2011;22:484–490. doi: 10.1097/MCA.0b013e32834b67bb. [DOI] [PubMed] [Google Scholar]
- [119].Ernault AC, Meijborg VMF, Coronel R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. Journal of the American College of Cardiology . 2021;78:1730–1745. doi: 10.1016/j.jacc.2021.08.037. [DOI] [PubMed] [Google Scholar]
- [120].Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, et al. Electroanatomical Remodeling of the Atria in Obesity: Impact of Adjacent Epicardial Fat. JACC. Clinical Electrophysiology . 2018;4:1529–1540. doi: 10.1016/j.jacep.2018.08.014. [DOI] [PubMed] [Google Scholar]
- [121].Lin YK, Chen YC, Chen JH, Chen SA, Chen YJ. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic Research in Cardiology . 2012;107:293. doi: 10.1007/s00395-012-0293-1. [DOI] [PubMed] [Google Scholar]
- [122].Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM, et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. Journal of the American College of Cardiology . 2015;66:1–11. doi: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- [123].Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA . 2013;310:2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- [124].Abed HS, Nelson AJ, Richardson JD, Worthley SG, Vincent A, Wittert GA, et al. Impact of weight reduction on pericardial adipose tissue and cardiac structure in patients with atrial fibrillation. American Heart Journal . 2015;169:655–662.e2. doi: 10.1016/j.ahj.2015.02.008. [DOI] [PubMed] [Google Scholar]
- [125].Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. Journal of the American College of Cardiology . 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- [126].Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The Association Between Diabetes Mellitus and Atrial Fibrillation: Clinical and Mechanistic Insights. Frontiers in Physiology . 2019;10:135. doi: 10.3389/fphys.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhang Q, Liu T, Ng CY, Li G. Diabetes mellitus and atrial remodeling: mechanisms and potential upstream therapies. Cardiovascular Therapeutics . 2014;32:233–241. doi: 10.1111/1755-5922.12089. [DOI] [PubMed] [Google Scholar]
- [128].Lorenzo-Almorós A, Casado Cerrada J, Álvarez-Sala Walther LA, Méndez Bailón M, Lorenzo González Ó. Atrial Fibrillation and Diabetes Mellitus: Dangerous Liaisons or Innocent Bystanders? Journal of Clinical Medicine . 2023;12:2868. doi: 10.3390/jcm12082868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chao TF, Suenari K, Chang SL, Lin YJ, Lo LW, Hu YF, et al. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. The American Journal of Cardiology . 2010;106:1615–1620. doi: 10.1016/j.amjcard.2010.07.038. [DOI] [PubMed] [Google Scholar]
- [130].Kearney K, Tomlinson D, Smith K, Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovascular Diabetology . 2017;16:34. doi: 10.1186/s12933-017-0515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lemkes BA, Hermanides J, Devries JH, Holleman F, Meijers JCM, Hoekstra JBL. Hyperglycemia: a prothrombotic factor? Journal of Thrombosis and Haemostasis: JTH . 2010;8:1663–1669. doi: 10.1111/j.1538-7836.2010.03910.x. JTH. [DOI] [PubMed] [Google Scholar]
- [132].Pechlivani N, Ajjan RA. Thrombosis and Vascular Inflammation in Diabetes: Mechanisms and Potential Therapeutic Targets. Frontiers in Cardiovascular Medicine . 2018;5:1. doi: 10.3389/fcvm.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Burstein SA. Cytokines, platelet production and hemostasis. Platelets . 1997;8:93–104. doi: 10.1080/09537109709169324. [DOI] [PubMed] [Google Scholar]
- [134].Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation . 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- [135].Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation . 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- [136].Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C, et al. Protective Effects of Sodium-Glucose Transporter 2 Inhibitors on Atrial Fibrillation and Atrial Flutter: A Systematic Review and Meta- Analysis of Randomized Placebo-Controlled Trials. Frontiers in Endocrinology . 2021;12:619586. doi: 10.3389/fendo.2021.619586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovascular Diabetology . 2019;18:165. doi: 10.1186/s12933-019-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovascular Diabetology . 2020;19:98. doi: 10.1186/s12933-020-01071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovascular Diabetology . 2018;17:6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. The New England Journal of Medicine . 2020;382:20–28. doi: 10.1056/NEJMoa1817591. [DOI] [PubMed] [Google Scholar]
- [141].Fernandez-Sola J, Estruch R, Grau JM, Pare JC, Rubin E, Urbano-Marquez A. The relation of alcoholic myopathy to cardiomyopathy. Annals of Internal Medicine . 1994;120:529–536. doi: 10.7326/0003-4819-120-7-199404010-00001. [DOI] [PubMed] [Google Scholar]
- [142].Alam AB, Toledo-Atucha E, Romaguera D, Alonso-Gómez AM, Martínez-Gonzalez MA, Tojal-Sierra L, et al. Associations of Alcohol Consumption With Left Atrial Morphology and Function in a Population at High Cardiovascular Risk. Journal of the American Heart Association . 2024;13:e031915. doi: 10.1161/JAHA.123.031915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hung CL, Gonçalves A, Lai YJ, Lai YH, Sung KT, Lo CI, et al. Light to Moderate Habitual Alcohol Consumption Is Associated with Subclinical Ventricular and Left Atrial Mechanical Dysfunction in an Asymptomatic Population: Dose-Response and Propensity Analysis. Journal of the American Society of Echocardiography: Official Publication of the American Society of Echocardiography . 2016;29:1043–1051.e4. doi: 10.1016/j.echo.2016.07.014. [DOI] [PubMed] [Google Scholar]
- [144].Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation . 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- [145].Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm . 2011;8:1416–1423. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- [146].Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GYH, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. European Heart Journal . 2015;36:1660–1668. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- [147].January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation . 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- [148].Li M, Ning Y, Tse G, Saguner AM, Wei M, Day JD, et al. Atrial cardiomyopathy: from cell to bedside. ESC Heart Failure . 2022;9:3768–3784. doi: 10.1002/ehf2.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. Journal of the American College of Cardiology . 2015;65:2239–2251. doi: 10.1016/j.jacc.2015.03.557. [DOI] [PubMed] [Google Scholar]
- [150].Dubrey S, Pollak A, Skinner M, Falk RH. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: evidence for atrial electromechanical dissociation. British Heart Journal . 1995;74:541–544. doi: 10.1136/hrt.74.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Packer M. Characterization, Pathogenesis, and Clinical Implications of Inflammation-Related Atrial Myopathy as an Important Cause of Atrial Fibrillation. Journal of the American Heart Association . 2020;9:e015343. doi: 10.1161/JAHA.119.015343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Diener HC, Easton JD, Hart RG, Kasner S, Kamel H, Ntaios G. Review and update of the concept of embolic stroke of undetermined source. Nature Reviews. Neurology . 2022;18:455–465. doi: 10.1038/s41582-022-00663-4. [DOI] [PubMed] [Google Scholar]
- [153].Jalini S, Rajalingam R, Nisenbaum R, Javier AD, Woo A, Pikula A. Atrial cardiopathy in patients with embolic strokes of unknown source and other stroke etiologies. Neurology . 2019;92:e288–e294. doi: 10.1212/WNL.0000000000006748. [DOI] [PubMed] [Google Scholar]
- [154].Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. The New England Journal of Medicine . 2018;378:2191–2201. doi: 10.1056/NEJMoa1802686. [DOI] [PubMed] [Google Scholar]
- [155].Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. The New England Journal of Medicine . 2019;380:1906–1917. doi: 10.1056/NEJMoa1813959. [DOI] [PubMed] [Google Scholar]
- [156].Wolsk E, Lamberts M, Hansen ML, Blanche P, Køber L, Torp-Pedersen C, et al. Thromboembolic risk stratification of patients hospitalized with heart failure in sinus rhythm: a nationwide cohort study. European Journal of Heart Failure . 2015;17:828–836. doi: 10.1002/ejhf.309. [DOI] [PubMed] [Google Scholar]
- [157].Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. The New England Journal of Medicine . 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- [158].Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circulation Research . 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- [159].Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. Journal of the American College of Cardiology . 2014;63:2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- [160].Sugumar H, Prabhu S, Voskoboinik A, Young S, Gutman SJ, Wong GR, et al. Atrial Remodeling Following Catheter Ablation for Atrial Fibrillation-Mediated Cardiomyopathy: Long-Term Follow-Up of CAMERA-MRI Study. JACC. Clinical Electrophysiology . 2019;5:681–688. doi: 10.1016/j.jacep.2019.03.009. [DOI] [PubMed] [Google Scholar]
- [161].Pump A, Di Biase L, Price J, Mohanty P, Bai R, Santangeli P, et al. Efficacy of catheter ablation in nonparoxysmal atrial fibrillation patients with severe enlarged left atrium and its impact on left atrial structural remodeling. Journal of Cardiovascular Electrophysiology . 2013;24:1224–1231. doi: 10.1111/jce.12253. [DOI] [PubMed] [Google Scholar]
- [162].Rivner H, Mitrani RD, Goldberger JJ. Atrial Myopathy Underlying Atrial Fibrillation. Arrhythmia & Electrophysiology Review . 2020;9:61–70. doi: 10.15420/aer.2020.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Cochet H, Scherr D, Zellerhoff S, Sacher F, Derval N, Denis A, et al. Atrial structure and function 5 years after successful ablation for persistent atrial fibrillation: an MRI study. Journal of Cardiovascular Electrophysiology . 2014;25:671–679. doi: 10.1111/jce.12449. [DOI] [PubMed] [Google Scholar]
- [164].Kronenberger R, Tanaka K, de Asmundis C, Meir M. Stiff left atrial syndrome with pulmonary veins occlusion after percutaneous radiofrequency ablation: a life-long complication that can lead to heart transplantation. Journal of Cardiothoracic Surgery . 2023;18:181. doi: 10.1186/s13019-023-02193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger FX, et al. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evidence . 2022;1:EVIDoa2200141. doi: 10.1056/EVIDoa2200141. EVIDoa. [DOI] [PubMed] [Google Scholar]